Abstract
Background:
Treatment of osteochondral talar defects (OCDs) after failed previous surgery is challenging. Promising short-term results have been reported with use of a metal resurfacing inlay implant.
Purpose:
To evaluate the midterm clinical effectiveness of the metal implant for OCDs of the medial talar dome after failed previous surgery.
Study Design:
Case series; Level of evidence, 4.
Methods:
We prospectively studied all patients who met the inclusion criteria and received a metal resurfacing inlay implant between 2007 and 2014. The primary outcome measure was implant survival, as measured by reoperation rate. Secondary outcome measures were numeric rating scales for pain at rest and during walking, running, and stair climbing; the Foot and Ankle Outcome Score (FAOS); the American Orthopaedic Foot and Ankle Society Ankle Hindfoot Scale; the 36-Item Short Form Health Survey (SF-36); return to work and sports; and radiographic evaluation.
Results:
This study included 38 patients with a mean age of 39 years (SD, ±13 years) and a mean follow-up of 5.1 years (SD, ±1.5 years). Two patients (5%) underwent revision surgery by means of an ankle arthrodesis (2 and 6 years postoperatively). In 8 patients, computed tomography scanning was conducted to assess postoperative complaints. These scans showed impression of the tibial plafond (n = 4), a small tibial cyst (<2.5 mm; n = 1), and cyst formation around the implant screw (n = 4). A total of 21 reoperations were performed, including medial malleolar screw removal (n = 12), arthroscopic removal of bony anterior impingement (n = 7), and calcaneal realignment osteotomy (n = 2). All secondary outcome measures improved significantly, apart from pain at rest, the FAOS symptoms subscale, and the SF-36 mental component scale. The mean time for return to sport was 4.1 months (SD, ±3 months), and 77% of patients resumed sporting activities postoperatively. Only 1 patient did not return to work postoperatively. Radiographs at final follow-up showed cyst formation (n = 2), subchondral periprosthetic radiolucency (n = 2), and non-preexisting joint space narrowing (n = 2).
Conclusion:
This study shows that the metal implant is an effective technique when assessed at midterm follow-up for OCDs of the medial talar dome after failed previous surgery.
Keywords: osteochondral defect, ankle, resurfacing implant, implant failure
Most osteochondral defects (OCDs) located in the ankle occur after an ankle fracture (up to 70%) or a lateral ankle ligament rupture (up to 7%).15 These lesions, including both articular hyaline cartilage and subchondral bone, are mainly located on the medial talar dome.10,37 Not all OCDs heal and stabilize. They often progress to a cystic lesion, resulting in deep ankle pain during activity, prolonged swelling, diminished range of motion, and synovitis.33
Despite good to excellent long-term results of primary surgical treatment of OCDs (up to 76%) by treatment such as debridement and bone marrow stimulation, not all patients experience relief of symptoms.11,20,30,32,37 Secondary treatment options (with reported success percentages >90%), often required in larger lesions (>15 mm diameter), consist of tissue transplant techniques such as osteochondral autograft transfer system (OATS),3,7 bone graft,9 autologous chondrocyte implantation (ACI),3,19 and matrix-induced autologous chondrocyte implantation (MACI).13,27,32,36,37 However, such options require a donor site, and a surgeon may not want to damage a fully healthy joint to potentially save another. A final and definitive solution is an ankle fusion.
Transplant techniques have shown a success rate of about 84% with a follow-up of 1 to 10 years.36 The literature overall lacks evidence, and studies are unclear regarding whether results include only patients receiving secondary treatment or whether a given study population also includes patients receiving primary treatment. A metal resurfacing implant (HemiCAP; Arthrosurface Inc), used in the humeral and femoral heads and even adapted for metatarsal head resurfacing, has been introduced as a secondary treatment option for medial talar OCDs.6,18,30,35 This implant has shown promising results in the resurfacing of large talar OCDs, with a 0% implant failure in 20 patients over a mean follow-up of 3 years.30 However, the follow-up time of the previous study was short and the number of patients small.
The purpose of the current study was to evaluate the effectiveness of the metal implant at 2 to 5 years for OCDs of the medial talar dome in a larger cohort after failed primary surgical treatment.
Methods
This study included patients with an OCD of the medial talar dome with failed previous surgery (ie, complaints for more than a year after previous surgical treatment and a persistent talar OCD on a computed tomography [CT] scan); the largest OCD diameter was 12 to 20 mm as measured on CT. Patients were excluded if they were younger than 18 years; if they had undergone a combined surgical procedure; if they had ankle osteoarthritis grade III,34 another ankle injury (eg, tibial osteochondral defect, ankle instability), advanced osteoporosis, infection, known allergy to implant material, or diabetes mellitus; or if they could not independently fill out the study questionnaire.2,30 The current report includes all eligible patients treated between 2007 and 2014, providing a minimum follow-up duration of 2 years.
This study was approved by the local medical ethics committee. All patients provided informed consent before participation in the study.
Operative Technique
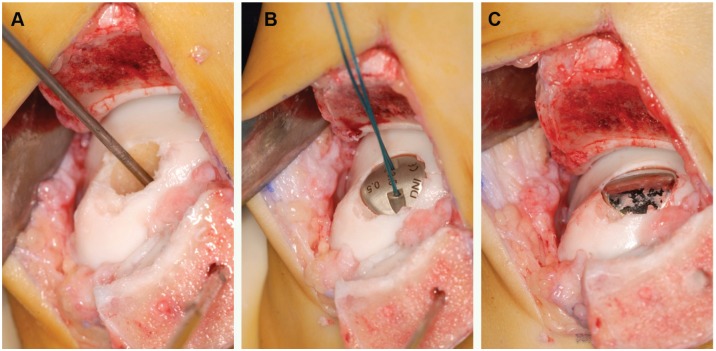
All procedures were performed by the senior author (C.N.v.D.) using a previously described technique.31 Briefly, an oblique medial malleolar osteotomy was performed to expose the talus.28,29 The OCD was debrided until a stable cartilage rim remained. Using a drill guide, the surgeon placed a guide pin into the center of the defect, perpendicular to the curvature of the medial talar dome. Subsequently, a cannulated screw was inserted. To determine the radius of curvature in the sagittal and coronal planes, a contact probe was used to allow for a precise fit of the articular component against the existing articular surface. A matching reamer was used to prepare the site for placement of the articular component. To provide final verification of fit, a trial cap with corresponding offsets was provided. The HemiCAP implant is available in 15 articular component offset sizes, based on the surface anatomic features of the medial talar dome. The final articular component was impacted on the screw, thereby engaging the taper interlock (Figure 1). Sufficient recession (0.5 mm) of the implant relative to the surrounding talar cartilage, to avoid protrusion as the cartilage deforms during weightbearing and the implant does not, was determined by direct inspection.31 The malleolar osteotomy was fixed and held in place by 2 lag screws via predrilled holes.
Figure 1.
(A) Guide pin in reamed defect. (B) Trial cap. (C) Final articular component placed on the screw.
Postoperatively, patients received a plaster cast for 2 weeks followed by a walker cast (Walker; Össur) for 4 weeks. If a walker was not tolerated well because of its size, a removable cast was chosen. Plantarflexion and dorsiflexion exercises (15 minutes twice daily) were encouraged during the period when the walker and removable plaster cast were used (weeks 2-6 postoperatively). For the first 6 weeks, patients were not allowed to bear weight on the operated ankle. To confirm consolidation of the malleolar osteotomy, standard anteroposterior mortise and lateral radiographs were taken 6 weeks postoperatively. At this point, physical therapy was prescribed to assist in functional recovery and facilitate the return to full weightbearing over approximately 1 month. Return to normal weightbearing and walking was typically accomplished 10 weeks after surgery. Impact activities, such as running, were allowed when no signs of prosthetic loosening and migration were seen after 6 months of follow-up. Noncontact sports were allowed after 9 months of follow-up and contact sports 1 year after surgery.22
Outcome Assessment
Patients were assessed by members of the research team (independent from the surgeon) preoperatively and at 2 weeks, 6 weeks, 3 months, 6 months, annually until 6 years postoperatively, and every 2 years thereafter. Preoperatively, a CT scan was obtained of all affected ankles to measure the 3-dimensional size.5,25 The primary outcome measure was implant survival measured by revision surgery. Implant survival was defined as the implant remaining in place without revision to a total ankle prosthesis or ankle arthrodesis.
Secondary outcome measures were the Numeric Rating Scale (NRS) for pain, the Foot and Ankle Outcome Score (FAOS),23 the 36-Item Short Form Health Survey (SF-36),1 the American Orthopaedic Foot and Ankle Society (AOFAS)17 Ankle Hindfoot Scale, return to work and sports, and radiographic assessment. The NRS for pain is an 11-point scale ranging from no pain (0 points) to the worst pain imaginable (10 points) and was used to assess pain at rest, during walking, during stair climbing, and during running. If patients were not able to perform one of these activities, the respective score was not assessed.24 The FAOS (Dutch language version)26 is a validated questionnaire assessing pain, other symptoms (swelling, locking, mobility), function (activities of daily living), sports, and foot- and ankle-related quality of life using 5 subscales ranging from 0 to 100. The SF-36 has been validated in Dutch and assesses general quality of life. The normative SF-36 value of the Dutch population is 49.2 for the physical component scale and 50.7 for the mental component scale.1 The AOFAS Ankle Hindfoot Scale has subjective and objective components and provides a score with a maximum of 100 points. Of the 100 points, 40 are assigned to pain, 50 to function, and 10 to alignment. This score was completed by the researcher after a patient interview and physical examination of the ankle.
Complications and time to return to work and sports were recorded during the patient interview and physical examination. Additionally, patients were asked to indicate their level of sports (1, highly competitive; 2, well trained and frequently active in sport; 3, sometimes active in sport; 4, not active in sport), whether they would undergo the procedure again, and whether they would recommend the procedure to friends and family.
At all follow-up visits after (and including) 6 weeks postoperatively, weightbearing radiographs were obtained (anteroposterior mortise and lateral views). Both an independent radiologist and 1 of the first 5 authors (G.V., M.L.R., C.v.B., I.v.E., R.M.G.), who did not perform the surgery, reviewed the radiographs for evidence of implant loosening (periprosthetic osteolysis, subsidence, migration and disengagement), cyst formation and sclerosis around the implant, malunion or nonunion of the medial malleolar osteotomy, and signs of osteoarthritis such as osteophytes and joint space narrowing.
Statistical Analysis
Statistical analyses were performed by use of SPSS software v23 (SPSS Inc). Categorical data are presented as frequency and continuous data as mean with SD or median with range, depending on their distribution. Normality was checked by use of the Shapiro-Wilk test and confirmed by observation. When data were normally distributed, the paired t test was used to compare scores at different moments in time; if data were skewed, the Wilcoxon signed rank test was used. Repeated-measures analysis of variance with Bonferroni correction was used to assess score changes over time for the NRS, FAOS, and SF-36. To minimize the effect of declining numbers of patients with longer follow-up time, the outcome data were analyzed in the following follow-up categories: preoperative, 2 years, 3-5 years, and 6-8 years. The last available follow-up assessment was included in the analyses, and the number of patients in each category is reported. A P value less than .05 was considered significant.
Results
A total of 44 patients underwent the procedure at our institution between October 2007 and September 2014. Six of these were excluded from the study: 3 patients were excluded because the resurfacing implant was their primary OCD treatment, 1 patient had diabetes mellitus, 1 patient underwent a combined procedure because of multiple ankle injuries, and 1 patient was not able to fill out the questionnaire independently.
The remaining 38 patients were included in this study. They had a mean age of 38.6 years (SD, ±13.2 years) at the time of inclusion and a mean body mass index of 26.5 kg/m2 (SD, ±3.8 kg/m2), and 65% were female. The mean follow-up was 5.1 years (SD, ±1.5 years). Seven patients (18%) missed the last 2 follow-up appointments and were analyzed with reduced follow-up (ie, 2 years). Additionally, some patients had incomplete questionnaires at some follow-up points, causing variation in the number of patients per follow-up interval. Apart from these missing data, there was no loss to follow-up.
Sixteen patients (42%) had undergone a total of 1 prior surgical intervention, 12 patients (32%) had undergone 2 prior surgical interventions, 7 patients (18%) had undergone 3 prior surgical interventions, and 1 patient (3%) had undergone 5 prior surgical interventions (Table 1). Two patients had received primary treatment outside our hospital, and the type and number of previous treatments were not retrieved. The mean lesion size was 15.7 mm (SD, ±3.4 mm) in the anterior-posterior direction, 10.5 mm (SD, ±2.1 mm) in the medial-lateral direction, and 9.3 mm (SD, ±3.0 mm) in the superior-inferior direction.
Table 1.
Previous Operation Rate and Type of Operation a
| First Previous Operation | Second Previous Operation | Third Previous Operation | Fourth Previous Operation | Fifth Previous Operation | No. of Times Performed | |
|---|---|---|---|---|---|---|
| Arthroscopic debridement | 8 | 6 | 2 | 1 | 1 | 18 |
| Arthroscopic debridement and MF | 18 | 9 | 5 | 32 | ||
| Open debridement | 1 | 1 | 1 | 3 | ||
| Open debridement and MF | 3 | 1 | 4 | |||
| Bone grafting | 4 | 3 | 7 | |||
| Open debridement and MF through MMO | 2 | 2 | ||||
| Total No. of previous operations | 36 | 20 | 8 | 1 | 1 | 66 |
MF, microfracture; MMO, medial malleolus osteotomy.
Implant Survival and Complications
Two ankle fusions (5%) were performed: 1 because of persisting deep ankle pain at 2-year follow-up, and 1 because of deep ankle pain in combination with radiographic loosening at 6-year follow-up (Figures 2 and 3). Additionally, 21 reoperations were performed including medial malleolar screw removal (n = 12), arthroscopic removal of bony anterior impingement (n = 7), and calcaneal realignment osteotomy (n = 2).
Figure 2.
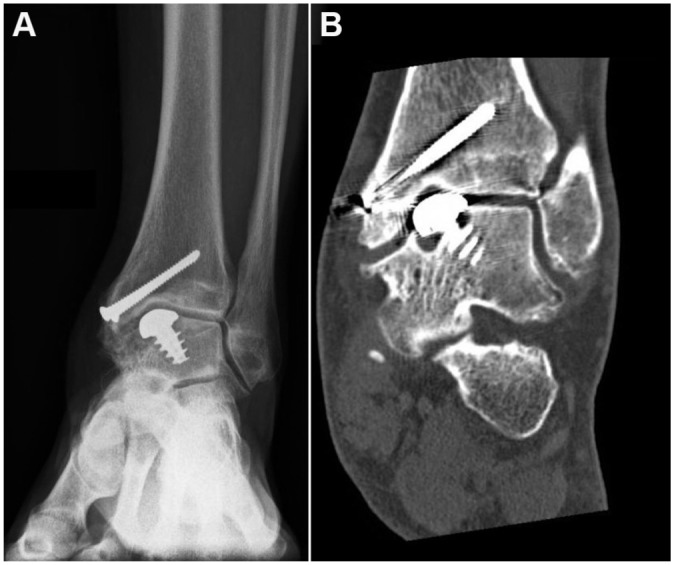
Development of a cyst around the implant, first diagnosed on a (A) regular radiograph and confirmed on (B)computed tomography scan. The images show no signs of loosening around the screw. The implant was removed, and the ankle joint was fused.
Figure 3.
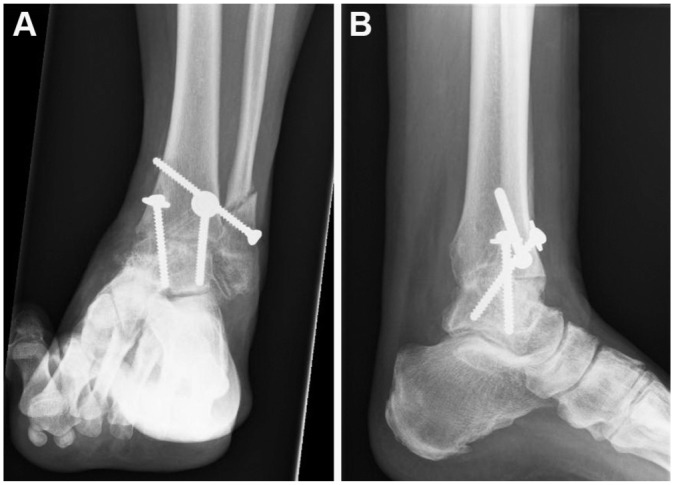
(A) Anteroposterior and (B) lateral view after talar fusion.
Nine (24%) patients reported complications. Complications were mainly reported between the date of surgery and the sixth week of follow-up, and all were resolved during the study. Reported complications consisted of disturbed sensibility (n = 6) and superficial wound dehiscence (n = 3).
Functional Outcome
The NRS at rest did not improve significantly over time (P = .219) (Figure 4). Other NRS scores showed significant improvement from the preoperative assessment to final follow-up: walking, from 6.2 (±1.7) to 3.6 (±2.8) (F3,97 = 7.3, P < .001); running, from 8.8 (±1.5) to 5.8 (±4.2) (F3,85 = 11.0, P < .001); and stair climbing, from 6.1 (2.1) to 2.7 (±2.9) (F3,97 = 11.7, P < .001). Post hoc pairwise comparisons using the Bonferroni correction showed significant improvement between preoperative NRS scores and all follow-up time intervals of running and stair climbing (Figure 4).
Figure 4.
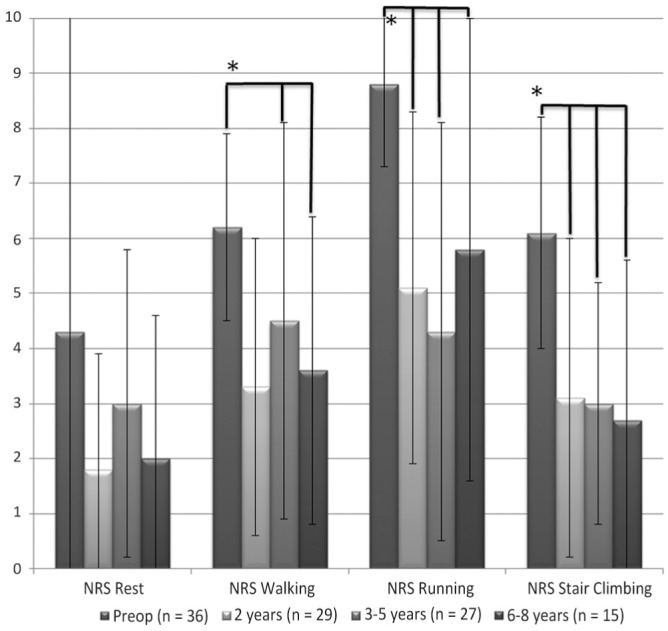
Numeric Rating Scale (NRS) for pain during rest, walking, running, and stair climbing (mean score and SD). *P < .05. The line above the graph represents significant difference with the preoperative assessment.
The FAOS pain, activities of daily living, sports, and quality of life subscales showed significant improvements from preoperative status to final follow-up (P ≤ .001) (Figure 5). The FAOS pain score improved from 51.2 (±15.5) preoperatively to 72.6 (±20.2) at 2-year follow-up and 73.2 (±20.9) at 3- to 5-year follow-up (F3,101 = 9.1, P = .001), with no significant improvement at the 6- to 8-year follow-up. The FAOS activities of daily living score improved from 61.3 (±16.8) preoperatively to 77.0 (±20.9) at the last follow-up (F3,101 = 9.6, P < .001). The FAOS sports score improved from 28.3 (±17.4) preoperatively to 54.1 (±29.2) at 2-year follow-up and 58.8 (±32.2) at 3- to 5-year follow-up, without significant improvement at final follow-up (F3,91 = 7.2, P < .001). The FAOS quality of life score improved from 18.7 (±15.0) preoperatively to 45.6 (±29.1) at final follow-up (F3,102 = 9.2, P < .001). The FAOS symptoms score did not show a significant improvement (P = .357).
Figure 5.
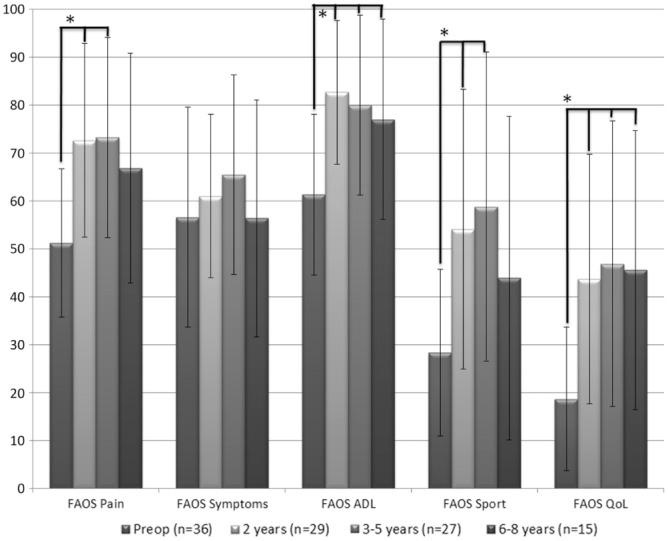
Score changes on the Foot and Ankle Outcome Score (FAOS) subscales. *P < .05. The line above the graph represents significant difference with the preoperative assessment. ADL, activities of daily living; QoL, quality of life.
The AOFAS showed a significant mean score increase of 56.9 (±16.8) preoperatively to 79.8 (±19.4) at 2 years postoperatively and 80.3 (±19.2) at 3 to 5 years postoperatively (F3,85 = 11.0, P < .001) (Figure 6).
Figure 6.
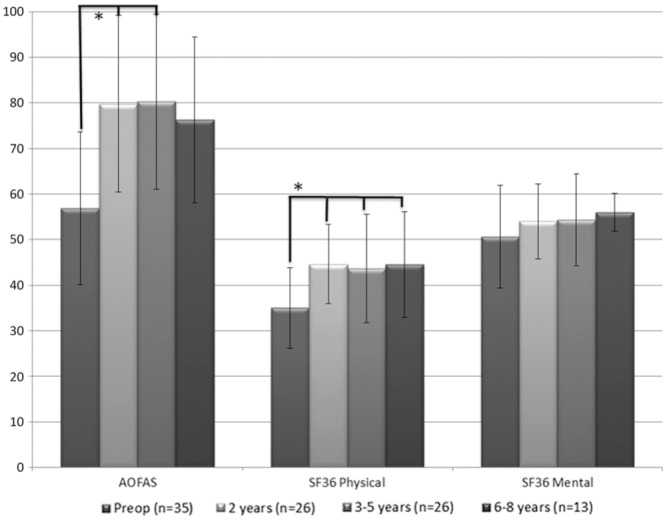
American Orthopaedic Foot and Ankle Society (AOFAS) Ankle Hindfoot Scale and 36-Item Short Form Health Survey (SF-36) questionnaire scores. *P < .05. The line above the graph represents significant difference with the preoperative assessment.
The SF-36 physical component scale showed significant improvement from 35.0 (±8.8) to 44.6 (±11.6) (F3,95 = 6.2, P = .001) at final follow-up with significant score improvement for each time interval. The mental component scale did not show a significant improvement (F3,96 = 1.4, P = .240) (Figure 6).
Return to Sports and Work
Twenty-seven of the 38 patients indicated that they had participated in sports before injury, and 19 (50%) indicated that they had participated in low-level sporting activities preoperatively. Twenty-one patients returned to some level of sports after their surgery: 9 resumed a higher level of sports compared with preoperatively, 5 stayed at the same level of sports participation, and 7 participated at a lower level. The mean time to sport resumption was 4.1 months (SD, ±3 months), and 84% of patients resumed sport within the first 6 months after surgery. This mainly entailed return to low-impact activities such as walking, cycling, and fitness, as other types were not allowed by the rehabilitation protocol. After 1 year, 1 patient returned to marathon running. At the final follow-up, 56% (n = 15/27) patients still participated in sports, of whom 5 participated often and considered themselves well trained.
Thirty-one patients worked before the operation, and 30 patients resumed work postoperatively after a median of 2 months (range, 0-51 months); of these, 27 (90%) resumed work within the first 6 months after surgery.
Patient Satisfaction
Of all patients who participated in this study, 80% indicated that they would undergo the surgery again if they had to make the same choice at their last follow-up point based on their current experience with the implant. Additionally, 88% would recommend the surgery to others.
Radiography
Overall, minimal changes were observed on the postoperative radiographs. Cyst formation was seen in 2 patients (5%), subchondral periprosthetic radiolucency in 2 patients (5%) (1 case of which was suspected of implant loosening), and non-preexisting joint space narrowing in 2 patients (5%) that did not seem to be related to the implant the side of the resurfacing implant. The patients in whom radiolucency was seen had matching complaints such as persistent pain.
In 8 patients, CT scanning was conducted to further assess complaints. These scans showed impression of the tibial plafond (n = 4), a small tibial cyst (<2.5 mm; n = 1), and cyst formation around the implant screw (n = 4). In 2 patients, no signs of implant degradation or implant-related complications were found. In the patient at risk of implant loosening (n = 1), ankle fusion was chosen to resolve complaints. Due to the low number of patients with cysts and/or joint space narrowing, these conditions were not assessed statistically.
Discussion
Treatment of OCDs using focal metal resurfacing implants after failed primary surgical treatment is a relatively new procedure, and the literature on this topic is scarce. In the current study, we found a high patient satisfaction rate with an implant survival rate of 95%. This prospective study provides an update of previously published results30 and includes a larger cohort and longer follow-up period. It shows that the clinical results are maintained over a period of 2 to 8 years. Although the clinical scores improved well, the reoperation rate was high. Two patients underwent a lateral shift calcaneal osteotomy to unload the medial talar dome. Furthermore, screw removal was performed in 12 patients and arthroscopic removal of bony impingement was performed in 7 patients due to postoperative bone growth on the anterior tibial rim; these were combined procedures. After these additional procedures, patients reported fewer complaints.
NRS scores for walking, running, and stair climbing improved over time. A possible reason why the NRS score at rest did not improve is because the preoperative score was relatively low (mean 4) and therefore the room for improvement was smaller due to a floor effect. The FAOS assesses symptoms through 5 questions about swelling, crepitations, locking, ability to fully dorsiflex, and ability to fully plantarflex. After open surgery with a medial malleolus osteotomy, swelling, decreased range of motion, and crepitations are often persistent. However, the FAOS symptoms subscale does not include pain, which is the main preoperative symptom, and therefore is less likely to improve the symptom subscale. The scores for the mental component scale of the SF-36 did not significantly change. However, the final mental component scale score was higher than that of a normal population.1 This indicates that the patients included in the current study were mentally healthy.
The results of the present and previous studies show promising effects of the resurfacing implant at both short- and midterm follow-up. As this study concerns a metal implant, durability is an important outcome factor, especially in a weightbearing joint such as the ankle. It is certain that durability is limited, but the failure time remains unknown. At the conclusion of this study, only 2 patients had received subsequent surgery due to prosthetic failure—at 2 and 6 years of follow-up, respectively. In both patients, complaints were resolved by removing the implant and performing an ankle fusion. Currently, no reliable options other than fusion are available after implant failure given the large taper interlock (10.3-17.4 mm) and concomitant bone loss; OATS requires multiple large plugs, and a prosthesis may not have enough purchase. When a resurfacing implant is used, some cartilage damage might not be covered and will remain untreated. Often this lesion fills with new fibrocartilaginous tissue, as seen during follow-up treatment. To strengthen the results from this report, a long-term follow-up assessment is required including more patients receiving a resurfacing implant.
Most outcomes showed significant differences between preoperative and postoperative scores, but many of the patients still had symptoms. Significant changes in assessment scores do not always correspond with clinically relevant differences in complaints. All NRS pain scores decreased 15% or more (although the decreased score for pain at rest was not statistically significant), which is a clinically relevant change.24 Scores for the SF-36 physical component scale, the AOFAS scale, and the FAOS subscales for pain, activities of daily living, and quality of life also demonstrated both statistical significance and clinically relevant changes.1,8,26 However, although the score for the FAOS sports scale demonstrated a statistically significant change, the change was not clinically relevant.26
Currently, no superior treatment for OCDs after failed primary surgery has been identified.19 Current secondary treatment options include OATS, autogenous cancellous bone graft, ACI, and MACI.36 However, these techniques are associated with donor site morbidity or involve 2-stage surgery. Although the resurfacing implant procedure has potential pitfalls such as wear and loosening, this procedure prevents donor site morbidity and the need for 2-stage surgery. After failed allograft or autograft, depending on the defect size, a resurfacing implant may still be considered as an extra step before a replacement prosthesis or fusion. Comparing these options with the resurfacing implant shows many similarities. All these techniques require an osteotomy (as only 17% of medially located talar OCDs can be accessed without an osteotomy), are suitable for large lesions, and show good clinical outcomes postoperatively.14,21 The risk of using autografts involves donor site complications such as pain, dysesthesia, and symptomatic scar tissue (3.6%-5.7%), which are not uncommon.12 These complications may be avoided using allografts, especially in larger lesions.16 Complications of allografts, which are not seen after use of a resurfacing implant, entail incongruent grafts, graft dislocation, or graft necrosis (1.1%-3.6%).12 Overall reported complication rates vary widely. However, most studies do not report on complications or report only intraoperative complications.4,3,27 An overall complication rate of 41% was found for OATS,12 whereas our study of the resurfacing implant found a complication rate of 24% and a reoperation rate of 55%. Gobbi et al14 concluded there were no differences between chondroplasty and OATS. Given these results, the resurfacing implant seems to be a valid option for the treatment of OCDs after failed primary treatment, given that the resurfacing implant entails no donor site morbidity and possibly has a lower complication rate compared with other secondary treatment options. Complications are still common despite precautions taken with the resurfacing implant, such as avoiding incongruency by shaping the implant for the talus, using a test implant for fitting, and using different curvatures for different implant sizes. Our cohort required a total of 21 reoperations, including 2 fusions, and radiographic changes were noted in 15% of patients.
The results of this study are limited, as no gold standard or reference therapy is available for failed secondary surgery for OCDs to allow comparison of our results. Additionally, our results do not show significant score improvements between the preoperative assessment and the 6- to 8-year follow-up for the FAOS pain, sports, and quality of life scales and the AOFAS scale despite significant score changes at earlier follow-up points. This may be related to a lower number of patients due to a shorter follow-up, which decreases the study power. The period studied (2007-2014) meant that not enough patients reached the 6- to 8-year follow-up (n = 15) compared with the initial power calculation of 20 patients,30 and thus the study had a lack of power. This 6- to 8-year follow-up was included to provide an indication of long-term outcome. A final limitation is that the technique requires a medial malleolar osteotomy to reach the defect, automatically excluding lateral lesions from this treatment option.
In conclusion, the metal resurfacing implant provides a low implant failure rate and good clinical outcomes but a high reoperation rate after failed primary talar OCD treatment at midterm follow-up. To determine long-term outcomes concerning implant failure and patient satisfaction, more cases and a longer follow-up period are needed.
Acknowledgments
The authors thank I. Sierevelt (SCORE, MC Slotervaart, Amsterdam) for statistical support.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: A travel grant was received from Arthrosurface Inc, Franklin, Massachusetts, to present the preliminary results of the current study at the AOFAS and ISAKOS congresses.
References
- 1. Aaronson NK, Muller M, Cohen PD, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055-1068. [DOI] [PubMed] [Google Scholar]
- 2. Athanasiou KA, Fleischli JG, Bosma J, et al. Effects of diabetes mellitus on the biomechanical properties of human ankle cartilage. Clin Orthop Relat Res. 1999;368:182-189. [PubMed] [Google Scholar]
- 3. Badekas T, Takvorian M, Souras N. Treatment principles for osteochondral lesions in foot and ankle. Int Orthop. 2013;37(9):1697-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baltzer AW, Arnold JP. Bone-cartilage transplantation from the ipsilateral knee for chondral lesions of the talus. Arthroscopy. 2005;21(2):159-166. [DOI] [PubMed] [Google Scholar]
- 5. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41:988-1020. [PubMed] [Google Scholar]
- 6. Bilge O, Doral MN, Yel M, Karalezli N, Miniaci A. Treatment of osteonecrosis of the femoral head with focal anatomic-resurfacing implantation (HemiCAP): preliminary results of an alternative option. J Orthop Surg Res. 2015;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Braun BJ, Brandenburg LO, Braun C. Treatment of a partial avascular necrosis of a metacarpal head (Morbus Mauclaire Dieterich’s Disease) utilizing the ostechondral autograft transfer system (OATS) technique [in German]. Handchir Mikrochir Plast Chir. 2012;44(1):35-39. [DOI] [PubMed] [Google Scholar]
- 8. Dawson J, Doll H, Coffey J, Jenkinson C; Oxford and Birmingham Foot and Ankle Clinical Research Group. Responsiveness and minimally important change for the Manchester-Oxford foot questionnaire (MOXFQ) compared with AOFAS and SF-36 assessments following surgery for hallux valgus. Osteoarthritis Cartilage. 2007;15(8):918-931. [DOI] [PubMed] [Google Scholar]
- 9. de l’Escalopier N, Barbier O, Mainard D, Mayer J, Ollat D, Versier G. Outcomes of talar dome osteochondral defect repair using osteocartilaginous autografts: 37 cases of Mosaicplasty®. Orthop Traumatol Surg Res. 2015;101(1):97-102. [DOI] [PubMed] [Google Scholar]
- 10. Elias I, Zoga AC, Morrison WB, Besser MP, Schweitzer ME, Raikin SM. Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int. 2007;28(2):154-161. [DOI] [PubMed] [Google Scholar]
- 11. Ferkel RD, Chams RN. Chronic lateral instability: arthroscopic findings and long-term results. Foot Ankle Int. 2007;28(1):24-31. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira C, Vuurberg G, Oliveira JM, et al. Good clinical outcome after osteochondral autologous transplantation surgery for osteochondral lesions of the talus but at the cost of a high rate of complications: a systematic review [published online May 6, 2016]. J ISAKOS Joint Dis Orthop Sports Med. doi: 10.1136/jisakos-2015-000020 [DOI] [Google Scholar]
- 13. Giza E, Howell S. Allograft juvenile articular cartilage transplantation for treatment of talus osteochondral defects. Foot Ankle Spec. 2013;6(2):141-144. [DOI] [PubMed] [Google Scholar]
- 14. Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006;22(10):1085-1092. [DOI] [PubMed] [Google Scholar]
- 15. Hannon CP, Smyth NA, Murawski CD, et al. Osteochondral lesions of the talus: aspects of current management. Bone Joint J. 2014;96(2):164-171. [DOI] [PubMed] [Google Scholar]
- 16. Kadakia AR, Espinosa N. Why allograft reconstruction for osteochondral lesion of the talus? The osteochondral autograft transfer system seemed to work quite well. Foot Ankle Clin. 2013;18(1):89-112. [DOI] [PubMed] [Google Scholar]
- 17. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353. [DOI] [PubMed] [Google Scholar]
- 18. Kline AJ, Hasselman CT. Resurfacing of the metatarsal head to treat advanced hallux rigidus. Foot Ankle Clin. 2015;20(3):451-463. [DOI] [PubMed] [Google Scholar]
- 19. Lambers KTA, Dahmen J, Reilingh ML, van Bergen CJA, Stufkens SAS, Kerkhoffs G. No superior surgical treatment for secondary osteochondral defects of the talus [published online July 7, 2016]. Knee Surg Sports Traumatol Arthrosc. doi: 10.1007/s00167-017-4629-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murawski CD, Kennedy JG. Operative treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2013;95(11):1045-1054. [DOI] [PubMed] [Google Scholar]
- 21. O’Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38(2):392-404. [DOI] [PubMed] [Google Scholar]
- 22. Reilingh ML, van Bergen CJ, Gerards RM, van Eekeren IC, van Dijk CN. HemiCAP for secondary treatment for osteochondral talar defects. In Arthroscopy. Berlin, Germany: Springer; 2016:1013-1022. [Google Scholar]
- 23. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794. [DOI] [PubMed] [Google Scholar]
- 24. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283-291. [DOI] [PubMed] [Google Scholar]
- 25. Scranton PE, Jr, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br. 2006;88(5):614-619. [DOI] [PubMed] [Google Scholar]
- 26. Sierevelt IN, Beimers L, van Bergen CJ, Haverkamp D, Terwee CB, Kerkhoffs GM. Validation of the Dutch language version of the Foot and Ankle Outcome Score. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2413-2419. [DOI] [PubMed] [Google Scholar]
- 27. Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2013;41(3):519-527. [DOI] [PubMed] [Google Scholar]
- 28. van Bergen CJ, Tuijthof GJ, Reilingh ML, van Dijk CN. Clinical tip: aiming probe for a precise medial malleolar osteotomy. Foot Ankle Int. 2012;33(9):764-766. [DOI] [PubMed] [Google Scholar]
- 29. van Bergen CJ, Tuijthof GJ, Sierevelt IN, van Dijk CN. Direction of the oblique medial malleolar osteotomy for exposure of the talus. Arch Orthop Trauma Surg. 2011;131(7):893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Bergen CJ, van Eekeren IC, Reilingh ML, Sierevelt IN, van Dijk CN. Treatment of osteochondral defects of the talus with a metal resurfacing inlay implant after failed previous surgery: a prospective study. Bone Joint J. 2013;95(12):1650-1655. [DOI] [PubMed] [Google Scholar]
- 31. van Bergen CJ, Zengerink M, Blankevoort L, van Sterkenburg MN, van Oldenrijk J, van Dijk CN. Novel metallic implantation technique for osteochondral defects of the medial talar dome: a cadaver study. Acta Orthop. 2010;81(4):495-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Dijk CN. Ankle Arthroscopy: Techniques Developed by the Amsterdam Foot and Ankle School. Berlin, Germany; Springer-Verlag; 2014. [Google Scholar]
- 33. van Dijk CN, Reilingh ML, Zengerink M, van Bergen CL. Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc. 2010;18(5):570-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Dijk CN, Tol JL, Verheyen CC. A prospective study of prognostic factors concerning the outcome of arthroscopic surgery for anterior ankle impingement. Am J Sports Med. 1997;25(6):737-745. [DOI] [PubMed] [Google Scholar]
- 35. Visco A, Vieira LA, Goncalves FB, et al. Surface arthroplasty for treating primary and/or secondary shoulder osteoarthrosis by means of the HemiCAP-Arthrosurface® system. Rev Bras Ortop. 2011;46(3):288-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zanon G, Di Vico G, Marullo M. Osteochondritis dissecans of the talus. Joints. 2014;2(3):115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]