Abstract
The immune system plays a major role in cancer surveillance. Harnessing its power to treat many cancers is now a reality that has led to cures in hopeless situations where no other solutions were available from traditional anticancer drugs. These spectacular achievements rekindled the oncology community’s interest in extending the benefits to all cancers including breast cancer. The first section of this article reviews the biological foundations of the immune response to different subtypes of breast cancer and the ways cancer may overcome the immune attack leading to cancer disease. The second section is dedicated to the actual immune treatments including breast cancer vaccines, checkpoint inhibitors, monoclonal antibodies, and the “unconventional” immune role of chemotherapy.
Keywords: Breast cancer, immune dormancy, immunotherapy
Introduction
Breast cancer is the most common cancer in women with an estimated 268 670 new cases in the United States in 2018.1 It is also the second leading cause of cancer death in women. Fortunately, with advances in detection and treatment, death rates from breast cancer are declining. More recent advancements in breast cancer therapy using novel mechanisms involving actionable cancer mutations and the body’s immune system have opened up new avenues for reducing the death rate further. Many of the obvious successes in immunotherapy have been in the field of melanoma, renal cancer, lung cancer, and others that have traditionally been known to be immunogenic. However, these are not the only cancers in which strides in immunotherapy are being made. Breast cancer is one cancer that, although not originally thought to be immunogenic, has had many encouraging results in the past few years. We aim to provide a succinct overview of breast cancer immunotherapy as well as possible future directions.
Background
The basis for immunotherapy in cancer has revolved around the concept of immunogenicity. For a long time, breast cancer has been considered nonimmunogenic. However, the role of the immune system in the emergence of breast cancer has been firmly established.2-8 Random or inherited genetic and epigenetic abnormalities confer proliferative and/or survival advantages on certain cells. These incipient cancer cells face internal and external control mechanisms including those from the immune system. By targeting the new antigens created by these genetic changes, the immune system plays a central role in cancer control that can be host-protective or tumor promoting. A mutated gene leads to the production of a neoantigen when it is transcribed then translated, highlighting the autoantigenicity of self-antigens as observed in model protein antigens.9
Epitopes from the neoantigen are presented after processing by the mammary epithelial cells in association with major histocompatibility complex (MHC) class I (MHC-I) on their surface. When an antigen-presenting cell (APC) encounters a neoantigen released from debris of cancer cells or secreted in the environment, it internalizes it via several mechanisms including endocytosis. The antigen resurfaces again after processing on the MHC class II (MHC-II) receptors and can be recognized by helper T-cell receptors (TCR). Helper T (TH) cells stimulate and drive cytotoxic T cells (Tc) and B cells to further maturation. T-cell maturation, proliferation, and survival require antigen-independent costimulatory signals from APCs. If the costimulatory signal is lacking, then the process of activation will be ineffective and may lead to Tc anergy. Once activated, Tc can attack the target cell by several mechanisms, including TCR-MHC-I recognition and binding. This leads to secretion of cytotoxic granules including perforin that result in cell lyses and demise.10 Another mechanism by which Tc can attack target cells is via FAS receptors on Tc that bind FASL on the target cell leading to caspase 3 and 8 activation in the target cell and eventually apoptosis.11 To ensure effective immune regulation, the very same APCs that send costimulatory signals (through CD27, CD28, CD40, OX40, 4-1BB, GITR, and ICOS surface proteins on T cells) to intensify the activation of naïve T cells also send inhibitory signals (through CTLA-4, PD-1, and LAG-3) to the already activated T cells and natural killer (NK) cells when the immune response has to wind down. The activated T cell starts synthesizing CTLA-4, which has higher affinity for B7 and competes with the stimulatory B7-CD28 binding.12 This mechanism prevents overstimulation by transient T-cell activation (Figure 1).
Figure 1.
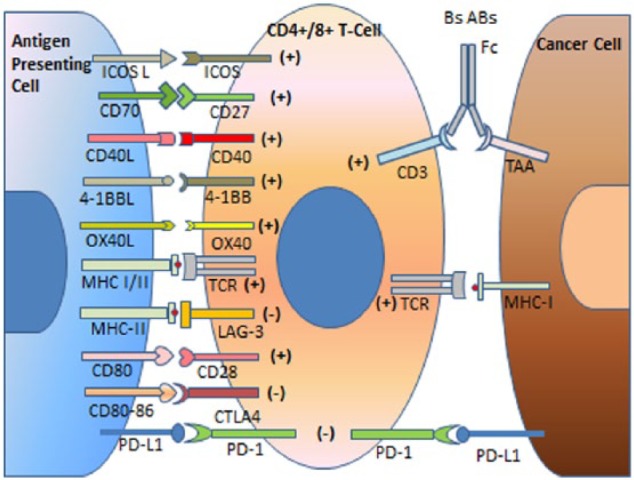
Antigen-presenting cells (APCs) process tumor-associated antigens (TAAs) and present them in combination with MHC surface proteins to T-cell receptor (TCR) on T-helper cells (MHC-II) and some CD8+ T cells (MHC-I). In the beginning of the immune reaction, costimulatory signals (CD27, CD28, CD40, OX40, 4-1BB, GITR, and ICOS surface proteins on T cells) help intensify the activation of naïve helper or cytotoxic T cells. As the immune reaction reaches its goal of eliminating the transformed cells, inhibitory signals (CTLA-4, PD-1, and LAG-3) help wind down T-cell activation. The expression of the inhibitory molecules by the transformed cells or other cells in the tumor microenvironment leads to local immunosuppression and the persistence of cancer cells. Inhibitory monoclonal antibodies (MAbs) targeting CTLA-4, PD-1, or PD-L1 have opened the way to a new era in IO. Targeting stimulatory pathways with agonist MAbs is being explored by multiple clinical trials (see below). Bispecific antibodies (BsABs) target T-cell surface receptors such as CD3 and TAA and recruit other effector cells through the Fc receptor such as macrophages or natural killer cells.
The interaction between the immune system and incipient cancer cells, also called immunoediting, goes through 3 phases: elimination, equilibrium, and escape.13-15 Elimination is supported by a wealth of experimental evidence in animals and humans. The innate and adaptive arms of the immune system recognize incipient cancer cells by the new antigens (resulting from mutations or translocations) presented on their surface in association with MHC-I or by the distress signals usually expressed by transformed cells that have undergone chromosomal changes (aneuploidy or hyperploidy)16,17 and eliminate them. Equilibrium is reached when the immune system fails to eliminate the transformed cells but stops them from progressing further. This can be conceived as the dormancy phase of primary cancer development. This phase is mediated by equilibrium between cells and cytokines that promote elimination (IL-12, IFN-γ, TNF-α, CD4 TH1, CD8+ T cells, NK cells, γδT cells) and those that promote persistence of the nascent tumor (IL-23, IL-6, IL-10, TGF-β, NKT cells, CD4 Th2, Foxp3+ regulatory T [Treg] cells, and MDSCs).18-20 Monocytes play an important role in this process. Under the influence of the tumor microenvironment (TME), they may differentiate into pro-inflammatory M1 or anti-inflammatory M2 types.21,22 Immune escape of cancer cells occurs by different mechanisms. In HR-positive breast cancer, the absence of strong tumor antigens and low expression of MHC-I allow the tumor to progress unnoticed by the immune system.23 Estrogen plays an immunosuppressive role in the TME that promotes tolerance of the weakly immunogenic cancer. Most immune cells including macrophages, T and B lymphocytes, and NK cells express estrogen receptor (ER).24 In presence of estrogen, the immune response is polarized to Th2− rather than Th1-effector immune response.25 In HER2-positive cancer cells, MHC-I presentation is inversely correlated with HER2 expression.26 Triple-negative breast cancers (TNBC) exhibit a spectrum of MHC-I presentation and strong tumor antigen expression, but immune escape in this subtype is mostly related to the development of the immunosuppressive TME (Tregs, MDSCs, PD-1/PD-L1).
Although it is true that single-transformed cells may remain dormant in inactive noncycling state, some transformed cells may continue dividing until they reach equilibrium between the newly produced cells and those undergoing cell death. These 2 models (of dormant single cells or a small cluster of transformed cells that remain microscopic and stay dormant) may also apply to disseminated tumor cells that land in a new environment and have to renegotiate a license to grow with the new TME.27
Factors promoting immune escape
Many factors play a role in tilting the balance established during the equilibrium phase toward tumor progression. Aging is associated with reduced production of new B and T lymphocytes in the bone marrow and the thymus, respectively, and with decreased function of the existing mature lymphocytes.28 Systemic inflammation associated with aging and the local pro-inflammatory microenvironment in the breast is incriminated in promoting the cancerous transformation of mammary stem cells that have been primed by losing tumor suppressor genes.20,29 Pro-inflammatory cytokines (TNF-α and IL-6) are associated with overexpression of COX2 and the aromatase enzyme,30 which lead to increased local concentrations of estrogens. Estrogens induce the expansion of Tregs and MDSCs, as well as the inhibition of antigen-presenting cells.31-34 In addition to the gradual decline of the immune system, dietary, commensal microbiota, use of antibiotics, procreational and hormonal factors, all play some role of variable importance in tilting the balance from equilibrium to escape.35-38
Immune escape, the angiogenic switch, and cancer cell dissemination
The immune escape and angiogenic switch may be closely connected. Tumor-associated macrophages (TAMs; and other immune and microenvironment cells) release proangiogenic factors that play an important role in triggering the angiogenic switch that leads to the expansion of the tumor mass (Figure 2).39
Figure 2.
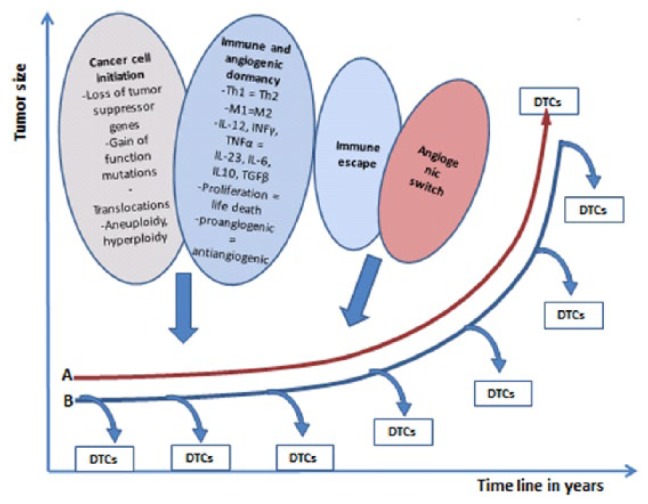
Immune and angiogenic dormancy in maintaining the cancer at a microscopic size. (A) Once the tumor undergoes immune escape and the angiogenic switch is turned on, the tumor grows locally and spreads metastases. In this model, DTCs are released at a later stage because DTCs do not gain access to the bloodstream until the tumor has acquired its own vasculature. (B) Another model stipulates that dissemination of cancer cells may occur very early in the beginning of the nascent cancer and continues throughout its growth and development. In this model, the role of immune escape is more important than the role of the angiogenic switch because microscopic tumors (and even in situ tumors) may spawn DTCs/micrometastasis before the angiogenic switch has taken place. DTCs indicate disseminated tumor cells.
Tumor progression is traditionally conceived in a linear fashion suggesting that once the tumor has undergone immune escape and turned the angiogenic switch on, it will grow locally and release cancer cells (disseminated tumor cells = DTCs) into the circulation (Figure 2A). Accumulating evidence suggests that DTCs may be released with the very early stages of transformation even during the in situ phase and before the tumor has established its own vasculature or before becoming clinically detectable (Figure 2B).40-42
Breast cancer stem cells and immune escape
Two models have been proposed to explain the emergence of breast cancer and its dissemination. The stochastic model stipulates that all cancer cells in a tumor are potentially capable of being tumor-initiating cells and the hierarchical model that stipulates the presence of a distinct cell population called cancer stem cells (CSCs; the concept of CSC is controversial43,44) endowed with the self-renewal capacity and tumor initiation function is necessary for the emergence and progression of cancer. The hierarchical model states that CSCs are capable of asymmetric division that gives rise to CSCs that join the stem cell compartment and to other cells that continue proliferating and differentiating allowing the expansion of the tumor mass while losing their self-renewal potential (transit-amplifying cells).45 Cancer stem cells are characterized by being metabolically quiescent and long-lived. They are responsible for the propagation and dissemination of cancer and resistance to classical cytotoxic treatments, contrary to the rest of tumor cells that are short-lived and possess limited proliferative capacity and are sensitive to cytotoxic agents.46-48 Recently, the discovery of phenotypic plasticity allows reconciling these 2 models by establishing the reversibility of differentiated cells to a stem cell phenotype in certain conditions. Based on the phenotypic plasticity model, stem cell function is the result of the interaction between the genetic make-up of a cancer cell and its microenvironment or niche.44
The immune system as a part of the CSC niche
Cancer stem cell niche is a distinct part of the TME.49 It plays a major role in protecting CSCs from the immune system and in maintaining their plasticity. The frequent success of transplantation experiments of cancer cells (regardless of their stemness characteristics or the size of the inoculate) in extremely immune-deficient models and their failure in immune-competent animals suggest a major role for the immune system in facilitating or hindering cancer cell growth, respectively.50
This fascinating concept suggests that the niche (of which the competence the immune system is an important part) shapes cancer cell stemness45 and determines the fate of dormant DTCs or microscopic tumors. Myeloid-derived suppressor cells (MDSCs), tumor- TAMs and tumor-associated neutrophils (TANs) recruited by cancer-released cytokines and chemokines participate in the local immune suppression in TME.51,52 The TAMs and TANs secrete TNF-α that induces the expression of Slug, Snail, and Twist transcription factors responsible for the induction of epithelial–mesenchymal transition in transformed epithelial cells that acquire CSC characteristics. Hypoxia, resulting from the increase in the tumor mass beyond certain size, has a CSC-protective effect against chemotherapy and radiotherapy. It activates the hypoxia-inducible factor 1α (HIF1α) pathway, vascular endothelial growth factor (VEGF), and TGF-β. The expression of TGF-β in the TME and the activation of WNT lead to an undifferentiated state in tumor cells,53 whereas the expression of VEGF stimulates tumor angiogenesis. The importance of the permissive role of the innate and adaptive immune systems in establishing distant metastases by DTCs is increasingly recognized.49
Assessment of Breast Cancer Immunogenicity
Traditional pathology and immunohistochemistry, gene expression profiling, RNA sequencing, and combined scores have been used to assess the immunogenicity of breast cancer. Traditional pathology tools allow the assessment of breast cancer immunogenicity by studying the presence of tumor-infiltrating lymphocytes (TILs) and assessing their types and correlation with survival and recurrence. While TILs were not found to have a prognostic value in the overall breast cancer population or ER-positive/human epidermal growth factor receptor 2–negative (ER+/HER2−) patients, TILs were found to have a prognostic value for disease-free survival (DFS) and overall survival (OS) in TNBC.54 In patients with TNBC who had residual disease after neoadjuvant chemotherapy, the presence of TILs was found to be associated with better OS as well as with metastasis-free survival. In ER-negative breast cancers, TILs, specifically CD8+ lymphocytes, were associated with better breast cancer–specific survival.54,55 The presence of CD8+ lymphocytes in patients with ER-negative breast cancers was also related to longer DFS.55 In general, the presence of TILs was positively correlated with MHC-I expression and inversely correlated with ER expression. The more immunogenic the breast cancer, the higher the concentration of TILs will be. Hence, it is not surprising that HR-positive breast cancer is considered the least immunogenic.
Mutational load and neoantigens
Recent advances in genomics and proteomics allow the detection of neoantigens that underlie immunogenicity in breast cancer and shed light on possible targets for therapy.56,57 Immunogenicity of a tumor is evaluated by the assessment of its antigenicity and the latter is evaluated by assessing its mutagenicity. Mutational load, the average number of somatic mutations per cancer cell, is associated with antigenicity and is, in general, lower in breast cancer compared with other tumors such as melanoma or lung cancer. However, major differences exist between different subtypes of breast cancer; TNBC has the highest mutational load compared with HR-positive breast cancers58,59 and high mutational load is associated with better prognosis in TNBC and HER2+ compared with low mutational load in the same type of breast cancer (see below). Conversely, higher mutational load is associated with higher concentrations of TILs and with poor prognosis in HR-positive breast cancer. Mutational load continues increasing in metastatic breast cancer (MBC) but TILs, PD-1, and PDL-1 expression decreases, very likely as a result of immune exhaustion and not because of decreased immunogenicity in advanced disease as suggested by Luen et al.60 Some specific mutations in DNA repair mechanisms such as those in the BRCA1/2 and MMR genes are associated with high mutational loads that can be localized (kataegis) or generalized.61,62 High mutational load is associated with high rates of neoantigens, which predict OS and response to checkpoint inhibitors.56,57,63-65
TILs and immune response
In assessing response to neoadjuvant treatment, the benefit of the presence of TILs can be seen here as well. Breast cancers with higher levels of TILs have better responses to neoadjuvant chemotherapy.7 In patients with HER2+ or TNBC, those with >60% TILs treated with an anthracycline plus taxane combination were more likely to have a pathologic complete response (pCR) and the rates of pCR were even higher when carboplatin was added to the treatment regimen.8 The ER-negative breast cancers that are lymphocyte-rich have far greater pCR rates when treated with neoadjuvant anthracycline-based chemotherapy compared with patients with lymphocyte-poor ER breast cancers.66 HER2+ breast cancers with TILs were associated with better DFS as well as OS in response to treatment with anthracyclines.2 There was a significantly associated decreasing risk of distant recurrence in patients being treated with adjuvant chemotherapy simultaneously with trastuzumab in HER2+ breast cancer for every 10% increase in TILs.3 Moreover, irrespective of whether or not a patient received systemic adjuvant chemotherapy, TILs and immune signatures were associated with better prognosis in HER2+ breast cancer.67 In patients with HER2 overexpression, a higher CD8+ infiltrate was seen after chemotherapy and this was associated with improved relapse-free survival.68
Strategies to Harness the Power of the Immune System
Several strategies have been used to harness the power of the immune system and redirect it to eradicate breast cancer or to induce immune dormancy:
Breast cancer vaccines;
Monoclonal antibodies (MAbs);
Antibody-drug conjugates (ADCs);
Checkpoint inhibitors;
Stimulatory molecule agonist antibodies;
Combination immunotherapy trials;
Enhance the immune-mediated effect of chemotherapy.
Breast cancer vaccines
Breast cancer vaccines are used for primary or secondary prevention and some are therapeutic. Several strategies have been used including peptide vaccines, recombinant protein vaccines, dendritic cell (DC) vaccines, whole tumor cell vaccines, DNA vaccines, and recombinant viral vectors vaccines.
They are all designed to stimulate an intrinsic antitumor response targeting tumor-associated antigens (TAAs). Tumor-associated antigens that are specifically recognized by T cells include HER2, mucin 1 (MUC1), carcinoembryonic antigen, sialyl-Tn (STn), human telomerase reverse transcriptase (hTERT), Wilms tumor gene (WT1), and tumor-associated carbohydrate antigens (TACAs).69 The antigens where current studies are primarily focused around include HER2, MUC1, and TACAs. DNA vaccines will not be discussed in this review.
HER2-targeted vaccines
As for the use of HER2 in vaccine developments, there have been a few attempts involving the E75, GP2, and AE37 peptides. Nelipepimut-S (NeuVax) is a combination of E75, a peptide from the extracellular domain of HER2 and granulocyte-macrophage colony-stimulating factor (GM-CSF); it stimulates cytotoxic T lymphocytes and CD8+ memory cells with high affinity for HLA-A2/A3. However, the immunity induced by the E75 vaccine waned after 6 months from initial vaccination requiring a booster given at 6 months from completion of the primary vaccination.70 NeuVax was tested in a phase I/II trial and showed improvement of DFS in HER2-positive breast cancer patients.71 The study enrolled 187 patients with early-stage breast cancer deemed at high risk for recurrence. Patients received 6 injections of NeuVax after tumor resection with standard of care (chemotherapy or radiation therapy [RT]) as indicated. The 5-year DFS was 89.7% for the vaccinated group versus 80.2% for the controls (P = .08). When the optimally dosed cohort was considered, DFS was increased to 94.8% versus 80.2% (P = .05). Apparently, the induction of cytotoxic T lymphocytes was crucial for the response to NeuVax as only 1 recurrence was observed in 30 patients (3%) who achieved cytotoxic T lymphocytes above the mean, compared with 8 of 56 (14%) for patients with levels of cytotoxic T lymphocytes below the mean.72 A phase III registration PRESENT trial is evaluating E75 in 758 early-stage, node-positive HLA-A2/A3 patients with low to intermediate HER2 expression with no evidence of disease after standard treatment. Patients are randomized to GM-CSF with E75 or GM-CSF with placebo, receiving 6 monthly injections, followed by a booster vaccination every 6 months for 3 years. The primary end point is DFS at 3 years.73
Work with the GP2 peptide is currently ongoing in a phase II clinical trial where vaccines containing GP2, a class I epitope derived from the HER2 transmembrane domain, is combined with GM-CSF and then compared with treatment of patients with GM-CSF only. Interim analysis presented in 2009 was already showing a decreased recurrence rate at 17.9 months in a group of patients treated with GP2 and GM-CSF (VG) versus GM-CSF alone (CG), 7.4% (2/27) compared with 13% (3/23), respectively (P = .65).74 At 34-month (1-60) median follow-up, DFS was compared in the intent to treat (ITT) (85% VG versus 81% CG, P = .57) and per treatment (PT) (94% VG versus 85% CG, P = .17) populations. In patients with HER2 overexpression (51 VG and 50 CG) DFS was 94% VG versus 89% CG, P = .86 (ITT) and 100% VG versus 89% CG, P = .08 (PT).75
The premise behind the AE37 vaccine is that it stimulates a CD4+ T-lymphocyte response that could potentially result in a more sustained immune response. The current data from clinical trials suggest that this vaccine has an effect on the risk of recurrence.76 The trial enrolled 298 patients: 153 received AE37 + GM-CSF and 145 received GM-CSF alone. At the time of the primary analysis, the recurrence rate in the vaccinated group was 12.4% versus 13.8% in the control group (relative risk reduction = 12%, hazard ratio [HR] = 0.885, 95% confidence interval (CI) = 0.472-1.659, P = .70). The Kaplan-Meier–estimated 5-year DFS rate was 80.8% in vaccinated versus 79.5% in control patients. In planned subset analyses of patients with IHC 1+/2+ HER2-expressing tumors, 5-year DFS was 77.2% in vaccinated patients (n = 76) versus 65.7% in control patients (n = 78) (P = .21). In patients with TNBC (HER2 IHC 1+/2+ and hormone receptor negative), DFS was 77.7% in vaccinated patients (n = 25) versus 49.0% in control patients (n = 25) (P = .12).77 Although the trial was negative for the whole population, the results in the triple-negative subset of patients were encouraging and warrant further investigation. Another approach to HER2-positive early breast cancer was the use of the patients’ stimulated DCs. Monocytic DC precursors harvested by apheresis are pulsed with 6 HER2 MHC class II–binding peptides then given back to the patients as injections into the lymph nodes, the breast lesions, or both, weekly for 6 weeks prior to surgery. A total of 54 patients (44 with ductal carcinoma in situ [DCIS] and 12 with invasive ductal carcinoma) were enrolled in the study. There was no difference in the immune response by injection site. Patients with DCIS reached higher rate of pCR (28.6%) compared with those with invasive cancer (8.3%).78 The failure of therapeutic vaccines and the success of this vaccine and other cancer prevention vaccines are attributed to the robustness of the immune system in the early stage or the preinvasive setting, making their use for breast cancer prevention in high-risk populations a promising next step.79
MUC1-targeted vaccine
The presence of high levels of antibodies to specific glycoforms of the MUC1 antigen has been shown to be associated with reduced rates and delay to metastasis in patients who have early-stage breast cancer.80 One of these particular glycoforms, STnMUC1, has already been used in a phase III trial in the form of the vaccine Theratope (STnMUC1, keyhole limpet hemocyanin, and the adjuvant Detox-B). Given as a single agent, Theratope did not show any improvement in survival. However, when given along with endocrine therapy, there was a demonstrated improvement in time to progression and OS.81 The reactivity of antibodies to MUC1 glycoforms might still be deceptive and can be related to an artifact rather than a true immune response to MUC1. The example of anti-Gal alpha (1,3) Gal antibodies is instructive. These antibodies are observed to react with mucin 1 (MUC1) found on the surface of human breast cancer cells.82 Natural occurring anti-Gal alpha (1,3) Gal antibodies found in all human serum can react with self-peptides (MUC1) expressed in large amounts on the surface of tumor cells, but not on normal cells. These findings are of interest and serve to explain reported findings that human cells can, at times, express Gal alpha (1,3) Gal; in reality, such expression is suggested as an artifact in that anti-Gal alpha (1,3) Gal antibodies react with mucin peptides.82 However, some antibodies display exquisite specificity, like those directed toward the Thomsen-Friedenreich (TF) antigen.83 The TF antibodies may arise in the postpartum period against carbohydrate structures expressed on the cell walls of the gastrointestinal flora and, presumably, may provide an early barrier against TF-carrying tumor cells.
Tumor-associated carbohydrate antigens
The widely used regimen of neoadjuvant chemotherapy is demonstrated to stimulate the immune response to TACA in some patients.84 Small retrospective studies have suggested that postchemotherapy lymphocyte infiltrates could be associated with better outcomes in patients who did not reach pCR.84 The high levels of anti-TF antibody before surgery is another example in which antibody targeting is associated with a better survival of patients with stage II breast cancer.85 This may indicate that the selection of immunopotentiating regimens of neoadjuvant chemotherapy might be beneficial for the host in conjunction with the functional activity of natural anticancer antibodies.
Because tumor tissue rejection is the goal of cancer immunotherapies, broad-spectrum TAAs, such as TACAs, are plausible targets once the problem of their low immunogenicity is solved.86 The fact that multiple proteins and lipids on the cancer cell are modified with the same carbohydrate structure creates a powerful advantage for TACAs as cancer targets in immunotherapy strategies. Thus, targeting TACAs has the potential to broaden the spectrum of target pathways recognized by the immune response, thereby lowering the risk of developing escape variants due to the loss of a given protein or carbohydrate antigen. Although TACAs are poor immunogens, certain investigators succeeded in eliciting cytotoxic antibodies reactive with naturally occurring forms of TACA using molecular mimicry to generate peptide mimotopes of TACA (carbohydrate mimetic peptides [CMPs]). Vaccination of mice with TACA peptide mimotopes reduced tumor growth and prolonged host survival in a murine tumor model.87 The first reports of this strategy in humans are promising and trials exploring their role in different types of breast cancer are underway.88
Multivalent vaccines comprising 2 or more candidate proteins are considered to substantially enhance the efficacy of vaccination against breast tumors. The enhancement in antitumor effect using a multivalent vaccination approach would be achieved on 2 levels: (1) by increasing the strength of immune response against arising tumor due to activation of a larger T-cell repertoire comprising multiple T-cell lineages reactive to more than one tumor-specific target and (2) by covering a broader range of tumors, including those that do not express the target protein by a univalent vaccination approach such as HER2 or MUC1. In addition, a multivalent vaccine will have the potential to target tumors that have lost or downregulated expression of one or more proteins or acquired expression of alternate proteins due to transcriptional dysregulation during their evolution from normal to dysplastic, to carcinoma in situ, to invasive, and to metastatic stages of breast tumor evolution. In other words, a multivalent vaccine approach could apply greater multitarget immunological pressure both on early and evolving tumors. It will thereby cover a larger tumor variety and increase efficacy of prevention as well as provide more effective therapy by lowering the probability of tumor escape and generation of resistance to the vaccine. Such approaches are heading to the clinic.
In contrast to a multivalent approach, a pan-immunogen that elicits responses to several antigens but as a univalent vaccine can achieve the same end as a multivalent vaccine. Tumor-associated carbohydrate antigens are among the most challenging of clinical targets for cancer immunotherapy, but this difficulty can be overcome by CMPs. Carbohydrate mimetic peptides are sufficiently potent to activate broad-spectrum antitumor reactivity. However, the activation of immune responses against terminal mono- and disaccharide constituents of TACA raises concerns regarding the balance between “tumor destruction” and “tissue damage,” as mono- and disaccharides are also expressed on normal tissue. To support the development of CMPs for clinical trial testing, we have demonstrated in preclinical safety assessment studies in mice that vaccination with CMPs can enhance responses to TACAs without mediating tissue damage to normal cells expressing TACA89 and are pursuing such an approach in multiple phase II trials. Particularly important is that these CMP-induced antibodies can overcome resistance to anoikis and drug resistance against breast cancer and enhance the efficacy of taxanes. This aspect might suggest that immunization with such CMPs can change the clinical paradigm in the neoadjuvant/adjuvant setting.
Monoclonal antibodies
Monoclonal antibodies are an integral part of our armamentarium in the fight against cancer. They can be divided into those that target the immune system (checkpoint inhibitors) and those that target oncogenic membrane receptors (HER2) or other surface molecules of unknown function (CD20). Trastuzumab is a standard component of the treatment of HER2-positive breast cancer. Its development in the 1990s was considered a landmark achievement in the field of targeted therapy. When combined with chemotherapy, it improves progression-free survival (PFS) and OS in metastatic HER2-positive breast cancer and DFS and OS in early-stage HER2-positive breast cancer. These conclusions have been consistently proven by multiple phase III clinical trials in the metastatic and adjuvant settings.90-95 First generation of small-molecule tyrosine kinase inhibitors (TKIs) were not found effective in the adjuvant setting, whereas second-generation TKIs had modest activity in the adjuvant setting (see below). The failure of TKIs to make a significant difference in the outcomes of these patients suggests that blocking the oncogenic stimulation of HER2 might not be the main mechanism of action of HER2-targeting MAbs.
Indeed, trastuzumab’s mechanism of action remains elusive. It targets HER2 and leads to its internalization and degradation. It inhibits downstream signaling pathways leading to decreased proliferation and increased apoptosis of cancer cells. Recently, its role in activating the immune system against tumor cells emerged as the main mechanism of action. The FinHer investigators found that every 10% increase in TILs was associated with decreased distant recurrence2 and other studies found that TILs had a prognostic and predictive value as their presence predicted for higher pCR to trastuzumab-containing chemotherapy and better DFS.19,96 A meta-analysis of neoadjuvant RCTs showed that the pCR rate was significantly higher in patients with lymphocyte-predominant breast cancer in HER2-positive breast cancer settings, with an absolute difference of 33.3% (95% CI = 23.6%-42.7%).97
The nature of tumor-infiltrating immune cells is more important than the mere presence or absence of TILs. Using CIBERSORT (leukocyte gene matrix LM22) to characterize immune cell composition of 7270 unrelated breast cancer samples from their gene expression profiles, Bense et al showed that the composition of the immune cell types differed per breast cancer subtype and interacted with the treatment. Increased fraction of Treg cells in HER2-positive tumors was associated with a lower pCR rate (odds ratio [OR] = 0.15) as well as shorter DFS (HR = 3.13) and OS (HR = 7.69). Increased fraction of γδT cells in all patients with breast cancer was associated with a higher pCR rate (OR = 1.55), prolonged DFS (HR = 0.68) and, in HER2-positive tumors, with prolonged OS (HR = 0.27). A higher fraction of activated mast cells was associated with worse DFS (HR = 5.85) and OS (HR = 5.33) in HER2-positive tumors. Furthermore, a high CD8+ T-cell exhaustion signature score was associated with shortened DFS in patients with ER-positive tumors regardless of HER2 status (HR = 1.80).98
The implications of these findings are substantial. Sorting out the antioncogenic from the immune stimulating roles of trastuzumab may be very difficult. However, the available data from the ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization) study suggest that interrupting HER2 downstream signaling using lapatinib does not add any benefit in early-stage breast cancer.99 The role of extended HER2 inhibition with neratinib, a second-generation HER2-targeting TKI, after completing trastuzumab therapy in the patients with HER2-positive breast cancer was tested in the ExteNET trial. This trial showed a modest benefit of 2% improvement in invasive DFS leading the Food and Drug Administration (FDA) to approve the drug.100 It is not clear whether all TKIs will behave like lapatinib and neratinib. The challenge for future development of novel drugs is to capitalize on the immune mechanism.
Antibody-drug conjugates
Antibody-drug conjugates are MAbs targeting a cancer-specific antigen and are linked to a payload of a cytotoxic drug by a linker.101 Antibody-drug conjugates are stable in the systemic circulation and most of the drugs in use to date have cleavable linkers that, after enzymatic cleavage or the exposure to a reduced pH (potential of hydrogen) or reduction by cytosolic thiols, release the cytotoxic drug inside the antigen-expressing cells.102 Antibody-drug conjugates using noncleavable linkers require a thorough catabolism in the lysosomes leading to the release of their cytotoxic drug that should exit the lysosome to cause cell death. The example of the latter is TDM1. The most commonly used payloads target either tubulins or DNA. TDM1 employs DM1, a strong microtubule inhibitor of the maytansinoid class. The selection of the surface targets depends on their high expression on cancer cells and low or no expression on normal cells. Rapid internalization of the ADCs after antibody binding is a prerequisite to their activity.
TDM1 or ado-trastuzumab emtansine is now FDA approved for patients with HER2-positive MBC whose disease has progressed on trastuzumab and a taxane based on the results of the EMILIA (emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer) trial. The trial randomly assigned the 991 patients enrolled in the study to capecitabine and lapatinib (control, n = 496) or trastuzumab emtansine (n = 495). The study had 2 coprimary end points: PFS and OS. Both end points were reached and were statistically superior in patients receiving ado-trastuzumab emtansine (difference in PFS medians of 3.2 months, HR = 0.65 [95% CI = 0.55-0.77], P < .0001 and difference in OS medians of 5.8 months, HR = 0.68 [95% CI = 0.55-0.85], P = .0006).103 Thrombocytopenia (14%), increased aspartate aminotransferase (5%), and anemia (4%) were the most frequently reported grade 3 adverse events on the TDM1 arm and diarrhea (21%) followed by palmar-plantar erythrodysesthesia syndrome (18%) in the control arm. Despite the success of TDM1 in HER2-positive breast cancer, 50% of the patients did not respond on the EMILIA trial. To meet the need of those patients, other ADCs targeting HER2 are being investigated. Three ADCs targeting HER2 are using trastuzumab, DS-8201a (drug/target: exatecan/topoisomerase I), SYD985 (drug/target: duocarmycin/DNA), and ADCT-502 (drug/target: pyrrolobenzodiazepine dimer/DNA). The other ADCs targeting HER2 are using different MAbs and different drugs and targets.102 Three ADCs are being developed for TNBC (Table 1).
Table 1.
ADCs in development for triple-negative breast cancer.
| Name | ADC target | Drug class/target | Latest development stage | Sponsor/trial ID |
|---|---|---|---|---|
| Sacituzumab govitecan IMMU-132 | Trophoblast cell surface antigen 2 (TROP2) | Irinotecan/topoisomerase I | Metastatic TNBC Phase III |
Immunomedics NCT02574455 |
| Glembatumumab vedotin CDX-011 CR011-vc-MMAE | Glycoprotein nonmetastatic b (GPNMB) | Auristatin/tubulin | Metastatic TNBC Phase II |
Celldex Therapeutics NCT01997333 |
| SAR566658 anti-CA6-DM4 | CA6 sialoglycotope of MUC1 | Maytansinoid/tubulin | Metastatic TNBC Phase II |
Sanofi NCT02984683 |
Abbreviations: ADC, antibody-drug conjugate; TNBC, triple-negative breast cancer.
Checkpoint inhibitors
Targeting programmed death-1 and programmed death-ligand 1 (PD-1/PD-L1) in breast cancer appears increasingly appealing after the success of such an approach in other cancers. The PD-1 receptor inhibits innate and adaptive immunity when upregulated on immune cells and engaged by its ligand, PD-L1.104 Cancers take advantage of this mechanism to induce a local immunosuppression by overexpressing PD-L1. The prognostic significance of PD-L1 is still unclear, as some studies have described its value as a positive and other as negative prognostic factor.105,106 Regardless, the inhibition of the PD-1/PD-L1 pathway is based on the idea of “inhibiting the inhibition” of the immune system. The agents being tried in breast cancer draw from those already being used in melanoma and other malignancies including nivolumab and pembrolizumab (anti-PD-1 antibodies) and atezolizumab (PD-L1 inhibitor). Currently, results from a phase Ib study in heavily pretreated patients with TNBC who received pembrolizumab demonstrated an acceptable toxicity and good safety profile.107 The KEYNOTE-086 trial is a phase II study with pembrolizumab in patients with metastatic TNBC as first-line (cohort B; n = 52; 100% were positive for PD-L1 expression) and subsequent line therapies (cohort A; n = 170; 60% were positive for PD-L1 expression).108,109 Overall response rate (ORR) was 4.7% in cohort A and 23% in cohort B. The 1- and 2-year OS rates were 37% and 18% in cohort A and 63% and 47% in cohort B, respectively. Similar results for ORR were obtained using single-agent atezolizumab in frontline (first line 23%) and subsequent line settings (second line 4% and third line 8%).110
Combinations of checkpoint inhibitors with chemotherapy were found to be promising in preclinical studies. The combination of pembrolizumab and eribulin was tested in a phase Ib/II study in 39 patients with TNBC, half of them were chemo-naïve and 43.6% were PD-L1 positive.111 Overall response rate with the combination was 33.3% (95% CI = 19.5%-48.1%). The CR rate was 2.6%. Stable disease for ⩾8 weeks was seen in 28.2% of patients. KEYNOTE-355, a phase III trial is underway to explore the safety and efficacy of pembrolizumab plus chemotherapy as first-line therapy in 858 patients with locally recurrent inoperable or metastatic TNBC (NCT02819518).112 The combination of atezolizumab and nab-paclitaxel in first-line metastatic TNBC showed an ORR at 46% with 8% of patients achieving complete response.113 Based on the results of this study, a phase III trial was launched (NCT02425891).114 When pembrolizumab was added to standard neoadjuvant chemotherapy using anthracycline and paclitaxel, it increased pCR rates from 20% (15/21) in the control arm to 71% (16/83) in the pembrolizumab arm in a group of patients with TNBC.111
More trials using PD-1/PD-L1 inhibitors are being planned in TNBC as this is the breast cancer subtype in which PD-1+ TILs and PD-L1+ cancer cells are more commonly seen.115 A randomized, phase III trial to evaluate the efficacy and safety of pembrolizumab as adjuvant therapy for TNBC with ⩾1 cm residual invasive cancer or positive lymph nodes (ypN+) after neoadjuvant chemotherapy started accruing patients in November 2016.116
CTLA-4 is another immune checkpoint that is being targeted in breast cancer. Similar to the PD-1/PD-L1 inhibitors, most ongoing clinical trials involving CTLA-4 generally revolve around melanoma. Ipilimumab is a CTLA-4 MAb FDA approved for the treatment of unresectable melanoma.117 It is currently being used in a phase I study examining its safety in combination with a new anti-B7-H3 mAb, enoblituzumab, to patients with multiple refractory cancers, including TNBC.118 Ipilimumab is also being combined with entinostat and nivolumab in a phase I study for metastatic HER2-negative breast cancer as well as with just nivolumab in a phase II study for patients with recurrent stage IV HER2-negative breast cancer.119 There are other ongoing trials evaluating the combination of a CTLA-4 inhibitor, with additional treatments. There is a phase II study of tremelimumab (CTLA-4 inhibitor) with a PD-L1 inhibitor, MEDI4736, in patients with HER2-negative breast cancer to look for the safety and efficacy of this regimen.120 A phase I study has already been completed with the combination of tremelimumab and exemestane in patients with hormone-responsive advanced breast cancer.121 Besides demonstrating that this treatment regimen is tolerable, the study showed that there was an associated increase in T cells with inducible costimulators (ICOS) and that more of the patients with stable disease tended to express higher levels of ICOS+ T cells versus the patients with progressive disease.121 CTLA-4 inhibitors have been evaluated in combination with other interventions as well. A phase I trial evaluating preoperative intervention in the form of ipilimumab and/or cryoablation in early-stage breast cancer showed these treatments to be safe and tolerable and plans are being made for a phase II trial with this regimen.122
Future development of these treatments should balance their benefit with their potential toxicity. CTLA-4 mAbs have been shown to have immune-related adverse events mostly affecting the skin and gastrointestinal tract.117 Other toxicities include hepatitis, thyroiditis, colitis, and hypophysitis.123 Compared with treatments targeting CTLA-4, therapies targeting PD-1/PD-L1 appear to have a lower frequency of immune-related adverse events.124 The combinations of anti-PD-1/PD-L1 mAbs and anti-CTLA-4 mAbs are more effective than single agents but they may be associated with increased incidence of pneumonitis that responds to holding the drug and/or using immunosuppressive agents; the rate of pneumonitis was 5% in one study.125
Lymphocyte activation gene-3 (LAG-3) is expressed on activated T cells, NK cells, and DCs and has more affinity for the MHC-II than CD4. It suppresses activation and proliferation of these cells allowing the immune reaction to wind down. IMP321 is a soluble form of LAG-3. It prevents the inhibition of LAG-3–bearing cells. It was evaluated in combination with paclitaxel in patients with MBC in a phase I/II trial. Overall response rate was 50% and clinical benefit was 90% at 6 months.126 A randomized phase II trial is enrolling (NCT02614833).
Stimulatory molecule agonist antibodies
The optimal immune response requires the engagement of costimulatory receptors expressed by CTLs, NK cells, CD4+T cells, or APCs. The most relevant receptors are CD27, CD28, CD40, OX40, 4-1BB, GITR, and ICOS. In addition to activating the proliferation and function of the cells carrying these receptors, their activation is associated with suppression of Tregs. The MAbs and fusion proteins were produced to target these receptors and many of them are now in phase I or II trials.127
MEDI6469, OX40 agonist MAb with strong stimulatory activity of T cells, is now in clinical investigation. It is being studied with stereotactic body RT in patients with breast cancer with metastatic lesions.128
4-1BB:4-1BBL is expressed on T cells and APCs, respectively. The engagement of the receptor by its ligand leads to stimulation of CD8+ T cells and NK cells with increased ADCC (antibody-dependent cell-mediated cytotoxicity), pro-inflammatory cytokines, and cytolytic activity.129 Urelumab (BMS-663513), a 4-1BB agonist MAb, was found to have a synergistic effect with trastuzumab in murine xenotranplant model of human breast cancer.130
CD40 can be expressed on immune cells and breast cancer cells. CD40 is expressed on APCs and its ligation is necessary to increase their antigen presentation and cytokine production that are culminated by increased T-cell activation.131 Although increased cytoplasmic expression of CD40 is the highest in hormone receptor–positive breast cancer,132 its membrane expression is higher on TNBC cell lines such as MDA-MB 231.133 The use of recombinant human CD40 ligand (rhCD40L) in cell culture of a TNBC cell line inhibited proliferation and synergized with the addition of interferon gamma and doxorubicin.134 Hence, CD40 agonist antibodies may exert their antitumor effect directly by targeting cancer cells and indirectly by activating the effector arm of the immune system targeting the tumor.131
Combination immunotherapy trials with other therapies
The PD-1 MAbs (nivolumab and pembrolizumab) and PD-L1 MAbs (atezolizumab, durvalumab, and avelumab) are being tested in many combination clinical trials (Tables 2 and 3). Some trials are exploring combinations with chemotherapy and others with biological agents targeting HER2-positive or hormone receptor–positive breast cancers. However, most of these studies are designed for TNBC due to its known immunogenicity and results from single-agent checkpoint inhibitors that showed efficacy in this subtype. Likewise, many IO (immuno-oncology) combination trials have been designed in the neoadjuvant and adjuvant settings using different combinations of checkpoint inhibitors with other checkpoint inhibitors, with chemotherapy, cytokines, or with vaccines (Table 4).
Table 2.
Ongoing PD-1 MAb combination trials in MBC.
| Study phase | Immunotherapy type | Other therapies | Breast cancer type | Clinicaltrials.org number |
|---|---|---|---|---|
| PD-1 | ||||
| I | Nivolumab | Nab-paclitaxel ± gemcitabine or carboplatin | MBC, pancreatic cancer, and NSCLC | NCT02309177 |
| II | Nivolumab | Cabozantinib (MET inhibitor) | TN-MBC | NCT03316586 |
| Ib/II | Pembrolizumab | Eribulin | TN-MBC | NCT02513472 |
| II | Pembrolizumab | Carboplatin | MBC | NCT03213041 |
| II | Pembrolizumab | Anthracycline or endocrine therapy | HR+ and TN-MBC | NCT02648477 |
| II | Pembrolizumab | Nab-paclitaxel | TN-MBC | NCT02752685 |
| Expl. | Pembrolizumab | Nab-paclitaxel and carboplatin | TN-MBC | NCT03121352 |
| II | Pembrolizumab | Eribulin | HR+ MBC | NCT03051659 |
| II | Pembrolizumab | Cyclophosphamide | TN-MBC | NCT02768701 |
| II | Pembrolizumab | With carboplatin versus carboplatin alone | Chest wall breast cancer | NCT03095352 |
| II | Pembrolizumab | Capecitabine | TN-MBC | NCT03044730 |
| Expl. | Pembrolizumab | Paclitaxel | HR+ and TN-MBC | NCT03018080 |
| II | Pembrolizumab | IBC | NCT02411656 | |
| II | Pembrolizumab with | Vorinostat and tamoxifen | HR+ MBC | NCT02395627 |
| II | Pembrolizumab | Chemotherapy | TNBC | NCT02734290 |
| II | Pembrolizumab | Carboplatin and gemcitabine | TN-MBC | NCT02755272 |
| II | Pembrolizumab | XRT | TN-MBC | NCT02730130 |
| II | Pembrolizumab | Imprime PGG | TN-MBC and metastatic melanoma | NCT02981303 |
| II | Pembrolizumab | BRCA-mutated MBC | NCT03025035 | |
| II | Pembrolizumab | XRT | HR+ MBC | NCT03051672 |
| II | Pembrolizumab | Selective androgen receptor modulator (SARM) GTX-024 | TN-MBC | NCT02971761 |
| II | Pembrolizumab | Abemaciclib | HR+ MBC | NCT02779751 |
| Ib | Pembrolizumab | TDM1 | HER2+ MBC | NCT03032107 |
| II | Pembrolizumab | BGB324 | TN-MBC or LABC | NCT03184558 |
| II | Pembrolizumab | Letrozole and palbociclib | HR+ MBC | NCT02778685 |
| I/II | Pembrolizumab | Binimetinib | TN-MBC or LABC | NCT03106415 |
| I | Pembrolizumab | JAK2 inhibition | TNBC | NCT03012230 |
| II | Pembrolizumab | BGB324 | TN-MBC or LABC | NCT03184558 |
| II | Pembrolizumab | Letrozole and palbociclib | HR+ MBC | NCT02778685 |
Abbreviations: IBC, inflammatory breast cancer; LABC, locally advanced breast cancer; MBC, metastatic breast cancer; NSCLC, non–small-cell lung cancer; TNBC, triple-negative breast cancer; TN-MBC, triple-negative metastatic breast cancer.
Table 3.
Ongoing PD-L1 MAb combination trials in MBC.
| Study phase | Immunotherapy type | Other therapies | Breast cancer type | Clinicaltrials.org number |
|---|---|---|---|---|
| PD-L1 | ||||
| II | Atezolizumab | Cobimetinib (MEK inhibitor) | MIBC | NCT03202316 |
| II | Atezolizumab | Carboplatin | TN-MBC | NCT03206203 |
| IIA | Atezolizumab | Paclitaxel, trastuzumab, and pertuzumab | HER2+ MBC | NCT03125928 |
| III | Atezolizumab | Chemotherapy | TN, recurrent LABC, or MBC | NCT03371017 |
| Ib | Atezolizumab | TDM1 or TP | HER2+ BC | NCT02605915 |
| II | Atezolizumab | Veliparib either alone or in combination | HDR-deficient TNBC | NCT02849496 |
| Ib/II | Atezolizumab | With or without entinostat | TN-MBC | NCT02708680 |
| I | Durvalumab | Hypofractionated XRT and tremelimumab | MBC, metastatic lung, melanoma, and pancreatic cancer | NCT02639026 |
| I/II | Durvalumab | Olaparib or cediranib | TN-MBC or LABC, metastatic lung, prostate, and CRC | NCT02484404 |
| II | Avelumab | Palbociclib and fulvestrant | HR+/HER2− MBC, after CDK and endocrine therapy (PACE) | NCT03147287 |
Abbreviations: CRC, colorectal cancer; LABC, locally advanced breast cancer; MBC, metastatic breast cancer; NSCLC, non–small-cell lung cancer; TNBC, triple-negative breast cancer; TN-MBC, triple-negative metastatic breast cancer.
Table 4.
Ongoing neoadjuvant and adjuvant combination immune-oncology trials in breast cancer.
| Study phase | Immunotherapy type | Other therapies | Breast cancer type | Clinicaltrials.org number |
|---|---|---|---|---|
| PD-1 | ||||
| Expl. | Pembrolizumab | Nab-paclitaxel | HR+ BC | NCT02999477 |
| III | Pembrolizumab | CTx | TNBC | NCT03036488 |
| III | Pembrolizumab | CTx | TNBC | NCT02819518 |
| II | Pembrolizumab | Hormonal therapy | Adjuvant HR+ | NCT02971748 |
| II | Pembrolizumab | Decitabine | HR+/HER2− TNBC |
NCT02957968 |
| PDL-1 | ||||
| II | Atezolizumab | Carboplatin and paclitaxel | TNBC | NCT02883062 |
| Expl. | Durvalumab | Tremelimumab | HR+ | NCT03132467 |
| I/II | Durvalumab | Weekly nab-paclitaxel and ddAC | TNBC | NCT02489448 |
| II | MPDL3280A | Nab-paclitaxel | TNBC | NCT02530489 |
| III | Atezolizumab | Paclitaxel | TNBC | NCT03125902 |
| III | Atezolizumab | CTx | TNBC | NCT03281954 |
| III | Atezolizumab | Anthracycline/nab-paclitaxel | TNBC | NCT03197935 |
| Cytokines | ||||
| Ib/II | IRX 2 | Preoperative early-stage BC | NCT02950259 | |
| Expl. | Anakinra (IL-1R antagonist) + | Chemotherapy + dendritic cell vaccine | TNBC | NCT02018458 |
| Vaccine | ||||
| I/II | Talimogene laherparepvec | CTx | TNBC | NCT02779855 |
| I | Personalized polyepitope DNA vaccine | Adjuvant TNBC with persistent disease following neoadjuvant chemotherapy |
NCT02348320 | |
| I | Personalized synthetic long-peptide breast cancer vaccine | Adjuvant TNBC with persistent disease following neoadjuvant chemotherapy |
NCT02427581 | |
| II | Herceptin and the HER2 vaccine E75 | Adjuvant HER2+ BC |
NCT01570036 | |
| III | Pembrolizumab | Adjuvant TNBC |
NCT02954874 | |
| I/II | Pembrolizumab ± XRT | TNBC | NCT02977468 | |
| II | Nivolumab | Ipilimumab | BC (also in ovarian and gastric cancer) | NCT03342417 |
| IB | Mammaglobin-A DNA vaccine | Neoadjuvant hormonal therapy | HR+ BC | NCT02204098 |
| Ib | PVX-410 vaccine | Alone and in combination with durvalumab | Adjuvant in stage II and III TNBC | NCT02826434 |
| II | Nelipepimut-S + GM-CSF (NeuVax) | Trastuzumab | HER2+ BC (high risk) | NCT02297698 |
Abbreviation: TNBC, triple-negative breast cancer.
The immune-mediated effect of chemotherapy
Traditionally, the effect of chemotherapy has been explained by the induction of apoptosis of cancer cells after interrupting their cell cycle apparatus. However, alternative mechanisms involving the immune system have been recently invoked.135,136 Taxanes, doxorubicin, and cyclophosphamide, which are standard chemotherapeutic agents in the treatment of breast cancer, are known to have major effects on the immune system in animals and human experiments.136-141 For example, taxanes, as a class, increase serum IFN-γ, IL-2, IL-6, and GM-CSF levels as well as reducing the levels of IL-1 and TNF-α.142 Paclitaxel given neoadjuvantly increases the levels of TILs within the tumor itself.143
The immune effects of chemotherapy may be summarized by (1) rendering dying cancer cells more visible to the immune system by exposing their TAAs, (2) stimulating the innate immune system, (3) stimulating T-cell differentiation, (4) promoting a cytokine profile that increases the likelihood of TH1 polarization, (5) inhibition of MDSCs and M2 macrophages, and (6) suppression of FOXP3+ Treg cells.141 Acknowledging these mechanisms is of major importance to optimize their benefit and minimize toxicity to the immune system that becomes an important executioner of chemotherapy effect. Furthermore, integrating chemotherapy with vaccines or checkpoint inhibitors is promising.144,145
Conclusions and Future Directions
Immunogenicity of breast cancer is subtype dependent with a spectrum that spans from the most immunogenic to the nonimmunogenic subtypes. On one hand, TNBC is the most immunogenic with high mutation and neoantigen load and high MHC-I expression. The immune system is already activated against the cancer as attested by the high TILs, but the cancer is counterattacking by creating an immune suppressive environment (Tregs, MDSCs) or expressing checkpoint immune inhibitory molecules (CTLA-4, PD-1/PD-L1). On the other hand, luminal A is the least immunogenic with the lowest mutation and neoantigen load and the loss or downregulation of the expression of TAAs. MHC-I expression is significantly reduced or absent. Hence, infiltration with TILs is minimal if any. High local concentrations of estrogen stimulate growth and maintain a local immune suppression by attracting Tregs and MDSCs. The other breast cancer subtypes fall in between these 2 extremes.
The overall goal of cancer immunotherapy is the activation of the immune system against the cancer. Vaccination has traditionally been to boost the latent immune response to tumor-specific antigens. Approaches have included cell-based protocols involving immunization with whole autologous or allogeneic tumors, as well as antigen-based strategies involving immunization with proteins or peptides overexpressed in tumors and underexpressed in normal tissues. HER2 and MUC1 are the predominant antigens used in human breast cancer vaccine trials. Although vaccination using these antigens may demonstrate tumor-reducing effects, neither antigen provides any tissue or tumor specificity because both are expressed in a variety of normal tissues and tumors raising concerns about the possibility of off target damage if a robust immune response is developed. However, despite the lack of inherent tissue specificity of HER2 and MUC1, these concerns about systemic autoimmune sequelae have not been substantiated so far. Tumor-associated carbohydrate antigens are pan-immunogens that elicit responses to several antigens, thus achieving the same goal as a multivalent vaccine. To overcome their low immunogenicity, investigators have used CMPs that seem to elicit a broad-spectrum antitumor reactivity. Here again, the activation of immune responses against TACAs raises concerns regarding the balance between “tumor destruction” and “tissue damage,” as TACAs are also expressed on normal tissues. The evidence gleaned from phase I and II trials is reassuring. It is not clear which subtype of breast cancer would benefit from this approach.
Monoclonal antibodies are an integral part of our armamentarium in the fight against cancer. They can be divided into those that target the immune system and those that target oncogenic membrane receptors (HER2) or other surface molecules of unknown function (CD20). Anti-HER2 antibodies have changed the outlook of this disease. The failure of small molecules that inhibit the oncogenic stimulation of HER2 and the lack or minimal response to these antibodies in tumors that lack TILs suggest that their action is more immune mediated than oncogenic mediated.
Monoclonal antibodies that inhibit checkpoints (checkpoint inhibitors) are changing the paradigm of care in many solid tumors. The first results of their use in breast cancer suggest that they are the most effective in TNBC. Their use is being investigated in the other subtypes. Due to the low immunogenicity of luminal A and B breast cancers, a combination strategy using vaccines to stimulate the immune response followed by checkpoint inhibitors is rational but its clinical usefulness remains to be proven.
Finally, the immune mechanism of chemotherapy is being increasingly recognized. Its contribution in the total effect of chemotherapy relative to the direct cytotoxic effect is not known. Any further development of chemotherapy in the future should take this aspect into consideration to maximize the immune stimulatory effect and minimize the immune suppressive effect of chemotherapy.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by funds from the Laura F Hutchins, MD, Distinguished Chair for Hematology and Oncology.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: IM: study design, writing, figure design, literature review, manuscript editing.MA: writing, literature review.AA: writing, literature review.TK-E: writing, manuscript editing.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 3. Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 4. Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–2966. doi: 10.1200/jco.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dieci MV, Criscitiello C, Goubar A, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25:611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 8. Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 9. Kieber-Emmons T, Kohler H. Evolutionary origin of autoreactive determinants (autogens). Proc Natl Acad Sci U S A. 1986;83:2521–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milstein O, Hagin D, Lask A, et al. CTLs respond with activation and granule secretion when serving as targets for T-cell recognition. Blood. 2011;117:1042–1052. doi: 10.1182/blood-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andersen MH, Schrama D, thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126:32–41. [DOI] [PubMed] [Google Scholar]
- 12. Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. [DOI] [PubMed] [Google Scholar]
- 13. Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. [DOI] [PubMed] [Google Scholar]
- 15. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. [DOI] [PubMed] [Google Scholar]
- 16. Croxford JL, Tang ML, Pan MF, et al. ATM-dependent spontaneous regression of early Eµ-myc-induced murine B-cell leukemia depends on natural killer and T cells. Blood. 2013;121:2512–2521. doi: 10.1182/blood-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Senovilla L, Vitale I, Martins I, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science. 2012;337:1678–1684. doi: 10.1126/science.1224922. [DOI] [PubMed] [Google Scholar]
- 18. Wu X, Peng M, Huang B, et al. Immune microenvironment profiles of tumor immune equilibrium and immune escape states of mouse sarcoma. Cancer Lett. 2013;340:124–133. [DOI] [PubMed] [Google Scholar]
- 19. Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dieci MV, Griguolo G, Miglietta F, Guarneri V. The immune system and hormone-receptor positive breast cancer: is it really a dead end? Cancer Treat Rev. 2016;46:9–19. [DOI] [PubMed] [Google Scholar]
- 21. Baumgarten SC, Frasor J. Minireview: inflammation: an instigator of more aggressive estrogen receptor (ER) positive breast cancers. Mol Endocrinol. 2012;26:360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jinushi M, Komohara Y. Tumor-associated macrophages as an emerging target against tumors: creating a new path from bench to bedside. Biochim Biophys Acta. 2015;1855:123–130. [DOI] [PubMed] [Google Scholar]
- 23. Lee HJ, Song IH, Park IA, et al. Differential expression of major histocompatibility complex class I in subtypes of breast cancer is associated with estrogen receptor and interferon signaling. Oncotarget. 2016;7:30119–30132. doi: 10.18632/oncotarget.8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pierdominici M, Maselli A, Colasanti T, et al. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett. 2010;132:79–85. [DOI] [PubMed] [Google Scholar]
- 25. Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. 2004;3:97–104. [DOI] [PubMed] [Google Scholar]
- 26. Inoue M, Mimura K, Izawa S, et al. Expression of MHC class I on breast cancer cells correlates inversely with HER2 expression. Oncoimmunology. 2012;1:1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nature Reviews Cancer. 2009;9:274. [DOI] [PubMed] [Google Scholar]
- 28. Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonafe M, Storci G, Franceschi C. Inflamm-aging of the stem cell niche: breast cancer as a paradigmatic example. Bioessays. 2012;34:40–49. [DOI] [PubMed] [Google Scholar]
- 30. Irahara N, Miyoshi Y, Taguchi T, Tamaki Y, Noguchi S. Quantitative analysis of aromatase mRNA expression derived from various promoters (I. 4, I. 3, PII and I. 7) and its association with expression of TNF-α, IL-6 and COX-2 mRNAs in human breast cancer. Int J Cancer. 2006;118:1915–1921. [DOI] [PubMed] [Google Scholar]
- 31. Prieto GA, Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology. 2006;118:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res. 2006;84:370–378. [DOI] [PubMed] [Google Scholar]
- 33. Nadkarni S, McArthur S. Oestrogen and immunomodulation: new mechanisms that impact on peripheral and central immunity. Curr Opin Pharmacol. 2013;13:576–581. [DOI] [PubMed] [Google Scholar]
- 34. Svoronos N, Perales-Puchalt A, Allegrezza MJ, et al. Tumor cell-independent estrogen signaling drives disease progression through mobilization of myeloid-derived suppressor cells. Cancer Discov. 2017;7:72–85. doi: 10.1158/2159-8290.CD-16-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rossini A, Rumio C, Sfondrini L, et al. Influence of antibiotic treatment on breast carcinoma development in proto-neu transgenic mice. Cancer Res. 2006;66:6219–6224. doi: 10.1158/0008-5472.CAN-05-4592. [DOI] [PubMed] [Google Scholar]
- 36. Kassayova M, Bobrov N, Strojny L, et al. Preventive effects of probiotic bacteria Lactobacillus plantarum and dietary fiber in chemically-induced mammary carcinogenesis. Anticancer Res. 2014;34:4969–4975. [PubMed] [Google Scholar]
- 37. Velicer CM, Heckbert SR, Lampe JW, Potter JD, Robertson CA, Taplin SH. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291:827–835. [DOI] [PubMed] [Google Scholar]
- 38. Rutkowski MR, Stephen TL, Svoronos N, et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell. 2015;27:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 40. Rhim AD, Thege FI, Santana SM, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146:647–651. doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gruber IV, Hartkopf AD, Hahn M, et al. Relationship between hematogenous tumor cell dissemination and cellular immunity in DCIS patients. Anticancer Res. 2016;36:2345–2351. [PubMed] [Google Scholar]
- 42. Sänger N, Effenberger KE, Riethdorf S, et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer. 2011;129:2522–2526. [DOI] [PubMed] [Google Scholar]
- 43. Hill RP, Perris R. “Destemming” cancer stem cells. J Natl Cancer Inst. 2007;99:1435–1440. [DOI] [PubMed] [Google Scholar]
- 44. Chaffer CL, Weinberg RA. How does multistep tumorigenesis really proceed? Cancer Discov. 2015;5:22–24. doi: 10.1158/2159-8290.CD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. [DOI] [PubMed] [Google Scholar]
- 46. Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. [DOI] [PubMed] [Google Scholar]
- 47. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983-3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Al-Hajj M. Cancer stem cells and oncology therapeutics. Curr Opin Oncol. 2007;19:61–64. doi: 10.1097/CCO.0b013e328011a8d6. [DOI] [PubMed] [Google Scholar]
- 49. Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers. 2014;6:1670–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raggi C, Mousa H, Correnti M, Sica A, Invernizzi P. Cancer stem cells and tumor-associated macrophages: a roadmap for multitargeting strategies. Oncogene. 2016;35:671–682. [DOI] [PubMed] [Google Scholar]
- 53. Scheel C, Eaton EN, Li SH, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen K. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. PLoS ONE. 2016;11:e0152500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 56. Robbins PF, Lu Y, El-Gamil M, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Luen S, Virassamy B, Savas P, Salgado R, Loi S. The genomic landscape of breast cancer and its interaction with host immunity. Breast. 2016;29:241–250. [DOI] [PubMed] [Google Scholar]
- 61. Nik-Zainal S, Alexandrov LB, Wedge DC, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149:979–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haricharan S, Bainbridge MN, Scheet P, Brown PH. Somatic mutation load of estrogen receptor-positive breast tumors predicts overall survival: an analysis of genome sequence data. Breast Cancer Res Treat. 2014;146:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brown SD, Warren RL, Gibb EA, et al. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014;24:743–750. doi: 10.1101/gr.165985.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13:R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Perez EA, Thompson EA, Ballman KV, et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the north central cancer treatment group n9831 adjuvant trastuzumab trial. J Clin Oncol. 2015;33:701–708. doi: 10.1200/JCO.2014.57.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ladoire S, Mignot G, Dabakuyo S, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224:389–400. [DOI] [PubMed] [Google Scholar]
- 69. Criscitiello C. Tumor-associated antigens in breast cancer. Breast Care (Basel). 2012;7:262–266. doi: 10.1159/000342164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Downs-Holmes C, Silverman P. Breast cancer: overview & updates. Nurse Pract. 2011;36:20–26; quiz 7. doi: 10.1097/01.NPR.0000407602.29522.d7. [DOI] [PubMed] [Google Scholar]
- 71. Mittendorf EA, Clifton GT, Holmes JP, et al. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol. 2014;25:1735–1742. doi: 10.1093/annonc/mdu211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schneble EJ, Berry JS, Trappey FA, et al. The HER2 peptide nelipepimut-S (E75) vaccine (NeuVax™) in breast cancer patients at risk for recurrence: correlation of immunologic data with clinical response. Immunotherapy. 2014;6:519–531. [DOI] [PubMed] [Google Scholar]
- 73. Mittendorf EA. Efficacy and safety study of NeuVax™ (nelipepimut-S or E75) vaccine to prevent breast cancer recurrence (PRESENT). https://clinicaltrials.gov/ct2/show/study/NCT01479244. Updated 2015. Accessed January 2, 2017.
- 74. Clifton G, Holmes J, Perez S, et al. Interim analysis of a randomized phase II study of the novel HER2/neu peptide (GP2) vaccine to prevent breast cancer recurrence: United States Military Cancer Institute Clinical Trials Group Study I-05. Cancer Res. 2009;69:5110. [Google Scholar]
- 75. Schneble EJ, Perez SA, Murray JL, et al. Primary analysis of the prospective, randomized, phase II trial of GP2+GM-CSF vaccine versus GM-CSF alone administered in the adjuvant setting to high-risk breast cancer patients. J Clin Oncol. 2014;32:134. [Google Scholar]
- 76. Sears AK, Perez SA, Clifton GT, et al. AE37: a novel T-cell-eliciting vaccine for breast cancer. Expert Opin Biol Ther. 2011;11:1543–1550. [DOI] [PubMed] [Google Scholar]
- 77. Mittendorf EA, Ardavanis A, Symanowski J, et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann Oncol. 2016;27:1241–1248. doi: 10.1093/annonc/mdw150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lowenfeld L, Mick R, Datta J, et al. Dendritic cell vaccination enhances immune responses and induces regression of HER2(pos) DCIS independent of route: results of randomized selection design trial. Clin Cancer Res. 2017;23:2961–2971. doi: 10.1158/1078-0432.CCR. [DOI] [PubMed] [Google Scholar]
- 79. Finn OJ. The dawn of vaccines for cancer prevention. Nat Rev Immunol. 2018;18:183–194. [DOI] [PubMed] [Google Scholar]
- 80. Blixt O, Bueti D, Burford B, et al. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011;13:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ibrahim NK, Murray JL, Zhou D, et al. Survival advantage in patients with metastatic breast cancer receiving endocrine therapy plus sialyl tn-KLH vaccine: post hoc analysis of a large randomized trial. J Cancer. 2013;4:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sandrin MS, Vaughan HA, Xing P, McKenzie IF. Natural human anti-Gal alpha(1,3)Gal antibodies react with human mucin peptides. Glycoconj J. 1997;14:97–105. [DOI] [PubMed] [Google Scholar]
- 83. Butschak G, Karsten U. Isolation and characterization of Thomsen-Friedenreich-specific antibodies from human serum. Tumour Biol. 2002;23:113–122. [DOI] [PubMed] [Google Scholar]
- 84. Andre F, Dieci MV, Dubsky P, et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res. 2013;19:28–33. doi: 10.1158/1078-0432.CCR. [DOI] [PubMed] [Google Scholar]
- 85. Kurtenkov O, Klaamas K, Mensdorff-Pouilly S, Miljukhina L, Shljapnikova L, Chužmarov V. Humoral immune response to MUC1 and to the Thomsen-Friedenreich (TF) glycotope in patients with gastric cancer: relation to survival. Acta Oncol. 2007;46:316–323. [DOI] [PubMed] [Google Scholar]
- 86. Monzavi-Karbassi B, Pashov A, Kieber-Emmons T. Tumor-associated glycans and immune surveillance. Vaccines. 2013;1:174–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kieber-Emmons T, Saha S, Pashov A, Monzavi-Karbassi B, Murali R. Carbohydrate-mimetic peptides for pan anti-tumor responses. Front Immunol. 2015;5:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Makhoul I, Hutchins L, Emanuel PD, et al. Moving a carbohydrate mimetic peptide into the clinic: clinical response of a breast cancer patient after mimotope-based immunotherapy. Hum Vaccin Immunother. 2015;11:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hennings L, Artaud C, Jousheghany F, Monzavi-Karbassi B, Pashov A, Kieber-Emmons T. Carbohydrate mimetic peptides augment carbohydrate-reactive immune responses in the absence of immune pathology. Cancers. 2011;3:4151–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Slamon DJ, Eiermann W, Robert NJ, et al. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab in HER2-positive early breast cancer. Paper presented at: San Antonio Breast Cancer Symposium; December 8-12, 2015; San Antonio, TX. [Google Scholar]
- 93. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 94. Joensuu H, Kellokumpu-Lehtinen P, Bono P, et al. Trastuzumab in combination with docetaxel or vinorelbine as adjuvant treatment of breast cancer: the FinHer trial. Breast Cancer Res Treat. 2005;94:S5. [Google Scholar]
- 95. Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27:6129–6134. [DOI] [PubMed] [Google Scholar]
- 96. Ingold Heppner B, Untch M, Denkert C, et al. Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin Cancer Res. 2016;22:5747–5754. doi: 10.1158/1078-0432.CCR-15-2338. [DOI] [PubMed] [Google Scholar]
- 97. Carbognin L, Pilotto S, Nortilli R, et al. Predictive and prognostic role of tumor-infiltrating lymphocytes for early breast cancer according to disease subtypes: sensitivity analysis of randomized trials in adjuvant and neoadjuvant setting. Oncologist. 2016;21:283–291. doi: 10.1634/theoncologist.2015-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bense RD, Sotiriou C, Piccart-Gebhart MJ, et al. Relevance of tumor-infiltrating immune cell composition and functionality for disease outcome in breast cancer. J Natl Cancer Inst. 2016;109:djw192. doi: 10.1093/jnci/djw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Piccart-Gebhart M, Holmes E, Baselga J, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: results from the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J Clin Oncol. 2016;34:1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. [DOI] [PubMed] [Google Scholar]
- 101. Thomas A, Teicher BA, Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016;17:e254–e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Trail PA, Dubowchik GM, Lowinger TB. Antibody drug conjugates for treatment of breast cancer: novel targets and diverse approaches in ADC design. Pharmacol Ther. 2017;181:126–142. [DOI] [PubMed] [Google Scholar]
- 103. Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12:130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016;47:78–84. [DOI] [PubMed] [Google Scholar]
- 106. Muenst S, Schaerli A, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib keynote-012 study. J Clin Oncol. 2016;34:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Adams S, Schmid P, Rugo HS, et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. J Clin Oncol. 2017;35(15_suppl):1008. [Google Scholar]
- 109. Adams S, Loi S, Toppmeyer D, et al. Phase 2 study of pembrolizumab as first-line therapy for PD-L1-positive metastatic triple-negative breast cancer (mTNBC): preliminary data from KEYNOTE-086 cohort B. J Clin Oncol. 2017; 35(15_suppl):1088 [Google Scholar]
- 110. Schmid P, Cruz C, Braiteh FS, et al. Atezolizumab in metastatic TNBC (mTNBC): long-term clinical outcomes and biomarker analyses. Cancer Res. 2017; 77(13_supp):2986 DOI: 10.1158/1538-7445.AM2017-2986 [DOI] [Google Scholar]
- 111. Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): results from I-SPY 2. J Clin Oncol. 2017;35(15_Supp):506.28029304 [Google Scholar]
- 112. Cortes Castan J, Guo Z, Karantza V, Aktan G. 234TiPKEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab (pembro) chemotherapy (chemo) vs placebo (PBO) chemo for previously untreated, locally recurrent, inoperable or metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2017;(28) (5 Supplement), 1 September 2017, mdx364.016, https://doi.org/10.1093/annonc/mdx364.016
- 113. Adams S, Diamond JR, Hamilton EP, et al. Phase Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2016;34(15_Supp):1009. [Google Scholar]
- 114. Emens LA, Adams S, Loi S, et al. IMpassion130: a phase III randomized trial of atezolizumab with nab-paclitaxel for first-line treatment of patients with metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2016. [Google Scholar]
- 115. Gatalica Z, Snyder C, Maney T, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23:2965–2970. doi: 10.1158/1055-9965.EPI. [DOI] [PubMed] [Google Scholar]
- 116. Pusztai L. Pembrolizumab in treating patients with triple-negative breast cancer. https://clinicaltrials.gov/ct2/show/study/NCT02954874. Updated 2016. Accessed January 2, 2017.
- 117. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Urba WJ. Safety study of enoblituzumab (MGA271) in combination with ipilimumab in refractory cancer. https://clinicaltrials.gov/ct2/show/NCT02381314. Updated 2016. Accessed December 25, 2016.
- 119. Connolly R. Entinostat, nivolumab, and ipilimumab in treating patients with solid tumors that are metastatic or cannot be removed by surgery or locally advanced or metastatic HER2-negative breast cancer. https://clinicaltrials.gov/ct2/show/NCT02453620. Updated 2016. Accessed December 25, 2016.
- 120. Santa-Maria C. MEDI4736 and tremelimumab in treating patients with metastatic HER2 negative breast cancer. https://clinicaltrials.gov/ct2/show/NCT02536794. Updated 2016. Accessed December 25, 2016.
- 121. Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16:3485–3494. doi: 10.1158/1078-0432.CCR. [DOI] [PubMed] [Google Scholar]
- 122. Page DB, Diab A, Yuan J, et al. Pre-operative immunotherapy with tumor cryoablation (cryo) plus ipilimumab (ipi) induces potentially favorable systemic and intratumoral immune effects in early stage breast cancer (ESBC) patients. J Immunother Cancer. 2015;3:O6. [Google Scholar]
- 123. Cimino-Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncology. 2015;29:375–375. [PubMed] [Google Scholar]
- 124. Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy—inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18:6580–6587. doi: 10.1158/1078-0432.CCR. [DOI] [PubMed] [Google Scholar]
- 125. Naidoo J, Wang X, Woo K, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Brignone C, Gutierrez M, Mefti F, et al. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J Transl Med. 2010;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cabo M, Offringa R, Zitvogel L, Kroemer G, Muntasell A, Galluzzi L. Trial watch: immunostimulatory monoclonal antibodies for oncological indications. Oncoimmunology. 2017;6:e1371896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Naghavi AO, Johnstone PA, Kim S. Clinical trials exploring the benefit of immunotherapy and radiation in cancer treatment: a review of the past and a look into the future. Curr Probl Cancer. 2016;40:38–67. [DOI] [PubMed] [Google Scholar]
- 129. Cheuk AT, Mufti GJ, Guinn B. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Ther. 2004;11:215–226. [DOI] [PubMed] [Google Scholar]
- 130. Kohrt HE, Houot R, Weiskopf K, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest. 2012;122:1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131. Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Slobodova Z, Ehrmann J, Krejci V, Zapletalova J, Melichar B. Analysis of CD40 expression in breast cancer and its relation to clinicopathological characteristics. Neoplasma. 2011;58:189–197. [DOI] [PubMed] [Google Scholar]
- 133. Kim H, Kim Y, Bae S, et al. Direct interaction of CD40 on tumor cells with CD40L on T cells increases the proliferation of tumor cells by enhancing TGF-β production and Th17 differentiation. PLoS ONE. 2015;10:e0125742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang Z, Wang J, Ling W, Zhang X, Shi Q. Effects of CD40 ligation combined with chemotherapy drugs on human breast cancer cell lines. J Int Med Res. 2013;41:1495–1504. [DOI] [PubMed] [Google Scholar]
- 135. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. [DOI] [PubMed] [Google Scholar]
- 137. Sevko A, Michels T, Vrohlings M, et al. Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol. 2013;190:2464–2471. doi: 10.4049/jimmunol.1202781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ge Y, Domschke C, Stoiber N, et al. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother. 2012;61:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Schiavoni G, Sistigu A, Valentini M, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71:768–778. doi: 10.1158/0008-5472.CAN. [DOI] [PubMed] [Google Scholar]
- 140. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. [DOI] [PubMed] [Google Scholar]
- 141. Alizadeh D, Trad M, Hanke NT, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74:104–118. doi: 10.1158/0008-5472.CAN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87:21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Demaria S, Volm MD, Shapiro RL, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- 144. Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. 2011;33:369–383. [DOI] [PubMed] [Google Scholar]
- 145. Liu W, Fowler D, Smith P, Dalgleish A. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer. 2010;102:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]


