Abstract
Background:
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a laparoscopy-guided administration of aerosolized chemotherapy. PIPAC seems to improve objective tumor response, survival and quality of life in patients with peritoneal metastasis. We assessed feasibility and efficacy of PIPAC in patients with peritoneal metastasis (PM).
Methods:
Patients were included in a prospective PIPAC protocol. Patients with colorectal PM were treated with oxaliplatin, patients with other primary tumors were treated with cisplatin and doxorubicin. Any chemotherapy exposure for healthcare workers was monitored by environmental and biological sampling. Feasibility was quantified by completion and complication rates. Response evaluation was documented by the peritoneal regression grading score (PRGS) and by peritoneal lavage cytology. Biopsy sites were marked by clips. Quality of life questionnaires were collected at baseline and after 60, 120 and 180 days.
Results:
A total of 35 patients with PM were treated with a median of three PIPAC procedures (range 1–9). Intraperitoneal access and completion of PIPAC was achieved in all patients. Few complications and adverse events were noted. There was no risk of chemotherapy exposure for healthcare workers. The mean PRGS was reduced significantly and a reduction of the PRGS was seen in 67% of the patients. Conversion from positive to negative cytology was achieved in 23% of the patients. Quality of life was stabilized from baseline to day 60.
Conclusions:
PIPAC is feasible and well tolerated, may stabilize the quality of life in patients with end-stage PM and may induce histological and cytological regression.
This study is registered at www.clinicaltrials.gov [ClinicalTrials.gov identifier: NCT02320448].
Keywords: aerosol, carcinomatosis, intraperitoneal, metastasis, palliative treatment, peritoneum
Introduction
Peritoneal metastasis (PM) is a sinister denominator of various primary tumors, originating mainly from the gastrointestinal tract or ovaries. Patients with PM have a poor quality of life, short life expectancy and are troubled by deteriorating symptoms, such as abdominal pain, ascites and bowel obstruction.1–5 The standard treatment is palliative systemic chemotherapy or best supportive care, apart from highly selected patients with limited PM from ovarian or colorectal cancer, who are eligible for cytoreductive surgery.6,7 Systemic chemotherapy has a documented effect in a broad range of primary malignancies with disseminated disease (e.g. liver and lung metastases), but in contrast the evidence regarding patients with isolated PM is scarce and incomplete.5 This may be due to the patients’ poor performance status and the fact that small-volume PM and ascites are nonmeasurable lesions according to Response Evaluation Criteria In Solid Tumors (RECIST) criteria,8 which impedes response evaluation.
As PM is confined to the abdominal cavity, intraperitoneal administration of chemotherapy seems obvious. Due to low penetration rates, catheter-related complications and challenging treatment logistics,9–13 the results are still ambiguous. A novel intraperitoneal treatment approach has recently been introduced, where aerosolized chemotherapeutics are emitted within the peritoneal cavity during a standard laparoscopy at a capnoperitoneum of 12 mmHg, the so-called ‘pressurized intraperitoneal aerosol chemotherapy’ (PIPAC).14–16 PIPAC is an experimental treatment strategy, and there are no randomized, controlled trials confirming the first preliminary data, and according to recent reviews, the oncological efficacy must be upheld in prospective clinical trials.17,18 Still, administration of intraperitoneal chemotherapy by PIPAC seems well tolerated and able to induce histological regression, improve or stabilize quality of life and may even lead to prolonged survival.19–27 This is remarkable, as the dose of chemotherapy administered during PIPAC is substantially reduced, when comparing with the normal systemic dose, and the included patients are often heavily pretreated with platinum-based combination chemotherapy regimens. Still, the available studies are mostly preclinical or retrospective17 and constrained by lacking baseline histology, which obscures the influence of histological response to prior systemic chemotherapy.
Study rationale
PIPAC is a combined surgical and oncological palliative treatment modality in patients with isolated PM from any origin. The main outcome of this prospective clinical study, is to document feasibility of PIPAC. Second, we evaluate the PIPAC treatment by using strict predefined response evaluation criteria and report this new response evaluation strategy.
Materials and methods
Patients were included from March 2015 to October 2016 and the last PIPAC was completed in July 2017. Patients were eligible for data analysis from the day of admission for the first PIPAC procedure. Inclusion criteria were patients with isolated PM who (1) progressed on standard palliative systemic chemotherapy, or (2) refused systemic chemotherapy, or (3) patients who, at the discretion of the medical oncologist, were ineligible for further systemic chemotherapy, due to treatment-related side effects (e.g. fatigue, neuropathy, nausea). Patients with Eastern Cooperative Oncology Group (ECOG) performance status >2, age ⩽ 18 years, bowel obstruction (total parenteral nutrition, nasogastric tube), history of platinum allergy, impaired liver, renal, heart (New York Heart Association class >2) or bone marrow function, and patients with extraperitoneal metastases on contrast-enhanced multi-slice computed tomography (CT) of the thorax and abdomen were excluded. Prior to therapy, each patient was evaluated at the local multidisciplinary tumor (MDT) conference. Patients were scheduled for three PIPAC procedures (q4–6 weeks) before re-evaluation at the MDT conference. To rule out extraperitoneal dissemination, a CT of the thorax and abdomen was performed at baseline and after every third PIPAC treatment. Based on a combination of tolerability, visual, radiological, cytological and histological regression, additional PIPAC treatment was planned.
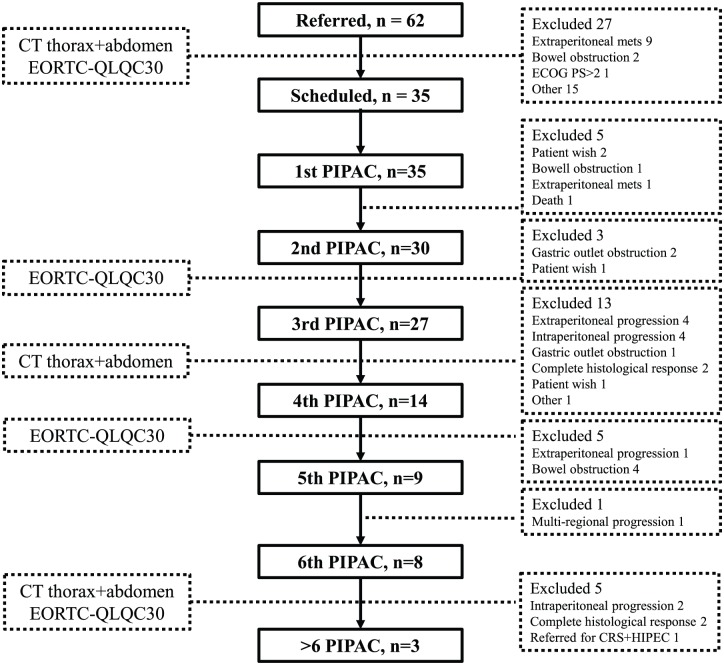
Overall, 60 patients were screened according to the predefined inclusion and exclusion criteria, and 35 patients were included (Figure 1). Primary endpoints were feasibility and response evaluation.
Figure 1.
Patient flow during PIPAC therapy.
CT, computed tomography; CRS, cyto-reductive surgery; ECOG PS, Eastern Cooperative Oncology Group performance status; EORTC-QLQC30, European Organisation for Research and Treatment of Cancer quality of life questionnaire; HIPEC, hyperthermic intraperitoneal chemotherapy.
PIPAC
PIPAC has been previously described,14,16 but in brief, PIPAC is a laparoscopy-controlled administration of pressurized intraperitoneal chemotherapy, performed in a standard operating room equipped with a ventilation system that complies with the requirements of ISO norm 14644-1 class 5. All procedures were performed by two certified PIPAC surgeons. Based on current evidence, patients with PM of colorectal or appendiceal origin were treated with oxaliplatin 92 mg/m2 in 150 ml dextrose, while patients with PM of other origin were treated with a combination of cisplatin 7.5 mg/m2 in 150 ml saline and doxorubicin 1.5 mg/m2 in 50 ml saline. After prophylactic antibiotics (3 g cefuroxime and 1.5 g metronidazole) and low-molecular heparin, two balloon safety trocars (5 and 12 mm; Applied Medical, Düsseldorf, Germany) were inserted through the abdominal wall guided by ultrasound, and a normothermic capnoperitoneum of 12 mmHg was obtained. After evacuation of ascites or peritoneal washing with 500 ml saline and mapping of the peritoneum according to Sugarbaker’s peritoneal cancer index (PCI),9 multiple (if possible up to four) peritoneal biopsies from PM were obtained and the biopsy sites marked by metal clips (Figure 2). A CE-certified nebulizer (CapnoPen®, Capnomed, Villingendorf, Germany) connected to a standard intravenous high-pressure injector (MEDRAD® salient dual contrast injector, Bayer HealthCare, Leverkusen, Germany) was inserted. The chemotherapy administration was remote controlled, since all personnel left the operating room before administration of the chemotherapeutics. At a flow rate of 30 ml/min and a maximum pressure of 200 pounds per square inch, chemotherapy was delivered within 5–10 min. After another 25 min of simple diffusion, the chemotherapy aerosol was evacuated with a closed line, through two sequential micro particle filters and the patient was closed with standard sutures. Postoperative pain was relieved by paracetamol (1 g × 4) and on-demand ibuprofen or morphine, if necessary. Postoperative nausea and vomiting (PONV) were minimized by perioperative dexamethasone and ondansetron (8 mg × 2) at day 0–2. If the postoperative period was uneventful, the patients were informed and discharged at the day of PIPAC or the first postoperative day. PONV were recorded at the morning of the first postoperative day and postoperative pain was evaluated on a visual analog scale of 0–10. Blood samples were analysed at day 1 and 10 after each PIPAC treatment to document toxicity. Screening for adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (National Cancer Institute) was performed according to 30 days definitions.
Figure 2.

(a) Punch biopsy of a PM. (b) The biopsy sites are marked by clips.
Feasibility
Feasibility was documented for both healthcare workers and patients. The environmental risk of chemotherapy exposure was documented through analysis of the air in the operating room (OR) and biologically, by taking blood samples of the two PIPAC surgeons, in order to detect platinum concentrations. The environmental measurements were performed by a national independent organization (Water and Environment, Life Science, Danish Technological Institute, Aarhus, Denmark).23
In terms of patient safety, PIPAC was feasible if (1) laparoscopic access was possible in 80% (28/35) of the patients, (2) if the PIPAC procedure was completed in 80% of the patients, (3) if there were no CTCAE grade 4 or 5 events, and (4) if 80% of the patients were discharged within 2 days after the procedure.
Response evaluation
Histology
If technically possible, four peritoneal quadrant biopsies were taken prior to each PIPAC procedure. The peritoneal biopsies were fixed in formalin (6–24 h) and embedded in paraffin. A total of three step sections were cut from the paraffin-embedded tissue blocks and stained with haematoxylin-eosin (H&E), followed by a section immunostained for epithelial cell adhesion molecule (Ep-CAM) and a final series of three step sections stained with H&E. If necessary, the pathologist used additional immunohistochemical stains, dependent on the particular biopsy and the type of primary tumor. For evaluation of the histological regression, the peritoneal regression grading score (PRGS) was used.28 The PRGS distinguishes between four grades of tumor regression, PRGS 1–4, depending on the ratio between regressive changes (inflammation, fibrosis, cell-free mucin lakes, accumulation of macrophages, infarct-like necrosis) and the presence of vital cancer cells or classical necrosis. PRGS 1 (complete response) is defined as no tumor cells present; PRGS 2 (major response) is regressive changes predominant over tumor cells; PRGS 3 (minor response) is predominance of tumor cells but regressive changes present, and PRGS 4 (no response) is no regressive changes present. The PRGS prior to the first PIPAC procedure is particularly important in order to document response to prior systemic chemotherapy, and since it forms a baseline for subsequent PIPAC response evaluation. As multiple biopsies are taken during each PIPAC procedure, it is recommended that the highest and the mean PRGS score should be reported in PIPAC publications.28
Cytology
A total of 150 ml of ascites or peritoneal lavage fluid was retrieved and analysed, while the remaining intraperitoneal fluid was evacuated and discarded. The fluid was centrifuged, and smears of the sediment were analysed using conventional cytology (Papanicolaou and May–Giemsa Grünwald staining). Leftovers of the sediment were embedded in paraffin wax. One section from the paraffin block was stained with H&E. If necessary, further sections were cut for immunocytochemical analyses for markers such as calretinin, CDX2, carcinoembryonic antigen (CEA), cytokeratin (CK) 7, CK20, Ep-CAM, Pax8 or maspin. For cytological evaluation, a five-tiered score was used: malignant cells, suspicious cells, atypical cells, no malignant cells, other. In the data analysis, only malignant cells were defined as positive cytology.
Quality of life
Quality of life was monitored by the European Organisation for Research and Treatment of Cancer quality of life questionnaire (EORTC-QLQC30)29 at baseline, day 60, day 120 and day 180.
Statistics
This was a descriptive study, and as such, a substantial number of patients were required to document response evaluation and feasibility. The study group did not perform a power calculation a priori, but estimated that 35 patients should be included. Values are given as means or medians where appropriate. Comparisons were performed using a Wilcoxon Mann–Whitney test for continuous data, p-values were two-tailed and a p-value of 0.05 was considered statistically significant. The statistical software Stata, version 13 (Stata Corp, Texas, USA) was used for statistical analysis.
Compliance with ethical standards
The study complies with the Helsinki Declaration and was approved by The Regional Committees on Health Research Ethics for Southern Denmark (Project-ID: S-20140211, www.clinicaltrials.gov NCT02320448), and all participants gave oral and written informed consent.
Results
During the study period, 35 patients with PM of any origin were treated with a total of 129 PIPAC procedures (median = 3, range 1–9). Primary tumor origin, previous systemic chemotherapy and demographic data are listed in Table 1. A total of 91% (32/35) of the patients had received palliative chemotherapy, and 3 patients had refused systemic chemotherapy. Overall, five patients (14%) received bidirectional chemotherapy, with an interval of 2 weeks from systemic chemotherapy to PIPAC, and 1 week from PIPAC to systemic chemotherapy. Among the 35 included patients, 30 and 27 patients were treated with two or three PIPAC procedures, respectively, and 14 patients received four or more PIPACs (Figure 1). One patient was excluded due to nontreatment-related death after the first PIPAC procedure. The patient had a nonresectable cholangiocarcinoma with internal drainage of the bile duct. She suffered from stent occlusion and cholangitis starting 3 weeks after PIPAC treatment and died 3 months later.
Table 1.
Demographic data and baseline characteristics.
| Demographic variables | |
| Age, years (range) | 65 (41–84) |
| Sex (male/female) | 19/16 |
| Months from diagnosis of PM (range) | 9.6 (1.5–130) |
| ECOG performance status 0/1 | 32 |
| ECOG performance status 2 | 3 |
| Previous treatment | no. patients |
| One-line palliative SC | 22 |
| Two-line palliative SC | 9 |
| >Two-line palliative SC | 1 |
| Combination PIPAC/SC | 5 |
| Primary tumor origin | no. patients |
| Stomach | 5 |
| Small bowel | 2 |
| Cholangiocarcinoma | 2 |
| Pancreas | 3 |
| Appendix incl. PMP | 4 |
| Colorectal | 12 |
| MPM | 1 |
| Ovarian | 5 |
| MUP | 1 |
| Primary tumor in situ | 14 |
| Baseline PM characteristics at PIPAC 1 | |
| PCI | Score (SD) |
| PCI when ⩾11 regions evaluated | 15.37 (11.56) |
| PCI when <11 regions evaluated | 6.6 (4.10) |
| PCI, total | 14.11 (11.20) |
| Ascites volume | no. patients |
| 0 ml | 22 |
| 1–500 ml | 7 |
| 501–1000 ml | 3 |
| >1000 ml | 3 |
ECOG, Eastern Cooperative Oncology Group; MPM, malignant peritoneal mesothelioma; MUP, metastasis of unknown primary; PCI, peritoneal cancer index; PIPAC, pressurized intraperitoneal aerosol chemotherapy; PM, peritoneal metastasis; PMP, pseudomyxoma peritoneii; SC, systemic chemotherapy; SD, standard deviation.
Feasibility
No detectable concentration of platinum particles was found at analysis of the air in the OR during two consecutive PIPAC procedures. No traces of cisplatin were detected in the blood samples from the surgeons.
Laparoscopic access and completion of PIPAC was achieved in all patients, both at primary and subsequent procedures. The procedure time did not change from the first to the third PIPAC procedure (Table 2). Postoperative nausea, vomiting and pain scores remained low throughout the course of therapy. 80% (28/35) and 89% (24/27) of the patients were discharged within 24 h after the first and the third PIPAC, respectively.
Table 2.
Feasibility and response evaluation data.
| Procedures | |||
| Total no. PIPAC procedures | 129 | ||
| PIPACs/pt, mean (SD) | 3.66 (1.95) | ||
| PIPACs/pt, median (range) | 3 (1–9) | ||
| Technical aspects | PIPAC 1 (n = 35) | PIPAC 3 (n = 27) | |
| Intraperitoneal access, no. patients (%) | 35 (100%) | 27 (100%) | |
| Procedure time, minutes (range) | 100 (71–156) | 92 (77–125) | |
| Days of admission, median (range) | 1 (1–4) | 1 (0–3) | |
| Discharge day 0/1, no. patients (%) | 28 (80%) | 24 (89%) | |
| Pain/PONV | |||
| Postoperative pain*, median (range) | 3 (0–10) | 3 (0–7) | |
| Postoperative nausea, no. patients | |||
| None | 24 | 14 | |
| Mild | 2 | 4 | |
| Moderate | 1 | 3 | |
| Severe | 8 | 5 | |
| Postoperative vomiting, no. patients | |||
| None | 26 | 18 | |
| 1 vomit | 2 | 1 | |
| 2–3 vomits | 3 | 2 | |
| >3 vomits | 4 | 5 | |
| Response evaluation | PIPAC 1 | PIPAC 3 | |
| PRGS, mean (SD) | 2.05 (0.66) | 1.54 (0.52) |
p = 0.0006 |
| PRGS, maximum value | 4 | 3 | |
| PRGS improvement, patients (%) | 18/27 (67%) | ||
| Malignant cells in peritoneal lavage | 13/22 (59%) | 9/22 (41%) | |
| Conversion nonmalignant → malignant | 1/22 (4.5%) | ||
| Conversion malignant → nonmalignant | 5/22 (23%) | ||
| No conversion | 8/22 (36%) |
Visual Analog Scale 1–10.
PIPAC, pressurized intraperitoneal aerosol chemotherapy; PONV, postoperative nausea and vomiting; PRGS, peritoneal regression grading score; Pt, patient; SD, standard deviation.
Grading of adverse events during the course of therapy is presented in Figure 3. One life-threatening adverse event was reported, as one of the patients had an iatrogenic perforation of the jejunum necessitating reoperation with primary suture on the first postoperative day. The perforation was just below the access site of the first trocar, and the bowel wall was otherwise intact, which makes a mechanical perforation most plausible. A total of four patients had severe adverse events: One patient had diarrhea necessitating hospitalization, another had a small bowel obstruction, a third had duodenal obstruction and the fourth patient had cholestasis. Most patients with moderate adverse events had urinary retention in the early postoperative period.
Figure 3.
Adverse events.
CTCAE, Common Terminology Criteria for Adverse Events.
Response evaluation
Based on the PRGS scores prior to PIPAC 1 and PIPAC 3, 67% of the patients had an objective tumor response and the overall mean PRGS score was reduced from 2.05 to 1.54 (p = 0.0006). Cytologically, data from both PIPAC 1 and PIPAC 3 were available, and free intraperitoneal tumor cells were eradicated in 5/22 (23%) patients (Table 2).
The quality of life according to the EORTC-QLQC30 global health score (Figure 4), and based on symptom and function scores, was stable between baseline and day 60, while there were not enough data from day 120 and day 180 for statistical analysis.
Figure 4.
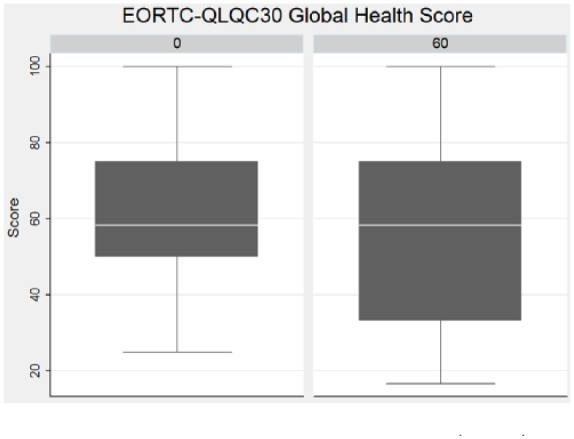
Global quality of life at baseline (day 0) and after 60 days according to EORTC-QLQC30.
EORTC-QLQC30, European Organisation for Research and Treatment of Cancer quality of life questionnaire.
Discussion
The PIPAC procedure was feasible and well tolerated for both healthcare workers and patients with no occupational exposure to chemotherapy.23 Laparoscopic access to the abdominal cavity and completion of PIPAC was achieved in all patients. PIPAC was repeatable in 30/35 patients with low toxicity and procedure related complications, but one life-threatening event occurred, due to unrecognized bowel lesion during open access to the abdomen, which is seen in 0.1–0.2% of all laparoscopies,30 and this was not related to the chemotherapy. This is the first prospective study assessing both cytological and histological response based on PRGS in a large series of 35 consecutive patients treated with PIPAC. PIPAC induced a significant histological response in patients with PM of different origin, and an objective tumor response with reduction of the mean PRGS score was observed in 67% of the patients. PIPAC eradicated free intraperitoneal tumor cells in 23% of the patients. Furthermore, PIPAC stabilized the patients’ quality of life, as documented by the EORTC-QLQC30 global health score, which has also been previously described.21,31
This study is important, since the results were obtained by adhering to a strict predefined strategy to evaluate the response to PIPAC. Biopsies and peritoneal lavage cytology were mandatory at baseline and during each PIPAC treatment, and the effect of both previous treatment strategies (i.e. systemic chemotherapy) and PIPAC could be monitored. The actual effect of systemic chemotherapy has been difficult to evaluate in previous PIPAC studies.17 In general, the effect of PIPAC has been defined and monitored by different methods (RECIST, PCI score, quality of life, histopathological tumor regression, median survival and changes in gene expression17), but none of these are without limitations. The proposed response evaluation strategy in the present study was based on repeated histological biopsies for PRGS assessment and peritoneal lavage cytology. All biopsy sites were marked by clips to reduce the risk of sampling error when taking the subsequent biopsies (Figure 2), but this method may also harbour some limitations. In a few cases, the clips were displaced, or the access to clip-marked elements were restricted by adhesions. Next, the distribution of the aerosolized chemotherapeutics might have been inhomogeneous, which in theory could have led to different histological regression scores, depending on the distance between the biopsy sites and the nebulizer.32 This supports future studies with both histological and cytological evaluation.
Eligibility for cytoreductive surgery is usually based on the PCI score,9 which PIPAC has been shown to improve.33 We find the PCI valuable to describe the study population at the index PIPAC procedure, but based on the adhesions caused by earlier resections, the two-trocar strategy and the treatment-induced peritoneal changes, PCI is a suboptimal tool for evaluation of response to PIPAC as the PCI cannot differ between macroscopic progression and treatment-induced fibrosis. Still, resectability assessment is important, since a fraction of patients may become candidates for cytoreductive surgery,34 and despite the mentioned visual limitations, the Dutch Seven Regions Count could serve as a simple macroscopic surveillance tool during the course of PIPAC therapy.35 CT of the thorax and abdomen is used to detect extraperitoneal lesions but has a low sensitivity for low-volume PM. However, future studies should investigate, whether diffusion-weighted gadolinium enhanced magnetic resonance imaging (MRI) is a useful additional response evaluation modality.36–38
While this study documents PIPAC treatment feasibility and efficacy in a broad range of referred patients with PM, its interpretation is limited due to the heterogeneous study population with 10 different primary tumors and 14 patients with a primary tumor in situ. Overall five patients were treated with a combination of systemic chemotherapy and PIPAC, which impedes isolated interpretation of the PIPAC treatment. The bidirectional concept of alternating PIPAC and systemic chemotherapy may improve overall outcome and based on the available data bidirectional therapy does not seem to carry an increased risk of complications.33,39
Preliminary survival data from PIPAC studies are interesting with reported survival rates in PM patients of 11–16 months,20,21,24–27,40 but study size, design and patient heterogeneity are major problems. The present study was not powered for subgroup analyses regarding survival. We chose to focus on an objective response evaluation based on data from the first and third PIPAC, as PIPAC treatment was planned in series of three. A total of 14 patients received more than three PIPACs, but due to the low patient number and different tumor types, the effect of more than three PIPAC procedures still has to be investigated.
Conclusion
Intraperitoneal chemotherapy administered through the PIPAC drug-delivery system was feasible and may be safely implemented at a tertiary referral center. Using a strict and uniform response evaluation process, PIPAC can induce statistically significant histological regression in a population of patients with PM, and an objective tumor response in two out of three patients. Cytologically, free intraperitoneal tumor cells are eradicated in selected patients, but the clinical impact of this finding in patients with visible PM is still unclear. More research in strategies and tools for objective response evaluation is needed, and the present data should be confirmed in larger PIPAC studies focusing on specific diseases before embarking on randomized multicenter trials.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Martin Graversen  https://orcid.org/0000-0001-5719-4916
https://orcid.org/0000-0001-5719-4916
Sönke Detlefsen  https://orcid.org/0000-0002-9466-2333
https://orcid.org/0000-0002-9466-2333
Contributor Information
Martin Graversen, Odense PIPAC Center (OPC) Odense Pancreas Center (OPAC) Odense Patient data Explorative Network (OPEN) Department of Surgery, Odense University Hospital, J.B. Winsloews Vej 4, Odense 5000, Denmark.
Sönke Detlefsen, OPC, OPAC, Department of Pathology, Odense University Hospital, Denmark.
Jon Kroll Bjerregaard, OPC, OPAC, Department of Oncology, Odense University Hospital, Denmark.
Claus Wilki Fristrup, OPC, OPAC, Department of Surgery, Odense University Hospital, Denmark.
Per Pfeiffer, OPC, OPAC, Department of Oncology, Odense University Hospital, Denmark.
Michael Bau Mortensen, OPC, OPAC, Department of Surgery, Odense University Hospital, Denmark.
References
- 1. Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000; 88: 358–363. [DOI] [PubMed] [Google Scholar]
- 2. Coccolini F, Gheza F, Lotti M, et al. Peritoneal carcinomatosis. World J Gastroenterol 2013; 19: 6979–6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 2014; 134: 622–628. [DOI] [PubMed] [Google Scholar]
- 4. Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist 2015; 20: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol 2016; 17: 1709–1719. [DOI] [PubMed] [Google Scholar]
- 6. Elferink MA, de Jong KP, Klaase JM, et al. Metachronous metastases from colorectal cancer: a population-based study in North-East Netherlands. Int J Colorectal Disease 2015; 30: 205–212. [DOI] [PubMed] [Google Scholar]
- 7. Bjerregaard JK, Mortensen MB, Schonnemann KR, et al. Characteristics, therapy and outcome in an unselected and prospectively registered cohort of pancreatic cancer patients. Eur J Cancer 2013; 49: 98–105. [DOI] [PubMed] [Google Scholar]
- 8. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 9. Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol 1998; 14: 254–261. [DOI] [PubMed] [Google Scholar]
- 10. Takahara N, Isayama H, Nakai Y, et al. Intravenous and intraperitoneal paclitaxel with S-1 for treatment of refractory pancreatic cancer with malignant ascites. Invest New Drugs 2016; 34: 636–642. [DOI] [PubMed] [Google Scholar]
- 11. Oman M, Blind PJ, Naredi P, et al. Treatment of non-resectable pancreatic cancer with intraperitoneal 5-FU and leucovorin IV. Eur J Surg Oncol 2001; 27: 477–481. [DOI] [PubMed] [Google Scholar]
- 12. Kim SW, Paek J, Nam EJ, et al. The feasibility of carboplatin-based intraperitoneal chemotherapy in ovarian cancer. Eur J Obstet Gynecol Reprod Biol 2010; 152: 195–199. [DOI] [PubMed] [Google Scholar]
- 13. Fung-Kee-Fung M, Provencher D, Rosen B, et al. Intraperitoneal chemotherapy for patients with advanced ovarian cancer: a review of the evidence and standards for the delivery of care. Gynecol Oncol 2007; 105: 747–756. [DOI] [PubMed] [Google Scholar]
- 14. Solass W, Kerb R, Murdter T, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014; 21: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Solass W, Herbette A, Schwarz T, et al. Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: proof of concept. Surg Endosc 2012; 26: 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solass W, Hetzel A, Nadiradze G, et al. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 2012; 26: 1849–1855. [DOI] [PubMed] [Google Scholar]
- 17. Grass F, Vuagniaux A, Teixeira-Farinha H, et al. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg 2017; 104: 669–678. [DOI] [PubMed] [Google Scholar]
- 18. Dueckelmann AM, Fink D, Harter P, et al. The use of PIPAC (pressurized intraperitoneal aerosol chemotherapy) in gynecological oncology: a statement by the German “Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR)”, the Swiss and Austrian AGO, and the North-Eastern German Society of Gynaecologic Oncology. Arch Gynecol Obstet 2018; 297: 837–846. [DOI] [PubMed] [Google Scholar]
- 19. Solass W, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol 2013; 20: 3504–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nadiradze G, Giger-Pabst U, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg 2016; 20: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Odendahl K, Solass W, Demtroder C, et al. Quality of life of patients with end-stage peritoneal metastasis treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Eur J Surg Oncol 2015; 41: 1379–1385. [DOI] [PubMed] [Google Scholar]
- 22. Blanco A, Giger-Pabst U, Solass W, et al. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol 2013; 20: 2311–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graversen M, Pedersen PB, Mortensen MB. Environmental safety during the administration of pressurized intraperitoneal aerosol chemotherapy. Pleura Peritoneum 2016; 1: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Demtroder C, Solass W, Zieren J, et al. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis 2016; 18: 364–371. [DOI] [PubMed] [Google Scholar]
- 25. Graversen M, Detlefsen S, Bjerregaard JK, et al. Peritoneal metastasis from pancreatic cancer treated with pressurized intraperitoneal aerosol chemotherapy (PIPAC). Clin Exp Metastasis 2017; 34: 309–314. [DOI] [PubMed] [Google Scholar]
- 26. Tempfer CB, Celik I, Solass W, et al. Activity of pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in women with recurrent, platinum-resistant ovarian cancer: preliminary clinical experience. Gynecol Oncol 2014; 132: 307–311. [DOI] [PubMed] [Google Scholar]
- 27. Tempfer CB, Winnekendonk G, Solass W, et al. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: a phase 2 study. Gynecol Oncol 2015; 137: 223–228. [DOI] [PubMed] [Google Scholar]
- 28. Solass W, Sempoux C, Detlefsen S, et al. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum 2016; 1: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85: 365–376. [DOI] [PubMed] [Google Scholar]
- 30. Ahmad G, Gent D, Henderson D, et al. Laparoscopic entry techniques. Cochrane Database Syst Rev 2015; (8): Cd006583. [DOI] [PubMed] [Google Scholar]
- 31. Teixeira Farinha H, Grass F, Kefleyesus A, et al. Impact of pressurized intraperitoneal aerosol chemotherapy on quality of life and symptoms in patients with peritoneal carcinomatosis: a retrospective cohort study. Gastroenterol Res Pract 2017; 2017: 4596176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khosrawipour V, Khosrawipour T, Diaz-Carballo D, et al. Exploring the spatial drug distribution pattern of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Ann Surg Oncol 2016; 23: 1220–1224. [DOI] [PubMed] [Google Scholar]
- 33. Alyami M, Gagniere J, Sgarbura O, et al. Multicentric initial experience with the use of the pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur J Surg Oncol 2017; 43: 2178–2183. [DOI] [PubMed] [Google Scholar]
- 34. Girshally R, Demtroder C, Albayrak N, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2016; 14: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Verwaal VJ, van Tinteren H, van Ruth S, et al. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br J Surg 2004; 91: 739–746. [DOI] [PubMed] [Google Scholar]
- 36. Michielsen K, Vergote I, Op de Beeck K, et al. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: a clinical feasibility study in comparison to CT and FDG-PET/CT. Eur Radiol 2014; 24: 889–901. [DOI] [PubMed] [Google Scholar]
- 37. Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 2012; 19: 1394–1401. [DOI] [PubMed] [Google Scholar]
- 38. Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 2015; 22: 1708–1715. [DOI] [PubMed] [Google Scholar]
- 39. Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol 2016; 14: 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tempfer CB, Rezniczek GA, Ende P, et al. Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin in women with peritoneal carcinomatosis: a cohort study. Anticancer Res 2015; 35: 6723–6739. [DOI] [PubMed] [Google Scholar]