Abstract
High-density lipoproteins (HDL) are naturally-occurring nanoparticles that are biocompatible, non-immunogenic and completely biodegradable. These endogenous particles can circulate for an extended period of time and transport lipids, proteins and microRNA from donor cells to recipient cells. Based on their intrinsic targeting properties, HDL are regarded as promising drug delivery systems. In order to produce on a large scale and to avoid blood borne pollution, reconstituted high-density lipoproteins (rHDL) possessing the biological properties of HDL have been developed. This review summarizes the biological properties and biomedical applications of rHDL as drug delivery platforms. It focuses on the emerging approaches that have been developed for the generation of biomimetic nanoparticles rHDL to overcome the biological barriers to drug delivery, aiming to provide an alternative, promising avenue for efficient targeting transport of nanomedicine.
KEY WORDS: Biomimetic nanocarrier, Reconstituted high- density lipoprotein, Biological barriers, Efficient targeting, Nanodrug delivery systems
Graphical abstract
This review summarizes the biological properties and biomedical applications of reconstituted high-density lipoproteins (rHDL) as drug delivery platforms with special focus on the emerging approaches that have been developed for the generation of biomimetic nanoparticles rHDL to overcome the biological barriers to drug delivery.
1. Introduction
High-density lipoproteins (HDL) are dynamic natural nanoparticles that are composed of diverse biological macromolecules. Many aspects of the nanoparticles such as size, shape and surface chemical composition play a key role in their multiple biological functions1. Compared with other lipoproteins, they have higher density and smaller size ranging from 8 to 12 nm in diameter2. The main protein constituent of HDL is apolipoprotein A-I (apoA-I) which comprises almost 70% of the protein mass in HDL and is occasionally accompanied by apoA-II, apoA-IV, apoA-V, etc.3, 4. ApoA-I, a 28 kDa protein, contains eight amphipathic α-helical domains of 22 amino acids each that is responsible for scaffolding the size and shape of natural HDLs4. In addition, apolipoprotein E (apoE) is also a pivotal component of HDL which occurs in much lower abundance than apoA. The interaction between apoA-I and lipids determines the final shape and size of HDL with the hydrophobic face of apoA-I mediating lipid interactions and the polar face interacting with water5. Natural HDL particles include discoidal subclass pre-β-HDL which is generated by exposure of lipid-free apoA-I to ATP-binding cassette transporters A1 (ABCA1) with the cholesterol/phospholipid transfer activity, and two spherical subclasses HDL2 and HDL3 which contain a neutral lipid core composed of cholesteryl ester and triglyceride. HDL2 and HDL3 represent the dominant HDL forms in human plasma6. HDL is referred to as “good cholesterol” by removing excess cholesterol from peripheral tissues and transporting it to the liver for catabolism or excretion via a process known as reverse cholesterol transport (RCT)7. In addition, HDL particles possess anti-inflammatory, anti-oxidative, anti-apoptotic and anti-infective properties. Therefore, HDL has been regarded as a key component which protects the cardiovascular system and reduces the risk of coronary artery disease8.
Recently, the potential of using HDL particles as drug delivery vehicles has been widely explored. Many biological properties of HDL can be harnessed for opening an attractive avenue towards optimal drug delivery. Firstly, these particles are completely biodegradable and have excellent biocompatibility. Secondly, as endogenous substances, they escape elimination by mononuclear phagocyte system (MPS) and do not trigger immunological responses. Furthermore, innate receptor-ligands present in HDL and the corresponding cellular receptors of these ligands have shown to help HDL exert their biological functions. HDL particles which contain apoA-I are known to bind many cellular receptors such as the scavenger receptor class B type 1 (SR-B1) and ATP-binding cassette transporter G1 (ABCG1)9. The SR-B1 and ABCG1 receptors have shown to mediate cholesterol transferring to HDL particles from the peripheral cells including foam cells. From the targeting delivery point of view, the SR-B1 receptor is abundant in hepatic cells, macrophages and cancer cells and it is thought to be a critical receptor for delivering HDL-cargo to those cells10. It has been reported that endogenous HDL can transport lipids, proteins and microRNA from donor cells to recipient cells, suggesting that the intrinsic and functional targeting capability of HDL makes it an ideal candidate for drug delivery and intracellular communication11.
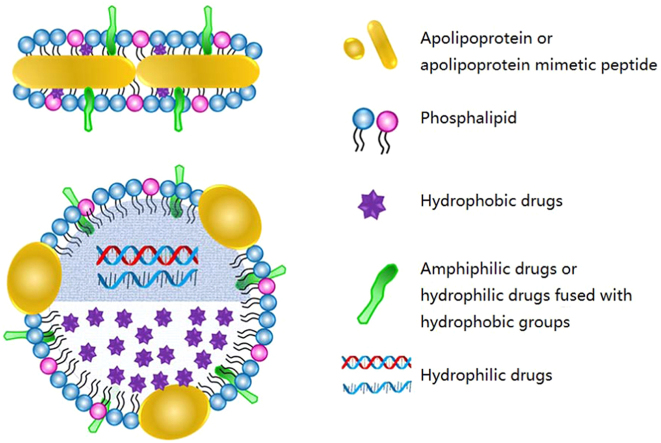
However, there are also several limitations for HDL-mediated drug delivery. Firstly, the source of endogenous HDL is restricted to the costly and laborious process of isolation and purification from human plasma, which is difficult on a large scale. Secondly, the safety concerns of blood borne pollution could be another major challenge for HDL as a universal drug delivery system. To address these issues, a great number of studies have focused on the development of rHDL particles which are artificially synthesized with phospholipids and apolipoproteins/apolipoprotein mimetic peptides as alternative therapeutics or drug delivery platforms. As the individual components of rHDL (lipid type, apolipoprotein choice and lipid/protein stoichiometry) can be flexibly adjusted, the physiochemical properties such as uniform size, zeta potential, core and surface loading of rHDL can be easily controlled. In terms of drug loading, hydrophobic drugs can be incorporated into the core of rHDL, amphiphilic drugs can be inserted into the lipid membrane and hydrophilic molecules can be carried either by fused with a hydrophobic group to insert into the surface of rHDL or by encapsulated into the core of rHDL with the help of certain hydrophobic components (Fig. 1)2, 12, 13. In addition, rHDL can not only harness the biology of HDL but also overcome the various biological barriers to drug delivery. In this review, we focus on the biological properties of these novel biomimetic nanoparticles and summarize the biomedical applications of rHDL as a drug delivery platform.
Figure 1.
The schematic of discoidal and spherical rHDL as drug delivery systems. As depicted, hydrophobic drugs can be incorporated into the core of rHDL, amphiphilic drugs can be inserted into the lipid membrane and hydrophilic molecules can be loaded either by fused with a hydrophobic group to insert into the surface of rHDL or by encapsulated into the core of rHDL with the help of certain hydrophobic components.
2. The biological properties of rHDL for drug delivery
2.1. Long circulation time and relative stability
Upon intravenous injection or absorption into the circulation, drug-loaded nanoparticles are exposed to a highly dynamic system in which the interaction between the particles and the blood proteins can largely influence their physicochemical properties and biofate. MPS, the macrophage system in the liver, spleen lymph nodes and bone marrow, has a profound impact on the circulation time and clearance rate of nanoparticles14. This process of sequestration begins with opsonization of nanoparticles, involving the adsorption of plasma proteins such as serum albumin, complement components and immunoglobulins, onto the surface of circulating nanoparticles15. Following opsonization, these nanoparticles are recognized and captured by the MPS which leads to their accumulation in the organs such as the liver and spleen and their consequent removal from circulation16. In order to achieve efficient delivery to the target sites, proper circulation time and plasma stability are the key prerequisites. In the case of rHDL, many of strategies have been developed to extend their systemic circulation time and maintain their stability in plasma.
The circulation lifetime of nanoparticles is dependent on opsonization and sequestration uptake by resident macrophages of the MPS. Polyethylene glycol (PEG), a type of hydrophilic polymer which has been approved for safety by the FDA for use in human pharmaceuticals17, 18, has been widely used as a strategy to endow nanoparticles with stealth properties by inhibiting serum protein binding to evade the MPS, improve pharmacokinetics19, 20, and enhance distribution to the target sites21. In the case of rHDL, as an HDL biomimetic, it is capable of escaping from elimination by MPS. Generally, the half-life of rHDL is comparable to the commonly used drug delivery system, pegylated liposomes. Recently, Murphy et al.22 found that pegylation of apoA-I in rHDL also markedly improved their plasma half-life. The formation of monopegylated apoA-I (via the N-terminus) increased the half-life of apoA-I approximately 7-fold in hypercholesterolemic ApoE−/− mice compared with apoA-I in nonpegylated rHDL. Furthermore, PEG-rHDL led to more pronounced suppression of bone marrow myeloid progenitor cell proliferation and monocytosis, reduced atherosclerosis and a stable plaque phenotype. However, the chain length as well as the density of PEG should be optimized for desirable effects because it may impact on the size, stability and toxicity of nanoparticles23. In addition, the utility of pegylation could be limited following repeated administration owing to the "accelerated blood clearance (ABC) phenomenon" induced by the spleen24.
Despite pegylation, altering lipid composition of rHDL could also change the circulation time and stability of rHDL in vivo. Lipid components account for approximately one-half the mass of total HDLs25, and 40%–60% of the total lipids are phospholipids which include phosphatidylcholines (PCs), phosphatidylinositol, sphingomyelin (SM), and lysophosphatidylcholine (LPC)26. Lipid components influence the density, size, shape, rigidity and surface charge of HDL particles, and further determine their biological and biophysical properties27. For example, SM enrichment has been shown to induce an ordered and rigid liquid–lipid bilayer environment in HDL particles and increase the circulation time and stability of liposome28, 29. In addition, Jayaraman et al.30 found that cholesterol, an essential constituent involved in the process of RCT, stabilized less stable lipoproteins by enhancing favorable packing interactions, but had less or opposite influence on the more stable complexes in which the packing interactions were already optimized.
Furthermore, both the size and geometry of nanoparticles are important for their flow, margination and adhesive properties in blood vessels under normal flow conditions, and also play a key role in determining the stability of rHDL in the circulation31. Interestingly, discoidal particles tend to exhibit a higher margination propensity compared to quasi-hemispherical and spherical particles, which would result in stronger interaction with vessel walls32. During circulation in the blood system, natural HDL particles exist in two different architectures including discoidal HDLs (d-HDLs) and spherical HDLs (s-HDLs). These two HDLs display different physical-chemical and biological properties. d-HDLs are referred to nascent HDLs and can be converted to mature s-HDLs catalyzed by lecithin-cholesterol acyltransferase (LCAT)33. The remodeling effect of LCAT is the major mechanism that mediates the instability of d-HDLs in their metabolic process and results in the leakage of encapsulated drugs before they reach the targeted cells34. Several approaches have been developed for alleviating drug leakage of d-rHDLs induced by LCAT. Wang et al.35 stably incorporated paclitaxel (PTX), a lipophilic anti-cancer agent, into d-rHDLs and decreased PTX leakage by covalently attaching mono-cholesteryl succinate (CHS) to apoA-I to form CHS-modified rHDL. The resulting CHS-modified rHDL did not remodel in the presence of LCAT, and showed longer circulation time in blood, alleviated unnecessary drug leakage and delivered more PTX to the cancer cells.
Cross-linking strategy can also enhance the stability of nanolipoprotein particles under physiological conditions. Smith et al.36 found that inserting a gadolinium-binding chitosan fastener on the liposome surface followed by covalent cross-linking of the lipid bilayer provided a useful method to anchor the functional units to the liposome surface and stabilized liposomes under physiological conditions. Recently, Gilmore et al.37 also developed lipid cross-linking nanolipoprotein particles (NLPs) to enhance their serum stability by incorporating a polymerizable lipid into the lipid bilayer. Compared with the non-cross-linking NLPs, cross-linking NLPs exhibited greater stability in 100% serum with no degradation over 48 h. This method of intermolecular cross-linking did not significantly affect the size of nanoparticles but powerfully enhanced their in vivo stability.
2.2. rHDL-mediated targeting delivery
2.2.1. The innate targets and receptors for rHDL
The mechanisms by which HDLs interact with their specific cellular receptors control the function and eventual fate of endogenous HDL as well as rHDL particles. ApoA-I is the most abundant protein in plasma HDL, and apoA-I/apoA-I mimetic peptides are most commonly used for the construction of rHDL. rHDL particles which contain the apoA-I/apoA-I mimetic peptides are known to bind majorly to SR-B1, an integral membrane protein mainly expressed in the liver and steroidogenic tissues38. As the endogenous receptor of HDL, SR-B1 is best known for its role in facilitating the uptake of cholesteryl esters from HDL to the liver and steroidogenic tissues. SR-B1 is also crucial in lipid soluble vitamin uptake39. In addition, the overexpressed SR-B1 is a relatively consistent marker in cancerous tissues. While SR-B1 normally mediates the transfer of cholesterol between HDL and healthy cells, it also facilitates the selective uptake of cholesterol in malignant cells. In this way, the upregulated of SR-B1 receptor becomes an attractive target for tumor targeting as well40.
Another relatively common apolipoprotein component of HDL is apoE, which has also been utilized for the synthesis of rHDL. In peripheral tissues, apoE is primarily produced by the liver and macrophages, and mediates cholesterol metabolism in an isoform-dependent manner41. In the central nervous system (CNS), apoE is mainly produced by astrocytes, mostly in the form of HDL, and transports cholesterol to neurons via apoE receptors41. ApoE is known to bind to members of the LDL receptor (LDLR) family that includes more than 10 different receptors to mediate endocytosis and uptake of the holo-particle. As the major receptor of apoE, LDLR occurs in all nucleated cells, mainly in the liver, also in the CNS and tumor tissues. In the process of cellular internalization, LDLR are clustered in clathrin-coated pits, and coated pits pinch off from the surface to form coated endocytic vesicles that carry lipoprotein into the cells. After internalization, the receptors dissociate from their ligands when they are exposed to lower pH in endosomes. After dissociation, the receptor folds back on itself to obtain a closed conformation and recycles to the cell surface. The rapid recycling of LDLR provides an efficient mechanism for delivery of cholesterol to the targeted cells42.
2.2.2. rHDL for overcoming the blood–brain barrier
The existence of the blood–brain barrier (BBB) is a major limiting factor for efficient delivery of therapeutic agents into the CNS. BBB is constituted by endothelial cell tight junctions in the brain microvessels and has a complex and highly organized multicellular structure43, 44. The BBB effectively protects the brain from most harmful substances in the external environment, and also excludes from the brain ∼100% of large-molecule neurotherapeutics and more than 98% of all small-molecule drugs45. Therefore, although acting as both morphological and functional barrier, the BBB also hinders effective drug delivery to the brain and impedes the therapy of various neurological disorders. Herein, development of nanocarriers that possess BBB permeability is of great importance.
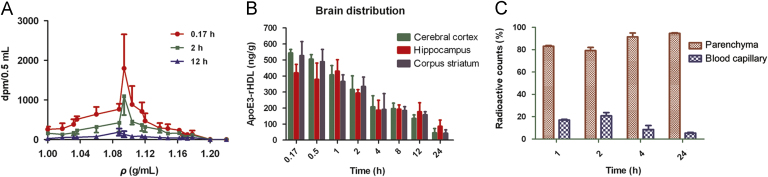
To address the limitation of crossing the BBB, many strategies have been investigated. Receptor-mediated transcytosis has been utilized as a key strategy for drug delivery across the BBB. The LDLR on the brain endothelial cell surface is a major apoE receptor that regulates the amount of apoE in the CNS46. Recent studies found that targeting LDLR provided a rational strategy for efficient transport across the BBB via receptor-mediated transcytosis and subsequent drug release in the brain47. It has been found that the bioactive apoE peptides were capable of binding to LDL receptor-related protein 1 (LRP1) tightly in a purified system and apoE could bind to LDLR family only after being incorporated into lipids48. Therefore, apoE-rHDL holds great promise for possessing BBB-penetrating activity and brain drug delivery via LDLR-mediated transcytosis. For instance, Song et al.49 developed apoE-rHDL as a biomimetic nanocarrier for the treatment of Alzheimer's disease. They utilized apoE3, one of the predominant apoE isoforms for construction of apoE-rHDL in which the N-terminal domain of apoE3 interacted with LDLR to mediate transcytosis. This biologically inspired nanocarrier successfully crossed the BBB (Fig. 2), promoted microglial and astroglial degradation of amyloid beta (Aβ) and rescued memory deficits in Alzheimer model mice.
Figure 2.
(A) Radioactivity in the brain homogenate at different density fractions after ultracentrifugation at 0.17, 2, and 12 h after intravenous administration of 125I-apoE3-rHDL. (B) Brain distribution of 125I-apoE3-rHDL at 0.17, 0.5, 1, 2, 4, 8, 12, and 24 h after intravenous administration. (C) Percentage of 125I-apoE3-rHDL accessing the brain parenchyma compared with that remained in the brain blood capillary at 1, 2, 4, and 24 h after intravenous administration. (Reproduced with permission from Ref. 48. Copyright 2014, American Chemical Society.
2.2.3. rHDL for penetrating plasma membrane
Many physiological barriers exist following systemic delivery of therapeutic agents. Once the nanoparticles reach the target cells, they need to penetrate plasma membranes and deliver cargo to targeted subcellular sites. Plasma membranes, possessing a dynamic structure, are critical borders between cells and the external environment.
2.2.3.1. Receptor-mediated rHDL cellular internalization
SR-B1, the major receptor for HDL, mediates transport of selective cholesterol esters from HDL to cytosol50. SR-B1-mediated interactions between rHDL and target cells have been investigated as an ideal strategy for drug delivery through the plasma membrane barrier. For example, Rui et al.51 developed rHDL nanoparticles that co-delivered PTX and doxorubicin (Dox) for improving anticancer efficacy. Co-delivery of multiple chemotherapeutics has become a versatile strategy in recent cancer treatment, but has been limited by the differing physiochemical properties of the agents. In order to solve this problem, rHDL nanoparticles that were capable of interacting with the receptor SR-B1 on the cell surface during cellular internalization were used to combine the two anticancer drugs into one delivery system by passively incorporating hydrophobic PTX, and subsequently remotely loading hydrophilic Dox into the same nanoparticles. This biomimetic nanocarrier successfully enhanced the accumulation of nanoparticles in the tumor region and increased their uptake in cancer cells. Both drugs were transported into the cytoplasm of cancer cells through the non-aqueous “channel” of SR-B1. This prominent strategy improved drug delivery into cells without endocytosis and degradation. In addition to intracellular delivery of small molecular therapeutics, SR-B1 also mediates efficient transmembrane delivery of macromolecular drugs. For example, Shahzad et al.52 incorporated siRNA into rHDL and successfully delivered siRNA in vivo to effectively inhibit tumor cell proliferation. Ding et al.53 also used rHDL to transport cholesterol-conjugated siRNA (Chol-siRNA) across the plasma membrane for direct cytosolic delivery via the SR-B1-mediated process.
2.2.3.2. Cell penetrating peptides-mediated rHDL cellular internalization
In addition to SR-B1-mediated cellular internalization, cell penetrating peptides (CPPs) have also been utilized as an effective strategy to help rHDL overcome the cellular plasma membrane54. It has been reported that CPPs possess the ability to interact with various cell surface molecules including membrane lipids and proteoglycans55. CPPs, which can themselves serve as carriers to transport protein, nucleic acids and nanoparticles into cells, have been extensively exploited for cancer therapy56. By taking advantage of the pH gradient between the tumor milieu (pH 6.4) and physiological environment (pH 7.4)57, pH-responsive CPPs can respond to tumor acidic microenvironment and have therefore been useful for mediating specific and efficient intracellular delivery to tumor cells. TH peptide [AGYLLGHINLHHLAHL(Aib)HHIL–NH2], an α-helical CPP that can efficiently enter cells under acidic environments, was found to significantly increase the tumor cells internalization of the pH-responsive CPP-modified drug delivery system, effectively inhibited tumor growth and largely improved the therapeutic efficacy58. R6H4 (RRRRRRHHHH), a pH-sensitive CPP with the ability of pH-responsive cellular uptake owing to histidine and cell penetration owing to arginine, has been used to improve direct cytosolic delivery of agents loaded by liposome59. Ding et al.60 recently designed a dual-functional biomimetic nanocarrier pH-responsive CPP-functionalized rHDL (cp-rHDL) by incorporating R6H4 into apoA-I rHDL, in which apoA-I enables attractive tumor-homing property, and the lipophilic anchored R6H4 offered a pH-controlled penetrating ability. The use of coumarin-6 as the fluorescent probe showed this hydrophobic molecular probe encapsulated in cp-rHDL to be efficiently transferred into the cytoplasm of tumor cells.
2.2.4. rHDL for delivery to subcellular targets
Targeting delivery of nanocarriers at the subcellular level has now become cumulatively important and is of increasing interest as more subcellular targets are identified. Once internalized, the nanocarriers are sorted and may then be exocytosed out of the cells or trapped in endosomes. The trapped carriers in the endosomes would subsequently be transported to lysosomes that are highly acidic and rich with enzymes, and finally result in degradation with little drug release into the cytosol61. To overcome intracellular delivery barrier, the common strategies are to deliver drugs into cytosolic organelles by promoting endosomal/lysosomal avoidance or escape.
2.2.4.1. rHDL with endosomal/lysosomal avoidance capacity
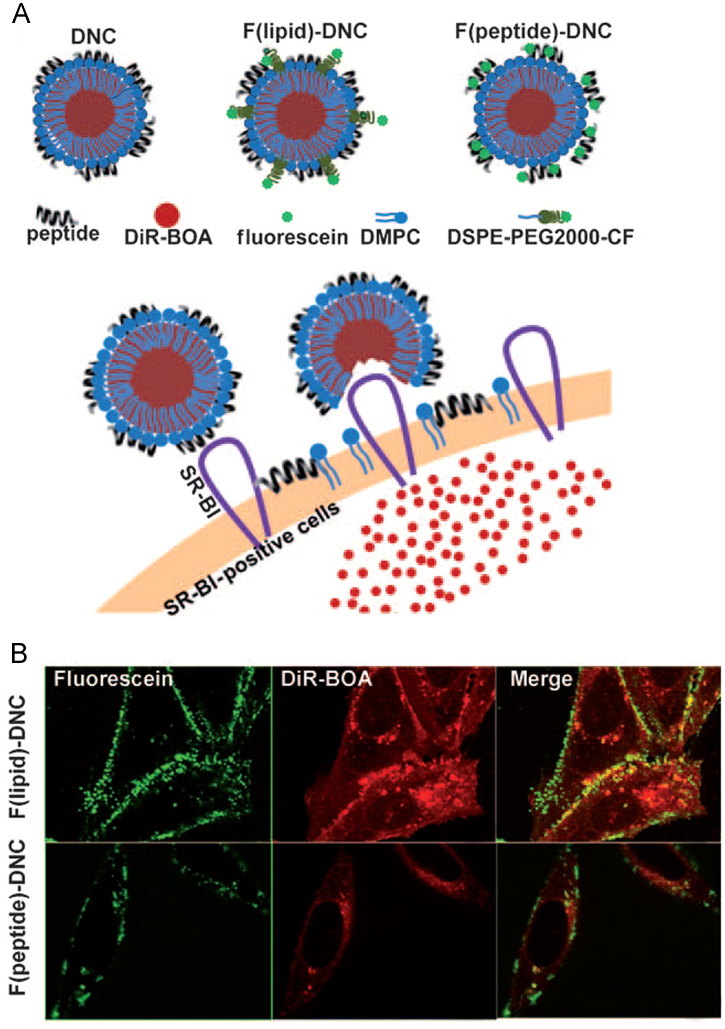
It is important to transport therapeutic molecules into cytosolic compartments because cytosolic organelles are usually the sites of action62. A delivery strategy for targeting at the subcellular level has been developed based on the fact that cholesterol esters are transported from HDL to cells in a SR-B1-mediated process in which a hydrophobic channel formed in the cell membrane. To further exploit this unique non-endocytic uptake mechanism for the direct cytosolic delivery of therapeutics, Zhang et al.62 created a peptide-phospholipid nanocarrier (NC) and trapped DiR-BOA, a hydrophobic fluorescent molecule, in the core-shell of NC, finding that the cargo DiR-BOA highly accumulated in the tumor cell cytosol (Fig. 3). Furthermore, this nanocarrier was highly biocompatible, exhibited long circulation half-life in serum and the size could be controlled precisely, providing a useful nanoplatform for efficient delivery of either small or large molecules. This carrier has been used to deliver a lipophilic drug, paclitaxel oleate, to attenuate its toxicity to non-targeted cells63.
Figure 3.
Proposed direct cytosolic delivery mechanism60. (A) Proposed a DiR-BOA-loaded peptide-phospholipid nanocarrier (DNC) structures and mechanism for the SR-BI-mediated cytosolic delivery of DNC cargo. (B) Confocal images of ldlA (mSR-BI) (SR-BI+) cells showing the fluorescein-labeled lipid (top) and peptide (bottom) localized on the cell surface and the DiR-BOA cargo in the cytosol. Scale bar is 10 μm. (Reproduced with permission from Ref. 60. Copyright 2009, Wiley.
The same approach has been utilized for the delivery of nucleic acids, especially for RNAi. The main challenge for RNAi therapeutics lies in systemic delivery of siRNA to the correct tissues and transporting them into the cytoplasm of target cells at safe and therapeutic levels. The NC described above was further developed for siRNA delivery in which siRNA was modified with cholesterol and the chol–siRNA was efficiently loaded by inserting into the lipid membrane of NC. The resulting nanoformulation demonstrated direct cytosolic delivery of siRNA in vitro, thereby bypassing endosomal trapping. It prolonged the blood circulation time of chol–siRNA by a factor of four, improved its biodistribution and facilitated its uptake in SR-B1-overexpressed tumors. The nanoformulation carrying BCL-2 siRNA efficiently downregulated BCL-2 protein, induced enhanced apoptosis in tumor cells and significantly inhibited tumor growth64. Shahzad et al.52 loaded siRNA into the core of rHDL by preincubation with oligolysine, and also achieved SR-B1-mediated highly efficient systemic delivery of siRNA in vivo.
SR-B1-mediated endosomal/lysosomal avoidance has also been exploited for addressing the intracellular hurdles of protein delivery. Kim et al.65 developed a biomimetic nanocarrier composed of 1,2-dioleoyl-trimethylammonium (DOTAP), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and apoA-I to enable successful protein delivery to intracellular targets. By conjugating cytochrome c (cytc) with a membrane permeable sequences peptide to enhance protein association with the lipid bilayer of rHDL, cytc was efficiently loaded and delivered to the cytosol of cancer cells. Kuai et al.66 demonstrated that high-density lipoprotein-mimicking nanodiscs coupled with antigen (Ag) peptides and adjuvants markedly improved Ag/adjuvant co-delivery to lymphoid organs and sustained Ag presentation on dendritic cells. The strategy was based on rHDL nanodiscs, composed of phospholipids and apoA-I-mimetic peptides, in which the nanodiscs elicited up to 47-fold greater frequencies of neoantigen-specific cytotoxic T-lymphocytes (CTLs) than soluble vaccines and even 31-fold greater than perhaps the strongest adjuvant in clinical trials.
2.2.4.2. rHDL with endosomal/lysosomal escape capacity
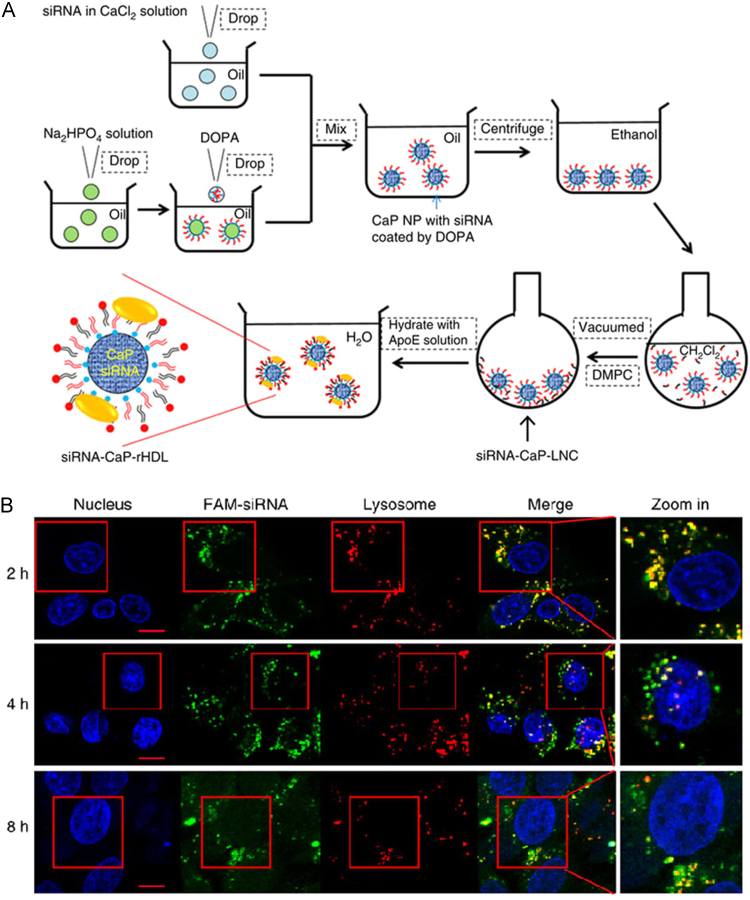
rHDL constructed with apoA-I and apoA-I mimetic peptide possesses innate endosomal/lysosomal avoidance capacity. In contrast, apoE-based rHDL undergo the endosomal–lysosomal intracellular pathway. In our recent work, siRNA entrapped by calcium phosphate (CaP) nanoparticles were introduced as a solid core of apoE-rHDL to enable high siRNA loading and efficient lysosome escape. Such nanocarriers achieved highly specific and efficient accumulation in glioblastoma cells via Ras activation-dependent macropinocytosis. To determine the time course of siRNA escaping from late-endosomes/lysosomes, we determined the colocalization between FAM–siRNA and LysoTracker Red, and found increasing fluorescence of FAM–siRNA spreading into the cytoplasm of target cells over the elapsed time (Fig. 4)13. Thus, the nanocarrier was able to release siRNA that was trapped in the late-endosomes/lysosomes.
Figure 4.
ApoE-rHDL with a calcium phosphate core (CaP-rHDL) to enable high siRNA loading and efficient lysosome escape. (A) The outline for the preparation of siRNA-loaded CaP-rHDL (siRNA-CaP-rHDL). (B) Lysosome escape of FAM–siRNA (green) loaded by CaP-rHDL after incubation for 2, 4 and 8 h at the siRNA concentration of 100 nmol/L in C6 glioblastoma cells. Lysosome was indicated by LysoTracker Red. Nucleus was stained by Hoechst33342 (blue). (Reproduced with permission from Ref. 13. Copyright 2017, Nature.
Another strategy for endowing nanoparticles with the ability to fuse with endosomal membranes consists of a process which mimics the viral mechanism of cellular entry by utilizing membrane-based envelope or viral fusogenic peptides. Harashima et al.67, 68 utilized a fusogenic peptide to modify rHDL for efficient accumulation in lung and delivery of encapsulated siRNA to the cytoplasm via endosomal membrane fusion. The peptide (GALA) enhanced endosomal escape by a mechanism similar to that of HA2 in the influenza virus. Furthermore, Kim et al.69 developed cationized HDL (catHDL) by utilizing cationic peptide and lipids, in which the complexation of catHDL and anionic block copolymer made it achievable for nanoparticles to be stable at neutral pH in plasma and cargos to successfully escape endosomal entrapment. Some neutral helper lipids such as DOPE, also demonstrate the ability to regulate a hexagonal phase in the endosome lumen for enhancing endosomal escape by disrupting the endosomal membrane65. Herein, by mimicking the endogenous shape and structure of native HDL, adjusting the composition of lipids and the ratio of different lipids opens new horizons to overcome the limitations of endosome/lysosome entrapment for achieving successful intracellular delivery.
The emerging approaches that utilize biomimetic nanoparticles rHDL for overcoming biological drug delivery barriers are summarized in Table 1.
Table 1.
The emerging strategies that utilized reconstituted HDL nanoparticles for overcoming biological drug delivery barriers.
| The biological barrier | Strategy | Strategy Cargo | Delivery outcome | Ref. |
|---|---|---|---|---|
| The mononuclear phagocyte system (MPS) clearance and instability in the circulation | Monopegylation of apoA-I in HDL particles | Pegylated apoA-I | A ≈7-fold increased plasma half-life of pegylated apoA-I in rHDL | 22 |
| Altering sphingomyelin levels in HDL particles | An apoA-I mimetic peptide, 5A | A sphingomyelin-induced ordered and rigid liquid bilayer environment in HDL | 26 | |
| Covalently attaching mono-cholesteryl succinate (CHS) to apoA-I to form CHS- modified rHDL | Paclitaxel (PTX) | Reduced drug leakage induced by LCAT, optimal tumor targeting properties and anti-cancer efficacy | 35 | |
| Curcumin | Enhanced ability to inhibit HepG2 cell growth and to induce apoptosis in Jeko cells | 73, 74 | ||
| Cross-linking of the lipid bilayer in HDL particles | Gadolinium | More effective delivery of gadolinium and dramatically enhanced MR signal per dose of liposome | 36 | |
| DiynePC phospholipids | No degradation over 48 h in 100% serum and greater uptake into the human bladder cancer cell of cross-linked rHDL | 37 | ||
| Hindrance by the blood-brain barrier (BBB) | LDL receptor (LDLR)-mediated transcytosis | ApoE3 | About 0.4% ID/g of ApoE3-rHDL gained access to the brain after one hour administration | 49 |
| Hindrance by the plasma membrane | SR-B1 receptor-mediated cellular internalization | Paclitaxel (PTX) and doxorubicin (Dox) | Increased the ratiometric accumulation of drugs in cancer cells and enhanced antitumor response at synergistic drug ratios of rHDL | 51 |
| siRNA | Highly efficient systemic delivery of siRNA and effective silencing the expression of two proteins | 52 | ||
| Chol-siRNA-VEGF | Direct cytosolic delivery for target-specific anti-angiogenic therapy in breast cancer. | 53 | ||
| Cell penetrating peptides (CPPs)- mediated cellular internalization | TH peptide, paclitaxel (PTX) | The rate of 86.3% for the tumor inhibition on C26 tumor-bearing mice | 58 | |
| R6H4 peptide, gambogic acid | Approximately 5-fold increase in IC50 compared to free GA, superior tumor accumulation and significant inhibition of tumor growth | 59 | ||
| Endosomal/lysosomal entrapment | SR-B1-mediated endosomal/lysosomal avoidance | Hydrophobic molecules | Direct transport of payload molecules into the cell cytoplasm without entire particles internalization | 62 |
| Paclitaxel oleate (PTXOL) | A 57% decreased tumor volume of nontargeted cells by PTXOL treatment but a 1220% increased tumor volume by PTXOL HPPS | 63 | ||
| BCL-2-siRNA | Decreased BCL-2 protein, enhanced apoptosis (2.5-fold) in tumors and significantly inhibited tumor growth with no adverse effect | 64 | ||
| Cytochrome c (cytc), membrane permeable sequence peptide | A 64–75% loading efficiency, induced massive apoptosis and specific tumor targeting effect | 65 | ||
| Antigen peptides and adjuvants | Markedly improved Ag/adjuvant co-delivery to lymphoid organs and sustained Ag presentation on dendritic cells | 66 | ||
| Calcium phosphate-mediated endosomal/ lysosomal escape | ATF5 siRNA | Remarkable RNA-interfering efficiency, increased glioblastoma cell apoptosis and inhibited tumor cell growth | 13 | |
| Viral fusogenic peptide GALA-mediated fusing with endosomal membrane | siRNA | Targeting the lung endothelium, delivering encapsulated siRNA to the cytoplasm, and eradicating lung metastasis | 68 |
3. The applications of rHDL for drug delivery
3.1. rHDL for cancer therapy
The following requirements should be considered for achieving efficient tumor-targeting drug delivery. First, the desirable nanocarrier should remain stable during circulation and efficiently accumulate in tumor. Second, it should have ability to penetrate within the tumor regions and be efficiently taken up by the tumor cells. Moreover, it should release the drug once taken up by tumor cells35. Based on the biomimetic design, rHDL maintains the proper proportions of phospholipids and apolipoproteins and takes the advantage of HDL to have high biocompatibility and relative long circulation time. Given the high expression of SR-B1 and LDLR in tumors compared with normal tissues, rHDL can be efficiently taken up by tumor cells through the receptor-mediated mechanism and mediate efficient cargo release afterwards, providing an excellent potential platform for cancer therapy.
rHDL has been utilized to deliver cytolytic peptides for cancer therapy. The lytic property of melittin is attributed to its ability to overcome tumor drug resistance, and the C-terminus of melittin contains a cytotoxic domain which may lead to various adverse effects in vivo. Huang et al.70 developed a hybrid cytolytic peptide α-melittin by linking the N-terminus of melittin to the C-terminus of α-peptide via a GSG linker. This hybrid cytolytic peptide interacted with phospholipids to form HDL-mimicking nanoparticles (α-melittin-NP) through the process of self-assembly. More than 80% α-melittin was found encapsulated in the nanoparticles. Furthermore, α-melittin-NP exhibited an ultrasmall size which enhanced solid tumors penetration. Once taken up by tumor cells, melittin was released into the cytosol. More recently, as a versatile drug delivery vehicle, rHDL nanoparticles have also been utilized to encapsulate fenretinide for addressing the limitation of off target toxicity. This formulation showed higher therapeutic efficiency than free fenretinide for neuroblastoma therapy71. For ovarian cancer, rHDL was applied to carry highly hydrophobic drug valcubicin (AD-32) to overcome the solubility obstacles for improving efficient intravesicular transport72. Also, d-rHDLs have been studied as efficient delivery vehicles carrying curcumin, a hydrophobic molecule with anti-inflammatory activity, to improve its anti-cancer effect on HepG2 cell73, 74, 75. In general, this biomimetic nanocarrier exhibits promising tumor-targeting delivery capacity and holds great potential for clinical therapy of solid tumors.
For tumor cells without SR-B1 expression, tumor targeting delivery of rHDL remains a challenge. One approach to address this is to modify targeting ligands on the rHDL nanoparticles. For example, adding targeting ligands to epidermal growth factor receptor (EGFR) to HDL-mimetic nanocarriers was a desirable strategy to protect therapeutic molecules during circulation and to enhance tumor targeting76. The EGFR ligand-modified nanocarriers were composed of phospholipids, amphipathic α-helical peptides and hydrophobic cargo through the self-assembled interaction, contributing to higher tumor accumulation and tumor cell internalization via an EGFR-mediated mechanism. Other studies have demonstrated that conjugating tumor-targeting molecules to the protein constituents of rHDL made it achievable to reroute lipoprotein nanoparticles from their native receptors to other disease-specific receptors. For instance, folic acid was used to conjugate to the Lys residues of apolipoprotein in lipoprotein for targeting lipoprotein to folate receptor (FR) versus its native receptor77. This strategy endowed lipoprotein nanoparticles with higher targeting efficiency to cancer cells and lower accumulation in hepatocytes.
3.2. rHDL for theranostics of atherosclerotic cardiovascular disease
Atherosclerosis is presently a worldwide cardiovascular disease which has developed into a leading cause of death and disability. This devastating chronic disease is characterized by the development of atheromatous plaques in the arterial walls and requires novel therapeutic strategies for efficient anti-atherosclerosis treatment78. Recent results suggest that RCTmediated by HDL promotes the efflux of cholesterol from the lipid-laden plaque macrophages and transports cholesterol to the liver for metabolism and excretion79. It is well-known that macrophages are involved in the progression of atherosclerosis and regarded as specific markers for imaging the vulnerable plaques80. Recently, Sigalov et al.81 developed rHDL with methionine oxidized apoA-I peptides to load Gd-based contrast agents for efficient macrophage imaging in vitro and in vivo. Another strategy to detect vulnerable plaques was to target macrophage apoptosis by imaging the collapse of mitochondrial membrane. Marrache et al.82 entrapped quantum dots in the core of rHDL for optical imaging and modified the surface of rHDL with triphenylphosphonium cations to target mitochondrial membrane. As for delivering therapeutic drugs to the atherosclerotic plaques, it was found that many polymeric materials have been used in nanoparticles to achieve targeting delivery and controlled release of the therapeutic drugs83. Poly lactic-co-glycolic acid (PLGA), a biodegradable polymer, can be employed for sustained drug release. Sanchez-Gaytan et al.84 developed a HDL-mimetic nanoparticle with its hydrophobic core incorporating PLGA as a new biomimetic platform. Combining the advantages of the intrinsic atherosclerotic plaque targeting property of HDL and sustained drug release profile of PLGA, PLGA-HDL hybrid nanoparticles displayed macrophage targeting and cholesterol efflux abilities, delivered therapeutic agents to atherosclerotic plaques and achieved controlled release of drug cargos. In in vivo studies, PLGA-HDL hybird nanoparticles were found colocalized with macrophages that accumulated at the atherosclerotic plaques, providing a new nanoplatform for atherosclerosis-targeting drug delivery and controlled drug release.
Inflammation is a key feature of atherosclerosis and a target for therapy. Statins possess potent anti-inflammatory properties but cannot be fully exploited due to their low systemic bioavailability. Duivenvoorden et al.85 utilized biomimetic nanocarrier rHDL to deliver statins to atherosclerotic plaques. The statin-loaded rHDL nanoparticles protected statins from degradation, accumulated in atherosclerotic lesions and produced inflammatory cytokines to efficiently inhibit plaque inflammation without exerting toxic effects in kidney, liver and myocytes. Considering inflammation as a main feature and target for atherosclerosis therapy, this new anti-atherosclerotic therapy is worth exploring and has high potential for clinical translation.
3.3. rHDL for brain disease therapy
Brain diseases such as Alzheimer's disease (AD), stroke and brain tumors have now become severe public health challenges which urgently need novel therapeutic strategies. One of the main limitations for brain disease therapy is restricted drug delivery. Many recent preclinical studies showed that rHDL holds great potential for efficient drug delivery and is therefore worth exploring their potential in brain disease therapy.
Glioblastoma is the most aggressive cancer that begins in the brain86. Many drugs for glioblastoma treatment are limited by inefficient brain delivery. Our group recently developed a novel nanoplatform apoE-rHDL that specifically and efficiently delivered tumor-targeting siRNA to glioblastoma cells via Ras activation-associated macropinocytosis13. This biomimetic nanocarrier achieved efficient tumor-targeting drug delivery via the following mechanism: Firstly, apoE3 interacted with the receptors that overexpressed in the BBB, so that apoE-rHDL possessed BBB permeability to reach the tumor regions. Next, Ras-dependent macropinocytosis provided a desirable mechanism for glioblastoma-targeting cellular uptake of the nanocarrier. Moreover, high siRNA loading was achieved by entrapping siRNA into a CaP core of apoE-rHDL. The resulted apoE-rHDL with a CaP core protected siRNA from degradation, and underwent efficient late endosome/lysosomes escape and direct cytosol siRNA release. Addition of activating transcription factor-5 (ATF5) siRNA as the cargo permitted the nanoformulation to specifically and efficiently stimulate apoptosis in glioblastoma cells. Taken together, this biomimetic nanocarrier shows great promise in overcoming the physiological barriers to efficient drug delivery and provides a new strategy for the therapy of brain tumors.
Alzheimer's disease (AD), a highly prevalent neurodegenerative disorder, exerts a heavy burden on modern society due to its complex pathological changes and lack of effective therapy strategies. It has been shown that accumulation of Aβ in the brain is crucial in AD pathogenesis, and accelerating Aβ clearance is a disease modifying strategy in AD therapy. By mimicking HDL in the CNS, we constructed a biologically inspired nanostructure apoE3-rHDL. This agent, which showed BBB permeability and high binding affinity to Aβ, may serve as a novel nanomedicine for disease modification in AD by accelerating Aβ clearance. ApoE3-rHDL was further developed as an efficient nanocarrier with Aβ-targeting ability for the delivery of α-mangostin (α-M), a polyphenolic xanthone derivative from mangosteen87. The resulted nanoformulation efficiently penetrated the BBB, accumulated at the surrounding of Aβ aggregation in both cortex and hippocampus, and more efficiently facilitated Aβ degradation. The treatment rescued memory impairment88. Furthermore, we incorporated monosialotetrahexosylganglioside (GM1) into lipid membrane of apoE3-rHDL to construct a new nanocarrier GM1-rHDL to further enhance the affinity to Aβ and accelerate Aβ degradation in microglia cells89. GM1-rHDL presented excellent brain distribution following intranasal administration. By incorporating NAP (NAPVSIPQ), a neuroprotective peptide which exerts protective function for damaged neurons, into the surface of GM1-rHDL, the resulting nanoformulation not only facilitated Aβ clearance but also alleviated neurologic changes, providing a multifunctional nanoplatform for the combination therapy of AD.
In addition to apoE-rHDL, apoA-I-rHDL has also been shown to lower Aβ levels following intravenous administration in symptomatic APP/PS1 mice, a well-characterized preclinical model of amyloidosis. Robert et al.90 demonstrated that in an acute study, rHDL containing human apoA-I decreased soluble brain Aβ levels after a single dose within 24 h. Caveolae-located SR-B1-mediated transcytosis is thought to account for the apical-to-basolateral transport of apoA-I-HDL holoparticles91. In addition, Handattu et al.92 reported that D-4F, an apoA-I mimetic peptide, had the ability to bind Aβ and inhibit Aβ deposition after oral administration. Herein, D-4F rHDL provides a promising alternative for cognitive function repair and AD treatment.
4. Conclusions
The development of biomimetic nanoparticulate drug delivery systems is an emerging field and holds great potential for efficient drug delivery by exploiting of the exceptional delivery mechanisms in mammalian cells. There is a growing realization that the sequential biological barriers usually limit the accumulation of therapeutic molecules at diseased sites. Extended circulation time and relative stability of nanoparticles in plasma are critical for clinical applications and are often affected by MPS-mediated clearance. In addition, the blood system barrier, the plasma membrane barrier for cellular internalization, intracellular endosome/lysosome escape and intracellular organelles targeting all have an impact on efficient and adequate targeting transport of drugs. Herein, advances in the development of novel biomimetic drug delivery technologies have attracted increased attention. The resulting nanoplatforms, which are distinct from conventional nanoparticle formulations, provide great potential for overcoming these biological hurdles.
rHDL nanoparticles are mimics of natural lipoprotein which are easy for mass-production and scale-up. A better understanding of the beneficial biological properties and the overall dynamics of rHDL is important for utilizing rHDL as drug delivery systems. We have summarized the key biological factors of rHDL to overcome the physiological barriers for optimized drug delivery. A series of studies have demonstrated that rHDL combined physiological properties of native HDL and emerging strategies to achieve following requirements: First, the enhanced stability of these nanoparticle carriers upon exposure to blood extends their circulatory half-life. Secondly, delivery of the agents through plasma membrane barriers to target intracellular sites of disease. Finally, avoidance or escape from endosomal and lysosomal degradation of these agents produces efficient cytosolic drug release. Incorporation of innovative design features into rHDL can successively overcome each of biological barriers and create a new generation of biomimetic nano-drug delivery systems.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos. 81373351, 81573382, and 81722043), grant from Shanghai Science and Technology Committee (15540723700), and “Shu Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (15SG14).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Damiano M.G., Mutharasan R.K., Tripathy S., McMahon K.M., Thaxton C.S. Templated high density lipoprotein nanoparticles as potential therapies and for molecular delivery. Adv Drug Deliv Rev. 2013;65:649–662. doi: 10.1016/j.addr.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Kuai R., Li D., Chen Y.E., Moon J.J., Schwendeman A. High-density lipoproteins: nature's multifunctional nanoparticles. ACS Nano. 2016;10:3015–3041. doi: 10.1021/acsnano.5b07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonsen J.B. Evaluation of reconstituted high-density lipoprotein (rHDL) as a drug delivery platform—a detailed survey of rHDL particles ranging from biophysical properties to clinical implications. Nanomedicine. 2016;12:2161–2179. doi: 10.1016/j.nano.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Mo Z.C., Ren K., Liu X., Tang Z.L., Yi G.H. A high-density lipoprotein-mediated drug delivery system. Adv Drug Deliv Rev. 2016;106:132–147. doi: 10.1016/j.addr.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Sikorski J.A. 6.20-atherosclerosis/lipoprotein/cholesterol metabolism. In: Taylor J.B., Triggle D.J., editors. Comprehensive medicinal chemistry II. Elsevier; Oxford: 2007. pp. 459–494. [Google Scholar]
- 6.Davidson W.S., Thompson T.B. The structure of apolipoprotein A-I in high density lipoproteins. J Biol Chem. 2007;282:22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- 7.Camont L., Chapman M.J., Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17:594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Kontush A., Chapman M.J. Antiatherogenic function of HDL particle subpopulations: focus on antioxidative activities. Curr Opin Lipidol. 2010;21:312–318. doi: 10.1097/MOL.0b013e32833bcdc1. [DOI] [PubMed] [Google Scholar]
- 9.Zannis V.I., Chroni A., Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med. 2006;84:276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 10.Trigatti B.L., Krieger M., Rigotti A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:1732–1738. doi: 10.1161/01.ATV.0000091363.28501.84. [DOI] [PubMed] [Google Scholar]
- 11.Vickers K.C., Palmisano B.T., Shoucri B.M., Shamburek R.D., Remaley A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H., Cruz W., Chen J., Zheng G. Learning from biology: synthetic lipoproteins for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:298–314. doi: 10.1002/wnan.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J.L., Jiang G., Song Q.X., Gu X., Hu M., Wang X.L. Lipoprotein-biomimetic nanostructure enables efficient targeting delivery of siRNA to Ras-activated glioblastoma cells via macropinocytosis. Nat Commun. 2017;8:15144. doi: 10.1038/ncomms15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minchin R.F., Martin D.J. Minireview: nanoparticles for molecular imaging—an overview. Endocrinology. 2010;151:474–481. doi: 10.1210/en.2009-1012. [DOI] [PubMed] [Google Scholar]
- 15.Szeto G.L., Lavik E.B. Materials design at the interface of nanoparticles and innate immunity. J Mater Chem B Mater Biol Med. 2016;4:1610–1618. doi: 10.1039/C5TB01825K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song G., Petschauer J.S., Madden A.J., Zamboni W.C. Nanoparticles and the mononuclear phagocyte system: pharmacokinetics and applications for inflammatory diseases. Curr Rheumatol Rev. 2014;10:22–34. doi: 10.2174/1573403x10666140914160554. [DOI] [PubMed] [Google Scholar]
- 17.Harris J.M., Chess R.B. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 18.Bhadra D., Bhadra S., Jain P., Jain N.K. Pegnology: a review of PEG-ylated systems. Pharmazie. 2002;57:5–29. [PubMed] [Google Scholar]
- 19.Mortimer G.M., Butcher N.J., Musumeci A.W., Deng Z.J., Martin D.J., Minchin R.F. Cryptic epitopes of albumin determine mononuclear phagocyte system clearance of nanomaterials. ACS Nano. 2014;8:3357–3366. doi: 10.1021/nn405830g. [DOI] [PubMed] [Google Scholar]
- 20.Wang T., Wang D., Liu J., Feng B., Zhou F., Zhang H. Acidity-triggered ligand-presenting nanoparticles to overcome sequential drug delivery barriers to tumors. Nano Lett. 2017;17:5429–5436. doi: 10.1021/acs.nanolett.7b02031. [DOI] [PubMed] [Google Scholar]
- 21.Milla P., Dosio F., Cattel L. PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Curr Drug Metab. 2012;13:105–119. doi: 10.2174/138920012798356934. [DOI] [PubMed] [Google Scholar]
- 22.Murphy A.J., Funt S., Gorman D., Tall A.R., Wang N. Pegylation of high-density lipoprotein decreases plasma clearance and enhances antiatherogenic activity. Circ Res. 2013;113:e1–e9. doi: 10.1161/CIRCRESAHA.113.301112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S.-D., Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145:178–181. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida T., Ichihara M., Wang X., Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J Control Release. 2006;115:243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Kontush A., Lhomme M., Chapman M.J. Unraveling the complexities of the HDL lipidome. J Lipid Res. 2013;54:2950–2963. doi: 10.1194/jlr.R036095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwendeman A., Sviridov D.O., Yuan W., Guo Y., Morin E.E., Yuan Y. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J Lipid Res. 2015;56:1727–1737. doi: 10.1194/jlr.M060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer N.O., Weilhammer D.R., Dunkle A., Thomas C., Hwang M., Corzett M. Evaluation of nanolipoprotein particles (NLPs) as an in vivo delivery platform. PLoS One. 2014;9:e93342. doi: 10.1371/journal.pone.0093342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen T.M., Hansen C., Rutledge J. Liposomes with prolonged circulation times: factors affecting uptake by reticuloendothelial and other tissues. Biochim Biophys Acta. 1989;981:27–35. doi: 10.1016/0005-2736(89)90078-3. [DOI] [PubMed] [Google Scholar]
- 29.Martínez-Beamonte R., Lou-Bonafonte J.M., Martínez-Gracia M.V., Osada J. Sphingomyelin in high-density lipoproteins: structural role and biological function. Int J Mol Sci. 2013;14:7716–7741. doi: 10.3390/ijms14047716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayaraman S., Benjwal S., Gantz D.L., Gursky O. Effects of cholesterol on thermal stability of discoidal high density lipoproteins. J Lipid Res. 2010;51:324–333. doi: 10.1194/jlr.M000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanco E., Shen H., Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentile F., Chiappini C., Fine D., Bhavane R.C., Peluccio M.S., Cheng M.M. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech. 2008;41:2312–2318. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Tsompanidi E.M., Brinkmeier M.S., Fotiadou E.H., Giakoumi S.M., Kypreos K.E. HDL biogenesis and functions: role of HDL quality and quantity in atherosclerosis. Atherosclerosis. 2010;208:3–9. doi: 10.1016/j.atherosclerosis.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 34.He H., Liu L., Bai H., Wang J., Zhang Y., Zhang W. Arachidonic acid-modified lovastatin discoidal reconstituted high density lipoprotein markedly decreases the drug leakage during the remodeling behaviors induced by lecithin cholesterol acyltransferase. Pharm Res. 2014;31:1689–1709. doi: 10.1007/s11095-013-1273-3. [DOI] [PubMed] [Google Scholar]
- 35.Wang J., Jia J., Liu J., He H., Zhang W., Li Z. Tumor targeting effects of a novel modified paclitaxel-loaded discoidal mimic high density lipoproteins. Drug Deliv. 2013;20:356–363. doi: 10.3109/10717544.2013.834418. [DOI] [PubMed] [Google Scholar]
- 36.Smith C.E., Kong H. Cross-linkable liposomes stabilize a magnetic resonance contrast-enhancing polymeric fastener. Langmuir. 2014;30:3697–3704. doi: 10.1021/la500412r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilmore S.F., Blanchette C.D., Scharadin T.M., Hura G.L., Rasley A., Corzett M. Lipid cross-linking of nanolipoprotein particles substantially enhances serum stability and cellular uptake. ACS Appl Mater Interfaces. 2016;8:20549–20557. doi: 10.1021/acsami.6b04609. [DOI] [PubMed] [Google Scholar]
- 38.Silver D.L., Tall A.R. The cellular biology of scavenger receptor class B type I. Curr Opin Lipidol. 2001;12:497–504. doi: 10.1097/00041433-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Valacchi G., Sticozzi C., Lim Y., Pecorelli A. Scavenger receptor class B type I: a multifunctional receptor. Ann NY Acad Sci. 2011;1229:E1–E7. doi: 10.1111/j.1749-6632.2011.06205.x. [DOI] [PubMed] [Google Scholar]
- 40.Mooberry L.K., Sabnis N.A., Panchoo M., Nagarajan B., Lacko A.G. Targeting the SR-B1 receptor as a gateway for cancer therapy and imaging. Front Pharmacol. 2016;7:466. doi: 10.3389/fphar.2016.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown M.S., Anderson R.G., Goldstein J.L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983;32:663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- 43.Sarkar A., Fatima I., Jamal Q.M., Sayeed U., Khan M.K., Akhtar S. Nanoparticles as a carrier system for drug delivery across blood–brain barrier. Curr Drug Metab. 2017;18:129–137. doi: 10.2174/1389200218666170113125132. [DOI] [PubMed] [Google Scholar]
- 44.Zhao X., Chen R., Liu M., Feng J., Chen J., Hu K. Remodeling the blood–brain barrier microenvironment by natural products for brain tumor therapy. Acta Pharm Sin B. 2017;7:541–553. doi: 10.1016/j.apsb.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pardridge W.M. The blood–brain barrier: bottleneck in brain drug development. Neurorx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basak J.M., Verghese P.B., Yoon H., Kim J., Holtzman D.M. Low-density lipoprotein receptor represents an apolipoprotein E-independent pathway of Aβ uptake and degradation by astrocytes. J Biol Chem. 2012;287:13959–13971. doi: 10.1074/jbc.M111.288746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molino Y., David M., Varini K., Jabès F., Gaudin N., Fortoul A. Use of LDL receptor-targeting peptide vectors for in vitro and in vivo cargo transport across the blood–brain barrier. FASEB J. 2017;31:1807–1827. doi: 10.1096/fj.201600827R. [DOI] [PubMed] [Google Scholar]
- 48.Croy J.E., Brandon T., Komives E.A. Two apolipoprotein E mimetic peptides, ApoE(130-149) and ApoE(141–155)2, bind to LRP1. Biochemistry. 2004;43:7328–7335. doi: 10.1021/bi036208p. [DOI] [PubMed] [Google Scholar]
- 49.Song Q., Huang M., Yao L., Wang X., Gu X., Chen J. Lipoprotein-based nanoparticles rescue the memory loss of mice with Alzheimer's disease by accelerating the clearance of amyloid-β. ACS Nano. 2014;8:2345–2359. doi: 10.1021/nn4058215. [DOI] [PubMed] [Google Scholar]
- 50.Acton S., Rigotti A., Landschulz K.T., Xu S., Hobbs H.H., Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 51.Rui M., Xin Y., Li R., Ge Y., Feng C., Xu X. Targeted biomimetic nanoparticles for synergistic combination chemotherapy of paclitaxel and doxorubicin. Mol Pharm. 2017;14:107–123. doi: 10.1021/acs.molpharmaceut.6b00732. [DOI] [PubMed] [Google Scholar]
- 52.Shahzad M.M.K., Mangala L.S., Han H.D., Lu C., Bottsford-Miller J., Nishimura M. Targeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticles. Neoplasia. 2011;13:309–319. doi: 10.1593/neo.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding Y., Wang Y., Zhou J., Gu X., Wang W., Liu C. Direct cytosolic siRNA delivery by reconstituted high density lipoprotein for target-specific therapy of tumor angiogenesis. Biomaterials. 2014;35:7214–7227. doi: 10.1016/j.biomaterials.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6:268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakase I., Takeuchi T., Tanaka G., Futaki S. Methodological and cellular aspects that govern the internalization mechanisms of arginine-rich cell-penetrating peptides. Adv Drug Deliv Rev. 2008;60:598–607. doi: 10.1016/j.addr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Wang F., Wang Y., Zhang X., Zhang W., Guo S., Jin F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J Control Release. 2014;174:126–136. doi: 10.1016/j.jconrel.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 57.Gerweck L.E., Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 58.Zhang Q., Tang J., Fu L., Ran R., Liu Y., Yuan M. A pH-responsive α-helical cell penetrating peptide-mediated liposomal delivery system. Biomaterials. 2013;34:7980–7993. doi: 10.1016/j.biomaterials.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Jiang T., Zhang Z., Zhang Y., Lv H., Zhou J., Li C. Dual-functional liposomes based on pH-responsive cell-penetrating peptide and hyaluronic acid for tumor-targeted anticancer drug delivery. Biomaterials. 2012;33:9246–9258. doi: 10.1016/j.biomaterials.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 60.Ding Y., Wang Y., Opoku-Damoah Y., Wang C., Shen L., Yin L. Dual-functional bio-derived nanoparticulates for apoptotic antitumor therapy. Biomaterials. 2015;72:90–103. doi: 10.1016/j.biomaterials.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 61.Chou L.Y., Ming K., Chan W.C. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Z., Cao W., Jin H., Lovell J.F., Yang M., Ding L. Biomimetic nanocarrier for direct cytosolic drug delivery. Angew Chem Int Ed Engl. 2009;48:9171–9175. doi: 10.1002/anie.200903112. [DOI] [PubMed] [Google Scholar]
- 63.Yang M., Chen J., Cao W., Ding L., Ng K.K., Jin H. Attenuation of nontargeted cell-kill using a high-density lipoprotein-mimicking peptide–phospholipid nanoscaffold. Nanomed (Lond) 2011;6:631–641. doi: 10.2217/nnm.11.10. [DOI] [PubMed] [Google Scholar]
- 64.Lin Q., Chen J., Jin H., Ng K.K., Yang M., Cao W. Efficient systemic delivery of siRNA by using high-density lipoprotein-mimicking peptide lipid nanoparticles. Nanomedicine. 2012;7:1813–1825. doi: 10.2217/nnm.12.73. [DOI] [PubMed] [Google Scholar]
- 65.Kim S.K., Foote M.B., Huang L. The targeted intracellular delivery of cytochrome C protein to tumors using lipid-apolipoprotein nanoparticles. Biomaterials. 2012;33:3959–3966. doi: 10.1016/j.biomaterials.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hatakeyama H., Ito E., Akita H., Oishi M., Nagasaki Y., Futaki S. A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J Control Release. 2009;139:127–132. doi: 10.1016/j.jconrel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Kusumoto K., Akita H., Ishitsuka T., Matsumoto Y., Nomoto T., Furukawa R. Lipid envelope-type nanoparticle incorporating a multifunctional peptide for systemic siRNA delivery to the pulmonary endothelium. ACS Nano. 2013;7:7534–7541. doi: 10.1021/nn401317t. [DOI] [PubMed] [Google Scholar]
- 69.Kim H., Okamoto H., Felber A.E., Polomska A., Morone N., Heuser J.E. Polymer-coated pH-responsive high-density lipoproteins. J Control Release. 2016;228:132–140. doi: 10.1016/j.jconrel.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Huang C., Jin H., Qian Y., Qi S., Luo H., Luo Q. Hybrid melittin cytolytic peptide-driven ultrasmall lipid nanoparticles block melanoma growth in vivo. ACS Nano. 2013;7:5791–5800. doi: 10.1021/nn400683s. [DOI] [PubMed] [Google Scholar]
- 71.Sabnis N., Pratap S., Akopova I., Bowman P.W., Lacko A.G. Pre-clinical evaluation of rHDL encapsulated retinoids for the treatment of neuroblastoma. Front Pediatr. 2013;1:6. doi: 10.3389/fped.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sabnis N., Nair M., Israel M., McConathy W.J., Lacko A.G. Enhanced solubility and functionality of valrubicin (AD-32) against cancer cells upon encapsulation into biocompatible nanoparticles. Int J Nanomed. 2012;7:975–983. doi: 10.2147/IJN.S28029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghosh M., Singh A.T., Xu W., Sulchek T., Gordon L.I., Ryan R.O. Curcumin nanodisks: formulation and characterization. Nanomedicine. 2011;7:162–167. doi: 10.1016/j.nano.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh A.T., Ghosh M., Forte T.M., Ryan R.O., Gordon L.I. Curcumin nanodisk-induced apoptosis in mantle cell lymphoma. Leuk Lymphoma. 2011;52:1537–1543. doi: 10.3109/10428194.2011.584253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 76.Zhang Z., Chen J., Ding L., Jin H., Lovell J.F., Corbin I.R. HDL-mimicking peptide-lipid nanoparticles with improved tumor targeting. Small. 2010;6:430–437. doi: 10.1002/smll.200901515. [DOI] [PubMed] [Google Scholar]
- 77.Zheng G., Chen J., Li H., Glickson J.D. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc Natl Acad Sci U S A. 2005;102:17757–17762. doi: 10.1073/pnas.0508677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 79.Wang N., Lan D., Chen W., Matsuura F., Tall A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choudhury R.P., Lee J.M., Greaves D.R. Mechanisms of disease: macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2005;2:309–315. doi: 10.1038/ncpcardio0195. [DOI] [PubMed] [Google Scholar]
- 81.Sigalov A.B. Nature-inspired nanoformulations for contrast-enhanced in vivo MR imaging of macrophages. Contrast Media Mol Imaging. 2014;9:372–382. doi: 10.1002/cmmi.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marrache S., Dhar S. Biodegradable synthetic high-density lipoprotein nanoparticles for atherosclerosis. Proc Natl Acad Sci U S A. 2013;110:9445–9450. doi: 10.1073/pnas.1301929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamaly N., Xiao Z., Valencia P.M., Radovic-Moreno A.F., Farokhzad O.C. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanchez-Gaytan B.L., Fay F., Lobatto M.E., Tang J., Ouimet M., Kim Y. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug Chem. 2015;26:443–451. doi: 10.1021/bc500517k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duivenvoorden R., Tang J., Cormode D.P., Mieszawska A.J., Izquierdo-Garcia D., Ozcan C. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. doi: 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bleeker F.E., Molenaar R.J., Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neuro-Oncol. 2012;108:11–27. doi: 10.1007/s11060-011-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yao L., Gu X., Song Q., Wang X., Huang M., Hu M. Nanoformulated α-mangostin ameliorates Alzheimer's disease neuropathology by elevating LDLR expression and accelerating amyloid-β clearance. J Control Release. 2016;226:1–14. doi: 10.1016/j.jconrel.2016.01.055. [DOI] [PubMed] [Google Scholar]
- 88.Song Q., Song H., Xu J., Huang J., Hu M., Gu X. Biomimetic apoE-reconstituted high density lipoprotein nanocarrier for blood–brain barrier penetration and amyloid β-targeting drug delivery. Mol Pharm. 2016;13:3976–3987. doi: 10.1021/acs.molpharmaceut.6b00781. [DOI] [PubMed] [Google Scholar]
- 89.Huang M., Hu M., Song Q., Song H., Huang J., Gu X. GM1-modified lipoprotein-like nanoparticle: multifunctional nanoplatform for the combination therapy of Alzheimer's disease. ACS Nano. 2015;9:10801–10816. doi: 10.1021/acsnano.5b03124. [DOI] [PubMed] [Google Scholar]
- 90.Robert J., Stukas S., Button E., Cheng W.H., Lee M., Fan J. Reconstituted high-density lipoproteins acutely reduce soluble brain Aβ levels in symptomatic APP/PS1 mice. Biochim Biophys Acta. 2016;1862:1027–1036. doi: 10.1016/j.bbadis.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 91.Balazs Z., Panzenboeck U., Hammer A., Sovic A., Quehenberger O., Malle E. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood–brain barrier model. J Neurochem. 2004;89:939–950. doi: 10.1111/j.1471-4159.2004.02373.x. [DOI] [PubMed] [Google Scholar]
- 92.Handattu S.P., Garber D.W., Monroe C.E., van Groen T., Kadish I., Nayyar G. Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer's disease. Neurobiol Dis. 2009;34:525–534. doi: 10.1016/j.nbd.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]