Abstract
Background/Aims:
Pokeweed antiviral protein (PAP) has been reported to downregulate Wnt/Jnk pathway and attenuate liver fibrosis. This study was designed to intensively explore the mechanism of anti-fibrosis effect of PAP.
Materials and Methods:
Hepatic stellate cell (HSC) activation was induced by high concentration of glucose. Cell viability was detected at different time points after PAP treatment. Meanwhile, hepatic fibrosis models in mice were induced by CCl4 injection. In the end, liver pathology was observed and contents of alanine transaminase, aspartate transaminase, lactic dehydrogenase, hyaluronic acid (HA), and laminin (LN) in serum together with hydroxyproline (Hyp) in liver were measured. The mRNA and protein expressions of HK2, PFKP, PCK1, and FBP1 as well as Jnk expression in HSC-T6 cells and liver tissue were detected by qPCR and western-blot, respectively.
Results:
Compared with high glucose, PAP reduced viability and expressions of HK2, PFKP, α-SMA, and Col1A1, where as enhanced the expressions of PCK1 and FBP1 in HSC-T6 cells (P < 0.05) respectively. PAP attenuated liver pathology, improved liver function, and reduced collagen deposition in liver tissue compared with the model group (P < 0.05) respectively. Moreover, PAP reduced expressions of HK2, PFKP, α-SMA, and Col1A1 where as increased the expression of PCK1 and FBP1 in the liver of mice compared with the model group (P < 0.05) respectively. Most importantly, PAP reduced the phosphorylation of Jnk both in cells and liver tissue compared with the model group (P < 0.05) respectively.
Conclusions:
Our results demonstrated that PAP attenuated liver fibrosis by regulating Wnt/Jnk-mediated glucose metabolism. It provided us a new target for the treatment of liver fibrosis.
Keywords: Glucose metabolism, liver fibrosis, PAP, Wnt/Jnk
INTRODUCTION
Liver fibrosis has been considered as a wound-healing response of liver to repeated liver injuries such as hepatitis, drugs, and alcohol, and it is characterized by an excessive deposition of extracellular matrix (ECM). Hepatic stellate cell (HSC) is the main source of ECM and its activation is the key event in liver fibrosis.[1] As many signaling pathways and cytokines participate in HSC activation, the mechanism involved in HSC activation and liver fibrogenesis remains poorly understood.
Among various pathways involved in HSC activation, Wnt signaling pathway has been regarded as the most important.[2] Furthermore, Wnt signaling has been mainly divided into two specific pathways according to the transducer it depends on –the canonical pathway and the noncanonical signaling pathway.[3] Wnt/Jnk pathway was noncanonical, which has been reported to promote HSC activation.[4] Moreover, Wnt/Jnk pathway has various cellular functions including cell proliferation and gene expression. Recent studies showed that this signaling predominated metabolism such as glucose metabolism.[5,6] Unfortunately, whether Wnt/Jnk-mediated glucose metabolism participates in liver fibrogenesis and its molecular mechanism has not been completely elucidated.
Pokeweed antiviral protein (PAP), an extraction from traditional Chinese herb, has been demonstrated to have various pharmaceutical activities including inhibiting hepatitis B replication and ameliorating liver fibrosis.[7,8] Many other studies also proved the inhibitory effects of PAP on Jnk signaling pathway.[9] However, whether the anti-fibrosis effect of PAP is related to the inhibition of Wnt/Jnk signaling remains unclear. This study was designed to further investigate the molecular mechanism involved in the suppressive effects of PAP on liver fibrosis and found that PAP attenuated liver fibrosis in rats through mediating Wnt/Jnk mediated glucose metabolism.
MATERIALS AND METHODS
Materials
Kits for determining serum alanine transaminase (ALT), aspartate transaminase (AST), lactic dehydrogenase (LDH), and hydroxyproline (Hyp) were all bought from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Cyto Tox 96 Nonradioactive Cytotoxicity assay for cell viability was obtained from Promega (USA). TGF-β1 was purchased from R&D Systems (USA). The primary antibodies were as following: mouse antibody against HK-2(TA500856, Origene), mouse antibody against PFKP (TA503979, Origene), mouse antibody against PCK1 (ab87340, Abcam), rabbit anti-FBP1 (ab109020, Abcam), rabbit anti-Jnk (#9252, Cell signaling), rabbit anti-pJnk (Thr 183/Tyr 185,#4668, Cell signaling), mouse anti-α-SMA (ab5694, Abcam), and mouse anti-β-actin (ab8227, Abcam). Secondary antibodies of anti-mouse and rabbit IgG conjugated with horseradish peroxidase were obtained from GE Healthcare (UK).
Cell culture
HSC line HSC-T6 cells were purchased from the Cancer Institute and Hospital, Chinese Academy of Medical Sciences. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, California, USA) containing 10% new bovine serum (Hangzhou, China), 100 U/ml penicillin, and 100ug/ml streptomycin, under a humidified 5% (v/v) CO2 atmosphere at 37°C. Cells were divided into 4 groups: normal group, cells treated with DMEM containing 1000mg/L glucose; control group, cells treated with DMEM containing 1000mg/L glucose together with pXF3H-neo transfection; treatment group, cells treated with DMEM containing 4500mg/L glucose together with pXF3H-PAP transfection; model group, cells treated with DMEM containing 4500mg/L glucose without transfection.
Animal treatments
Male mice (body weight 18–22g) were provided by the Experiment Animal Center of Anhui Medical University (Hefei, China). Mice were housed at the Animal House Facility of Anhui Provincial Hospital, Anhui Medical University. All animal procedures were performed under the guidelines set by the Animal Care and Use Committee of Anhui Medical University.
Mice were randomly divided into four groups (n = 25 to 30mice per group). Mice in model group (n = 17) were injected subcutaneously with CCl4 at a dose of 3 ml/kg twice a week for 8 weeks. At the same time, mice in the treatment group (n = 25) were treated with 50 ug of pXF3H-PAP by hydrodynamic injection through the tail vein in combination with CCl4. Those in control group (n = 19) received equal volume of pXF3H-neo plus CCl4. Mice in normal group (n = 22) received equal volume of vehicle solution without CCl4.
At the end of the experiment, mice were anesthetized with 10% chloral hydrate and killed. Serum samples were collected from mice and stored at −80°C for ELISA kits. Liver tissue was harvested 3 days after the last injection for 3 purposes: (1) fixed in 10% formalin for histological examinations; (2) stored at −80°C for HYP determination; and (3) preserved in RNA later (Invitrogen, USA) for RNA isolation.
Biochemical determination
The serum levels of ALT, AST, and LDH in mice were detected by ELISA kits, and the content of HA and LN in serum of mice were measured by radioimmunoassay.
Hyp content test
Hyp content in liver tissue was evaluated by spectrophotometric method according to the instructions. Results were expressed as Hyp (mg)/wet liver weight (g).
Liver pathology
Liver tissues from mice were fixed in 10% formalin, embedded in paraffin and cut at a thickness of 5 μm. Hematoxylin and eosin (H and E) staining was employed to examine the changes in liver pathology. Masson's trichrome staining was engaged to assess the collagen distribution in liver tissue. Fibrosis score were determined by two independent pathologists blindly according to the score system described by Hou et al.[10]
Plasmid preparation
Expression plasmid pXF3H-PAP driven by a CMV promoter was presented by Prof. Yongwen He (Union Hospital, Wuhan China), the plasmid was amplified, purified, and resuspended in ddH2O at a final concentration of 1ug/ul.
Cell viability assay
HSC-T6 viability was tested by CytoTox 96 Nonradioactive Cytotoxicity assay. Cells were seeded into 96 well plate at a density of 5 × 104 cells with 200ul culture media. Cells were incubated in culture media with or without PAP transfection for 12, 24, 36, and 48 h. Then cells were harvested and lysed by 30ul lysis solution, followed by incubation at 37°C for 50 min. Supernatants were transferred to a fresh 96-well plate. Reconstituted Substrate Mix and stop solution were added to the sample according to the instruction, respectively. The plate was read at 560nm absorbance.
Quantitative real-time polymerase chain reaction
RNA was extracted from cells and mice liver tissue using RNA easy mini kit (Qiagen) according to manufacturer's instructions. The RNA was reverse transcribed into cDNA by First Strand cDNA Synthesis kit (Toyobo, Japan). The qPCR was carried in a 7500 Fast Real-Time PCR System (Applied Biosystems). Amplification was done in a total volume of 20ul for 35 cycles and the production was detected by SYBR green Plus reagent kit (Roche Applied Science, Germany). GAPDH was used as the reference gene and the samples were run in triplicate. The primers were as follows:
-
GAPDH (Invitrogen, Shanghai):
Forward, 5'- ACCACAGTCCATGCCATCAC-3';
Reverse, 5'- TCCACCACCCTGTTGCTGTA-3'
-
α-SMA (Invitrogen, Shanghai):
Forward, 5'- GATCACCATCGGGAATGAACGC-3';
Reverse, 5'- CTTAGAAGCATTTGCGGTGGAC-3'
-
Col 1A1(Invitrogen, Shanghai):
Forward,5'-ACTGCAACATGGAGACAGGTCA GA-3';
Reverse, 5'-ATCGGTCATGCTCTCTCCAAACCA-3'
-
HK2 (Invitrogen, Shanghai):
Forward, 5'- TCAAAGAGAACAAGGGCGAG -3';
Reverse, 5'- AGGAAGCGGACATCACAATC-3'
-
PFKP (Invitrogen, Shanghai):
Forward, 5'- ACTGAAGGGCTCCCACGGCA-3';
Reverse, 5'- GGCCGCAGTTTCAGCCACCA -3'
-
PCK1(Invitrogen, Shanghai):
Forward, 5'-AGTCACCATCACTTCCTGGAAGA-3';
Reverse, 5'- GGTGCAGAATCGCGAGTT -3'
-
FBP1(Invitrogen, Shanghai):
Forward, 5'- GACTGCCTCGCGTCCATCGG-3';
Reverse, 5'- GCTGCCACCAGGGTTCCTGCC-3'.
Western blot analysis
HSC-T6 cells and Matrigel treated T6 cells were seeded into 6-well plate at a density of 1 × 106 cells/well. Next day, cells were transfected with pXF3H-PAP. At 48h after transfection, cells were harvested and lysed in 1ml lysis buffer. The extracted protein was separated by SDS gel and PVDF membrane (Roche, USA). The membranes were incubated with antibodies at 4°C overnight. Next day, the membranes were probed with a HRP coupled secondary antibody. In the end, membranes were developed with the ECL Plus Western Blotting Detection System (Amersham Life Science, UK). β-Actin was used as reference gene.
Statistical analysis
Results were depicted as mean ± SEM. Data were analyzed by Student's t-test when only two groups were compared, and analysis of variance (ANOVA) when three or more than three groups were involved. P < 0.05 was considered to be significant.
RESULTS
Effect of PAP on liver fibrosis in mice induced by CCl4
As shown in Table 1, plasma levels of ATL, AST, and LDH in CCl4-treated mice increased significantly compared with control mice, while they were obviously decreased by PAP treatment compared with model group. Moreover, HA and LN levels in the liver tissue of PAP treatment group were remarkably higher than control group, but significantly lower than model group. There were no significant differences in ATL, AST, LDH, HA, and LN levels between the control and normal groups. These results indicated that PAP could improve the liver function of mice.
Table 1.
Levels of ATL, AST, LDH, HA and LN in serum and Hyp in liver tissue

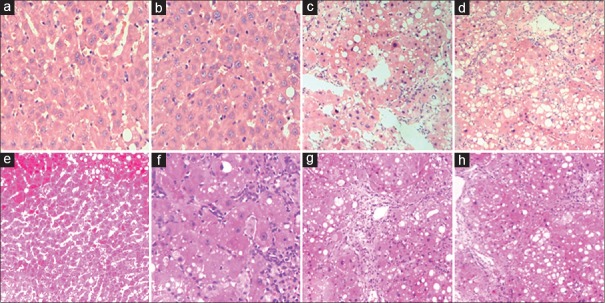
At the end of the experiment, H and E staining and Masson's trichrome staining were used to examine the changes in liver pathology. Liver tissue samples from the model group showed that PAP not only ameliorated adipose degeneration of hepatocytes but also reduced the inflammatory cell infiltration. As shown by Masson in Figure 1, the collagen fibers in CCl-4 treated mice were significantly more than those in normal mice, while they were markedly decreased by PAP treatment compared with the model group (P < 0.05).
Figure 1.
Liver pathology (×200) by H and E (a-d) and Masson's staining (e-h). Liver samples from normal group showed normal liver architecture, few infiltrated inflammatory cells and trace collagen (a and e). On the contrary, there were much more inflammatory cells and collagen in model group than normal group, the liver structure was disordered (d and h). The liver condition was significantly improved by PAP treatment (b and f) compared with model group. There was no significant difference in cell infiltration and collagen deposition between model group (d and h) and control group (c and g)
In addition, Hyp content assay was also used to evaluate the collagen content in the livers [Table 1]. The Hyp content was obviously higher in CCl4 treated group than in control group (P < 0.05), but lower than PAP treatment group (P < 0.05).
Effect of PAP on cell viability of HSC-T6 cells
The effect of PAP on T6 cells was examined by cytotoxicity assay. As shown by Figure 2, viability of T6 cells of control and treatment group showed a lower viability than the model group (P < 0.05, respectively). There was no significant difference in the cell viability between cells from normal, treatment, and control groups (P > 0.05, respectively), suggesting that PAP reduced glucose-induced HSC activation.
Figure 2.
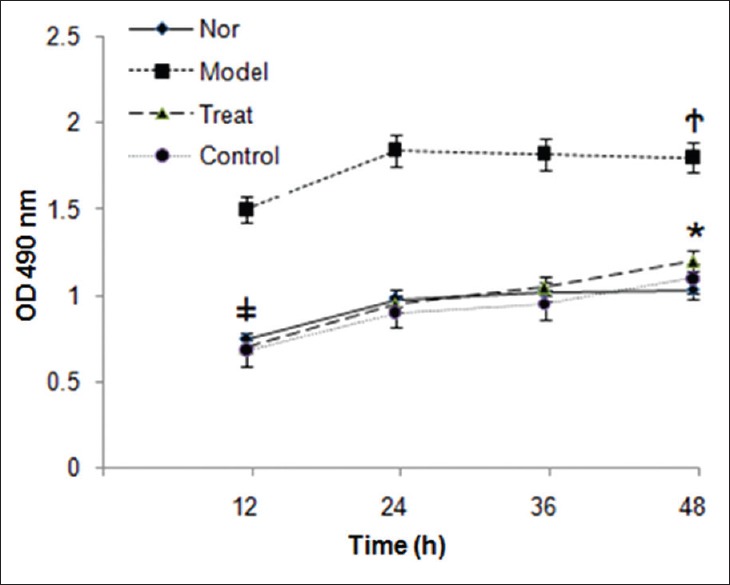
Viability of HSC-T6 cells. High glucose up-regulated viability of HSC-T6 cells, while it was significantly reduced by PAP treatment. There was no significant difference among normal, treatment and normal group. * P > 0.05 compared with normal group. †P < 0.05 compared with treatment group. ‡P > 0.05 compared with control group
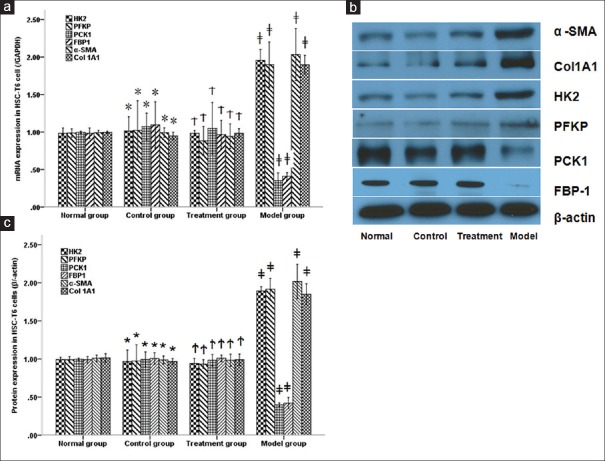
Effect of PAP on mRNA and protein expressions of HK2, PFKP, PCK1, FBP1, α-SMA, and Col1A1 in T6 cells and liver tissue
To observe the effect of PAP on Wnt/Jnk-mediated glucose metabolism and liver fibrosis. qPCR and western-blot were used to observe the mRNA and protein expressions of key enzymes and fibrosis genes in T6 cells and liver tissue, respectively. As shown in Figure 3, both mRNA and protein expressions of HK2, PFKP, α-SMA, and Col1A1 increased significantly in T6 cells from model group compared with normal group, while they were remarkably reduced by PAP treatment (P < 0.05, respectively). On the contrary, mRNA expressions of PCK1 and FBP1 were lower in T6 cells from the model group than T6 cells from the normal group, whereas they were remarkably increased by PAP treatment (P < 0.05, respectively).
Figure 3.
mRNA (a) and protein (b, c) expressions of HK2, PFKP, PCK1, FBP1, α-SMA and Col1A1 in HSC-T6 cells. PAP significantly reduced both mRNA and protein expressions of HK2, PFKP, α-SMA and Col1A1 while increased PCK1 and FBP1 in HSC-T6 cells treated by high glucose * P > 0.05 compared with normal group. †P > 0.05 compared with treatment group. ‡P < 0.05 compared with control group
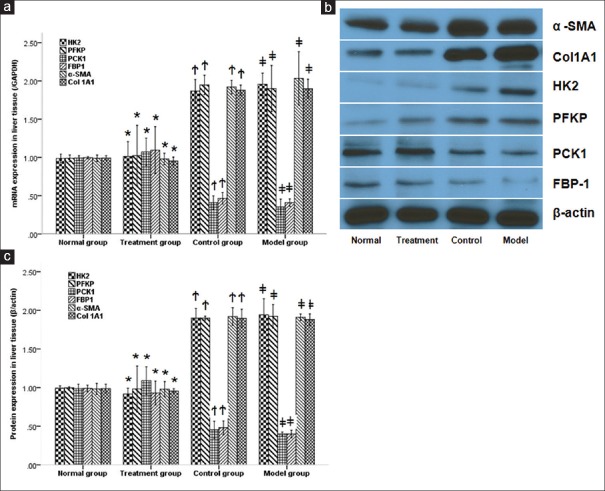
When it comes to CCl4 treated mice, mRNA and protein expressions of HK2, PFKP, α-SMA, and Col1A1 increased significantly in the model group compared with the normal group, while they were remarkably reduced by PAP treatment (P < 0.05, respectively). Similar to T6 cells, shown by Figure 4, mRNA expressions of PCK1 and FBP1 were lower in the model group than normal group, while they were both remarkably increased by PAP treatment (P < 0.05, respectively). There were no significant differences in the mRNA and protein expression of HK2, PFKP, PCK1, FBP1, α-SMA, and Col1A1 between the model and control groups (P > 0.05, respectively).
Figure 4.
mRNA (a) and protein (b, c) expressions of HK2, PFKP, PCK1, FBP1, α-SMA and Col1A1 in liver tissue. PAP significantly reduced both mRNA and protein expressions of HK2, PFKP, α-SMA and Col1A1 while increased PCK1 and FBP1 in liver tissue of mice induced by CCl4. * P > 0.05 compared with normal group. †P < 0.05 compared with treatment group. ‡P > 0.05 compared with control group
Effect of PAP on Wnt/Jnk pathway in T6 cells and liver tissue
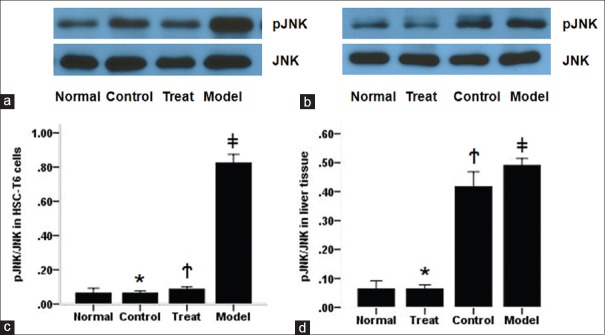
To investigate the effects of PAP on Jnk signaling, we tested the expression of total Jnk and pJnk in HSC-T6 cells and liver tissue by western-blot. As shown in Figure 5, the pJNK expression increased significantly in HSC-T6 cells from the model group compared with the normal group, while it was remarkably reduced by PAP treatment (P < 0.05, respectively). However, there is no significant difference in the expression of total Jnk among the four groups (P > 0.05, respectively). Interestingly, the pJNK levels in the normal, treatment, and control groups showed no significant difference (P > 0.05, respectively), indicating that PAP treatment could remarkably reduce glucose-induced HSC activation through downregulating Wnt/Jnk pathway.
Figure 5.
Expression of Jnk/pJnk in HSC-T6 cells and liver tissue. PAP reduced phosphorylation of Jnk in HSC-T6 cells treated with high glucose (a and c), and liver tissue induced by CCl4 (b and d). c, * P > 0.05 compared with normal group, †P > 0.05 compared with control group, ‡P < 0.05 compared with treatment group; d, *P > 0.05 compared with normal group, †P < 0.05 compared with treatment group, ‡P > 0.05 compared with control group
Regarding the expression of Jnk in liver tissue, CCl4 induced the phosphorylation of Jnk in liver tissue compared with normal group, while it was obviously reduced by PAP treatment compared with the model group (P < 0.05, respectively). Similar to HSC-T6 cells, there was no significant difference in total JNK expression among mice from four groups as well as pJNK level between the normal and control groups (P > 0.05, respectively). These results suggested that PAP inhibited liver fibrosis in mice by suppressing the phosphorylation of Jnk.
DISCUSSION
In general, liver fibrosis represents the wound-healing response of liver to various liver injures including hepatitis, alcohol, and immune compounds. Hepatitis virus and alcohol occupied the leading position in the etiology of liver fibrosis over the past decades and went down with the progress of medical science and society. On the contrary, at present, metabolic disturbance has risen to the front place as the cause of liver fibrosis. Interesting, recent studies demonstrated that disorder of glucose metabolism plays an important role in liver fibrosis and that many liver fibrosis patients also have diabetes mellitus.[11] Thus, researchers have tried to explore the interaction among cytokines, liver fibrosis, and glucose metabolism.
Liver fibrosis has been regarded as the excessive deposition of ECM in the liver.[12] Owing to the repeated liver injures, HSC became activated and transferred into myofibroblast cells to produce more ECM in liver, resulting in liver fibrosis.[13] Most importantly, activated HSC can convert into quiescent-like stellate cells to avoid immune clearance and necrosis,[14] but these quiescent-like cells can produce more ECM than activated ones when exposed to stimulus.[14] Therefore, suppression of HSC activation may prevent and reverse liver fibrosis.
Liver fibrosis has also been regarded as a chronic inflammation associated disease, which involves the interplay of different inflammatory mediators, cytokines, and signaling pathways.[15,16,17] That is, cytokines or signaling mediators may exert their influence on liver fibrosis through regulating glucose metabolism. Wnt/Jnk has been demonstrated to be one of the most important pathways contributing to liver fibrosis. Furthermore, Wnt/Jnk signaling has also been reported to participate in various physiological processes such as glucose metabolism.[18,19,20,21] Further, PAP is known as an anti-fibrosis agent and has been shown to have numerous pharmaceutical properties, including regulating Wnt/Jnk pathway and inhibiting liver fibrosis.[22,23] In this study, PAP was shown to significantly downregulate Wnt/Jnk pathway both in HSC-T6 cells and mice.
Moreover, HK2, PFKP, PCK1, and FBP1 are important enzymes regulating glucose metabolism. It has also been reported that high concentration of glucose could induce HSC activation.[24] To testify the effect of glucose metabolism on HSC activation, we treated HSC with high concentration of glucose. Consequently, glucose increased viability and enhanced the mRNA and protein expressions of α-SMA together with glycolysis enzymes such as HK2 and PFKP in HSC-T6 cells compared with normal cells, which were all reduced by PAP treatment. Most importantly, PAP treatment also reduced Jnk phosphorylation compared with model group, suggesting that PAP inhibited glucose-induced HSC activation by regulating Jnk mediated glycolysis. These results were consistent with previous studies.[25,26]
CCl-4-induced liver fibrosis in mice is a widely accepted research model.[27] Using this model, our study has demonstrated that PAP significantly inhibited CCl4-induced liver fibrosis in mice.[8] Contents of HA and LN in serum and Hyp in liver have been regarded as accurate indices reflecting liver fibrosis score.[28,29,30] In this study, the contents of HA and LN in serum and Hyp in liver tissue were all higher in the model group than that in the control group, while they were significantly reduced by PAP treatment, suggesting that PAP inhibited liver fibrosis. Moreover, mRNA and protein levels of α-SMA and Col1A1in the liver of mice were remarkably increased in the model group compared with the control group, while they were obviously reduced by PAP treatment, suggesting that PAP had a strong anti-fibrosis effect. Furthermore, we tested the expressions of key proteins of glucose metabolism in liver tissue and found that, with increasing liver fibrosis score, the levels of key proteins involved in glycolysis including HK2 and PFKP in liver tissue from mice increased while the levels of PCK1 and FBP1 decreased. On the contrary, compared to the model group, PAP decreased mRNA and protein expressions of HK2 and PFKP while increased PCK1 and FBP1 expressions, suggesting that PAP inhibited liver fibrosis by regulating glucose metabolism.
In addition, Jnk is well known as an important subgroup of mitogen-activated protein kinases (MAPKs), which are highly conserved kinases. Jnk has been reported to participate in many physiological processes including cell proliferation, apoptosis, and gene expression. Importantly, Jnk has also been demonstrated to be a vital mediator of noncanonical Wnt pathways and linked with diabetes and obesity[31,32,33] and liver fibrosis.[34] However, the roles of Wnt/Jnk in glucose metabolism and liver fibrosis remain enigmatic. Interestingly, our study suggested that PAP reduced the phosphorylation of Jnk in activated HSC-T6 cells and liver tissue from CCl-4-induced mice, indicating that PAP inhibited liver fibrosis through suppressing Wnt/Jnk-mediated glucose metabolism.
Nevertheless, our study has some limitations, the non inclusion of study in mice model with diabetes mellitus, the lack of observation of effect of insulin on HSC activation and liver fibrosis. These limitations may make it difficult to elucidate the interaction between diabetes mellitus and liver fibrosis. To verify the mechanism involved in diabetes mellitus-induced liver fibrosis, further precise studies are warranted.
CONCLUSION
In conclusion, this study demonstrated that PAP suppressed HSC activation and attenuated CCl4-induced liver fibrosis, and its anti-fibrosis effect was related to the inhibition of glycolysis through regulating Wnt/Jnk pathway. As liver fibrosis is a complicated process, further studies are needed to explore the underlying mechanisms involved in anti-fibrosis effect of PAP.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was supported by Natural Science Foundation of Anhui Province (No. 1508085MH172), Tianqin Liver Diseases Research Fund (No. TQGB20140075), WBE Liver Fibrosis Foundation (No. CFHPC20161005) and International Cooperative Project of Anhui Province (No. 1704e1002231).
Compliance with ethical standards.
REFERENCES
- 1.Kim IH. The Potential Role of Elk-3/Egr-1 Signaling Pathway in the Epithelial-Mesenchymal Transition during Liver Fibrosis. Gut Liver. 2017;11:11–2. doi: 10.5009/gnl16564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong Y, Shen X, He M, Wu Z, Zheng Q, Wang Y, et al. Activation of the JNK-c-Jun pathway in response to irradiation facilitates Fas ligand secretion in hepatoma cells and increases hepatocyte injury. J Exp Clin Cancer Res. 2016;35:114. doi: 10.1186/s13046-016-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran FH, Zheng JJ. Modulating the wnt signaling pathway with small molecules. Protein Sci. 2017;26:650–61. doi: 10.1002/pro.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seki E, Brenner DA, Karin M. A Liver Full of JNK: Signaling in Regulation of Cell Function and Disease Pathogenesis, and Clinical Approaches. Gastroenterology. 2012;143:307–20. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Yu X. Emerging role of JNK in insulin resistance. Curr Diabetes Rev. 2013;9:422–8. doi: 10.2174/15733998113099990074. [DOI] [PubMed] [Google Scholar]
- 6.Pal M, Febbraio MA, Lancaster GI. The roles of c - Jun NH2 - terminal kinases (JNKs) in obesity and insulin resistance. J Physiol. 2016;594:267–79. doi: 10.1113/JP271457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He YW, Guo CX, Pan YF, Peng C, Weng ZH. Inhibition of hepatitis B virus replication by pokeweed antiviral protein in vitro. World J Gastroenterol. 2008;14:1592–7. doi: 10.3748/wjg.14.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Zhu C, Chen X, Li Y, Gao R, Wu Q. Pokeweed antiviral protein down-regulates Wnt/β-catenin signalling to attenuate liver fibrogenesis in vitro and in vivo. Dig Liver Dis. 2011;43:559–66. doi: 10.1016/j.dld.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Chan Tung KW, Mansouri S, Hudak KA. Expression of pokeweed antiviral protein in mammalian cells activates c-Jun NH2-terminal kinase without causing apoptosis. Int J Biochem Cell Biol. 2008;40:2452–61. doi: 10.1016/j.biocel.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Hou JL. Fibrosis assessment: Impact on current management of chronic liver disease and application of quantitative invasive tools. Hepatol Int. 2016;10:448–61. doi: 10.1007/s12072-015-9695-0. [DOI] [PubMed] [Google Scholar]
- 11.Isoda H, Takahashi H, Eguchi Y, Kojima M, Inoue K, Murayama K, et al. Re-evaluation of glycated hemoglobin and glycated albumin with continuous glucose monitoring system as markers of glycemia in patients with liver cirrhosis. Biomed Rep. 2017;6:51–6. doi: 10.3892/br.2016.808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Natarajan V, Harris EN, Kidambi S. SECs (Sinusoidal Endothelial Cells), Liver Microenvironment, and Fibrosis. Biomed Res Int. 2017:4097205. doi: 10.1155/2017/4097205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senoo H, Mezaki Y, Fujiwara M. The stellate cell system (vitamin A-storing cell system) Anat Sci Int. 2017;92(4):387–455. doi: 10.1007/s12565-017-0395-9. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Deng X, Liang J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp Cell Res. 2017;352:420–6. doi: 10.1016/j.yexcr.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 15.Li P, He K, Li J. The role of Kupffer cells in hepatic diseases. Mol Immunol. 2017;85:222–9. doi: 10.1016/j.molimm.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Xu F, Liu C, Zhou D, Zhang L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J Histochem Cytochem. 2016;64:157–67. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, et al. Hedgehog Controls Hepatic Stellate Cell Fate by Regulating Metabolism. Gastroenterology. 2012;143:1319–29. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papa S, Bubici C. Linking apoptosis to cancer metabolism: Another missing piece of JuNK. Mol Cell Oncol. 2016;3:e1103398. doi: 10.1080/23723556.2015.1103398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aminzadeh A. Protective effect of tropisetron on high glucose induced apoptosis and oxidative stress in PC12 cells: Roles of JNK, P38 MAPKs, and mitochondria pathway. Metab Brain Dis. 2017;32:819–26. doi: 10.1007/s11011-017-9976-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee SY, Lee J, Lee H, Kim B, Lew J, Baek N, et al. MicroRNA134 Mediated Upregulation of JNK and Downregulation of NFkB Signalings Are Critically Involved in Dieckol Induced Antihepatic Fibrosis. J Agric Food Chem. 2016;64:5508–14. doi: 10.1021/acs.jafc.6b01945. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Zheng Y, Zhang H, Sun H. Targeting cancer cell metabolism: The combination of metformin and 2-Deoxyglucose regulates apoptosis in ovarian cancer cells via p38 MAPK/JNK signaling pathway. Am J Transl Res. 2016;8:4812–21. [PMC free article] [PubMed] [Google Scholar]
- 22.Chan Tung KW, Mansouri S, Hudak KA. Expression of pokeweed antiviral protein in mammalian cells activates c-Jun NH2-terminal kinase without causing apoptosis. Int J Biochem Cell Biol. 2008;40:2452–61. doi: 10.1016/j.biocel.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Zhu C, Chen X, Li Y, Gao R, Wu Q. Pokeweed antiviral protein down-regulates Wnt/β-catenin signalling to attenuate liver fibrogenesis in vitro and in vivo. Dig Liver Dis. 2011;43:559–66. doi: 10.1016/j.dld.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Tu W, Ye J, Wang ZJ. Embryonic liver fordin is involved in glucose glycolysis of hepatic stellate cell by regulating PI3K/Akt signaling. World J Gastroenterol. 2016;22:8519–27. doi: 10.3748/wjg.v22.i38.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cisternas P, Salazar P, Silva-Álvarez C, Barros LF, Inestrosa NC. Activation of Wnt Signaling in Cortical Neurons Enhances Glucose Utilization through Glycolysis. J Biol Chem. 2016;291:25950–64. doi: 10.1074/jbc.M116.735373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo L, Qiao Y, Zhang L, Pan Q. Protective Role of Glucagon-Like Peptide-1 Against High-Glucose-Induced Endothelial Oxidative Damage. Medicine (Baltimore) 2015;94:e2055. doi: 10.1097/MD.0000000000002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delire B, Stärkel P, Leclercq I. Animal Models for Fibrotic Liver Diseases: What We Have, What We Need, and What Is under Development. J Clin Transl Hepatol. 2015;3:53–66. doi: 10.14218/JCTH.2014.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuman MG, Cohen LB, Nanau RM. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin Biochem. 2016;49:302–15. doi: 10.1016/j.clinbiochem.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Mak KM, Mei R. Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. Anat Rec (Hoboken) 2017 doi: 10.1002/ar.23567. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Ebrahimi H, Naderian M, Sohrabpour AA. New Concepts on Pathogenesis and Diagnosis of Liver Fibrosis; A Review Article. Middle East J Dig Dis. 2016;8:166–78. doi: 10.15171/mejdd.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ning C, Wang X, Gao S, Mu J, Wang Y, Liu S, et al. Chicory inulin ameliorates type 2 diabetes mellitus and suppresses JNK and MAPK pathways in vivo and in vitro. Mol Nutr Food Res. 2017;61(8) doi: 10.1002/mnfr.201600673. [DOI] [PubMed] [Google Scholar]
- 32.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–9. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Temkin V, Karin M. From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: The receptor-interacting protein 1 odyssey. Immunol Rev. 200;;20:8–21. doi: 10.1111/j.1600-065X.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Jin Q, Wu YL, Sun P, Jiang S, Zhang Y, et al. Tetrandrine regulates hepatic stellate cell activation via TAK1 and NF-κB signaling. Int Immunopharmacol. 2016;36:263–70. doi: 10.1016/j.intimp.2016.04.039. [DOI] [PubMed] [Google Scholar]