Abstract
Background/Aims:
The role of endoscopic preoperative biliary drainage (PBD) for pancreatic head cancer is controversial because of the high incidence of stent occlusion before surgery. This study was performed to evaluate the feasibility and safety of PBD using a fully covered self-expandable metallic stent (FCSEMS).
Patients and Methods:
This multicenter prospective study involved 26 patients treated for pancreatic head cancer with distal bile duct obstruction from April 2011 to March 2013. An FCSEMS was endoscopically placed in 24 patients. Among these, 7 patients were diagnosed with unresectable cancer, and 17 underwent surgery at a median of 18 days after FCSEMS placement. The main outcome measure was preoperative and postoperative adverse events.
Results:
Two adverse events (cholecystitis and insufficient resolution of jaundice) occurred between FCSEMS placement and surgery (12%). Postoperative adverse events occurred in eight patients (47%). The cumulative incidence of stent-related adverse events 4 and 8 weeks after FCSEMS placement among the 24 patients who underwent this procedure were 19%.
Conclusions:
PBD using an FCSEMS is feasible in patients with resectable pancreatic head cancer. Placement of an FCSEMS can be an alternative PBD technique when surgery without delay is impossible. A larger randomized controlled trial is warranted.
Keywords: Pancreatic cancer, preoperative biliary drainage, self-expandable metallic stent
INTRODUCTION
Surgical resection is the only curative treatment option in patients with pancreatic cancer without evidence of major vascular invasion or distant metastasis. Obstructive jaundice is a common symptom in patients with pancreatic head cancer, and the performance and efficacy of preoperative biliary drainage (PBD) remains controversial.[1,2,3,4,5] A meta-analysis revealed that preoperative adverse events related to the drainage procedure itself outweigh the benefits of PBD.[6] A recent randomized controlled trial performed by van der Gaag et al.[7] showed that PBD was associated with an increased incidence of perioperative adverse events. However, PBD is still performed in clinical practice because immediate surgery without PBD cannot be scheduled in many centers.[8] Another flaw of the randomized study is the use of plastic stents (PSs) rather than self-expandable metallic stents (SEMSs). The major preoperative adverse events were cholangitis caused by PS occlusion, which might be resolved by the use of SEMSs.[9,10,11,12] While the efficacy of SEMS placement has been increasingly reported in patients receiving neoadjuvant treatment,[13,14] PS placement is still the standard treatment for PBD in patients with resectable pancreatic cancer without neoadjuvant treatment.[15]
The aim of this prospective study was to evaluate the feasibility and safety of PBD using a fully-covered SEMS (FCSEMS) for the treatment of resectable pancreatic head cancer.
PATIENTS AND METHODS
Study design
A single-arm prospective feasibility study was conducted in three academic centers in Japan; Tokyo University Hospital, Hokkaido University Hospital, and Saitama Medical University International Medical Center. The protocol was approved by the institutional review board at each institution. This study was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000007183).
Patients
The eligibility criteria were (i) suspicion of pancreatic head cancer based on clinical and imaging findings, (ii) obstructive jaundice [total bilirubin (T-Bil) level of >5.0 mg/dL] or an elevated aspartate aminotransferase level (>3 times the upper limit of normal), irrespective of symptoms such as pruritis or cholangitis, and (iii) a candidate for definitive surgery. The exclusion criteria were (i) prior endoscopic, percutaneous, or surgical biliary drainage; (ii) suspicion of tumor involvement to the orifice of the cystic duct (OCD); (iii) not suitable for endoscopic procedures such as gastric outlet obstruction; (iv) severe heart disease, hepatic insufficiency, renal dysfunction, endocrine disease, or coagulopathy; and (v) pregnancy or possibility of pregnancy. Written informed consent was obtained from all patients before the endoscopic procedure.
Placement of FCSEMS and surgery
All FCSEMSs placed were 10-mm-diameter WallFlex™ Biliary RX Fully Covered Stents (Boston Scientific, Natick, MA) [Figure 1]. The proximal end of the stent was placed at least 2 cm below the hepatic hilum, and the distal end was placed in the duodenum. Sphincterotomy was performed before FCSEMS placement at the discretion of each endoscopist. Prophylactic antibiotics were administrated in all cases. Surgery was performed after improvement of jaundice or elevated liver enzyme levels.
Figure 1.
Fully covered self-expandable metallic stent (WallFlex™ Biliary RX Fully Covered Stent; Boston Scientific)
Outcomes
The primary outcome was the incidence of preoperative and postoperative adverse events. Preoperative adverse events were diagnosed according to the TOKYO criteria 2014,[16] a standard reporting system for biliary stenting based on the 1991 consensus guidelines.[17] Insufficient resolution of jaundice was included as an adverse event of PBD. Postoperative adverse events up to 90 days after surgery were graded according to the International Study Group on Pancreatic Fistula (ISGPF) definition[18] and Clavien–Dindo classification.[19] Severe postoperative adverse events were defined as Clavien–Dindo grade III and IV. The cumulative occurrence of adverse events after FCSEMS placement both in patients undergoing surgical resection and in patients who were eventually diagnosed with unresectable cancer was evaluated by Kaplan–Meier analysis. Cases involving surgery without preoperative adverse events were censored at the time of surgery.
Sample size
Formal sample size calculation was not performed because this study was a pilot feasibility study. The sample size was determined to be 40 patients during the 2-year study enrollment based on the estimated annual number of patients with pancreatic head cancer amenable to surgical resection.
RESULTS
Twenty-six patients were enrolled in this study from April 2011 to March 2013. A flow chart of the study enrollment is shown in Figure 2. Two patients were excluded due to failed FCSEMS placement – one underwent percutaneous biliary drainage after failed endoscopic retrograde cholangiopancreatography (ERCP) and the other underwent PS instead of FCSEMS placement because of an unstable clinical condition during ERCP. FCSEMSs were successfully placed in the remaining 24 patients. Among these, seven patients were diagnosed with unresectable cancer after FCSEMS placement and did not undergo surgery. Finally, 17 patients underwent surgery for pancreatic cancer after FCSEMS placement.
Figure 2.
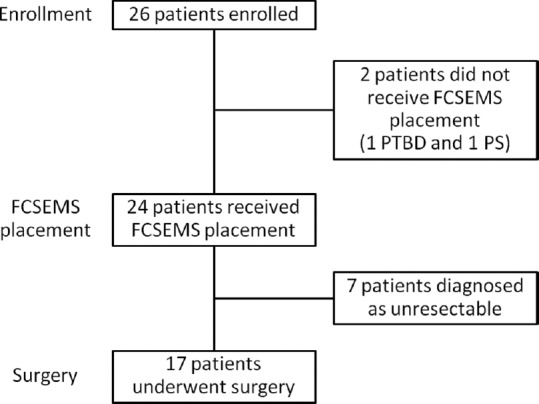
Flow chart of study enrollment FCSEMS, fully covered self-expandable metallic stent PTBD, percutaneous transhepatic biliary drainage PS, plastic stent
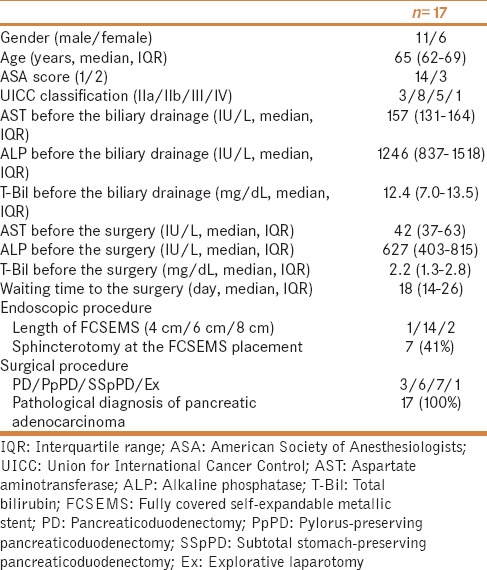
Patients' characteristics
The patients' characteristics are shown in Table 1. The patients comprised 11 males and 6 females with a median age of 65 years. The median T-Bil level before PBD was 12.4 mg/dL. Sphincterotomy was performed at the time of FCSEMS placement in seven patients. The surgery was performed at a median of 18 days after FCSEMS placement. The median T-Bil level just before the surgery was 2.2 mg/dL. The surgical procedures were pancreaticoduodenectomy (n = 3), pylorus-preserving pancreaticoduodenectomy (n = 6), subtotal stomach-preserving pancreaticoduodenectomy (n = 7), and explorative laparotomy (n = 1). Surgeons experienced no intraoperative difficulties attributable to preoperative FCSEMS placement. Pathological confirmation of pancreatic cancer was performed after the surgery in all cases.
Table 1.
Patient characteristics
Preoperative and postoperative adverse events in 17 patients who underwent surgery
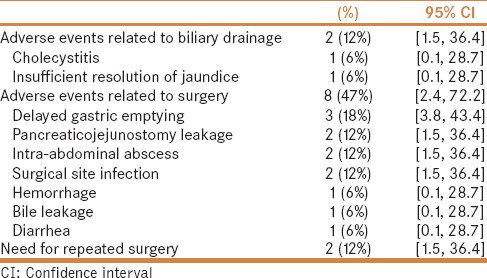
The perioperative adverse events that occurred in this study are shown in Table 2. Two (12%) preoperative adverse events and eight (47%) postoperative adverse events were observed.
Table 2.
Preoperative and postoperative adverse events in 17 patients who underwent surgery
Neither pancreatitis nor stent migration occurred during the preoperative period. One patient developed acute cholecystitis 15 days after FCSEMS placement, which was resolved by percutaneous drainage. This patient underwent curative surgery 16 days after the gallbladder drainage. The other patient required repeated biliary drainage because of insufficient resolution of jaundice without stent occlusion. Although an endoscopic nasobiliary catheter was temporarily inserted through the indwelling FCSEMS, jaundice did not resolve and the endoscopic nasobiliary drainage tube was removed 14 days after its placement. The T-Bil level then gradually decreased, and the patient underwent surgery 45 days after FCSEMS placement.
Twelve postoperative adverse events occurred in eight patients within the follow-up period of 90 days. Severe adverse events occurred in five patients. Pancreaticojejunostomy leakage occurred in two patients, but improved without any further intervention (ISGPF grade A). All patients were alive 90 days after the surgery. Postoperative adverse events occurred in 7/15 (47%) patients among the group without preoperative adverse events and in 1/2 (50%) among the group with preoperative adverse events. Median hospital stay after the surgery was 19 days (interquartile range, 15–26 days).
Stent-related adverse events in 24 patients who underwent FCSEMS placement
Four stent-related adverse events occurred in all 24 patients (17 surgical cases and 7 unresectable cases) [Table 3]. The cumulative incidence of adverse events at 4 and 8 weeks after FCSEMS placement was 19% (95% confidence interval, 1.1–34.1) [Figure 3].
Table 3.
Biliary drainage-related adverse events in 24 patients who underwent FCSEMS placement

Figure 3.
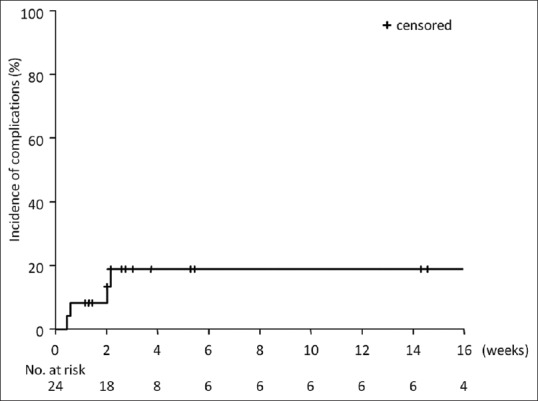
Cumulative incidence of stent-related adverse events in 24 patients who underwent fully covered self-expandable metallic stent placement
DISCUSSION
The role of PBD in the treatment of resectable pancreatic head cancer remains controversial. While early retrospective[20,21,22,23] and randomized studies[3,5] showed decreases in postoperative mortality and morbidity, other randomized studies[1,2,4] and more recent retrospective studies[24,25] showed no significant benefit of PBD. The major disadvantage of PBD is its high rate of preoperative adverse events. The reported adverse event rate of PBD using PSs is 42% to 46%,[7,26] which outweighs the benefit of PBD.[27] Therefore, the advantage of PBD can be maximized if preoperative adverse events are reduced. In the present prospective study, the preoperative adverse event rate was low (12%), consistent with the short- and long-term adverse event rates of 8% and 4%, respectively, in a retrospective study of SEMS placement for PBD.[28] A larger-diameter SEMS was associated with a lower incidence of premature stent occlusion than was observed in PSs, as shown in patients with unresectable malignant biliary obstruction.[29,30]
The optimal duration of PBD is also controversial. An experimental study suggested that recovery of liver function depends on both the duration of obstructive jaundice and the duration of PBD, and at least 4 weeks of PBD was needed for liver function recovery.[31] A recent meta-analysis suggested that the optimal drainage duration to reduce the overall morbidity rate was more than 4 weeks.[32] However, it is sometimes difficult to schedule major surgery for patients with pancreatic head cancer within 4 weeks in clinical practice. Another study showed that, when PSs were used for PBD, the rate of preoperative adverse events was >40%;[7] however, the present study demonstrated that FCSEMS placement can potentially reduce the cumulative incidence of preoperative adverse events (19% at 4- and 8-weeks). Thus, the superiority of FCSEMS over PS placement can be justified if the waiting time for surgery would be prolonged, such as in patients receiving neoadjuvant treatment.
The goal of PBD should be to reduce the morbidity and mortality associated with surgical resection. The use of a large-bore stent with self-expandable force can potentially cause inflammation and adhesion around the bile duct, which can hinder surgical resection.[33] However, SEMS placement reportedly does not affect surgical procedures compared with PS,[34] and the surgeons in the present study did not experience difficulties attributable to the FCSEMSs.
Cost is another issue associated with SEMS placement. Although a retrospective study of 366 patients undergoing pancreaticoduodenectomy concluded that SEMS should be used in selected cases because of its cost,[35] resectability is not readily confirmed at the time of initial biliary drainage. In another study, 20% and 26% of the patients with resectable pancreatic cancer exhibited liver metastasis and peritoneal dissemination, respectively, on preoperative radiological imaging.[36] In such cases, subsequent stent revision might be required since median patency of PS had been reported to be approximately 4 months[29,37] or palliative surgery such as hepaticojejunostomy is sometimes performed during laparotomy. Therefore, initial SEMS placement regardless of resectability is reportedly more cost-effective.[38,39]
Cholecystitis after SEMS placement is a well-known adverse event, and tumor involvement to the OCD is a reported risk factor of cholecystitis after SEMS placement.[40,41] Although patients with tumor involvement to the OCD were excluded from this study, acute cholecystitis occurred in 2 of 24 patients who underwent FCSEMS placement, which is similar to the incidence of 10% in a previous report involving the use of partially covered WallFlex™ stents.[42] Compression of the OCD caused by the flared ends or high axial force of the FCSEMS might result in the development of acute cholycystitis.[43] Acute cholecystitis after SEMS placement remains an unsolved problem, and further improvement of SEMSs is necessary to reduce this stent-related adverse event.
There are some limitations of this study. This was a single-arm study with a small sample size. In addition to this study, we have completed another pilot study of covered SEMS in cases undergoing neoadjuvant chemotherapy for pancreatic cancer (UMIN000011855). Given the increasing evidence of covered SEMS as PBD for pancreatic cancer, we aim to start a large scale, multicenter, randomized control trial of a covered SEMS and PS in cases with pancreatic cancer who would undergo upfront surgery or neoadjuvant chemotherapy. In addition, we were unable to enroll the originally planned number of patients during the study period. Data regarding the effects of FCSEMS placement on the surgical procedure, such as blood loss and operative duration, were lacking. However, the surgeons did not experience any intraoperative difficulties attributable to FCSEMS placement, as previously reported in neoadjuvant settings.[34,44]
CONCLUSIONS
FCSEMS placement is safe and feasible for PBD in patients with resectable pancreatic head cancer. FCSEMS placement can reduce the rate of preoperative adverse events such as stent occlusion, especially in patients undergoing elective surgery. A randomized controlled trial comparing FCSEMS with PS placement or no PBD is warranted to confirm our findings.
Financial support and sponsorship
Nil.
Conflicts of interest
Hiroyuki Isayama has received honoraria for speaking at an event organized by the Boston Scientific Japan Corporation and has received research funds from the Boston Scientific Japan Corporation for another research project.
Osamu Togawa, Hiroshi Kawakami, Yousuke Nakai, Dai Mohri, Tsuyoshi Hamada, Hirofumi Kogure, Kazumichi Kawakubo, Naoya Sakamoto, Kazuhiko Koike, and Hiroto Kita have no conflict of interests to disclose with regards to this work.
REFERENCES
- 1.Hatfield AR, Tobias R, Terblanche J, Girdwood AH, Fataar S, Harries-Jones R, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet. 1982;2:896–9. doi: 10.1016/s0140-6736(82)90866-2. [DOI] [PubMed] [Google Scholar]
- 2.McPherson GA, Benjamin IS, Hodgson HJ, Bowley NB, Allison DJ, Blumgart LH. Pre-operative percutaneous transhepatic biliary drainage: The results of a controlled trial. Br J Surg. 1984;71:371–5. doi: 10.1002/bjs.1800710522. [DOI] [PubMed] [Google Scholar]
- 3.Smith RC, Pooley M, George CR, Faithful GR. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice: A randomized, controlled trial examining renal function. Surgery. 1985;97:641–8. [PubMed] [Google Scholar]
- 4.Pitt HA, Gomes AS, Lois JF, Mann LL, Deutsch LS, Longmire WP., Jr Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545–53. doi: 10.1097/00000658-198505000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lygidakis NJ, van der Heyde MN, Lubbers MJ. Evaluation of preoperative biliary drainage in the surgical management of pancreatic head carcinoma. Acta Chir Scand. 1987;153:665–8. [PubMed] [Google Scholar]
- 6.Sewnath ME, Karsten TM, Prins MH, Rauws EJ, Obertop H, Gouma DJ. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17–27. doi: 10.1097/00000658-200207000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–37. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 8.Baron TH, Kozarek RA. Preoperative biliary stents in pancreatic cancer--proceed with caution. N Engl J Med. 2010;362:170–2. doi: 10.1056/NEJMe0908773. [DOI] [PubMed] [Google Scholar]
- 9.Tsujino T, Isayama H, Koike K. Preoperative drainage in pancreatic cancer. N Engl J Med. 2010;362:1343–4; author reply 1346. [PubMed] [Google Scholar]
- 10.Tol JA, van Hooft JE, Timmer R, Kubben FJ, van der Harst E, de Hingh IH, et al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut. 2016;65:1981–7. doi: 10.1136/gutjnl-2014-308762. [DOI] [PubMed] [Google Scholar]
- 11.Song TJ, Lee JH, Lee SS, Jang JW, Kim JW, Ok TJ, et al. Metal versus plastic stents for drainage of malignant biliary obstruction before primary surgical resection. Gastrointest Endosc. 2016;84:814–21. doi: 10.1016/j.gie.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Crippa S, Cirocchi R, Partelli S, Petrone MC, Muffatti F, Renzi C, et al. Systematic review and meta-analysis of metal versus plastic stents for preoperative biliary drainage in resectable periampullary or pancreatic head tumors. Eur J Surg Oncol. 2016;42:1278–85. doi: 10.1016/j.ejso.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Aadam AA, Evans DB, Khan A, Oh Y, Dua K. Efficacy and safety of self-expandable metal stents for biliary decompression in patients receiving neoadjuvant therapy for pancreatic cancer: A prospective study. Gastrointest Endosc. 2012;76:67–75. doi: 10.1016/j.gie.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 14.Kubota K, Sato T, Watanabe S, Hosono K, Kobayashi N, Mori R, et al. Covered self-expandable metal stent deployment promises safe neoadjuvant chemoradiation therapy in patients with borderline resectable pancreatic head cancer. Dig Endosc. 2014;26:77–86. doi: 10.1111/den.12049. [DOI] [PubMed] [Google Scholar]
- 15.Sasahira N, Hamada T, Togawa O, Yamamoto R, Iwai T, Tamada K, et al. Multicenter study of endoscopic preoperative biliary drainage for malignant distal biliary obstruction. World J Gastroenterol. 2016;22:3793–802. doi: 10.3748/wjg.v22.i14.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isayama H, Hamada T, Yasuda I, Itoi T, Ryozawa S, Nakai Y, et al. TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc. 2015;27:259–64. doi: 10.1111/den.12379. [DOI] [PubMed] [Google Scholar]
- 17.Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, et al. Endoscopic sphincterotomy complications and their management: An attempt at consensus. Gastrointest Endosc. 1991;37:383–93. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 18.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: An international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakayama T, Ikeda A, Okuda K. Percutaneous transhepatic drainage of the biliary tract: Technique and results in 104 cases. Gastroenterology. 1978;74:554–9. [PubMed] [Google Scholar]
- 21.Denning DA, Ellison EC, Carey LC. Preoperative percutaneous transhepatic biliary decompression lowers operative morbidity in patients with obstructive jaundice. Am J Surg. 1981;141:61–5. doi: 10.1016/0002-9610(81)90013-1. [DOI] [PubMed] [Google Scholar]
- 22.Norlander A, Kalin B, Sundblad R. Effect of percutaneous transhepatic drainage upon liver function and postoperative mortality. Surg Gynecol Obstet. 1982;155:161–6. [PubMed] [Google Scholar]
- 23.Gundry SR, Strodel WE, Knol JA, Eckhauser FE, Thompson NW. Efficacy of preoperative biliary tract decompression in patients with obstructive jaundice. Arch Surg. 1984;119:703–8. doi: 10.1001/archsurg.1984.01390180065011. [DOI] [PubMed] [Google Scholar]
- 24.Lai EC, Mok FP, Fan ST, Lo CM, Chu KM, Liu CL, et al. Preoperative endoscopic drainage for malignant obstructive jaundice. Br J Surg. 1994;81:1195–8. doi: 10.1002/bjs.1800810839. [DOI] [PubMed] [Google Scholar]
- 25.Heslin MJ, Brooks AD, Hochwald SN, Harrison LE, Blumgart LH, Brennan MF. A preoperative biliary stent is associated with increased complications after pancreatoduodenectomy. Arch Surg. 1998;133:149–54. doi: 10.1001/archsurg.133.2.149. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama H, Tsuyuguchi T, Sakai Y, Nisikawa T, Miyazaki M, Yokosuka O. Preoperative drainage for distal biliary obstruction: Endoscopic stenting or nasobiliary drainage? Hepatogastroenterology. 2013;60:231–4. doi: 10.5754/hge12621. [DOI] [PubMed] [Google Scholar]
- 27.Qiu YD, Bai JL, Xu FG, Ding YT. Effect of preoperative biliary drainage on malignant obstructive jaundice: A meta-analysis. World J Gastroenterol. 2011;17:391–6. doi: 10.3748/wjg.v17.i3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqui AA, Mehendiratta V, Loren D, Kowalski T, Fang J, Hilden K, et al. Self-expanding metal stents (SEMS) for preoperative biliary decompression in patients with resectable and borderline-resectable pancreatic cancer: Outcomes in 241 patients. Dig Dis Sci. 2013;58:1744–50. doi: 10.1007/s10620-012-2482-z. [DOI] [PubMed] [Google Scholar]
- 29.Isayama H, Yasuda I, Ryozawa S, Maguchi H, Igarashi Y, Matsuyama Y, et al. Results of a Japanese multicenter, randomized trial of endoscopic stenting for non-resectable pancreatic head cancer (JM-test): Covered Wallstent versus DoubleLayer stent. Dig Endosc. 2011;23:310–5. doi: 10.1111/j.1443-1661.2011.01124.x. [DOI] [PubMed] [Google Scholar]
- 30.Almadi MA, Barkun A, Martel M. Plastic vs. Self-Expandable Metal Stents for Palliation in Malignant Biliary Obstruction: A Series of Meta-Analyses. Am J Gastroenterol. 2017;112:260–73. doi: 10.1038/ajg.2016.512. [DOI] [PubMed] [Google Scholar]
- 31.Koyama K, Takagi Y, Ito K, Sato T. Experimental and clinical studies on the effect of biliary drainage in obstructive jaundice. Am J Surg. 1981;142:293–9. doi: 10.1016/0002-9610(81)90296-8. [DOI] [PubMed] [Google Scholar]
- 32.Sun C, Yan G, Li Z, Tzeng CM. A meta-analysis of the effect of preoperative biliary stenting on patients with obstructive jaundice. Medicine (Baltimore) 2014;93:e189. doi: 10.1097/MD.0000000000000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayaru L, Kurzawinski TR, Shankar A, Webster GJ, Hatfield AR, Pereira SP. Complications and diagnostic difficulties arising from biliary self-expanding metal stent insertion before definitive histological diagnosis. J Gastroenterol Hepatol. 2008;23:315–20. doi: 10.1111/j.1440-1746.2006.04562.x. [DOI] [PubMed] [Google Scholar]
- 34.Cavell LK, Allen PJ, Vinoya C, Eaton AA, Gonen M, Gerdes H, et al. Biliary self-expandable metal stents do not adversely affect pancreaticoduodenectomy. Am J Gastroenterol. 2013;108:1168–73. doi: 10.1038/ajg.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haapamaki C, Seppanen H, Udd M, Juuti A, Halttunen J, Kiviluoto T, et al. Preoperative biliary decompression preceding pancreaticoduodenectomy with plastic or self-expandable metallic stent. Scand J Surg. 2015;104:79–85. doi: 10.1177/1457496914543975. [DOI] [PubMed] [Google Scholar]
- 36.Doucas H, Sutton CD, Zimmerman A, Dennison AR, Berry DP. Assessment of pancreatic malignancy with laparoscopy and intraoperative ultrasound. Surg Endosc. 2007;21:1147–52. doi: 10.1007/s00464-006-9093-8. [DOI] [PubMed] [Google Scholar]
- 37.Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488–92. doi: 10.1016/0140-6736(92)92752-2. [DOI] [PubMed] [Google Scholar]
- 38.Chen VK, Arguedas MR, Baron TH. Expandable metal biliary stents before pancreaticoduodenectomy for pancreatic cancer: A Monte-Carlo decision analysis. Clin Gastroenterol Hepatol. 2005;3:1229–37. doi: 10.1016/s1542-3565(05)00886-4. [DOI] [PubMed] [Google Scholar]
- 39.Kahaleh M, Brock A, Conaway MR, Shami VM, Dumonceau JM, Northup PG, et al. Covered self-expandable metal stents in pancreatic malignancy regardless of resectability: A new concept validated by a decision analysis. Endoscopy. 2007;39:319–24. doi: 10.1055/s-2007-966263. [DOI] [PubMed] [Google Scholar]
- 40.Suk KT, Kim HS, Kim JW, Baik SK, Kwon SO, Kim HG, et al. Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastrointest Endosc. 2006;64:522–9. doi: 10.1016/j.gie.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 41.Isayama H, Kawabe T, Nakai Y, Tsujino T, Sasahira N, Yamamoto N, et al. Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2006;4:1148–53. doi: 10.1016/j.cgh.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Isayama H, Mukai T, Itoi T, Maetani I, Nakai Y, Kawakami H, et al. Comparison of partially covered nitinol stents with partially covered stainless stents as a historical control in a multicenter study of distal malignant biliary obstruction: The WATCH study. Gastrointest Endosc. 2012;76:84–92. doi: 10.1016/j.gie.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 43.Nakai Y, Isayama H, Kawakubo K, Kogure H, Hamada T, Togawa O, et al. Metallic stent with high axial force as a risk factor for cholecystitis in distal malignant biliary obstruction. J Gastroenterol Hepatol. 2014;29:1557–62. doi: 10.1111/jgh.12582. [DOI] [PubMed] [Google Scholar]
- 44.Singal AK, Ross WA, Guturu P, Varadhachary GR, Javle M, Jaganmohan SR, et al. Self-expanding metal stents for biliary drainage in patients with resectable pancreatic cancer: Single-center experience with 79 cases. Dig Dis Sci. 2011;56:3678–84. doi: 10.1007/s10620-011-1815-7. [DOI] [PubMed] [Google Scholar]


