Abstract
Taking inspiration from nature, the biomimetic concept has been integrated into drug delivery systems in cancer therapy. Disguised with cell membranes, the nanoparticles can acquire various functions of natural cells. The cell membrane-coating technology has pushed the limits of common nano-systems (fast elimination in circulation) to more effectively navigate within the body. Moreover, because of the various functional molecules on the surface, cell membrane-based nanoparticles (CMBNPs) are capable of interacting with the complex biological microenvironment of the tumor. Various sources of cell membranes have been explored to camouflage CMBNPs and different tumor-targeting strategies have been developed to enhance the anti-tumor drug delivery therapy. In this review article we highlight the most recent advances in CMBNP-based cancer targeting systems and address the challenges and opportunities in this field.
Abbreviations: CC, cancer cell; CMBNPS, cell membrane-based nanoparticles; CTC, circulating tumor cell; DOX, doxorubicin; DSPE, distearoyl phosphoethanolamine; EPR, enhanced permeability and retention; ICG, indocyanine green; NIR, near infrared; NPs, nanoparticles; PLGA, poly (lactic-co-glycolic acid); PM-NV, platelet membrane-coated nanovehicle; PTX, paclitaxel; RBC, red blood cell; TDDS, targeting drug delivery system; TRAIL, tumor necrosis factor-related apoptosis inducing ligand; VCAM1, vascular cell adhesion molecule-1
KEYWORDS: Cell membrane, Biomimetic nanoparticle, Drug delivery, Cancer targeting, Circulation, Molecular recognition
Graphical abstract
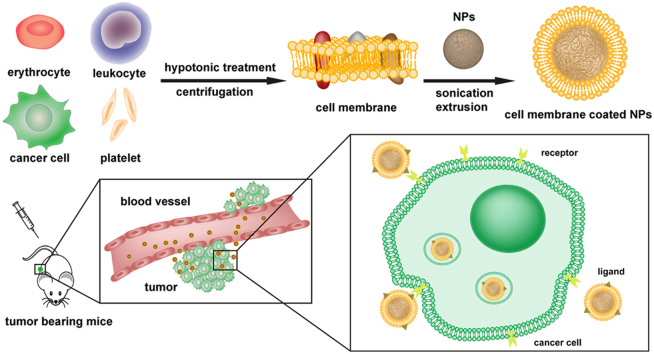
Nanoparticles can be cloaked with various types of cell membranes. The membrane-coating technology can prolong the circulation of nanomedicine in the bloodstream and show cancer-targeting potential by molecular recognition. This review provides an overview of cell membrane-based nanoparticles (CMBNPs) for tumor diagnosis and treatment.
1. Introduction
Cancer has long been a global threat and is the second leading cause of death1. As one of the most common strategies for the treatment of cancer, chemotherapy remains unsatisfactory due to the low targeting ability and severe adverse effects of anti-cancer drugs2., 3.. To address these problems, targeting drug delivery systems (TDDS), especially nanoparticle-based TDDS, have been intensively studied and developed4. The advantages of nanoparticles (NPs), such as high drug loading capacity, adjustable physio-chemical properties and flexibility to be modified, make them appropriate to encapsulate anti-cancer drugs and thereby alter their solubility, stability and in vivo behavior5. Moreover, the surface modification of NPs can prolong their circulation in the blood and provide specific targeting so as to increase efficacy while decreasing adverse effects6., 7.. However, there are still many drawbacks limiting NPs to meet clinical expectations. Most NPs are recognized and eliminated as a foreign substance by immune system. PEGylation of NPs can decrease the fast elimination by reticuloendothelial system. Some studies discovered that the repetitive administration of PEGylated NPs can induce an immune response which can lead to faster elimination of NPs8., 9.. In addition, the desired targeting capacity of NPs was especially dependent on the surface modification, which was complicated to fabricate and difficult to achieve10., 11.. Consequently, nanoparticle-based TDDS have not yet reached their full therapeutic potential. Seeking a safer and more effective approach is urgently demanded.
In the early 1980s, cells were exploited as carriers to deliver drugs or nanoparticles12., 13., which significantly enhanced the retention and targeting efficiency of these drugs. Although the use of live cell-based carriers flourished, some deficiencies remain. One of major concern is the activity of passenger drugs, since drugs may be digested by the lysosomes of the cell carrier14. Moreover, it is difficult to control the release of drug, which may be leaked or exocytosed during transport15. Confronted with these problems, scientists have recently found a clue from nature to design biomimetic, cell membrane-based nanoparticles (CMBNPs). Initially, the original CMBNPs were fabricated from a red blood cell (RBC) membrane shell and a poly (lactic-co-glycolic acid) (PLGA) core, via a co-extrusion process, forming a core-shell structure. Subsequently, various CMBNPs have been explored with the flexibility of choosing different membrane materials and different nanoparticle cores. The translocation of a natural cell membrane to a synthesized NP can combine the advantages of a biomimetic cell membrane surface and the tailored flexibility of material chemistry16., 17.. One of the most important profits is that the CMBNPs can be disguised as autogenous cells, so as to escape immune system elimination and prolong the circulation time in the blood, which is extremely necessary for the enhanced permeability and retention (EPR) effect for tumor targeting18.
In addition, the complex components of a natural cell membrane can be maintained in CMBNPs, which might endow the CMBNPs with some biological functions propitious to tumor targeting19. As reported, numerous cells are involved in or related to the development and progression of cancer, such as red blood cells, leukocytes, cancer cells and even sub-cellular platelets20, and different cells play different parts in the process. The membrane-based functions of cancer-related cells, including extravasation, chemotaxis, and cancer cell adhesion, inspired researchers to explore the CMBNPs to be a carriers for tumor-targeting drug delivery21., 22.. We classify CMBNPs according to the type of source cells including red blood cells, leukocytes, cancer cells and platelets. Different types of cell membranes could endow CMBNPs with various functions, which will lead to diverse in vivo biological behavior. This classification covers most of currently reported CMBNPs and shows the basic mechanism of the biomimetic strategy.
Besides the versatile capacity of the coated membrane, the core of CMBNPs are also flexibly designed for various applications, such as anti-cancer drug delivery, tumor imaging, and photothermal therapy. All these advantages make CMBNPs promising for translation from bench to bedside. Therefore, in order to direct the rational design and further improvement of CMBNPs, it is necessary to understand their structural concepts and targeting mechanisms. Herein, we provide an up-to-date review of various membrane-derived CMBNPs for the treatment of cancer, as well as the challenges and opportunities related to the application of CMBNPs in cancer therapy.
2. Red blood cell membrane-coated nanoparticle
Red blood cells are the most abundant cellular constituent of the blood with the total number in human body approaching 30 trillion23. Human blood transfusion was first performed in France in 1667 and around 50 million blood units are transfused every year in clinics24, which makes erythrocytes widely available. Furthermore, mature erythrocytes lack a cell nucleus and organelles, so the RBC membrane is convenient to extract and purify25.
An optimal nano-sized drug delivery system requires relatively long blood circulation to achieve effective tumor targeting and efficacy26., 27.. The immune system, however, can recognize foreign bodies according to determinants absent on host cells or “markers of self” normally present28. Red blood cells, expressing a variety of immunomodulatory markers on their cell membrane, can be recognized as a self-component and circulate for about 40 days in mice, and 3 months in the human body29. One of the most typical markers is CD47, a transmembrane protein, which can bind to the inhibitory receptor signal regulatory protein alpha and emit a “don't eat me” signal that inhibits phagocytosis of RBCs by immune cells. It was reported that RBCs lacking CD47 were rapidly cleared from the bloodstream by macrophages29. Therefore, RBC membrane-coated NPs, a biomimetic strategy, are able to integrate the unique advantages of natural erythrocytes, such as long circulation, with artificial nanoparticles, which can protect the encapsulated drug. Red blood cell membrane-coated PLGA NPs were first reported and laid the foundation for subsequent studies30. After that, many studies from different research groups were carried out to demonstrate the utility of the RBC membrane for cancer treatment.
In order to preserve the membrane as long as possible, researchers usually prepare RBC membrane-coated nanoparticles with a well-established top-down method. For example, poly(lactic-co-glycolic acid) (PLGA), a biodegradable polymer approved by FDA, is used to fabricate the nanoparticulate cores. The purified RBC membrane is then fused around the NPs surface via an extrusion method30. It was shown that compared with the bare cores, biomimetic NPs exhibited greatly prolonged circulation time due to the preservation of “markers of self” on the RBC membrane in a right-side-out orientation31. Furthermore, results indicated that the functionalized NPs demonstrated significantly enhanced accumulation at tumor sites in a subcutaneous tumor model due to an increased ability to utilize the EPR effect32. Thus, the biomimetic strategy is promising to be an alternative to polyethylene glycol (PEG) stealth coating in a more biocompatible way. Many studies relevant to cancer drug delivery were carried out after the pioneering work of the RBC membrane-disguised nanoparticles. Zhang et al.33 has reported that the chemotherapeutic drug, doxorubicin (DOX), could be efficiently loaded into PLGA cores, which was then cloaked with RBC membrane. The RBC membrane-coated, DOX-loaded nanoparticles exhibited significantly increased inhibition of tumor growth and excellent immunocompatibility compared with the free DOX, which brought new insight to chemotherapy.
Numerous studies have shown that active TDDS can selectively enter into tumor cells, results in better target-selectivity and finally achieves better therapeutic effect34. The red blood cell, however, lacks related targeting ligands and lacks the active targeting capacity for solid tumors, which would limit its application in cancer treatment. Facing this problem, targeting ligand, which is widely used in TDDS, may be combined with RBC membrane. Chemical synthesis is a common approach for ligand modification, while it may impair the integrity of RBC membrane. Since keeping the integrity of the membrane is extremely vital to maintain cellular function, the chemical method may not be appropriate. In this regard, a so-called lipid-insertion approach has been developed35. The folate acid ligand was first conjugated to the distearoyl phosphoethanolamine (DSPE) lipid and then ligand–linker–lipid conjugates were inserted into RBC membrane. The ligand-functionalized RBC membranes were used for nanoparticle coating to achieve active targeting ability. Both a small molecule folate (MW = 441 Da) and a nucleolin-targeting aptamer AS1411 (MW = 9000 Da) were successfully inserted on the RBC membrane-coated nanoparticle, and the results showed a significant targeting effect was achieved in model cancer cell lines in vitro. Fu et al. modified the membrane-coated nanoparticle with a typical tumor-targeting peptide RGD (Arg–Gly–Asp). Doxorubicin and paclitaxel were chosen as model drugs and co-encapsulated into the magnetic O-carboxymethyl-chitosan NP core. The tumor growth inhibition effect of the novel system was much stronger than that of the non-modified membrane-coated NPs36.
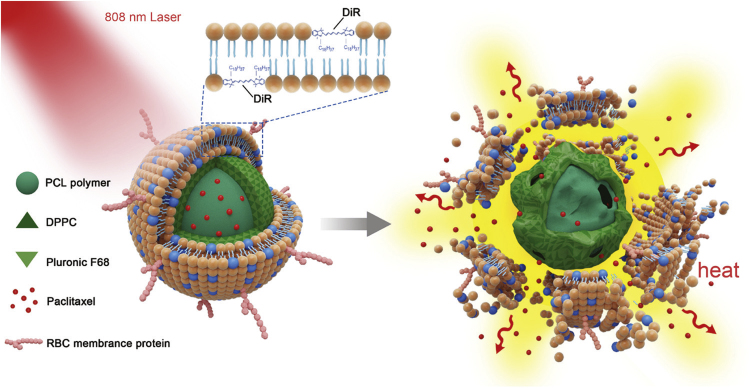
Besides long retention lifetime in circulation and selective targeting ability, controllable drug release is also essential for an ideal drug delivery system in cancer therapy. Some types of synthetic nanoparticles reveal excellent controlled release properties. For the biomembrane-coated NP system, it is relatively easy to choose different core materials to achieve various purposes19. As shown in Fig. 1, a new near infrared (NIR) laser-responsive RBC-mimetic NP system has been fabricated to realize long blood circulation, controlled drug release, and synergistic chemo/photothermal therapy37. DiR, a cyanine dye, was inserted into the RBC membrane shell and paclitaxel (PTX) was loaded in the thermo-responsive hybrid polymeric nanoparticle cores. The results have shown that the structure of the system could be destroyed by light-induced hyperthermia which then triggered rapid PTX release. The in vivo results suggested that it might be a promising delivery system to fight against metastatic breast cancer.
Figure 1.
The NIR‐driven drug release of the RBC‐mimetic NPs (PTX-PN@DiR-RV). Adapted with permission from Ref. 37. Copyright Wiley Online Library, 2016.
3. Leukocyte membrane-coated nanoparticle drug delivery system
White blood cells, commonly termed as leukocytes, are between 7–20 μm in diameter, which are larger than red blood cells. Most leukocytes can do amoeboid movement, which makes them easily migrate to and from the blood vessels to the extravascular tissues. Thus, leukocytes are widespread in the blood vessels and lymphatic vessels as well as other tissues. Chronic inflammation has been characterized as one of the main features of cancer38. A diverse population of inflammatory cells, including neutrophils, dendritic cells, macrophages, eosinophils and mast cells, as well as lymphocytes, are involved in progression of the tumor39. Tumor cells produce various cytokines and chemokines that attract leukocytes40. Unfortunately, most leukocytes subsequently become accomplices of tumor cells under the inflammatory microenvironment41. Such tumor-associated macrophages or fibroblasts will help the metastases or neovascularization of tumor which finally results in rapid tumor growth. Discarding the function of tumor accomplices, leukocytes would be promising drug delivery vehicles for tumor targeting because of their inflammation chemotaxis. Thus, leukocyte membrane-cloaked nanoparticles are a possibility19.
A large population of cancer-related leukocytes are macrophages42. Coated with macrophage membranes, nanoparticles could display long blood circulation time similar with RBC membrane-coated nanoparticles. Moreover, the macrophage-camouflaged nanoparticles could possess the capability of crossing vascular barriers and molecular recognition ability on tumor cells through functional proteins residing on the membranes43. The Tasciotti group first developed a macrophage membrane-coated porous silica particle. The membranes were coated onto the silica particles through electrostatic and hydrophobic interactions between the positively charged particles and negatively charged cell membranes44. The functional molecules, such as CD45, CD11a and glycans, were all maintained on the particle surface, which were helpful to prevent the internalization/uptake by macrophages, phagocytic cells or vein endothelial cells and preferentially bind to and transport through inflamed endothelium in tumors. Zhang et al.45 further characterized the tumor-targeting mechanism of macrophage membrane-coated nanoparticles. The inflammatory-related receptors on membranes were responsible for the tumor homing effect, since blocking LFA-1 or CXCR1 and CXCR2 on the membrane-coated nanoparticles could significantly inhibit the recruitment of nanoparticles by the inflammatory tissue. Besides drug delivery, the macrophage cloaking technology could also facilitate photothermal therapy, tumor imaging and diagnostics46., 47.. These papers indicated the outstanding tumor targeting or homing effect of macrophage-derived nanovectors compared with uncoated nanocarriers. However, the tumor homing mechanism is still debated. Although the macrophages would be recruited to the inflammatory site, the chemotaxis and extravasation process were highly complex48., 49.. The adhesion, cytomorphosis, and cell–cell interaction of macrophages are necessary for drug delivery. While the macrophage-camouflaged nanoparticles were not living cells, it was scarcely possible to maintain all the complex functions of macrophage cells. Therefore, the tumor-homing mechanism of macrophage membrane-coated nanoparticles should be well studied in the future, and not simply viewed as limited to chemotaxis.
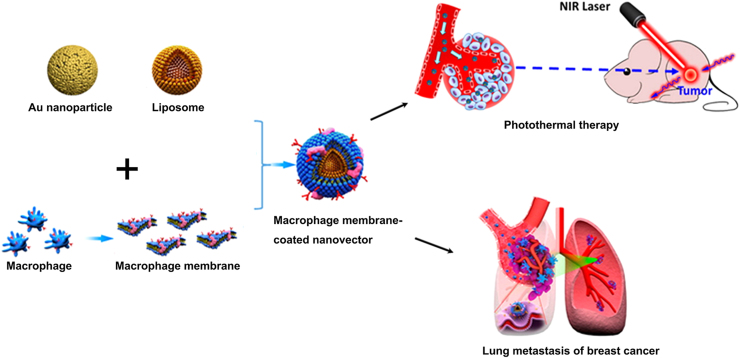
Besides homing to inflammation sites, macrophages could also actively bind to cancer cells via receptor interactions on membranes50. This binding would promote the metastasis of cancer cells and subsequently form metastatic lesions. Based on this binding effect, Li's group developed a macrophage membrane-coated liposome to target metastatic cancer cells via the interaction between α4 integrins of the macrophage membrane and the vascular cell adhesion molecule-1 (VCAM1) of cancer cells51. As they showed, the macrophage membrane decoration significantly enhanced cellular uptake in metastatic 4T1 breast cancer cells and suppressed lung metastasis of breast cancer (Fig. 2). To further determine the interaction of macrophage membrane-coated nanoparticles and tumor cells, He et al.52 developed a Janus nanoparticle with only half-side membrane cloaking. They found that it was the membrane coated hemisphere that adhered to and penetrated the surface of cancer cells. This study brought new insight and a new approach to explore the specific binding of membrane-coated nanoparticles.
Figure 2.
Schematic illustration of macrophage membrane-coated nanovectors for photothermal therapy in subcutaneous tumor or targeting lung metastasis of breast cancer. Adapted with permission from Refs. 47., 51. Copyright American Chemical Society, 2016.
Evidence showed that neutrophils and monocytes possessed both a circulating tumor cell (CTC) and niche-targeting property by the intrinsic cell adhesion molecules on membranes53., 54.. Ting Kang et al.55 developed a neutrophil membrane-coated nanoparticle for cancer metastasis prevention and therapy which could target both CTCs in circulation and premetastatic niche. When loaded with a proteasome inhibitor, carfilzomib, the nanoformulation facilitated selective CTC apoptosis in blood and prevented the formation of nodules at the early stage. Chan et al. also proved that the monocyte membrane-coated nanoghosts had higher affinity for metastatic MCF-7 breast cancer cell lines than their uncoated counterparts56.
Immunotherapy for cancer has drawn much attention recently. As immune cells, T-lymphocytes play an important role in tumor recognition and suppression. In this regard, lymphocyte membrane-camouflaged nanoparticles have also been studied, and shown to exhibit enhanced localization at the tumor site after low-dose irradiation just as is seen with cytotoxic CD8+ T cells57.
As shown in Table 1, a considerable number of leukocyte-derived nanoparticles have been developed. Compared with coating with RBC membrane, the leukocyte membrane decoration of nanoparticles could not only prolong the circulation in vivo, it could actively target to inflammatory sites and cancer cells through functional molecules on the membranes. Although it is promising for leukocyte membrane-coated formulations, there are still some limitations that need to be considered for their application. Leukocyte membranes are always obtained from immortal cell lines, which may not be as biocompatible as autogenous RBC membranes. Moreover, leukocytes are karyocytes which express specific main histocompatibility complex (MHC) molecules on their surface. Consequently, the immunogenicity of this formulation should be considered in the future58.
Table 1.
Examples of leukocyte membrane-coated nanoparticles for tumor therapy.
| Membrane source | Cancer model | Targeting mechanism | Drug-loading | Ref. | |
|---|---|---|---|---|---|
| LeukoLike Vectors (LLV) | THP-1 and J774 cell line | Melanoma | Inflammation adhesion | None | 44 |
| Macrophage cell membrane-camouflaged mesoporous silica nanocapsules (MSNCs) | RAW 264.7 | 4T1 Subcutaneous tumor | Unclear | DOX | 43 |
| Macrophage cell membrane- camouflaged AuNS (MPCM-AuNSs) | RAW 264.7 | 4T1 Subcutaneous tumor | Cancer cell recognition | Photothermal | 47 |
| Macrophage membrane-coated emtansine liposome (MEL) | RAW 264.7 | 4T1 metastasis lung cancer | Metastatic cancer cell binding | Emtansine | 51 |
| Neutrophil mimicking nanoparticles (NM-NPs) | Mouse primary neutrophils | Circulating tumor cells | Metastatic cancer cell binding | Carfilzomib | 55 |
| Monocyte cell membrane-derived nanoghosts | U937 | None | Cancer cell recognition | DOX | 56 |
| hCTL membrane-coated PLGA nanoparticles (TPNPs) | Human primary T cells | Gastric cancer | Immune recognition | PTX | 57 |
4. Cancer cell membrane-coated nanoparticle (CCNPs)
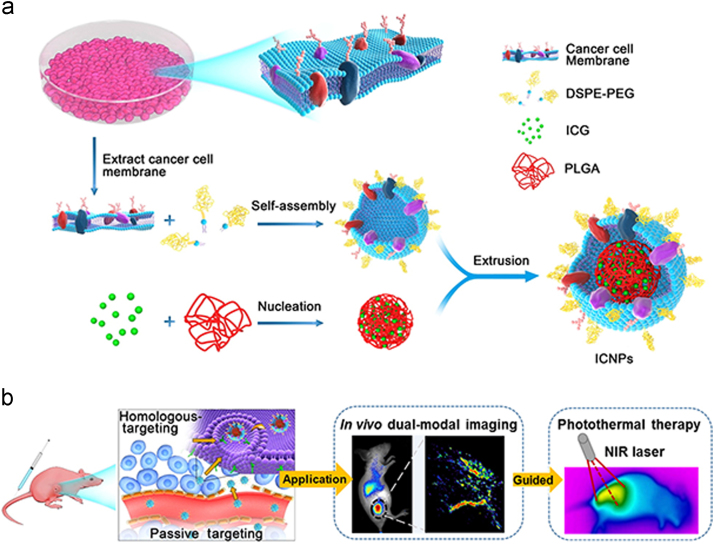
Cancer cells possess various peculiar properties compared with blood cells, such as limitless replicative potential, immune escape and homologous targeting abilities20. Instead of being obtained from patient autologous plasma or a donor, cancer cells can be easily obtained through in vitro cell culture, because of their proliferative ability59. During metastasis, homotypic cancer cell aggregation is critically important for establishing secondary lesions in distant tissues and organs60., 61.. It is reported that the aggregation process is based on surface adhesion molecules (e.g., N-cadherin, galectin-3, epithelial cell adhesion molecule (EpCAM)) on cancer cell membranes62. Utilization of natural cell membranes for vehicle surface functionalization offers the unique advantage of a complete replication of membrane surface protein diversity from the source cells onto the engineered nanoparticles. Inspired by the inherent immune escape and homologous adhesion properties of cancer cells, various cancer cell membrane-coated nanoparticles are designed for tumor targeting diagnosis and therapy. For example, a kind of novel cancer cell membrane-cloaked upconversion nanoprobe was developed, which exhibited low immunogenicity and homologous targeting effects63. As shown in Fig. 3, indocyanine green (ICG) was encapsulated in PLGA core as probe, and the cancer cell membrane was hybridized with DSPE-PEG to camouflage the nanoparticle (ICNP). Together with the remarkable NIR fluorescence emission performance of the upconversion core, the core-shell nanoparticle was used for highly specific in vivo tumor imaging. Different core materials can be chosen to achieve a versatile delivery system. The anticancer agent doxorubicin (DOX) has been incorporated into a gold nanocage to form the inner cores, which was further coated with membrane of 4T1 breast cancer cells. The system combined the advantages of both photothermal therapy and chemotherapy. It has shown that the nanoparticle exhibited the superior targeting efficiency of the 4T1 cells, higher accumulation in tumor tissue and hyperthermia-responsive drug release behavior64. Further, a cancer cell membrane-coated probe (indocyanine green, ICG)-loaded lipid polymer NPs has been shown to be an excellent nanosystem for homologous-targeting dual-modal imaging and imaging-guided photothermal therapy.
Figure 3.
Illustration of cancer cell membrane-biomimetic nanoparticles for targeting recognition of source cancer cell, dual-modal imaging, and photothermal therapy. Adapted with permission from Ref. 62. Copyright American Chemical Society, 2016.
The application of therapeutic cancer vaccines, an exciting strategy in the cancer immunotherapy field, has recently aroused great attention65. Different from conventional cytotoxic drugs, the goal of a cancer vaccine is to activate the immune system against cancer rather than directly kill tumor cells, and has shown unique strength. Nevertheless, stimulating an immune response against cancer with the use of vaccines still faces difficult challenges66. Specific short peptides were always used as vaccines to induce dendritic-cell activation, while it was less effective because those peptides vaccines have a short half-life and are difficult to reach the antigen-presenting cells. In addition, adjuvants were necessary to elevate the immune response, which was difficult to incorporate in peptide vaccines. Moreover, it is hard to select ideal tumor antigens to train the immune system without adjuvants. As a result, the immunization effect and therapeutic benefits of the traditional cancer vaccine were not satisfactory. Recently, adjuvant-loaded PLGA NPs coated with cancer cell membrane were developed as a novel cancer vaccine system, completely retaining all the surface antigen of source cells67. The results of the study showed that the platform enabled co-delivery of multiple cancer antigens and adjuvants in a stable nanoparticle form, and provided a promising way to deliver cancer vaccine.
5. Platelet membrane-coated nanoparticle
Platelets, the smallest circulating blood cells, are fragments of cytoplasm produced from mature megakaryocytes in bone marrow.68 There are about 150,000–350,000 platelets per microliter circulating in the blood to preserve the integrity of the vasculature. Their average life span is 8 to 9 days.69 It is well known that platelets play an important role in the process of hemostasis after vascular injury, wound healing, inflammatory reaction and thrombosis70. Recently, extensive studies show that the hemostatic properties of platelets crucially promote the metastatic progression of cancer in many different ways71, such as contribution to tumor angiogenesis, assistance of tumor survival in the bloodstream and promotion of tumor cell and vascular interactions. The recognition and interaction between circulating tumor cells (CTCs) and platelets has drawn wide attention. After activation, platelets would change shape, release granules containing growth factors, chemokines and proteases, and increase their adhesiveness to form heteroaggregates with CTCs and leukocytes72.
Based on the close interactions between platelets and tumor metastasis, biomimetic strategies were developed for tumor targeting drug delivery. Platelet transfusions have been extensively used to treat or prevent bleeding since 1950s73, therefore the source of platelets is reliable. Moreover, compared with other nuclear cells, pure platelets have fewer antigen and show lower immunogenicity74., 75..
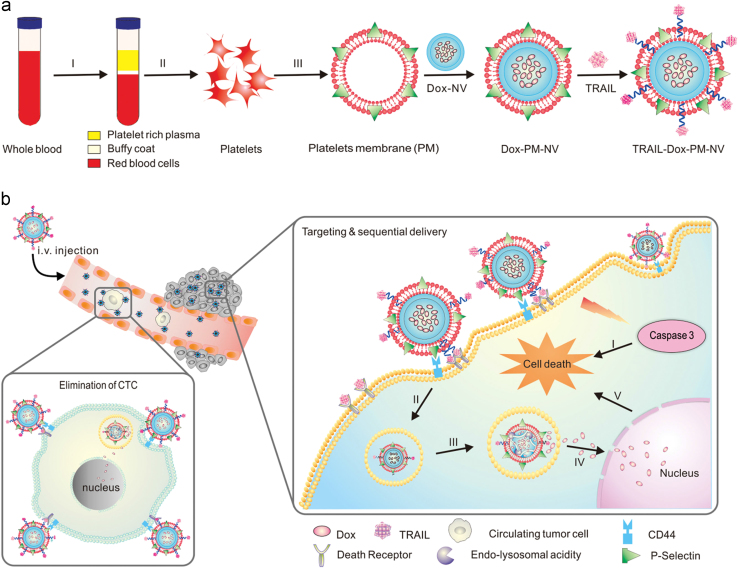
Recently, Hu et al.76 developed a platelet membrane-coated nanovehicle with tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) inserted onto the outer membrane and Dox loaded into the inner nanoparticles (Fig. 4). The results showed that platelet membrane-coated nanovehicle (PM-NV) had the strongest antitumor efficacy on an animal model with both a subcutaneous tumor and metastatic site76. The authors explained that the system could actively target CTCs based on the affinity between overexpressed P-selectin on the platelet membrane and CD44 receptors upregulated on the cancer cells, which could further facilitate the apoptosis effect on CTCs induced by TRAIL. Unfortunately, there were no related experiments in this article directly proving the mechanism. Platelet membrane-coated nanoparticles can be applied for tumor imaging as well. For example, a kind of platelet-mimicking magnetic nanoparticle was reported for enhanced cancer imaging and therapy. Fe3O4 magnetic nanoparticles was coated with platelet membrane collected from mice blood, inheriting the long blood circulation and cancer targeting capabilities from the platelets. The results revealed that this theranostic system can be used to enhance tumor magnetic resonance imaging and photothermal therapy for personalized diagnosis and therapy of cancers77.
Figure 4.
Schematic design of drug-loaded PM-NV for targeting and sequential drug delivery. Adapted with permission from Ref. 76. Copyright Wiley Online Library, 2015.
6. Conclusions
The CMBNP has shown the potential to significantly improve the function of current nanoparticle systems in cancer therapy. It can possess both unique functions exhibited by different cell types and flexible designs derived from various cores. As summarized in Table 2, CMBNPs are not limited to the four types that we reviewed above. Bacterial membranes, stem cell membranes and other bio-functional membranes have been explored for preparing CMBNPs in succession. The development of new type CMBNPs may further enrich tumor targeting strategies.
Table 2.
Summary of CMBNPs and their characteristics.
| Types | Membrane | Material core | Preparation method | Functions | Refs. |
|---|---|---|---|---|---|
| RBC-CMBNPs | RBC | PLGA/Au/Silicon/ | Extrusion/sonication | Long-circulation/detoxin/vaccine | 30., 37., 78. |
| WBC-CMBNPs | Leucocyte | PLGA/silicon/lipid | Extrusion/sonication | Inflammation targeting/ extravasations through inflamed endothelium | 44., 51. |
| Platelet-CMBNPs | Platelet | PLGA/ acryl amide nanogels | Extrusion | CTC-targeting/restenosis targeting | 76., 77. |
| CC-CMBNPs | Cancer cell | PLGA | Extrusion | Vaccine/ natural cancer-targeting | 62., 63., 67. |
| Bacterial membrane- CMBNPs | E. coli outer membrane | Au | Extrusion | Vaccine | 79 |
| MSC-CMBNPs | Stem cell | Gelatin nanogels | Extrusion | Cancer targeting | 80 |
One of the most common points of the inspiring strategies discussed above is the incredibly prolonged circulation after coating with the cell membrane. Just like the parent cells, the CMBNPs will be recognized as autogenous friends, which will reduce elimination by RES system. Beyond self-recognition, like exosomes81, the natural target functionality of cell membranes can facilitate extravasation, chemotaxis and specific cell–cell interactions. With breakthroughs in research on cell function in cancer78, more specific cell membrane coatings will be developed to achieve desired therapeutic benefits.
In addition to serving as vehicles for targeted drug delivery, the CMBNPs themselves may also play roles in cancer immune modulation. Zhang's group has recently reported RBC-coated CMBNPs for antivirulence vaccination by presenting bacterial-derived antigens82., 83.. This study raised a possibility to introduce cancer-related antigens onto CMBNPs to elevate cancer immune recognition. Moreover, to specifically induce an immune response, the specific cancer cell membrane might be isolated from tumor resection, which could facilitate postoperative immunotherapy by CMBNPs.
Despite the current progress in the research and development of CMBNPs, the field is still in its infancy. Several challenges are confronting the CMBNPs in translating from bench to bedside. Firstly, the source of cell membrane is quite limited. Except for RBC membrane, most cell membranes are isolated from cell lines and involve several isolation steps. The preparation process is complex and the yield of CMBNPs is low. In this regard, there is an urgent demand to simplify and expand the preparation process of CMBNPs for clinical study. Secondly, there are still some mysteries remaining to be explored to leverage this strategy in the future. For instance, plenty of proteins are presented on the cell membrane. Among them, some are responsible for targeting, while some are liable to induce immune responses. To identify the helpful proteins and remove the unwanted proteins will definitely promote the performance of CMBNPs in cancer therapy. Thirdly, unlike synthetic materials, the quality of CMBNPs is arduous to control and the safety of CMBNPs is a concern. To tackle these challenges, a shift in focus from discovery to process development and multi-disciplinary cooperation is needed. Overall, the emergence of biomimetic design has brought a paradigm shift in cancer treatment with nanomedicine. More efficacious and inspiring strategies will be developed to advance cancer treatment with cell membrane-based nanoparticles.
Acknowledgments
We thank for the financial support from National Natural Science Foundation of China (81773911, 81690263, 81673372, and 81361140344), National Basin Research Program of China (2013CB 932500), and Development Project of Shanghai Peak Disciplines–Integrated Medicine (No. 20150407).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Zhang S., Zhao P., Zeng H., Zou X. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26:48–58. doi: 10.3978/j.issn.1000-9604.2014.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W., Zheng R., Zeng H., Zhang S., He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 5.Mitragotri S., Burke P.A., Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owens D.E., III, Peppas N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Danhier F., Feron O., Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Chapman A.P. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv Drug Deliv Rev. 2002;54:531–545. doi: 10.1016/s0169-409x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- 9.Lubich C., Allacher P., de la Rosa M., Bauer A., Prenninger T., Horling F.M. The mystery of antibodies against polyethylene glycol (PEG)—what do we know? Pharm Res. 2016;33:2239–2249. doi: 10.1007/s11095-016-1961-x. [DOI] [PubMed] [Google Scholar]
- 10.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 11.Yu M.K., Park J., Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2:3–44. doi: 10.7150/thno.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLoach JR., Barton C., Culler K. Preparation of resealed carrier erythrocytes and in vivo survival in dogs. Am J Vet Res. 1981;42:667–669. [PubMed] [Google Scholar]
- 13.Georgieva R., Moya S., Hin M., Mitlöhner R., Donath E., Kiesewetter H. Permeation of macromolecules into polyelectrolyte microcapsules. Biomacromolecules. 2002;3:517–524. doi: 10.1021/bm010164n. [DOI] [PubMed] [Google Scholar]
- 14.Pang L., Zhang C., Qin J., Han L., Li R., Hong C. A novel strategy to achieve effective drug delivery: exploit cells as carrier combined with nanoparticles. Drug Deliv. 2017;24:83–91. doi: 10.1080/10717544.2016.1230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang X., Röcker C., Hafner M., Brandholt S., Dörlich R.M., Nienhaus G.U. Endo- and exocytosis of zwitterionic quantum dot nanoparticles by live HeLa cells. ACS Nano. 2010;4:6787–6797. doi: 10.1021/nn101277w. [DOI] [PubMed] [Google Scholar]
- 16.Luk B.T., Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220:600–607. doi: 10.1016/j.jconrel.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao W., Zhang L. Coating nanoparticles with cell membranes for targeted drug delivery. J Drug Target. 2015;23:619–626. doi: 10.3109/1061186X.2015.1052074. [DOI] [PubMed] [Google Scholar]
- 18.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 19.Fang R.H., Jiang Y., Fang J.C., Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83. doi: 10.1016/j.biomaterials.2017.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Iero M., Valenti R., Huber V., Filipazzi P., Parmiani G., Fais S. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2007;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 22.Azmi A.S., Bao B., Sarkar F.H. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metast Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Föeller M., Huber S.M., Lang F. Erythrocyte programmed cell death. IUBMB Life. 2008;60:661–668. doi: 10.1002/iub.106. [DOI] [PubMed] [Google Scholar]
- 24.Godfrin Y., Horand F., Franco R., Dufour E., Kosenko E., Bax B.E. International seminar on the red blood cells as vehicles for drugs. Expert Opin Biol Ther. 2012;12:127–133. doi: 10.1517/14712598.2012.631909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierigè F., Serafini S., Rossi L., Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60:286–295. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 26.Brigger I., Dubernet C., Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2012;64:24–36. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 27.Yoo J.-W., Chambers E., Mitragotri S. Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr Pharm Des. 2010;16:2298–2307. doi: 10.2174/138161210791920496. [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov R., Janeway Jr., CA Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 29.Oldenborg P.-A., Zheleznyak A., Fang Y.F., Lagenaur C.F., Gresham H.D., Lindberg F.P. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 30.Hu C.M., Zhang L., Aryal S., Cheung C., Fang R.H., Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu C.M., Fang R.H., Luk B.T., Chen K.N., Carpenter C., Gao W. 'Marker-of-self' functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale. 2013;5:2664–2668. doi: 10.1039/c3nr00015j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev. 2011;63:131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Luk B.T., Fang R.H., Hu C.M., Copp J.A., Thamphiwatana S., Dehaini D. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics. 2016;6:1004–1011. doi: 10.7150/thno.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farokhzad O.C. Using ligands to target cancer cells. Clin Adv Hematol Oncol. 2012;10:543–544. [PubMed] [Google Scholar]
- 35.Fang R.H., Hu C.M., Chen K.N., Luk B.T., Carpenter C.W., Gao W. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale. 2013;5:8884–8888. doi: 10.1039/c3nr03064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu Q., Lv P., Chen Z., Ni D., Zhang L., Yue H. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane. Nanoscale. 2015;7:4020–4030. doi: 10.1039/c4nr07027e. [DOI] [PubMed] [Google Scholar]
- 37.Su J., Sun H., Meng Q., Yin Q., Zhang P., Zhang Z. Bioinspired nanoparticles with NIR-controlled drug release for synergetic chemophotothermal therapy of metastatic breast cancer. Adv Funct Mater. 2016;26:7495–7506. [Google Scholar]
- 38.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 40.Pang L., Qin J., Han L., Zhao W., Liang J., Xie Z. J. Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget. 2016;7:37081–37091. doi: 10.18632/oncotarget.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sica A., Allavena P., Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 42.Franklin R.A., Liao W., Sarkar A., Kim M.V., Bivona M.R., Liu K. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xuan M., Shao J., Dai L., He Q., Li J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv Healthc Mater. 2015;4:1645–1652. doi: 10.1002/adhm.201500129. [DOI] [PubMed] [Google Scholar]
- 44.Parodi A., Quattrocchi N., van de Ven A.L., Chiappini C., Evangelopoulos M., Martinez J.O. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q., Ren Y., Mu J., Egilmez N.K., Zhuang X., Deng Z. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75:2520–2529. doi: 10.1158/0008-5472.CAN-14-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao L., He Z., Meng Q.F., Zhou Z., Bu L.L., Guo S.S. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles. J Biomed Mater Res A. 2017;105:521–530. doi: 10.1002/jbm.a.35927. [DOI] [PubMed] [Google Scholar]
- 47.Xuan M., Shao J., Dai L., Li J., He Q. Macrophage cell membrane camouflaged au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8:9610–9618. doi: 10.1021/acsami.6b00853. [DOI] [PubMed] [Google Scholar]
- 48.Middleton J., Patterson A.M., Gardner L., Schmutz C., Ashton B.A. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–3860. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 49.Garrood T., Lee L., Pitzalis C. Molecular mechanisms of cell recruitment to inflammatory sites: general and tissue-specific pathways. Rheumatology. 2005;45:250–260. doi: 10.1093/rheumatology/kei207. [DOI] [PubMed] [Google Scholar]
- 50.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao H., Dan Z., He X., Zhang Z., Yu H., Yin Q. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10:7738–7748. doi: 10.1021/acsnano.6b03148. [DOI] [PubMed] [Google Scholar]
- 52.He W., Frueh J., Wu Z., He Q. How leucocyte cell membrane modified janus microcapsules are phagocytosed by cancer cells. ACS Appl Mater Interfaces. 2016;8:4407–4415. doi: 10.1021/acsami.5b10885. [DOI] [PubMed] [Google Scholar]
- 53.Swierczak A., Mouchemore K.A., Hamilton J.A., Anderson R.L. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastas- Rev. 2015;34:735–751. doi: 10.1007/s10555-015-9594-9. [DOI] [PubMed] [Google Scholar]
- 54.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang T., Zhu Q., Wei D., Feng J., Yao J., Jiang T. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11:1397–1411. doi: 10.1021/acsnano.6b06477. [DOI] [PubMed] [Google Scholar]
- 56.Krishnamurthy S., Gnanasammandhan M.K., Xie C., Huang K., Cui M.Y., Chan J.M. Monocyte cell membrane-derived nanoghosts for targeted cancer therapy. Nanoscale. 2016;8:6981–6985. doi: 10.1039/c5nr07588b. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L., Li R., Chen H., Wei J., Qian H., Su S. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: a new approach to enhance drug targeting in gastric cancer. Int J Nanomed. 2017;12:2129–2142. doi: 10.2147/IJN.S126016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greene J.M., Wiseman R.W., Lank S.M., Bimber B.N., Karl J.A., Burwitz B.J. Differential MHC class I expression in distinct leukocyte subsets. BMC Immunol. 2011;12:39. doi: 10.1186/1471-2172-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherr C.J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 60.Glinsky V.V., Glinsky G.V., Glinskii O.V., Huxley V.H., Turk J.R., Mossine V.V. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–3811. [PubMed] [Google Scholar]
- 61.Khaldoyanidi S.K., Glinsky V.V., Sikora L., Glinskii A.B., Mossine V.V., Quinn T.P. MDA-MB-435 human breast carcinoma cell homo- and heterotypic adhesion under flow conditions is mediated in part by thomsen-friedenreich antigen-galectin-3 interactions. J Biol Chem. 2003;278:4127–4134. doi: 10.1074/jbc.M209590200. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z., Zhao P., Luo Z., Zheng M., Tian H., Gong P. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10:10049–10057. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
- 63.Rao L., Bu L.L., Cai B., Xu J.H., Li A., Zhang W.F. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv Mater. 2016;28:3460–3466. doi: 10.1002/adma.201506086. [DOI] [PubMed] [Google Scholar]
- 64.Sun H., Su J., Meng Q., Yin Q., Chen L., Gu W. Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer. Adv Funct Mater. 2017;27:1604300. [Google Scholar]
- 65.Schlom J. Therapeutic cancer vaccines: current status and moving forward. J Natl Cancer Inst. 2012;104:599–613. doi: 10.1093/jnci/djs033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg S.A. Cancer immunotherapy comes of age. Nat Clin Pract Oncol. 2005;2:115. doi: 10.1038/ncponc0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang R.H., Hu C.J., Luk B.T., Gao W., Copp J.A., Tai Y. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel S.R., Hartwig J.H., Italiano J.E., Jr. The biogenesis of platelets from megakaryocyte propatelets. J Clin Investig. 2005;115:3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harker L.A., Roskos L.K., Marzec N.M., Carter R.A., Cherry J.K., Sundell B. Effects of megakaryocyte growth and development factor on platelet production, platelet life span, and platelet function in healthy human volunteers. Blood. 2000;95:2514–2522. [PubMed] [Google Scholar]
- 70.Wagner D.D., Burger P.C. Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 71.Gay L.J., Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nash G.F., Turner L.F., Scully M.F., Kakkar A.K. Platelets and cancer. Lancet Oncol. 2002;3:425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 73.Stroncek D.F., Rebulla P. Platelet transfusions. Lancet. 2007;370:427–438. doi: 10.1016/S0140-6736(07)61198-2. [DOI] [PubMed] [Google Scholar]
- 74.Sintnicolaas K., van Marwijk Kooij M., van Prooijen H.C., van Dijk B.A., van Putten W., Claas F.H. Leukocyte depletion of random single-donor platelet transfusions does not prevent secondary human leukocyte antigen-alloimmunization and refractoriness: a randomized prospective study. Blood. 1995;85:824–828. [PubMed] [Google Scholar]
- 75.Eernisse J.G., Brand A. Prevention of platelet refractoriness due to HLA antibodies by administration of leukocyte-poor blood components. Exp Hematol. 1981;9:77–83. [PubMed] [Google Scholar]
- 76.Hu Q., Sun W., Qian C., Wang C., Bomba H.N., Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27:7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rao L., Bu L.L., Meng Q.F., Cai B., Deng W.W., Li A. Antitumor platelet-mimicking magnetic nanoparticles. Adv Funct Mater. 2017;27:1604774. [Google Scholar]
- 78.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao W., Fang R.H., Thamphiwatana S., Luk B.T., Li J., Angsantikul P. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15:1403–1409. doi: 10.1021/nl504798g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao C., Lin Z., Jurado-Sánchez B., Lin X., Wu Z., He Q. Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small. 2016;12:4056–4062. doi: 10.1002/smll.201600624. [DOI] [PubMed] [Google Scholar]
- 81.Ha D., Yang N., Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei X., Gao J., Wang F., Ying M., Angsantikul P., Kroll A.V. In situ capture of bacterial toxins for antivirulence vaccination. Adv Mater. 2017;29:1701644. doi: 10.1002/adma.201701644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang F., Fang R.H., Luk B.T., Hu C.M., Thamphiwatana S., Dehaini D. Nanoparticle-based antivirulence vaccine for the management of methicillin-resistant Staphylococcus aureus skin infection. Adv Funct Mater. 2016;26:1628–1635. doi: 10.1002/adfm.201505231. [DOI] [PMC free article] [PubMed] [Google Scholar]