Abstract
Background:
The efficacy and safety of fluticasone propionate/formoterol fumarate pressurized metered-dose inhaler (pMDI) (fluticasone/formoterol; Flutiform®; 100/10 µg b.i.d.) was compared with fluticasone propionate (Flixotide® Evohaler® pMDI; 100 µg b.i.d.) and fluticasone/salmeterol (Seretide® Evohaler® pMDI; 100/50 µg b.i.d.) in a pediatric asthma population (EudraCT number: 2010-024635-16).
Methods:
A double-blind, double-dummy, parallel group, multicenter study. Patients, aged 5–<12 years with persistent asthma ⩾ 6 months and forced expiratory volume in 1 s (FEV1) ⩽ 90% predicted were randomized 1:1:1 to 12 weeks’ treatment. The study objectives were to demonstrate superiority of fluticasone/formoterol to fluticasone and non-inferiority to fluticasone/salmeterol.
Results:
A total of 512 patients were randomized: fluticasone/formoterol, 169; fluticasone, 173; fluticasone/salmeterol, 170. Fluticasone/formoterol was superior to fluticasone for the primary endpoint: change from predose FEV1 at baseline to 2 h postdose FEV1 over 12 weeks [least squares (LS) mean difference 0.07 l; 95% confidence interval (CI) 0.03, 0.11; p < 0.001] and the first key secondary endpoint, FEV1 area under the curve over 4 hours (AUC0–4 h) at week 12 (LS mean difference 0.09 l; 95% CI: 0.04, 0.13; p < 0.001). Per a prespecified non-inferiority margin of −0.1 l, fluticasone/formoterol was non-inferior to fluticasone/salmeterol for the primary endpoint (LS mean difference 0.00 l; 95% CI −0.04, 0.04; p < 0.001) and first key secondary endpoint (LS mean difference 0.01; 95% CI −0.03, 0.06; p < 0.001). Fluticasone/formoterol was non-inferior to fluticasone/salmeterol for the second key secondary endpoint, change from predose FEV1 over 12 weeks (treatment difference −0.02 l; 95% CI −0.06, 0.02; p < 0.001), but was not superior to fluticasone for this endpoint (LS mean difference 0.03 l; 95% CI −0.01, 0.07; p = 0.091). All treatments elicited large improvements from baseline to week 12 for the Pediatric Asthma Quality of Life Questionnaire (LS mean change 0.76 to 0.85 units) and Asthma Control Questionnaire (LS mean change −1.03 to −1.13 units). Few severe exacerbations were seen (fluticasone/formoterol: two; fluticasone/salmeterol: two). All treatments were well tolerated.
Conclusions:
This study supports the efficacy and safety of fluticasone/formoterol in a pediatric asthma population and its superiority to fluticasone.
Keywords: asthma, children, combination therapy, fluticasone propionate, formoterol fumarate, ICS/LABA
Introduction
Inhaled corticosteroid (ICS) and long-acting β2-agonist (LABA) combinations are recommended as a Step 3 controller option in children aged 6–11 years in the Global Initiative for Asthma (GINA) guidelines,1 where asthma is uncontrolled on ICS alone, although the preferred step-up therapy in this age group is medium-dose ICS.
Single-inhaler combination ICS/LABA therapy has been shown to increase treatment adherence and may improve treatment outcomes compared with free combinations of ICS and LABA as it assures concomitant administration of ICS.2–4 To date, only two ICS/LABA combination therapies are available for use in children, namely fluticasone/salmeterol as both a dry powder inhaler (DPI) and pressurized metered-dose inhaler (pMDI) (Seretide® Accuhaler®/Evohaler®, respectively) and budesonide/formoterol DPI (Symbicort® Turbohaler®).
Fluticasone propionate is an effective, well-established ICS, providing sustained anti-inflammatory effects. Formoterol fumarate is the most rapid-acting LABA, with a speed of onset comparable to the short-acting β2-agonist, salbutamol. Flutiform®, fluticasone propionate and formoterol fumarate (fluticasone/formoterol) combination therapy via an HFA-propelled pMDI, has been evaluated in a number of studies in adults and/or adolescents with mild to severe asthma,5–14 and has been approved for use in this population in over 30 countries in Europe, Asia, and elsewhere. A single open-label study has also been conducted in pediatric asthmatic patients.15 The present study (FFLAIR: Fluticasone propionate/FormoteroL Assessed In pediatric asthma) was designed to further evaluate the efficacy and safety of fluticasone/formoterol in the pediatric population (EudraCT number: 2010-024635-16).
Methods
Participants
Male and female patients, aged 5 to <12 years, with persistent asthma for ⩾6 months, on a stable ICS dose for ⩾4 weeks, with predose forced expiratory volume in 1 s (FEV1) ⩾ 60% to ⩽90% predicted, ⩾15% FEV1 reversibility, and inadequate asthma control on an ICS alone at a dose of ⩽500 µg/day fluticasone (or equivalent) or controlled asthma on an ICS/LABA combination at an ICS dose of ⩽200 µg/day fluticasone (or equivalent), were eligible for enrolment.
Exclusion criteria were specified to ensure patient safety, for example, by excluding patients with potentially brittle asthma evidenced by life-threatening asthma within the past year, hospitalization or an emergency room visit for asthma within the past 6 months, systemic (injectable or oral) corticosteroid medication within 1 month and by excluding patients with current or prior nonresponse or partial response only to an ICS/LABA combination. Exclusion criteria were also specified to ensure disease stability at study entry, for example, by excluding patients with a clinically significant upper or lower respiratory infection within 4 weeks prior to study entry. Patients with coexistent pulmonary diseases (e.g. cystic fibrosis, bronchiectasis, tuberculosis) were also excluded.
Study design
This was a multicenter, randomized, double-blind, parallel-group study. Eligible patients entered a 14-day run-in period during which they received fluticasone pMDI (Flixotide® Evohaler®, GlaxoSmithKline, UK) 100 µg twice daily (b.i.d.). Salbutamol pMDI (Ventolin® Evohaler®, GlaxoSmithKline, UK) 100 µg was used as rescue medication. Patients completed an electronic diary daily to record rescue medication use, study medication use, asthma symptom scores, sleep disturbance due to asthma, and morning and evening peak flow (PEFR). The daytime and night-time symptom scales used are nonvalidated but have been employed in multiple prior studies.5–15 Peak flow manoeuvres were performed in triplicate each morning and evening with the maximum value obtained used in subsequent analyses. Asthma symptoms were scored from 0 (no symptoms) to 5 (asthma so severe you cannot carry out normal daily activities). Sleep disturbance was scored from 0 (slept through the night, no asthma) to 4 (could not sleep at all due to asthma). An asthma control day was defined as a day with no asthma symptoms, no sleep disturbance due to asthma, and no rescue medication use. Mild to moderate asthma exacerbations were defined as at least 2 consecutive days with predose morning PEFR > 30% below baseline, and/or awakening due to asthma, and/or ⩾4 inhalations of rescue medication/day. Severe asthma exacerbations were defined as a deterioration in asthma requiring additional therapy (e.g. systemic corticosteroids) and/or emergency room visit or hospitalization, as derived from the American Thoracic Society (ATS)/European Respiratory Society (ERS) definitions.16
At the end of the run-in period, patients underwent pre- and 2 h postdose spirometry [FEV1, forced vital capacity (FVC), forced expiratory flow at 25%, 50%, and 75% of FVC (FEF25, FEF50, FEF75 and FEF25–75) and PEFR] and 4 h serial spirometry [FEV1 area under the curve over 4 h (AUC0–4 h)] performed in accordance with ATS/ERS standards17 (and subjected to centralized over-reading), asthma control (ACQ) and health status (Pediatric Asthma Quality of Life; PAQLQ) questionnaires were completed, a 12 h overnight urine collection was performed and, in a subgroup of patients, fractional exhaled nitric oxide (FeNO) was assessed. Patients were included in this subgroup based on the availability of FeNO equipment at site.
At the end of the run-in period, only patients fulfilling the following criteria were eligible for randomization: FEV1 ⩽ 90% predicted (following appropriate withholding of study medication) and, during the last 7 days of the run-in period, rescue medication use for at least 3 days and at least one night with sleep disturbance (i.e. sleep disturbance score of ⩾1) and/or at least 3 days with asthma symptoms (i.e. a symptom score of ⩾1). Note that the run-in period could be extended to 28 days if a patient failed to meet the randomization criteria after the initial 14-day period.
Eligible patients were randomized 1:1:1 to one of three treatment groups: fluticasone pMDI (Flixotide Evohaler) 100 µg b.i.d., fluticasone/formoterol pMDI [Flutiform] 100/10 µg b.i.d. or fluticasone/salmeterol pMDI [Seretide Evohaler] 100/50 µg b.i.d. Randomization was performed by the study sponsor using a validated system that automates the random assignment of treatment groups to randomization numbers, and was stratified to ensure balanced allocation within the age groups 5 to <8 years and 8 to <12 years.
Patients received study medication for 12 weeks.
All patients received two inhalers during the treatment period: active or placebo fluticasone and a corresponding active or placebo ICS/LABA (fluticasone/formoterol or fluticasone/salmeterol). Thus, allocation to ICS or ICS/LABA was fully blinded, whilst potential allocation to fluticasone/formoterol or fluticasone/salmeterol was open label.
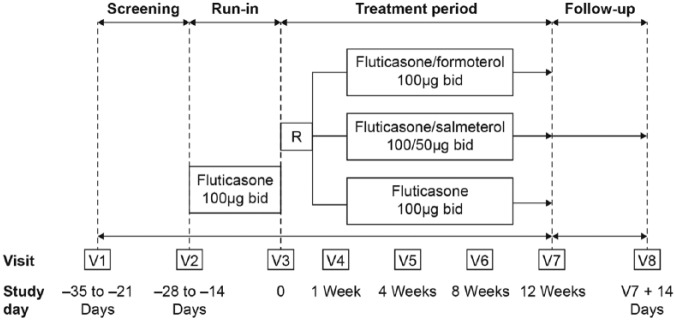
During the treatment period, patients attended four clinic visits (at weeks 1, 4, 8, 12) and completed their electronic diaries throughout the treatment period. Pre- and 2 h postdose lung function was assessed at each clinic visit. At week 12, 4 h serial spirometry was performed, a 12 h (overnight) urine collection was gathered (commencing the evening prior to the final clinic visit), ACQ and PAQLQ were completed, and FeNO was again assessed in a subgroup. The occurrence of adverse events (AEs) was monitored throughout the study whilst routine hematology and biochemistry were performed at screening and week 12. A 14-day follow-up period concluded the study (Figure 1).
Figure 1.
Study design.
b.i.d., twice daily; R, randomization; V, visit.
The study was performed in accordance with the Declaration of Helsinki, the International Council for Harmonization Harmonized Tripartite Guidelines for Good Clinical Practice, and approved by independent ethics committees (Table S1). All patients and their guardians/legally authorized representatives were provided with oral and written information describing the study. The patient’s guardian/legally authorized representative signed an informed consent form.
The primary endpoint was the change from predose FEV1 at baseline (day 1) to 2 h postdose FEV1 over the 12-week treatment period. The first key secondary endpoint was FEV1 AUC0–4h at week 12. The second key secondary endpoint was the change from predose FEV1 at baseline to predose FEV1 over the 12-week treatment period. These endpoints were selected on the basis of regulatory guidelines, following discussions with national regulatory authorities and the European Medicines Agency’s Pediatric Committee, and on the basis of the predictive value of pre- and postdose lung function for future exacerbation risk.16,18–21
Statistics
The sample size of 159 patients per group was based on an estimated difference between fluticasone/formoterol and fluticasone (the primary comparison) for the primary endpoint of 100 ml8,22,23 assuming a standard deviation of 274 ml,15 90% power, and a two-sided alpha (α) of 0.05.
The full analysis population (FAP) included all randomized patients who received at least one dose of study medication and had at least one valid efficacy (FEV1) assessment. The per protocol population (PPP) included all patients in the FAP without major protocol violations.
The primary and key secondary endpoints were analyzed using a repeated measures approach based on observed data. For these endpoints, the primary comparison between fluticasone/formoterol and fluticasone was tested for superiority, based on the FAP. The secondary comparison (fluticasone/formoterol versus fluticasone/salmeterol) was tested for non-inferiority, based on the PPP. Non-inferiority was concluded if the lower limit of the 95% confidence interval (CI) for the least squares (LS) mean difference between treatments was ⩾−0.1 l. Supportive analyses for each comparison were performed using the alternative population. To account for missing data, sensitivity analyses were performed using multiple imputation methods.
The primary endpoint was tested in a hierarchical (gate keeping) manner. The test procedure started with the fluticasone/formoterol versus fluticasone comparison (evaluating superiority) and continued to the fluticasone/formoterol versus fluticasone/salmeterol comparison (evaluating non-inferiority) in a confirmatory manner, only if the first comparison (fluticasone/formoterol versus fluticasone) was significant at the 0.05 α level. If both 2 h postdose FEV1 comparisons were significant at the 0.05 α level (i.e. showed superiority compared with fluticasone and non-inferiority compared with fluticasone/salmeterol), only then were the key secondary endpoints (FEV1 AUC at week 12 and change in predose FEV1 over 12 weeks) tested in pairs using a Hochberg closed-testing procedure. The treatment comparisons were therefore ordered, such that if the comparison with the larger p value was not significant at α = 0.05, but the lower p value was significant at α = 0.025, the treatment comparison associated with the lower p value was considered statistically significant. Only if both treatment comparisons of the first pair of secondary endpoints (i.e. for FEV1 AUC0–4h) were significant at the α = 0.05 level, would the next pair of secondary endpoints (i.e. for change in predose FEV1) be tested in a confirmatory manner. Therefore, p values would be considered confirmatory if observed as statistically significant in the hierarchical testing strategy. If the confirmatory testing was stopped, subsequent endpoints in the hierarchy, as with the statistical tests of all other endpoints, would be considered exploratory.
The primary endpoint was analyzed using a repeated-measures analysis of covariance (ANCOVA) with fixed terms for treatment, age group, predose FEV1 at baseline, visit and treatment by visit interaction, and center as a random effect. A similar model was employed to analyse the second key secondary endpoint (change in predose FEV1 from baseline over 12 weeks). FEV1 AUC0–4 h at week 12 was analyzed using an ANCOVA with fixed terms for treatment, age group, predose FEV1 at baseline, and center as a random effect.
For other efficacy endpoints, both treatment comparisons (fluticasone/formoterol versus fluticasone, fluticasone/formoterol versus fluticasone/salmeterol) were tested for superiority and based on the FAP (unless otherwise stated) in an exploratory manner.
Change from baseline in diary morning and evening predose PEFR, asthma symptom scores, sleep disturbance scores, percentage of symptom-free days, percentage of awakening-free nights, percentage of rescue-medication-free days, percentage of asthma-control days were analyzed using a similar repeated-measures ANCOVA as per the primary endpoint. The change in FeNO from baseline, PAQLQ and ACQ scores were analyzed using an ANCOVA as per FEV1 AUC0–4 h.
Post hoc analyses of the change from baseline in diary morning and evening predose FEV1 over 12 weeks were performed using a similar repeated-measures ANCOVA as per the primary endpoint. Post hoc analyses of the change in clinic PEFR from predose at baseline to predose over the 12-week treatment period, and from predose at baseline to 2 h postdose over the 12-week treatment period were also performed using a similar ANCOVA to that employed for the primary endpoint analysis [but with predose PEFR (rather than FEV1) at baseline as a fixed term].
The incidence of asthma exacerbations, the incidence of discontinuations due to lack of efficacy, the proportion of patients achieving a PAQLQ score increase ⩾ 0.5 units, and the proportion of patients achieving an ACQ score reduction ⩾ 0.5 units were analyzed using logistic regression. The annualized rate of asthma exacerbations was analyzed using a negative binomial model. The time to first asthma exacerbation was analyzed using a Cox proportional hazards model. The change in rescue medication puffs per day from baseline to each subsequent visit over the 12-week treatment period was analyzed using a repeated measures Friedman test. Other endpoints were summarized descriptively.
The analysis of safety data was based on the safety population, that is, all randomized patients who received at least one dose of study medication. Only descriptive summaries were generated.
Results
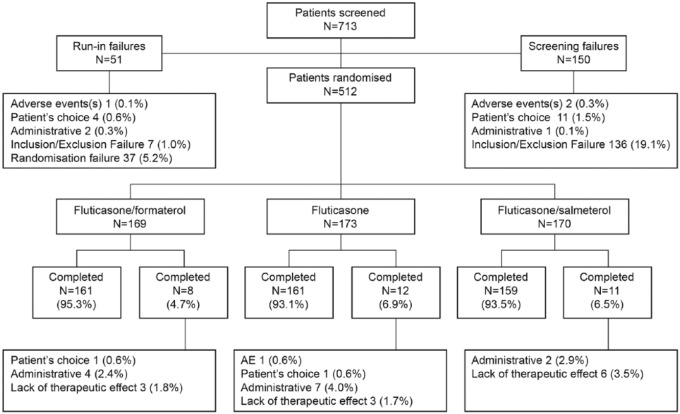
A total of 713 patients were screened and 512 randomized at 59 centers in 8 countries (Bulgaria, Czech Republic, Hungary, India, Poland, Romania, Russia and Ukraine). Of these, 481 patients (93.9%) completed the study. Slightly fewer patients discontinued in the fluticasone/formoterol group (8 patients) compared with the other treatment groups (fluticasone/salmeterol: 11; fluticasone: 12; Figure 2).
Figure 2.
Patient flow diagram.
Demographic and baseline asthma characteristics of all three treatment groups were similar (Table 1).
Table 1.
Demography and asthma characteristics at screening, full analysis population.
| Fluticasone/formoterol |
Fluticasone |
Fluticasone/salmeterol |
Total |
||
|---|---|---|---|---|---|
| (n = 167) | (n = 171) | (n = 168) | (n = 506) | ||
| Age (years) | Mean (SD) | 8.4 (1.81) | 8.4 (1.86) | 8.6 (1.80) | 8.5 (1.82) |
| Gender (n) | Male/female | 109/58 | 116/55 | 113/55 | 338/168 |
| Race [n (%)] | Caucasian | 164 (98.2) | 167 (97.7) | 165 (98.2) | 496 (98.0) |
| Asian | 3 (1.8) | 4 (2.3) | 3 (1.8) | 10 (2.0) | |
| Duration of asthma (years) | Mean (SD) | 3.5 (2.36) | 3.8 (2.50) | 3.5 (2.43) | 3.6 (2.43) |
| (n = 166) | (n = 167) | (n = 166) | (n = 499) | ||
| FEV1 presalbutamol (l) | Mean (SD) | 1.48 (0.361) | 1.44 (0.354) | 1.50 (0.360) | 1.47 (0.358) |
| % Predicted FEV1 | Mean (SD) | 73.8 (6.76) | 72.1 (7.17) | 73.5 (7.63) | 73.1 (7.22) |
| FEV1 reversibility (%) | Mean (SD) | 24.1 (10.49) | 26.1 (11.49) | 24.9 (9.75) | 25.0 (10.62) |
| Patients using ICS alone | n (%) | 118 (70.7) | 132 (77.2) | 129 (76.8) | 379 (74.9) |
| Median daily ICS dose | μg (min, max) | 200.0 (37.5, 500.0) | 200.0 (50.0, 500.0) | 200.0 (50.0, 500.0) | 200.0 (37.5, 500.0) |
| Patients using ICS and LABA | n (%) | 49 (29.3) | 39 (22.8) | 39 (23.2) | 127 (25.1) |
FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; SD, standard deviation.
Primary endpoint
Fluticasone/formoterol was superior to fluticasone for the change from predose FEV1 at baseline to 2 h post dose FEV1 over the 12-week treatment period. The LS mean difference (fluticasone/formoterol versus fluticasone) was 0.07 l [95% CI: 0.03, 0.11 l, p < 0.001 (FAP)] (Figure 3). Fluticasone/formoterol was non-inferior to fluticasone/salmeterol per the prespecified non-inferiority margin of −0.1 l: LS mean difference 0.00 l [95% CI: −0.04, 0.04 l, p < 0.001 (PPP)]. Very similar results were obtained for the alternate populations (PPP and FAP) and for the sensitivity analyses for both treatment comparisons.
Figure 3.
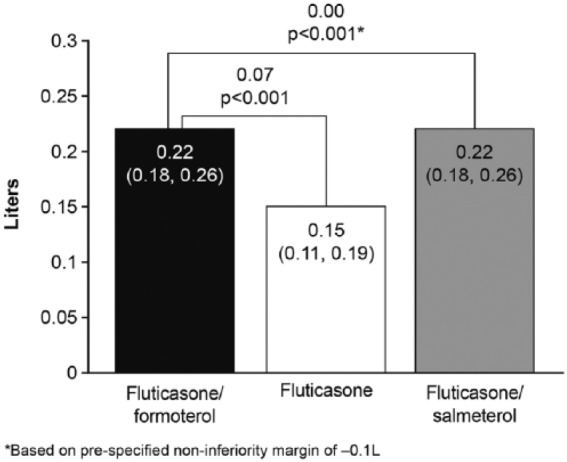
Least squares mean change from predose forced expiratory volume in 1 s at baseline to 2 h postdose over the 12-week treatment period, full analysis population.
Key secondary endpoints
Forced expiratory volume in 1 s area under the curve over 4 h at week 12
Fluticasone/formoterol was superior to fluticasone with regards to FEV1 AUC0–4h at week 12. The LS mean treatment difference (fluticasone/formoterol versus fluticasone) was 0.09 l [95% CI: 0.04, 0.13 l, p < 0.001 (FAP)] (Figure 4). Fluticasone/formoterol was non-inferior to fluticasone/salmeterol per the prespecified non-inferiority margin of −0.1 l: LS mean difference 0.01 l [95% CI: −0.03, 0.06, p < 0.001 (PPP)]. Similar results were obtained for the alternate populations (PPP and FAP) for both treatment comparisons.
Figure 4.
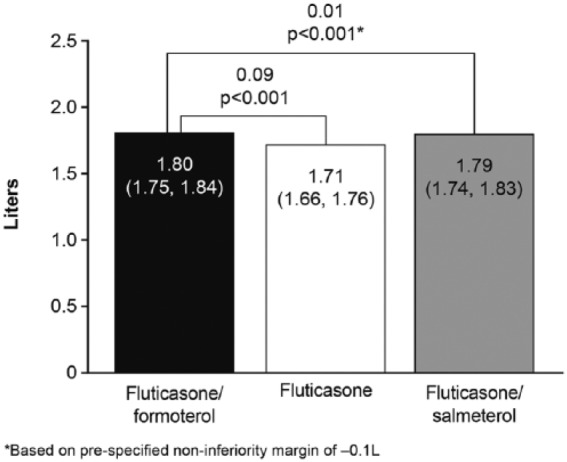
Forced expiratory volume in 1 s area under the curve over 4 h at week 12, full analysis population.
Change in predose area under the curve over 4 h from baseline over 12-week treatment period
A numerically greater effect was seen with fluticasone/formoterol compared with fluticasone, but the difference was not statistically significant: LS mean treatment difference 0.03 l [95% CI: −0.01, 0.07 l, p = 0.091 (FAP)] (Figure 5). Given the Hochberg testing procedure employed, comparison of fluticasone/formoterol versus fluticasone/salmeterol was still undertaken in a confirmatory statistical manner, although statistical significance was to be declared at the 2.5% significance level, given the failure to demonstrate superiority of fluticasone/formoterol over fluticasone. Non-inferiority of fluticasone/formoterol compared with fluticasone/salmeterol was shown: LS mean treatment difference −0.02 l [95% CI: −0.06, 0.02 l, p < 0.001 (PPP)]. Again, very similar results were obtained for the alternate populations (PPP and FAP) for both treatment comparisons.
Figure 5.
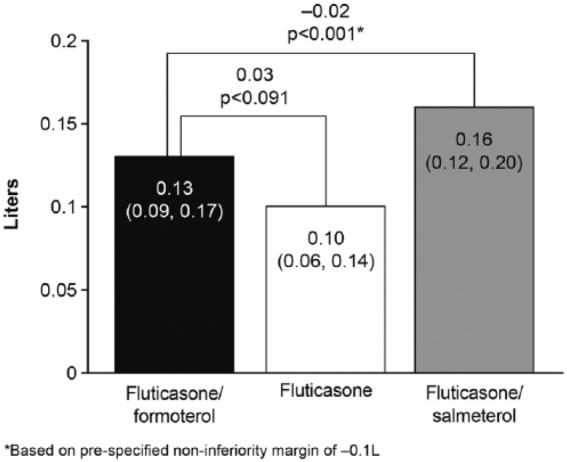
Change in predose forced expiratory volume in 1 s (litres) from baseline over the 12-week treatment period, full analysis population.
Other secondary endpoints
Lung function
FVC, FEF25, FEF50, FEF75 and FEF25–75 were summarized descriptively. Other than FVC, all these endpoints exhibited numerically greater predose and 2 h postdose changes from baseline with fluticasone/formoterol and fluticasone/salmeterol than with fluticasone monotherapy, with generally similar treatment effects observed with the two combination therapies (Table 2).
Table 2.
Change in forced vital capacity, forced expiratory flow at 25%, 50%, 75%, and between 25–75% at week 12: descriptive statistics, full analysis population.
| Predose, change from baseline* |
2 h postdose, change from baseline* |
|||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| FVC (l) | ||||
| Fluticasone/formoterol | 156 | 0.128 (0.2743) | 146 | 0.195 (0.2417) |
| Fluticasone | 149 | 0.151 (0.2625) | 148 | 0.193 (0.2679) |
| Fluticasone/ salmeterol | 156 | 0.170 (0.2344) | 153 | 0.196 (0.2544) |
| FEF25 (l/s) | ||||
| Fluticasone/formoterol | 156 | 0.570 (0.8283) | 146 | 0.941 (0.8069) |
| Fluticasone | 149 | 0.335 (0.6711) | 148 | 0.408 (0.6099) |
| Fluticasone/ salmeterol | 156 | 0.564 (0.7583) | 153 | 0.891 (0.7790) |
| FEF50 (l/s) | ||||
| Fluticasone/formoterol | 156 | 0.370 (0.6240) | 146 | 0.714 (0.5995) |
| Fluticasone | 149 | 0.176 (0.4986) | 148 | 0.282 (0.5091) |
| Fluticasone/salmeterol | 156 | 0.382 (0.5313) | 153 | 0.652 (0.5683) |
| FEF75 (l/s) | ||||
| Fluticasone/formoterol | 156 | 0.189 (0.3638) | 146 | 0.343 (0.3832) |
| Fluticasone | 149 | 0.077 (0.3780) | 148 | 0.134 (0.3485) |
| Fluticasone/salmeterol | 156 | 0.164 (0.4016) | 153 | 0.338 (0.4284) |
| FEF25–75 (l/s) | ||||
| Fluticasone/formoterol | 156 | 0.346 (0.5320) | 146 | 0.622 (0.5180) |
| Fluticasone | 149 | 0.139 (0.4583) | 148 | 0.243 (0.4461) |
| Fluticasone/salmeterol | 156 | 0.325 (0.5025) | 153 | 0.578 (0.5248) |
Change from predose measurement at the baseline visit.
FEF, forced expiratory flow; FEF25, forced expiratory flow at 25%; FVC, forced vital capacity; SD, standard deviation.
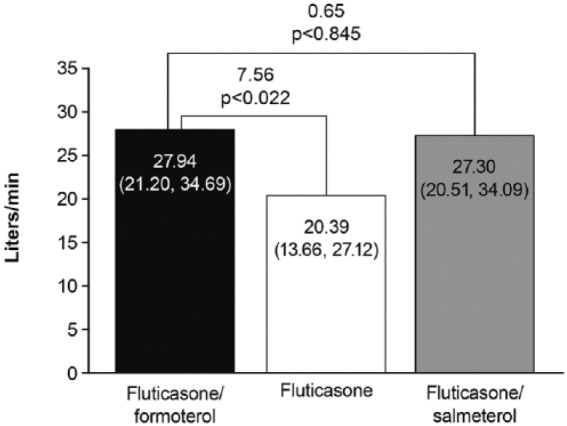
Changes in clinic PEFR from predose at baseline to predose (Figure 6) and 2 h postdose (Figure 7) over the 12-week treatment period were greater with fluticasone/formoterol and fluticasone/salmeterol than with fluticasone monotherapy, whilst effects with the combination therapies were similar to one another.
Figure 6.
Change from clinic predose peak flow (PEFR) at baseline to predose PEFR over the 12-week treatment period, full analysis population.
Figure 7.
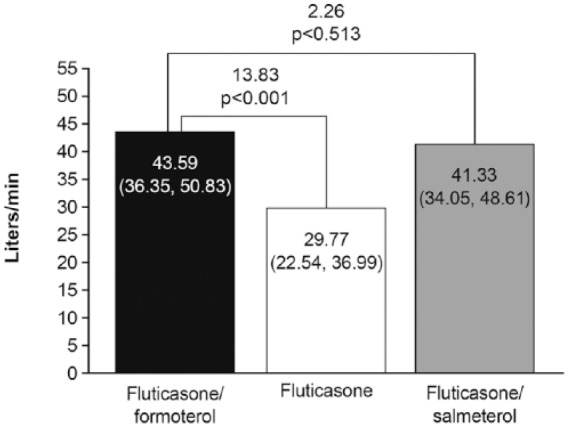
Change from clinic predose peak flow (PEFR) at baseline to 2 h postdose PEFR over 12-week treatment period, full analysis population.
Changes in daily (home) predose morning PEFR from baseline over the 12-week treatment period were greater with fluticasone/formoterol (16.49 l/min) than fluticasone (9.00 l/min): LS mean treatment difference 7.49 l/min [95% CI: 1.24, 13.75 l/min, p = 0.019 (FAP)]. Differences between fluticasone/salmeterol (12.67 l/min) and fluticasone, and between fluticasone/formoterol and fluticasone/salmeterol were nonsignificant at the 5% level. Results for the change from baseline in daily predose evening PEFR over 12 weeks were similar: fluticasone/formoterol versus fluticasone LS mean difference 6.54 l/min [95% CI: 0.37, 12.71 l/min, p = 0.038 (FAP)]. Again, differences between fluticasone/salmeterol and fluticasone, and between the combination treatments were nonsignificant at the 5% level (Table S2).
Symptoms
Substantial improvements in asthma symptom scores, the percentage of symptom-free days, sleep disturbance scores, the percentage of awakening-free nights, and the percentage of asthma control days were seen over the 12-week treatment period in all three treatment groups, with no between-group differences noted (Table 3).
Table 3.
Asthma symptoms, full analysis population.
| Baseline | End of study | Change from baseline | ||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Asthma symptom scores | ||||||
| Fluticasone/formoterol | 167 | 0.90 (0.498) | 164 | 0.21 (0.395) | 164 | −0.69 (0.564) |
| Fluticasone | 170 | 0.89 (0.577) | 168 | 0.22 (0.499) | 167 | −0.68 (0.717) |
| Fluticasone/salmeterol | 167 | 0.88 (0.517) | 165 | 0.21 (0.470) | 164 | −0.67 (0.608) |
| Percentage of symptom-free days | ||||||
| Fluticasone/formoterol | 167 | 29.26 (25.700) | 164 | 73.03 (33.419) | 164 | 43.76 (36.673) |
| Fluticasone | 170 | 31.01 (29.562) | 168 | 76.78 (31.410) | 168 | 45.65 (39.370) |
| Fluticasone/salmeterol | 167 | 29.69 (27.422) | 165 | 75.32 (33.328) | 165 | 45.71 (39.816) |
| Sleep disturbance scores | ||||||
| Fluticasone/formoterol | 167 | 0.66 (0.445) | 164 | 0.14 (0.327) | 164 | −0.52 (0.458) |
| Fluticasone | 170 | 0.69 (0.493) | 167 | 0.14 (0.396) | 166 | −0.56 (0.632) |
| Fluticasone/salmeterol | 167 | 0.67 (0.512) | 165 | 0.08 (0.234) | 164 | −0.60 (0.512) |
| Percentage of awakening-free nights | ||||||
| Fluticasone/formoterol | 167 | 40.55 (30.327) | 164 | 76.17 (29.264) | 164 | 35.14 (34.703) |
| Fluticasone | 170 | 37.65 (30.981) | 167 | 77.58 (27.718) | 167 | 39.77 (38.020) |
| Fluticasone/salmeterol | 167 | 40.55 (34.142) | 165 | 79.82 (24.385) | 165 | 39.90 (38.492) |
| Asthma control days | ||||||
| Fluticasone/formoterol | 167 | 11.21 (15.932) | 164 | 52.87 (36.360) | 164 | 41.45 (36.874) |
| Fluticasone | 170 | 10.93 (16.250) | 167 | 55.68 (35.007) | 167 | 44.64 (34.533) |
| Fluticasone/salmeterol | 167 | 11.13 (18.583) | 165 | 58.52 (36.148) | 165 | 47.26 (37.844) |
SD, standard deviation.
During the treatment period, a high proportion of patients (approximately 57%) across all treatment groups fulfilled the protocol definition of a mild/moderate asthma exacerbation, that is, at least 2 consecutive days with: asthma-related sleep disturbance, and/or ⩾4 puffs of rescue medication use, and/or >30% reduction in PEFR from baseline. It was observed that 59% of all mild/moderate exacerbation events qualified as such on the basis of sleep disturbance alone, that is, these events were not associated with either a concomitant deterioration in PEFR or with any use of rescue medication. This suggested that when many children awoke during the night for reasons other than asthma, these awakenings were wrongly recorded as being asthma-related awakenings in the patient diaries. A post hoc analysis of mild/moderate exacerbations was therefore undertaken in which the awakening criterion was omitted from the event definition. Per this definition, the event incidence was: fluticasone/formoterol 33.5%; fluticasone 28.1%; fluticasone/salmeterol 30.4%. There were no significant differences between treatment groups: fluticasone/formoterol versus fluticasone (odds ratio: 1.30; 95% CI: 0.81, 2.09), fluticasone/formoterol versus fluticasone/salmeterol (odds ratio: 1.20; 95% CI: 0.75, 1.92). The annualized rate of asthma exacerbations was 3.62 in the fluticasone/formoterol group, 3.91 in the fluticasone group (fluticasone/formoterol versus fluticasone rate ratio 0.97; 95% CI: 0.59, 1.58; p = 0.900), and 3.30 in the fluticasone/salmeterol group (fluticasone/formoterol versus fluticasone/salmeterol rate ratio 1.09; 95% CI: 0.66, 1.78; p = 0.738).
Four patients [none administered fluticasone/formoterol; two fluticasone (1.2%); two fluticasone/salmeterol (1.2%)] experienced a severe exacerbation during the treatment period, one of whom was hospitalized for asthma (in the fluticasone/salmeterol treatment group).
Similar improvements in PAQLQ score were observed in all treatment groups from a mean baseline of approximately 5.5 units to a mean of approximately 6.3 units, corresponding to ‘hardly any’ impairment of health status by week 12. Approximately 61% of patients across all treatment groups attained a clinically relevant improvement in health status (Table S3).
Mean ACQ scores at baseline were high (approximately 1.9 units) in all treatment groups, and in all groups decreased (improved) by approximately 1.0 unit by week 12, with no significant between-group differences. Approximately 75% of patients across treatment groups attained a clinically relevant reduction in ACQ score by week 12 (Table S4).
Safety
Overall, 125 (24.6%) patients experienced 203 on-treatment AEs. AEs were reported by slightly more patients in the fluticasone group [52 patients (30.2%)] than the fluticasone/formoterol group [38 patients (22.6%)] or the fluticasone/salmeterol group [35 patients (20.7%)]. The most commonly reported AEs are listed below in Table 4.
Table 4.
Most frequent adverse events, incidence (⩾2%) in any treatment group, safety population.
| Preferred term | Fluticasone/formoterol |
Fluticasone |
Fluticasone/salmeterol |
|---|---|---|---|
| (n = 168) |
(n = 172) |
(n = 169) |
|
| n (%) | n (%) | n (%) | |
| Patients with at least one AE | 38 (22.6) | 52 (30.2) | 35 (20.7) |
| Bronchitis | 3 (1.8) | 2 (1.2) | 4 (2.4) |
| Cough | 2 (1.2) | 4 (2.3) | 2 (1.2) |
| Nasopharyngitis | 4 (2.4) | 15 (8.7) | 13 (7.7) |
| Pharyngitis | 4 (2.4) | 7 (4.1) | 4 (2.4) |
| Rhinitis | 8 (4.8) | 4 (2.3) | 4 (2.4) |
| Viral rhinitis | 1 (0.6) | 2 (1.2) | 4 (2.4) |
AE, adverse event.
Most patients who reported AEs experienced mild events (80 patients, 15.7%) with only three patients experiencing a severe AE [bronchitis (fluticasone/formoterol), laryngitis (fluticasone) and upper limb fracture (fluticasone)]. The severe AEs of bronchitis and upper limb fracture were both classified as serious AEs (that is, requiring hospitalization), whilst the severe laryngitis led to discontinuation from the study. None of these were considered related to study medication by the investigator. Overall analyses of AEs, laboratory parameters and vital signs did not reveal any safety concerns or notable differences in the safety of profile of the three study treatments.
Discussion
The primary endpoint (2 h postdose FEV1 over 12 weeks) and first key secondary endpoint (FEV1 AUC0–4 at week 12) reflect both ICS and LABA treatment effects.20 Fluticasone/formoterol was shown to be superior to fluticasone and non-inferior to fluticasone/salmeterol for both of these endpoints.
A 4 h serial spirometry was employed in this study on the basis of earlier fluticasone/formoterol studies in which FEV1 AUC0–4 h and FEV1 AUC0–12 h were shown to be highly correlated (Pearson correlation coefficient, R > 0.9). The 4 h endpoint compared with 12 h serial spirometry is also advantageous because it means an inpatient stay for 4 h rather than 12, which is far more acceptable to pediatric patients and their carers. Additionally, approximately half the number of forced expiratory manoeuvres are required to define a 4 versus 12 h profile, thereby reducing the potential for fatigue and noncompliance to confound the resultant data. Prior to study commencement, the use of the 4 h endpoint was discussed and agreed with the European Medicines Agency’s Pediatric Committee.
The third endpoint in the testing hierarchy was predose FEV1 over 12 weeks, which measures ICS effect20 when LABA effects are at their lowest ebb over the dosing interval. Superiority of fluticasone/formoterol over fluticasone was not confirmed for this endpoint. However, for all closely analogous endpoints in the study (predose morning diary FEV1 over 12 weeks; predose clinic PEFR over 12 weeks; predose morning diary PEFR over 12 weeks) significant differences between fluticasone/formoterol and fluticasone were noted. Whilst non-inferiority between fluticasone/formoterol and fluticasone/salmeterol was confirmed for predose FEV1 over 12 weeks, per the prespecified non-inferiority margin of −0.1 l and Hochberg testing procedure, it should be acknowledged that this endpoint possessed limited assay sensitivity given the modest observed difference between fluticasone/formoterol and fluticasone. This limitation was indeed the reason for the a priori designation of the predose FEV1 endpoint in the third tier of the confirmatory testing hierarchy.
Results for multiple other secondary lung function endpoints supported the results for the primary efficacy endpoint; lung function effects with fluticasone/formoterol and fluticasone/salmeterol were in almost all cases similar to one another and numerically greater than those observed with fluticasone, whether predose or postdose.
By contrast, results for the symptom-related and exacerbation endpoints were very similar across all three treatment arms. Changes from baseline for symptomatic indices were large. For the ACQ and PAQLQ, mean treatment changes from baseline considerably exceeded the threshold for the minimum clinically relevant within-individual change.
Unlike for adults, in whom the additional symptomatic benefits of ICS/LABAs are clear, this study showed differences between ICS/LABA compared with ICS alone for lung function but not for symptom-based outcomes. This is well described in the literature: the majority of pediatric studies of ICS/LABAs have reported a similar pattern.2,25–28 The reason for the apparent difference in adult and pediatric populations may be the difficulties in obtaining a subjective assessment from young children, who do not possess a clear perception of time and whose recall of events is frequently not reliable.29 Thus, events occurring shortly before clinical examination may be those most prominent to younger children.30 In addition, younger children are less able to adequately verbalize their experiences,30 whilst the perceived stigma associated with illness and a reluctance to differ from their healthy peers may result in under-reporting of symptoms.29 Additionally, children subconsciously adapt their lifestyles to limit their symptom experience.31 These factors may explain why reports of symptoms and activity limitation appear to be non-normally distributed in asthmatic children (i.e. skewed towards being ‘healthy/normal’)32 and differ from the symptomatic impairment reported by adults with the same objective degree of lung function impairment.29 Such issues may also explain why parents underestimate the severity of their children’s symptoms: Kuehni and Frey reported that almost 40% of parents reported their child’s asthma control to be ‘excellent’ when in fact it was ‘poor’ per asthma guideline control criteria.31 Such factors may similarly explain why symptomatic benefits are generally not seen in children with other GINA Step 3 options, that is, medium-dose ICS32–35 or ICS/leukotriene combinations,36 when compared with low dose ICS.
It is less clear why, unlike in adults, ICS/LABAs do not appear to provide additional protection against severe exacerbations in children compared with ICS. It may be that severe events are also subject to the symptom-reporting issues described above, despite these events requiring therapeutic intervention, since the carer and physician must judge whether the child warrants review and then treatment. An alternative hypothesis is that stabilization of airway tone, a proposed mechanism by which LABAs reduce exacerbation risk,37 may be relatively less important in children than in adults.
Results from the present study are nonetheless reassuring in that they provide no support for the view, based on earlier reports, that ICS/LABAs may be associated with an increased risk of severe exacerbations in children compared with ICS monotherapy.38 Note that the majority of studies intimating the latter possibility did not assure coadministration of both drugs via a combination inhaler. Results from the recent US Food and Drug Administration mandated study by Stempel and colleagues also provide definitive evidence39 in this regard: over 6000 children aged 4–11 years were randomized to single inhaler ICS/LABA (fluticasone/salmeterol) versus ICS (fluticasone at the same dose). There was no difference in the occurrence of serious asthma-related events (hospitalization, intubation, or death) between treatments. Additionally, there was no difference in the occurrence of exacerbations requiring systemic steroids, although a trend in favour of ICS/LABA was evident [hazard ratio 0.86 (95% CI: 0.73 to 1.01)]. The data from Stempel and colleagues therefore support the view that any potentially deleterious effect of LABA monotherapy upon airway inflammation is mitigated by the coadministration of an ICS.40
In addition to the severe exacerbation data from the present 12-week study (no events in the fluticasone/formoterol group and two in each of the other arms), further reassurance is available from an earlier open-label, pediatric study of fluticasone/formoterol:15 of the 208 asthmatic children treated with fluticasone/formoterol over 36 weeks, none experienced an exacerbation requiring systemic corticosteroids or hospitalization.
Our study did not assess growth, nor did it include a higher dose ICS monotherapy comparator. However, in view of current GINA guidelines advocating medium doses of ICS as the preferred GINA Step 3 therapy, the results of the CAMP study and two recent meta-analyses are relevant. In CAMP, an ICS dose-dependent reduction in final adult height of 0.1 cm/μg/kg body weight was seen (p = 0.007).41 Similar findings were reported by Loke and colleagues42 and Pruteanu and colleagues43 in their respective meta-analyses. Although these growth impairment effects are relatively modest, they vary across children and warrant consideration when escalating ICS doses in pediatric patients.
Finally, the BADGER study offers perhaps the most useful recent insight into treatment escalation in pediatric asthma and the potential limitations of parallel-group designs in settings where individual patient responses vary considerably. In this double-blind, three-way crossover study, children uncontrolled on low-dose ICS were treated with three different step-up options [medium-dose ICS, add-on LABA, and add-on leukotriene receptor antagonist (LTRA)] in separate, 16-week study periods.44 For all pairwise treatment comparisons, a substantial proportion of patients responded better to the ‘less successful’ treatment. Thus, whilst a greater proportion of patients responded better to LABA add-on (54%) than to ICS dose escalation (p = 0.002), a large minority of 32% responded better to the latter. A very similar result was seen for the LABA versus LTRA comparison. The study model employed by Lemanske and coworkers thus illustrates the diversity of step-up responses in children, which may further contribute to the difficulty evidencing symptomatic treatment differences in this population. Similarly designed studies may represent a more informative model with which to evaluate asthma treatments in future pediatric studies.
Conclusion
Fluticasone/formoterol was superior to fluticasone and non-inferior to fluticasone/salmeterol in terms of effects upon lung function. All three treatments elicited large improvements in symptomatic indices, but no differences between treatments were evident for these outcomes, as in earlier ICS/LABA studies. Few severe exacerbations were seen in this 12-week study, with none observed on fluticasone/formoterol. Safety profiles were similar for all three study treatments. Overall, these results support the efficacy and safety of fluticasone/formoterol in children.
Supplementary Material
Supplementary Material, Supplementary_data for Efficacy and safety of fluticasone propionate/formoterol fumarate in pediatric asthma patients: a randomized controlled trial by Anna Płoszczuk, Miroslava Bosheva, Kay Spooner, Tammy McIver and Sanjeeva Dissanayake in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors would like to thank all study investigators and participants. Medical writing assistance was provided by Susan Chisholm and Evelin Kozma, Mundipharma Research Limited, in line with CONSORT guidelines, EMWA and Good Publication Practice guidelines.
Footnotes
Funding: The work was funded by Mundipharma Research Limited.
Conflict of interest statement: Kay Spooner is an employee of the study sponsor, Mundipharma Research Limited. Tammy McIver and Sanjeeva Dissanayake were employees of the study sponsor at the time this study was conducted.
Anna Ploszczuk and Miroslava Bosheva have no conflicts of interest to disclose.
Trademark statements: ®Flutiform is a registered trade mark of Jagotec AG.
Flixotide, Seretide, Ventolin, Evohaler, and Accuhaler are registered trademarks of Glaxo Group Limited.
Symbicort and Turbohaler are registered trade marks of AstraZeneca AB.
Contributor Information
Anna Płoszczuk, Prywatna Praktyka Lekarska, Gabinet Pediatryczno-Alergologiczny, Ul. Przejazd 2A, Białystok, Poland.
Miroslava Bosheva, University Hospital Plovdiv, Medical University of Plovdiv, Bulgaria.
Kay Spooner, Mundipharma Research Limited, Cambridge, UK.
Tammy McIver, Mundipharma Research Limited, Cambridge, UK.
Sanjeeva Dissanayake, Mundipharma Research Limited, Cambridge, UK.
References
- 1. Global Initiative for Asthma. GINA Global Strategy for Asthma Management and Prevention, 2016; http://ginasthma.org/
- 2. Ni Chroinin M, Lasserson TJ, Greenstone I, et al. Addition of long-acting beta-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev 2009; 8: CD007949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murphy K, Bender B. Treatment of moderate to severe asthma: patient perspectives on combination inhaler therapy and implications for adherence. J Asthma Allergy 2009; 2: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GlaxoSmithKline Briefing Document. Benefit risk assessment of salmeterol for the treatment of asthma in adults and children, 2008; https://wayback.archive-it.org/7993/20170405035641/https://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4398b1-04-GSK.pdf
- 5. Bodzenta-Lukaszyk A, Pulka G, Dymek A, et al. Efficacy and safety of fluticasone and formoterol in a single pressurized metered dose inhaler. Respir Med 2011; 105: 674–682. [DOI] [PubMed] [Google Scholar]
- 6. Bodzenta-Lukaszyk A, Dymek A, McAulay K, et al. Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study. BMC Pulm Med 2011; 11: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nathan RA, D’Urzo A, Blazhko V, et al. Safety and efficacy of fluticasone/formoterol combination therapy in adolescent and adult patients with mild-to-moderate asthma: a randomised controlled trial. BMC Pulm Med 2012; 12: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bodzenta-Lukaszyk A, Buhl R, Balint B, et al. Fluticasone/formoterol combination therapy versus budesonide/formoterol for the treatment of asthma: a randomized, controlled, non-inferiority trial of efficacy and safety. J Asthma 2012; 49: 1060–1070. [DOI] [PubMed] [Google Scholar]
- 9. Aalbers R, Brusselle G, McIver T, et al. Onset of bronchodilation with fluticasone/formoterol combination versus fluticasone/salmeterol in an open-label, randomized study. Adv Ther 2012; 29: 958–969. [DOI] [PubMed] [Google Scholar]
- 10. Corren J, Mansfield LE, Pertseva T, et al. Efficacy and safety of fluticasone/formoterol combination therapy in patients with moderate-to-severe asthma. Respir Med 2013; 107: 180–195. [DOI] [PubMed] [Google Scholar]
- 11. Kaiser K, Pertseva T. Long-term safety and efficacy of fluticasone propionate/formoterol fumarate combination therapy in patients with asthma. Prim Care Respir J 2013; 22: A1–A18. [Google Scholar]
- 12. Bodzenta-Lukaszyk A, Van Noord J, Schroder-Babo W, et al. Efficacy and safety profile of fluticasone/formoterol combination therapy compared to its individual components administered concurrently in asthma: a randomised controlled trial. Curr Med Res Opin 2013; 29: 579–588. [DOI] [PubMed] [Google Scholar]
- 13. Mansur AH, Kaiser K. Long-term safety and efficacy of fluticasone/formoterol combination therapy in asthma. J Aerosol Med Pulm Drug Deliv 2013; 26: 190–199. [DOI] [PubMed] [Google Scholar]
- 14. Papi A, Mansur AH, Pertseva T, et al. Long-term fluticasone propionate/formoterol fumarate combination therapy is associated with a low incidence of severe asthma exacerbations. J Aerosol Med Pulm Drug Deliv 2016; 29: 346–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emeryk A, Klink R, McIver T, et al. A 12-week open-label, randomized, controlled trial and 24-week extension to assess the efficacy and safety of fluticasone propionate/formoterol in children with asthma. Ther Adv Respir Dis 2016; 10: 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reddel HK, Taylor R, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 2009; 180: 59–99. [DOI] [PubMed] [Google Scholar]
- 17. Miller MR, Hankinson J, Brusasco V, et al. Series ATS/ERS task force: standardisation of lung function testing’ standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 18. Fuhlbrigge AL, Kitch BT, Paltiel AD, et al. FEV(1) is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol 2001; 107: 61–67. [DOI] [PubMed] [Google Scholar]
- 19. Fuhlbrigge AL Weiss ST Kuntz KM et al.;. CAMP Research Group. Forced expiratory volume in 1 second percentage improves the classification of severity among children with asthma. Pediatrics 2006; 118: e347–e355. [DOI] [PubMed] [Google Scholar]
- 20. Covar RA, Szefler SJ, Zeiger RS, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol 2008; 122: 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. European Medicines Agency/399193/2012. Flutiform assessment report pursuant to Article 29(4) of Directive 2001/83/EC, as amended, http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Flutiform_29/WC500134240.pdf
- 22. Malone R, La Force C, Nimmagadda S, et al. The safety of twice-daily treatment with fluticasone propionate and salmeterol in pediatric patients with persistent asthma. Ann Allergy Asthma Immunol 2005; 95: 66–71. And unpublished data in SAS30031. [DOI] [PubMed] [Google Scholar]
- 23. Tal A, Simon G, Vermeulen JH, et al. Budesonide/formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthma. Pediatric Pulmonol 2002; 34: 342–350. [DOI] [PubMed] [Google Scholar]
- 24. Dissanayake S, Grothe B, McIver T, et al. Validation of FEV1 spirometric endpoints as a measure of the anti-inflammatory and bronchodilatory effects of fluticasone/formoterol combination therapy. Eur Respir J 2013; 42: P4131. [Google Scholar]
- 25. Pohunek P, Kuna P, Jorup C, et al. Budesonide/formoterol improves lung function compared with budesonide alone in children with asthma. Pediatr Allergy Immunol 2006; 17: 548–465. [DOI] [PubMed] [Google Scholar]
- 26. Morice AH, Peterson S, Beckman O, et al. Efficacy and safety of a new pressurised metered-dose inhaler formulation of budesonide/formoterol in children with asthma: a superiority and therapeutic equivalence study. Pulm Pharmacol Ther 2008; 21: 152–159. [DOI] [PubMed] [Google Scholar]
- 27. Malone R, La Force C, Nimmagadda S, et al. The safety of twice-daily treatment with fluticasone propionate and salmeterol in pediatric patients with persistent asthma. Ann Allergy Asthma Immunol 2005; 95: 66–71. [DOI] [PubMed] [Google Scholar]
- 28. Tal A, Simon G, Vermeulen JH, et al. Budesonide/formoterol in a single inhaler versus inhaled corticosteroids alone in the treatment of asthma. Pediatr Pulmonol 2002; 34: 342–350. [DOI] [PubMed] [Google Scholar]
- 29. Santanello NC. Pediatric asthma assessment: validation of 2 symptom diaries. J Allergy Clin Immunol 2001; 107: S465–S472. [DOI] [PubMed] [Google Scholar]
- 30. Yawn BP, Brennemen SK, Allen-Ramey FC, et al. Assessment of asthma severity and asthma control in children. Pediatrics 2006; 118: 322–329. [DOI] [PubMed] [Google Scholar]
- 31. Kuehni CE, Frey U. Age-related differences in perceived asthma control in childhood: guidelines and reality. Eur Respir J 2002; 20: 880–889. [DOI] [PubMed] [Google Scholar]
- 32. Santanello NC, Davies G, Galant SP, et al. Validation of an asthma symptom diary for interventional studies. Arch Dis Child 1999; 80: 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang L, Axelsson I, Chung M, et al. Dose response of inhaled corticosteroids in children with persistent asthma: a systematic review. Pediatrics 2011; 127: 129–138. [DOI] [PubMed] [Google Scholar]
- 34. Verona E, Petrov D, Cserhati E, et al. Fluticasone propionate in asthma: a long term dose comparison study. Arch Dis Child 2003; 88: 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adams NP, Bestall JC, Jones P, et al. Fluticasone at different doses for chronic asthma in adults and children. Cochrane Database Syst Rev 2008; CD003534. [DOI] [PubMed] [Google Scholar]
- 36. Chauhan BF, Ben Salah R, Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids in children with persistent asthma. Cochrane Database Syst Rev 2013; 10: CD009585. [DOI] [PubMed] [Google Scholar]
- 37. Jackson CM, Lipworth B. Benefit-risk assessment of long-acting β2-agonists in asthma. Drug Safety 2004; 27: 234–270. [DOI] [PubMed] [Google Scholar]
- 38. Bisgaard H. Effect of long-acting beta 2 agonists on exacerbations rates of asthma in children. Pediatr Pulmonol 2003; 36: 391–398. [DOI] [PubMed] [Google Scholar]
- 39. Stempel DA, Szefler SJ, Pedersen S, et al. Safety of adding salmeterol to fluticasone propionate in children with asthma. New Engl J Med 2016; 375: 840–849. [DOI] [PubMed] [Google Scholar]
- 40. Dissanayake SB. Safety of β2-agonists in asthma: linking mechanisms, meta-analyses and regulatory practice. AAPS J 2015; 17: 754–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kelly HW, Sternberg AL, Lescher R, et al. Effect of inhaled glucocorticoids in childhood on adult height. New Engl J Med 2012; 367: 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Loke YK, Blanco P, Thavarajah M, et al. Impact of inhaled corticosteroids on growth in children with asthma: systematic review and meta-analysis. PLoS One 2015; 10: e0133428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pruteanu AI, Chauhan BF, Zhang L, et al. Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth. Cochrane Database Syst Rev 2014; 17: CD009878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lemanske RF, Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. New Engl J Med 2010; 362: 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material, Supplementary_data for Efficacy and safety of fluticasone propionate/formoterol fumarate in pediatric asthma patients: a randomized controlled trial by Anna Płoszczuk, Miroslava Bosheva, Kay Spooner, Tammy McIver and Sanjeeva Dissanayake in Therapeutic Advances in Respiratory Disease