Abstract
In diffuse large B-cell lymphoma (DLBCL), the clinical and biological significance of concordant and discordant bone marrow (BM) involvement have not been well investigated. We evaluated 712 de novo DLBCL patients with front-line rituximab-containing treatment, including 263 patients with positive and 449 with negative BM status. Compared with negative BM disease, concordant BM adversely impacted overall and progression-free survival, probably independent of the International Prognostic Index (IPI) and cell-of-origin classification. Once BM is concordantly involved, poor prognosis was not associated with the extent of BM involvement. Conversely, patients with discordant BM showed favorable overall survival similar to stage I-II DLBCL. A BM-adjusted IPI, using three parameters: concordant BM involvement, age >60 years, and performance status >1, improves the risk stratification for DLBCL with positive BM. Intensive immunochemotherapy seemingly rendered survival benefit for patients with concordant BM, as did rituximab maintenance for the discordant BM group. Frequently revealing adverse clinical and molecular characteristics, patients with concordant BM demonstrated gene expression signatures relevant to tumor cell proliferation, migration, and immune escape. In conclusion, clinical and biological heterogeneity is seen in DLBCL with positive BM but concordant BM involvement represents a distinct subset with unfavorable gene signatures, high-risk clinicopathologic features, and poor prognosis.
Keywords: Bone marrow involvement, concordant bone marrow involvement, discordant bone marrow involvement, diffuse large B-cell lymphoma, rituximab
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoma and is highly heterogeneous in regard to clinical manifestations, biological features, and prognosis. The introduction of rituximab (R) combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) has resulted in improved outcome in DLBCL patients; however, variable prognosis is present, and identifying distinct subsets for prognostication and therapeutic decisions is essential.1–3
Approximately 11–34% of patients have bone marrow (BM) involvement when DLBCL is initially diagnosed.4–6 In most patients, BM is involved by large B-cell lymphoma (concordant disease), but a subset of patients have small cell low-grade lymphoma in the BM (discordant disease).4, 7–10 BM involvement by lymphoma is clinically recognized as advanced disease and contributes to higher International Prognostic Index (IPI) scores. The IPI, a powerful clinical tool for risk-stratification of DLBCL, is calculated by using five clinical parameters: age >60 years, Ann Arbor stage III–IV, Eastern Cooperative Oncology Group (ECOG) performance status (PS) >1, elevated serum lactate dehydrogenase (LDH), and involvement of more than one extranodal site.11, 12 Of interest, some studies have reported that the impact of concordant versus discordant BM involvement on overall survival (OS) and progression-free survival (PFS) was different.4, 7–10 Due to the limited number of enrolled patients with positive BM and the dissimilarity of design and inclusion criteria in earlier studies, the consensus regarding the prognostic impact of concordant versus discordant BM is controversial and needs to be validated in a larger series. Furthermore, whether differences between concordant and discordant BM involvement can be optimized in an adjusted IPI for risk stratification is unknown.
In addition to the IPI, cell-of-origin (COO) classification defined by gene expression profiling (GEP) or immunohistochemistry (IHC) surrogates has been widely adopted, which classifies DLBCL cases into germinal center B-cell-like (GCB) subtype and activated B-cell-like/non-GCB subtype, with the former associated with better survival.13–17 Meanwhile, several other biomarkers, including CD5, p53, MYC expression, MYC/BCL2 co-expression, TP53 mutation, BCL2, MYC rearrangement, and MYC/BCL2 double hit, have been implicated in mechanisms of disease and have been suggested as predictors of poor prognosis in DLBCL.18–24 Thus far, no large studies have explored the relationship between these high-risk pathologic features and the various types of BM involvement in DLBCL patients.
To address these equivocal questions and elucidate the role of BM involvement in DLBCL patients, we conducted a large study of de novo DLBCL treated with immunochemotherapy. We analyzed gene expression profiling (GEP) and molecular analyses to better characterize the pathologic features of concordant BM involvement.
Materials and Methods
Patients
The study cohort included 263 de novo DLBCL patients with positive BM involvement and 449 patients with negative BM. This study is part of the International DLBCL Consortium Program. All patients needed to meet the following prerequisites: they were treated with front-line rituximab-containing standard protocols; age 16 years or older with a confirmed diagnosis of DLBCL according to 2016 WHO criteria25 on pathology review; they underwent a pretreatment bilateral or unilateral posterior superior iliac crest BM biopsy (with both biopsy and aspiration); they had available BM slides for a central review in case of positive for lymphoma in BM. Patients were excluded if they had primary central nervous system (CNS), cutaneous, or mediastinal DLBCL, HIV infection, an identified past history of lymphoma, or another malignancy that was uncontrolled. The study were reviewed and approved by the institutional review boards of each participating center, and the overall study was approved by the institutional review board of the University of Texas MD Anderson Cancer Center.
Disease staging and treatment response of all patients were assessed by the Ann Arbor system26 and the Revised International Working Group response criteria27, respectively. A modification of the Ann Arbor classification defined stage I-II as limited DLBCL and stage III–IV as advanced DLBCL.28 The interpretation of TP53 mutations by gene sequencing, MYC and BCL2 rearrangements by fluorescence in situ hybridization analyses, and p53, CD5, BCL2, and MYC expression by IHC staining were based on published data.17, 20, 22, 23 A cut-off value for a high Ki-67 index was considered to be 70%. GEP of specimens involved by DLBCL was performed and the COO classification was analyzed by GEP and the IHC algorithm based on Choi and Visco/Young’s algorithm methods.17
Definition and classification of BM involvement
BM trephine biopsies, clot sections, and aspirate smears from DLBCL patients who were reported to have positive BM were centrally reviewed. Utilizing morphology and IHC, the types and extent of BM involvement were identified.9, 29–31 Concordant BM disease was defined by the involved BM area consisting mostly large non-cleaved DLBCL cells; discordant BM was defined by the involved BM consisting mostly small low-grade lymphoma cells. We defined extensive BM involvement at 25% and higher replacement of the medullary space; less than 25% BM infiltration defined as limited/focal BM involvement.32 When bilateral BM specimens were available, determination of the extent of BM involvement depended on analysis of the sample with the greater degree of infiltration by lymphoma.
Statistical analyses
Clinicopathologic features were compared between the groups by using the independent samples t test for continuous variables and the χ2 test for categorical variables. PFS was calculated from the date of initial diagnosis to the time of recurrence, disease progression, or death from any cause. OS was measured from the date of initial diagnosis until death, regardless of the cause. Patients still alive were censored at the date of the last contact. PFS and OS were estimated by the Kaplan-Meier method and the log-rank test was used for comparison between groups. The Cox proportional hazard model was used for multivariate analysis to assess the independent effects of prognostic variables on survival. The data were analyzed by SPSS V.22.0 for Windows (SPSS Inc., Chicago, IL, USA). A two-sided P value of < .05 was considered statistically significant and multiple comparisons were assessed by Bonferroni correction.
Results
Patient characteristics
A total of 712 patients with DLBCL were enrolled in this study. 263 patients with BM positive for lymphoma consisted of 173 (65.8%) cases with concordant BM and 90 (34.2%) with discordant BM involvement. The remaining 449 patients had negative BM including 212 (47.2%) patients with advanced DLBCL (stage III–IV). Clinical and pathologic characteristics according to type of BM involvement were compared and summarized in Tables 1 and 2, respectively. Given that either concordant or discordant BM disease belongs to a category of advanced DLBCL, we considered advanced DLBCL but with negative BM as an independent group to exclude the influence of stage and highlight the role of BM involvement.
Table 1.
Clinical characteristics of patients grouped by type of BM involvement
| Characteristics | Negative BM (n = 449) No. (%) | Concordant BM (n = 173) No. (%) | Discordant BM (n = 90) No. (%) |
P value
|
Advanced DLBCL & Negative BM (n = 212) No. (%) |
P value
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Neg v Con | Neg v Dis | Con v Dis | Adv-Neg v Con | Adv-Neg v Dis | |||||
| Age (years) | |||||||||
|
| |||||||||
| Median | 62.6 | 63.0 | 63.0 | 0.841 | 0.715 | 0.838 | 62.0 | 0.950 | 0.814 |
|
| |||||||||
| Range | 16–89 | 25–91 | 31–89 | 17–89 | |||||
| Sex | |||||||||
|
| |||||||||
| Male | 267 (59.5) | 81 (46.8) | 54 (60.0) | 0.004 | 0.925 | 0.042 | 123 (58.0) | 0.029 | 0.749 |
|
| |||||||||
| Female | 182 (40.5) | 92 (53.2) | 37 (40.0) | 89 (42.0) | |||||
| IPI factors | |||||||||
|
| |||||||||
| Age >60 years | 255 (56.8) | 101 (58.4) | 51 (56.7) | 0.720 | 0.982 | 0.789 | 121 (57.1) | 0.911 | 0.948 |
|
| |||||||||
| LDH elevated | 240 (57.4) | 138 (80.2) | 34 (38.2) | <0.001 | 0.001 | <0.001 | 134 (66.0) | 0.003 | <0.001 |
|
| |||||||||
| Stage III–IV | 212 (47.9) | 173 (100.0) | 90 (100.0) | <0.001 | <0.001 | 1.000 | 212 (100.0) | 1.000 | 1.000 |
|
| |||||||||
| ECOG PS ≥2 | 53 (12.8) | 63 (36.6) | 10 (11.2) | <0.001 | 0.691 | <0.001 | 39 (19.7) | <0.001 | 0.078 |
|
| |||||||||
| Extranodal sites >1 | 63 (14.1) | 115 (66.5) | 55 (61.1) | <0.001 | <0.001 | 0.388 | 61 (28.9) | <0.001 | <0.001 |
|
| |||||||||
| IPI score | |||||||||
|
| |||||||||
| Low (0–1) | 173 (39.9) | 6 (3.5) | 11 (12.4) | <0.001 | <0.001 | <0.001 | 21 (10.2) | <0.001 | 0.861 |
|
| |||||||||
| Intermediate (2–3) | 214 (49.3) | 85 (49.4) | 58 (65.1) | 138 (67.4) | |||||
|
| |||||||||
| High (4–5) | 47 (10.8) | 81 (47.1) | 20 (22.5) | 46 (22.4) | |||||
|
| |||||||||
| Bulky mass ≥7cm | 108 (28.5) | 50 (30.1) | 28 (31.1) | 0.700 | 0.623 | 0.869 | 60 (32.8) | 0.586 | 0.781 |
|
| |||||||||
| B symptoms | 141 (32.4) | 100 (58.1) | 18 (20.0) | <0.001 | 0.02 | <0.001 | 97 (46.6) | 0.028 | <0.001 |
Abbreviations: Neg, negative BM involvement; Con, concordant BM involvement; Dis, discordant BM involvement; Adv-Neg, advanced DLBCL with negative BM involvement
Table 2.
Pathologic characteristics of patients grouped by type of BM involvement
| Characteristics | Negative BM (n =449) No. (%) | Concordant BM (n =173) No. (%) | Discordant BM (n =90) No. (%) |
P value
|
Advanced DLBCL & Negative BM (n =212) No. (%) |
P value
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Neg v Con | Neg v Dis | Con v Dis | Adv-Neg v Con | Adv-Neg v Dis | |||||
| COO | |||||||||
|
| |||||||||
| GCB | 233 (53.4) | 63 (51.6) | 50 (83.3) | 0.725 | <0.001 | <0.001 | 95 (46.3) | 0.354 | <0.001 |
|
| |||||||||
| Non-GCB | 203 (46.6) | 59 (48.4) | 10 (16.7) | 110 (53.7) | |||||
| Ki-67 index | |||||||||
|
| |||||||||
| ≥70 | 283 (65.4) | 91 (71.7) | 30 (54.5) | 0.185 | 0.115 | 0.025 | 143 (69.8) | 0.713 | 0.034 |
|
| |||||||||
| <70 | 150 (34.6) | 36 (28.3) | 25 (45.5) | 62 (30.2) | |||||
| CD5 expression | |||||||||
|
| |||||||||
| Positive | 17 (4.0) | 25 (19.1) | 6 (9.5) | <0.001 | 0.052 | 0.089 | 8 (3.9) | <0.001 | 0.081 |
|
| |||||||||
| Negative | 411 (96.0) | 106 (80.9) | 57 (90.5) | 196 (96.1) | |||||
| BCL2 expression | |||||||||
|
| |||||||||
| positive | 247 (57.4) | 92 (75.4) | 40 (87.0) | <0.001 | <0.001 | 0.104 | 127 (62.6) | 0.017 | 0.001 |
|
| |||||||||
| negative | 183 (42.6) | 30 (24.6) | 6 (13.0) | 76 (37.4) | |||||
| MYC expression | |||||||||
|
| |||||||||
| Positive | 132 (31.3) | 28 (58.3) | NA | 0.001 | NA | NA | 70 (35.7) | 0.004 | NA |
|
| |||||||||
| negative | 290 (68.7) | 20 (41.7) | NA | 126 (64.3) | |||||
| BCL2/MYC DPL | |||||||||
|
| |||||||||
| positive | 85 (19.9) | 19 (31.1) | 0 (0.0) | 0.045 | 0.266 | 0.139 | 47 (23.7) | 0.246 | 0.214 |
|
| |||||||||
| negative | 342 (80.1) | 42 (68.9) | 5 (100.0) | 157 (79.3) | |||||
| BCL2 rearrangement | |||||||||
|
| |||||||||
| positive | 65 (18.5) | 20 (28.6) | 6 (26.1) | 0.056 | 0.370 | 0.818 | 38 (22.8) | 0.342 | 0.722 |
|
| |||||||||
| negative | 286 (81.5) | 50 (71.4) | 17 (73.9) | 129 (77.2) | |||||
| MYC rearrangement | |||||||||
|
| |||||||||
| positive | 31 (11.0) | 21 (34.4) | 1 (16.7) | <0.001 | 0.662 | 0.377 | 17 (12.1) | <0.001 | 0.736 |
|
| |||||||||
| negative | 251 (89.0) | 40 (65.6) | 5 (83.3) | 124 (87.9) | |||||
| BCL2/MYC DHL | |||||||||
|
| |||||||||
| Positive | 9 (2.6) | 9 (12.9) | 0 (0.0) | <0.001 | 0.443 | 0.077 | 4 (2.4) | 0.001 | 0.460 |
|
| |||||||||
| negative | 336 (97.4) | 61 (87.1) | 22 (100.0) | 161 (97.6) | |||||
| p53 expression | |||||||||
|
| |||||||||
| positive | 129 (35.1) | 19 (45.2) | NA | 0.193 | NA | NA | 64 (37.4) | 0.352 | NA |
|
| |||||||||
| negative | 239 (64.9) | 23 (54.8) | NA | 107 (62.6) | |||||
| TP53 mutation | |||||||||
|
| |||||||||
| positive | 79 (20.8) | 10 (23.3) | NA | 0.707 | NA | NA | 40 (22.9) | 0.956 | NA |
|
| |||||||||
| negative | 301 (79.2) | 33 (76.7) | NA | 135 (77.1) | |||||
Abbreviations: Neg, negative BM involvement; Con, concordant BM involvement; Dis, discordant BM involvement; Adv-Neg, advanced DLBCL with negative BM involvement; NA, not available
The median age and the proportion of elderly patients (>60 years) were similar for those with negative BM, including patients with advanced DLBCL, as well as for patients with concordant or discordant BM involvement. The concordant BM group contained more females than males, which was significantly different from the other three groups. The concordant BM group was more likely to have aggressive clinical features such as elevated LDH, poor PS, B symptoms, and high IPI scores than the negative BM group, even compared with advanced DLBCL patients with negative BM. In contrast, patients with discordant BM were less likely to have an elevated LDH or B symptoms. Compared with patients with negative BM (including advanced DLBCL patients), the concordant BM group was more likely to express CD5 and MYC, harbor MYC rearrangement, co-express MYC and BCL2 (double positive lymphoma; DPL), and to be MYC/BCL2 double-hit lymphoma (DHL). BCL2 overexpression was significantly more common in patients with concordant or discordant BM disease than in patients with negative BM. The proportion of GCB subtype was higher in the discordant BM group than in the negative BM group, concordant BM group, or advanced DLBCL with negative BM (83.3% vs 53.4%, 51.6%, 46.3%, respectively; P < .001).
Positive BM involvement characteristics
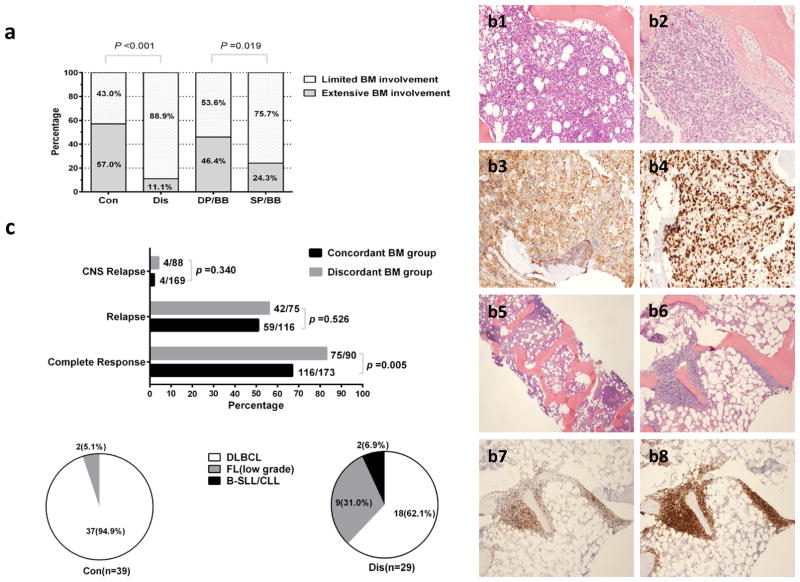
Concordant BM disease was more likely to be associated with extensive BM involvement than discordant disease was (57.0% vs 11.1%, P < 0.001; Figure 1a). 199 of 263 (75.7%) patients with BM positive for lymphoma underwent bilateral BM biopsies. In these patients, 125 (62.8%) were bilaterally positive and 74 (37.2%) were positive on a single side; patients with BM positive on a single side more often had limited BM involvement (75.7% vs 53.6% for bilateral involvement, P = .019; Figure 1a). In 90 patients with discordant BM involvement, precise histopathologic subtypes of BM samples of 78 patients were confirmed: 54 (69.2%) of 78 patients had low-grade follicular lymphoma (FL), 14 (17.9%) small lymphocytic leukemia (SLL)/chronic lymphocytic lymphoma (CLL), and 10 (12.8%) marginal zone lymphoma (MZL). Representative images of BM involvement are shown in Figure 1(b1–b8).
Figure 1. Characteristics of patients with BM positive for lymphoma.
(a) Percentage of limited vs extensive BM involvement in concordant (Con) vs discordant (Dis) groups and double-positive BM involvement in bilateral BM biopsies (DP/BB) vs single-positive BM involvement in bilateral BM biopsies (SP/BB) groups. (b) A representative illustration of morphologic and immunophenotypic profiling of BM specimens in Con and Dis groups. Two DLBCL patients with 70% (b1) and 100% (b2) BM involvement; DLBCL cells are positive for CD20 (b3) with proliferation index Ki-67 (b4) at 80%; one FL case with paratrabecular involvement (b5, b6). FL cells are positive for PAX-5 (b7) and BCL2 (b8). Magnification x20 and x40. (c) Percentage of complete response, relapse, and CNS relapse in Con vs Dis groups. Pie charts showing the proportions of different pathologic subtypes of second biopsy when relapse occurs in Con vs Dis groups.
In this study, most of patients were treated with front-line R-CHOP; 40 (23.1%) of 173 patients with concordant BM were initially treated with intensive regimens (R-Hyper-CVAD/MA [rituximab, hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine] or R-DA-EPOCH [rituximab, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin]); 14 (15.6%) of 90 patients with discordant BM lymphoma received rituximab maintenance for at least one year after R-CHOP therapy. CNS prophylaxis (i.e. intrathecal methotrexate and/or intravenous high-dose methotrexate with or without cytarabine) was given to 107 (61.8%) of patients with concordant BM and 13 (14.4%) of patients with discordant BM. As shown in Figure 1c, Complete response was achieved in 75 patients (83.3%) with discordant BM lymphoma, which was a higher rate than that achieved in 116 patients (67%) with concordant BM (P = .005). Nevertheless, there was no significant difference in the probability of disease relapse (P = .526) and CNS relapse (P = .340) between patients with discordant versus concordant BM involvement. In addition, compared with patients with negative BM, concordant BM disease had a similar CNS relapse rate (2.4% vs 1.6%, P = .503); no significant difference of CNS relapse rate (4.5% vs 1.6%, P = .072) was seen in patients with discordant BM involvement. 39 patients with concordant BM and 29 patients with discordant BM lymphoma underwent a second non-BM tissue biopsy when disease relapsed; the biopsy specimen was more likely to show low grade lymphoma in the discordant BM group than in the concordant BM group (37.9% vs 5.1%, P < .001).
Prognostic significance of BM involvement
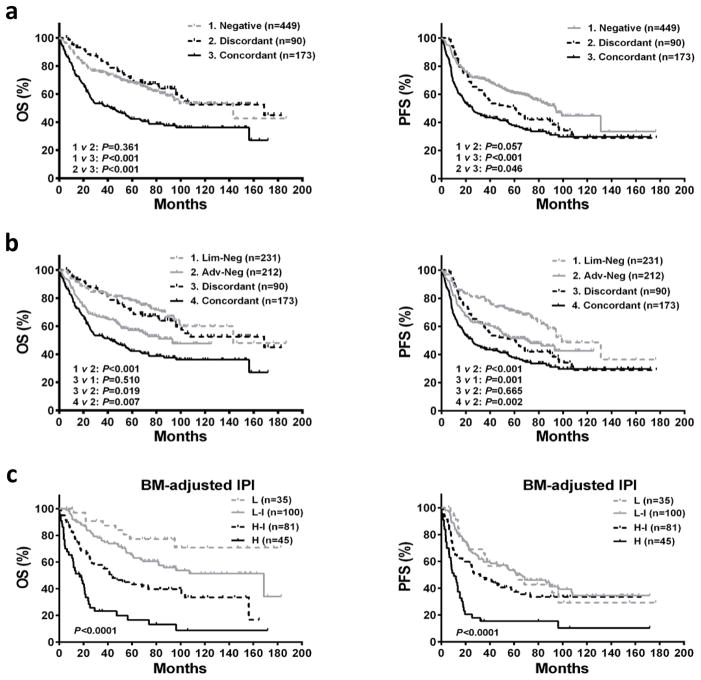
At the time of analysis, the median follow-up duration was 51.1 months (range: 0.23–186.7). The survival of patients with concordant BM involvement were significantly worse than not only patients with negative BM (5-year OS: 42.3% vs 67.7%, P < .001; 5-year PFS: 37.2% vs 60.6%, P < .001) but also advanced DLBCL with negative BM (5-year OS: 42.3% vs 57.2%, P = .007; 5-year PFS: 37.2% vs 51.5%, P = .002) (Figure 2a, 2b). As for patients with discordant BM involvement, the 5-year OS (70.0% vs 77.6%, P = .510) was comparable to patients with limited DLBCL (stage I–II), the 5-year PFS was similar to advanced DLBCL with negative BM (49.7% vs 51.5%, P = .665) (Figure 2b).
Figure 2. Survival curves for OS and PFS according to type of BM disease.
(a) Comparisons of OS and PFS among negative, discordant, and concordant BM involvement groups. (b) Comparisons of OS and PFS among limited DLBCL with negative BM involvement (Lim-Neg), advanced DLBCL with negative BM involvement (Adv-Neg), discordant BM, and concordant BM groups. (c) The BM-adjusted IPI for risk stratification in patients with positive BM involvement. L, low risk; L-I, low-intermediate risk; H-I, high-intermediate risk; H, high risk.
Prognostic significance of BM involvement incorporating IPI and COO
Multivariate analysis incorporating the IPI and COO subtypes was performed to illustrate the independent prognostic significance of different types of BM involvement (Supplementary Table S1). Concordant BM lymphoma retained a negative prognostic impact on OS and PFS, independent of the IPI and COO subtypes (OS: RR = 1.396, P = .032; PFS: RR = 1.497, P = .006). In contrast, discordant BM lymphoma had no prognostic effect on OS and PFS in the multivariate analysis. When examining the outcome of patients with high IPI scores or in non-GCB patients, the prognostic value of concordant lymphoma in the BM was evident (Supplementary Figure S1).
Development of BM-adjusted IPI
The univariate analysis of baseline features in 263 patients with concordant or discordant BM involvement revealed that age >60 years, PS >1, elevated LDH, B symptoms, concordant BM involvement, high Ki-67, and non-GCB subtype adversely affected OS, whereas extranodal sites >1 and bulky mass did not (Supplementary Figure S2). Following multivariate analysis, age >60 years, PS >1, and concordant BM involvement were independent predictors of OS (Table 3). Thereby, a BM-adjusted IPI for patients with positive BM was constructed by using three clinical parameters, each equal to one point; four risk categories were generated: low (0 point), low-intermediate (1 point), high-intermediate (2 points), and high (3 points). Based on this risk-stratification model, patients assigned to the low-risk group had relatively good outcomes (5-year OS: 77.3%), and high-risk patients experienced extremely poor outcomes (5-year OS: 16.5%)(Figure 2c). The similar results were observed when we randomly divided 263 patients with positive BM into the training (n=132) and validation (n=131) sets (Supplementary Figure S3).
Table 3.
Prognostic factors of OS according to multivariate selection in patients with positive BM involvement
| Positive BM involvement (n =263) | RR | 95% CI | P value | Score |
|---|---|---|---|---|
| Age >60 years | 2.376 | 1.432–3.943 | 0.001 | 1 |
|
| ||||
| Elevated LDH | 1.239 | 0.667–2.303 | 0.498 | |
|
| ||||
| ECOG PS ≥2 | 1.757 | 1.027–3.005 | 0.040 | 1 |
|
| ||||
| B symptoms | 1.549 | 0.914–2.626 | 0.104 | |
|
| ||||
| Concordant BMI | 1.997 | 1.079–3.696 | 0.028 | 1 |
|
| ||||
| Non-GCB | 0.925 | 0.553–1.546 | 0.766 | |
| High Ki-67 | 1.469 | 0.810–2.665 | 0.206 | |
RR indicates relative risk.
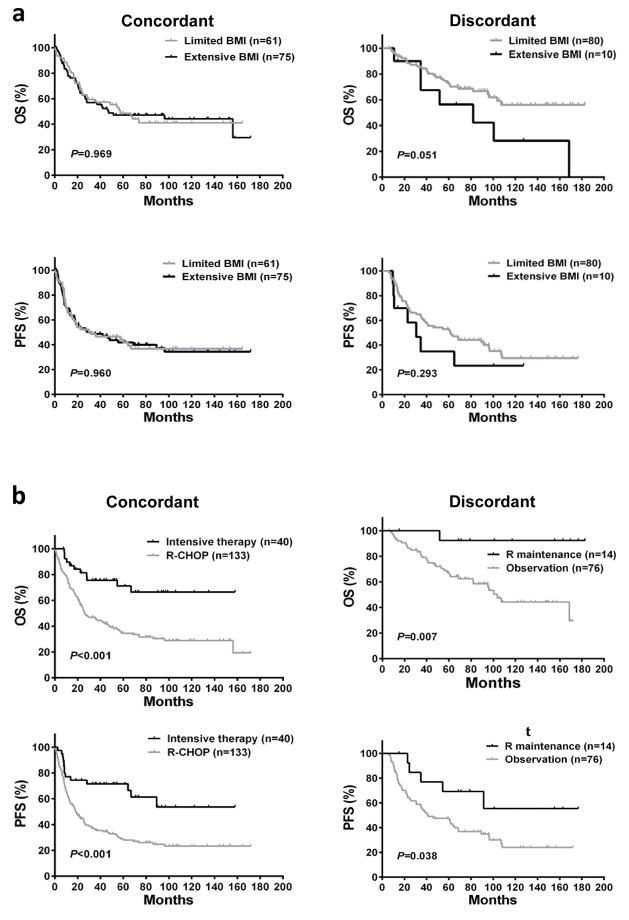
Prognostic significance of the extent of BM involvement and treatment in patients with concordant versus discordant BM disease
Extensive BM involvement compared with limited BM lymphoma did not lead to worse OS (P = .969) or PFS (P = .960) in the concordant BM subset (Figure 3a). However, in patients with discordant BM disease, limited BM involvement seemingly indicated a better OS (71.7% vs 50.0%, P = .051; Figure 3a). For concordant BM group, patients treated with intensive regimens had better 5-year OS (71.1% vs 34.5%, P < .001) and PFS (66.4% vs 28.0%, P < .001) than patients accepting R-CHOP (Figure 3b); after controlling for remaining two BM-adjusted IPI factors (age and PS), the survival benefit from intensive chemotherapy still existed (Supplementary Table S2). In patients with discordant BM, rituximab maintenance brought a 5-year OS (92.3% vs 65.8%, P = .007) and PFS (69.2% vs 46.1%, P = .038) benefit in comparison with observation after R-CHOP (Figure 3b).
Figure 3. Survival curves for OS and PFS according to the extent of BM involvement and treatment in concordant and discordant BM groups.
Limited BMI indicates limited BM involvement; Extensive BMI, extensive BM involvement; R maintenance, rituximab maintenance after induction therapy; Observation, observation after induction therapy.
Prognostic significance of biomarkers in concordant versus negative BM groups
We compared the prognostic significance of these adverse biomarkers independently and in combination summarized in Table 2 in concordant versus negative BM groups and estimated the prognostic power of concordant BM lymphoma relative to these biomarkers. Non-GCB/ABC, high Ki-67, CD5, p53 expression, and MYC/BCL2 DPL predicted significant or borderline inferior survival in both patients with concordant and negative BM, whereas BCL2, MYC expression, BCL2, MYC rearrangement, and TP53 mutation lost their prognostic power in concordant BM group. Concordant BM disease remained a significant negative predictor in each of above-mentioned biomarker-positive subsets except in MYC/BCL2 DHL subgroup (Supplementary Figure S4).
Gene expression signature of concordant BM involvement
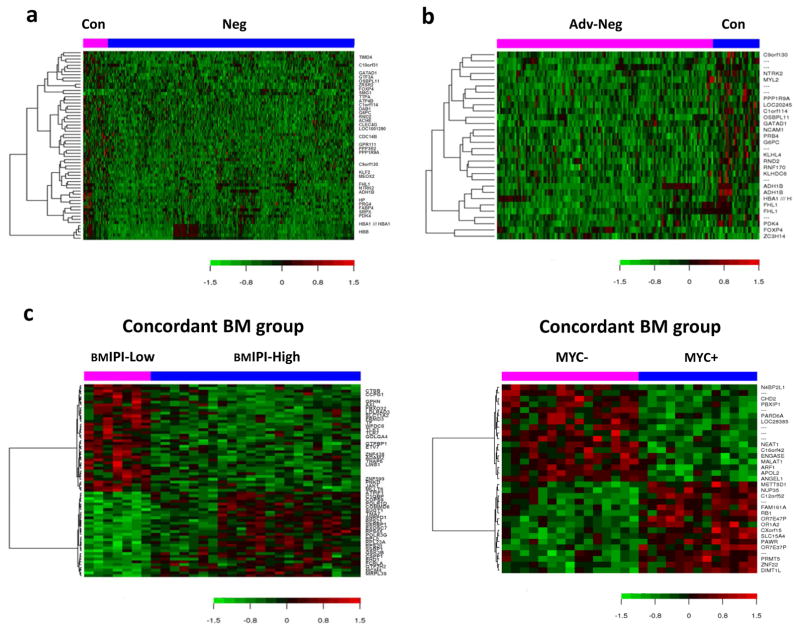
To unfold the potential molecular basis underlying the aggressive clinical course of patients with concordant BM, we profiled and compared the GEP results of DLBCL with concordant versus negative BM involvement (Figure 4a). 34 genes were differentially expressed, including 32 genes up-regulated and only 2 genes down-regulated in the concordant BM group (false discovery rate =0.1; P < .001; Table 4a). Among the 32 up-regulated genes, 10 genes were involved in cell metabolism; 6 participated in signaling pathways and cell cycle regulation; RND2, GPR111, PPP1R9A, and PRG4 encoded various proteins playing a key role in cellular adhesion or cytoskeletal reorganization; GATAD1, GTF3A, ZRSR2, SRPX, and MEOX2 were transcriptional regulators; and TIMD4, CLEC4G, and HP linked with immune response. We further compared the GEP results between DLBCL in patients with concordant BM involvement and patients with advanced DLBCL without BM involvement (Figure 4b). 17 genes differentially expressed were picked out (false discovery rate =0.15; P < .001; Table 4b); 12 (70.6%) of which were also identified in the former comparison.
Figure 4. GEP heatmap in concordant BM involvement group.
(a) GEP comparison between concordant and negative BM involvement groups. (b) GEP comparison between concordant BM group and advanced DLBCL with negative BM involvement group. (c) GEP comparison between high and low BM-adjusted IPI subsets in concordant BM group. GEP comparison between MYC+ and MYC− subsets in concordant BM group. Con indicates concordant BM involvement; Neg, negative BM involvement; Adv-Neg, advanced DLBCL with negative BM involvement; BMIPI-Low, low BM-adjusted IPI; BMIPI-High, high BM-adjusted IPI.
Table 4.
Genes differentially expressed in Con vs Neg and Con vs Adv-Neg
| Gene functional categories | Up-regulated genes
|
Down-regulated genes
|
||
|---|---|---|---|---|
| No. of genes | Representative genes | No. of genes | Representative genes | |
| (a) Con vs Neg | ||||
|
| ||||
| Metabolism | 10 | ATP4B, TTPA, OSBPL11, G6PC, ADH1B, FABP4, PDK4, ACHE, HBA1///HBA2, HBB | 0 | |
|
| ||||
| Signaling pathways, cell cycle regulation | 6 | DAB1, PPP3R2, NTRK2, CDC14B, KLF2,FHL1 | 0 | |
|
| ||||
| Extracellular matrix, adhesion and cytoskeletal organization | 4 | RND2, GPR111, PPP1R9A, PRG4 | 0 | |
|
| ||||
| Transcriptional regulation | 5 | GATAD1, GTF3A, ZRSR2, SRPX, MEOX2 | 2 | FOXP4, SMG1 |
|
| ||||
| Immune response | 3 | TIMD4, CLEC4G, HP | 0 | |
|
| ||||
| Others (including ncRNA, unknown) | 4 | C10orf31, C90rf130, LOC100129029, C1orf114 | 0 | |
| (b) Con vs Adv-Neg | ||||
|
| ||||
| Metabolism | 4 | OSBPL11*, G6PC*, ADH1B*, PDK4*, HBA1///HBA2* | 0 | |
|
| ||||
| Signaling pathways, cell cycle regulation | 3 | RNF170, NTRK2*, FHL1* | 0 | |
|
| ||||
| Extracellular matrix, adhesion and cytoskeletal organization | 6 | MYL2, NCAM1, KLHL4, PPP1R9A*, RND2*, KLHDC6 | 0 | |
|
| ||||
| Transcriptional regulation | 1 | GATAD1* | 2 | FOXP4*, ZC3H14 |
|
| ||||
| Immune response | 0 | 0 | ||
|
| ||||
| Others (including ncRNA, unknown) | 3 | PRB4, C90rf130*, C1orf114*, | 0 | |
Abbreviations: Con, concordant BM involvement; Neg, negative BM involvement; Adv-Neg, advanced DLBCL with negative BM involvement; ncRNA, non-coding RNA.
Genes differentially expressed in both Con vs Neg and Con vs Adv-Neg
We compared the GEP results between low (score: 1) and high BM-adjusted IPI (score: 2 or 3) subgroups and between MYC+ and MYC- subgroups with concordant BM involvement (Figure 4c). A total of 56 genes were differentially expressed in the former comparison and 26 differentially expressed genes were determined in the latter. Information about these genes is listed in Supplementary Table S3 and S4.
Discussion
In the current clinical practice, positive BM involvement (whether concordant or discordant) in DLBCL is generally recognized as high-risk advanced disease and is treated with the same immunochemotherapy regimen.33, 34 This study, enrolling the largest number of de novo DLBCL patients with positive BM involvement to date, allowed valuable and effective analysis and confirmed that there is heterogeneity in the positive BM cohort.
It is well-known that patients with concordant BM involvement have a poor prognosis; however, the impact of discordant BM on the outcome of DLBCL patients remains controversial and even contradictory.7, 8, 35 Our study showed distinct observations: the OS of patients with discordant BM involvement unexpectedly was comparable to that of patients with limited DLBCL; however, PFS remained poor, similar to that of advanced DLBCL with negative BM. Moreover, we found that concordant BM involvement demonstrated even poorer OS and PFS than did advanced DLBCL but with negative BM. These findings further emphasize the worse outcome of DLBCL patients with concordant BM and the different prognosis of patients with concordant versus discordant BM involvement. In addition, we noted that the difference in PFS curves between discordant and concordant BM groups seemingly disappeared at 10 years later; based on the second biopsy pathology, patients with discordant BM were more likely to have indolent or low-grade lymphoma recurrence when bone marrow was involved. These information are useful to help clinicians to design the BM status-based disease surveillance strategies.
Compared with the negative BM group, which has the largest proportion of low IPI scores, concordant BM large cell lymphoma is associated with a higher frequency of high IPI scores; patients with discordant BM involvement more often have intermediate IPI scores. This difference is attributed to groups’ differing probabilities of having poor-risk clinical features encompassed by the IPI. Along with the IPI, the COO classification is widely accepted as a biological predictor in clinical practice. The results of our study showed that there is a similar proportion of the non-GCB subtype between concordant and negative BM groups and a significantly greater proportion of the GCB subtype in the discordant BM group; these findings are not in agreement with those of Park et al7 of Korea, who observed a greater frequency of the non-GCB subtype in patients with concordant BM involvement. The discrepant results may be explained by the variation in ethnic groups and the size of the two studies. Although different types of BM involvement have a significant correlation with different IPI scores and COO subtypes, concordant BM involvement provides additional prognostic information beyond that conferred by the IPI and COO classification.
Considering the independent prognostic significance of concordant BM involvement, current IPI systems, including the standard IPI, the revised IPI, and the NCCN-IPI, have an inevitable defect, especially for DLBCL patients with a positive BM, for whom they equalize concordant and discordant BM involvement. Consequently, we used multivariate analysis in the context of patients with positive BM lymphoma to develop a BM-adjusted IPI, which includes three independent prognostic factors: concordant BM involvement, age >60 years, and PS >1; extranodal sites >1 and elevated LDH that are included in the current IPI systems are excluded because they have no independent prognostic significance. The BM-adjusted IPI is easy to use, is reasonable for DLBCL patients with positive BM involvement, and is potent in distinguishing the four risk groups.
In this study, extensive BM involvement was more often seen with concordant than with discordant BM disease and limited BM involvement was more likely in patients with unilateral involvement by lymphoma. Similar results were reported by Chuang et al4 in the pre-rituximab era, despite the different cut-off values for distinguishing extensive from limited BM involvement. Interestingly, when we explored the prognostic significance of the extent of BM involvement in the concordant BM group, patients with limited BM involvement had the same poor survival as did those with extensive BM disease; however, in the discordant BM group, patients with limited BM involvement had a significant trend for longer survival than did those with extensive BM involvement. These phenomena illustrate that 1) the extent of BM involvement should be considered as one of the key points of differential diagnosis between concordant and discordant BM disease; 2) the importance of bilateral bone marrow biopsies, as reported by Juneja et al36, should be emphasized once again because unilateral biopsies might “miss” positive BM involvement in some patients, in particular those with limited BM involvement; and 3) the prognostic significance of the extent of BM involvement in different types of BM disease may be divergent.
Besides more often having poor-risk clinical features, the concordant BM group has an increased probability for some unfavorable molecular characteristics, which may contribute to the dismal outcome of patients with concordant BM. Interestingly, concordant BM disease remained an adverse predictor in almost all of biomarker-positive subsets, whereas only part of frequently used biomarkers retained their prognostic significance for patients with concordant BM, as did for patients with negative BM. These findings suggest that concordant BM involvement may not be entirely surrogate for these molecular features, and other possible mechanisms to induce concordant BM involvement may underscore the adverse biological events that should not be neglected. Through the GEP analysis in this study, some differential expression genes were revealed in the concordant BM group: high expression level of metabolism-related genes and transcription-activating genes may reflect a high proliferation potency of lymphoma cells; although genes associated with cellular adhesion or migration have not often been described in lymphoma, such a signature in the metastases of solid tumors is well-established;37 TIMD4 is a member of the T-cell immunoglobulin and mucin domain gene family, which plays a critical role in immunoregulation, and a high level of TIMD4 has been reported as a negative regulator of antitumor immunity.38 Therefore, a potential molecular basis underlying the development of concordant BM disease may be related to tumor proliferation, cellular adhesion or migration, and immune tolerance or escape.
Kremer et al39 reported that a clonal relationship between the low-grade infiltrates in the BM and DLBCL was confirmed in the two-thirds of patients with discordant BM disease, with the remaining one-third exhibiting different clones as shown by comparatively analyzing IGH and BCL2 rearrangements. In our study, most patients with discordant BM disease had low-grade FL in their BM samples, and displayed the hallmark of DLBCL transformed from FL that the proportions of GCB subtype and BCL2 overexpression are significantly higher than in pure de novo DLBCL.40 These data further support the hypothesis that discordant BM disease is considered as either an occult indolent B-cell lymphoma from which DLBCL transformed or coincident indolent B-cell lymphoma in DLBCL.
Some authors have recommended intensive immunochemotherapy as the initial therapy for high-risk DLBCL, and some specific dose-intensive approaches such as R-Hyper-CVAD/MA or R-DA-EPOCH could partly conquer the inferior prognosis caused by MYC translocation, MYC/BCL2 co-expression, and MYC/BCL2 double hit.41–44 We found that intensive regimens seemingly resulted in longer survival than did R-CHOP for patients with concordant BM. Also, likely due to the above hypothesis for discordant BM involvement, the survival of this group appeared to be prolonged by rituximab maintenance, just as this strategy improved outcome in advanced indolent B-cell lymphoma.45, 46 It may be appropriate for patients with different types of BM disease to accept precision treatment strategies, whereas this assumption needs further study in future prospective clinical trials.
Although former studies have reported positive BM involvement associated with an increased likelihood of CNS relapse in DLBCL, universal application of CNS prophylaxis is not justified.47 Sehn et al10 reported that without routine CNS prophylaxis, concordant BM involvement was a significant risk factor for CNS relapse and there was a trend toward a slightly higher rate of CNS relapse in patients with discordant BM. However, our study showed different observation in which the frequency of CNS relapse was not escalated in patients with concordant BM disease, most of whom had received CNS prophylaxis. Therefore, the difference in the two studies suggests CNS prophylaxis may reduce the likelihood of CNS relapse.
In conclusion, this retrospective study provides a comprehensive summary of clinical and biological features in DLBCL with BM involvement in the rituximab era. Positive BM infiltration in DLBCL represents a heterogeneous group of disorders. The BM-adjusted IPI for patients with positive BM is an effective, valuable and potent prognostic model for risk stratification. Our findings suggest that DLBCL with concordant BM involvement constitutes a distinct subset with unfavorable gene expression signatures, high-risk clinicopathologic features, and poor prognosis.
Supplementary Material
Acknowledgments
This work was supported by National Cancer Institute/National Institutes of Health grants R01CA138688, R01CA187415 and 1RC1CA146299 to YL and KHY. This work was also partially supported by National Cancer Institute and National Institutes of Health grants P50CA136411 and P50CA142509, and by MD Anderson’s Cancer Center Support Grant CA016672. ZY and LD are the recipients of the Hematology/Oncology Scholarship Award. KHY is also supported by The University of Texas MD Anderson Cancer Center Institutional Research and Development Fund, an Institutional Research Grant Award, an MD Anderson Cancer Center Lymphoma Specialized Programs on Research Excellence (SPORE) Research Development Program Award, an MD Anderson Cancer Center Myeloma SPORE Research Development Program Award, a Gundersen Lutheran Medical Foundation Award, the University Cancer Foundation via the Sister institution network Fund at The University of Texas MD Anderson Cancer Center and is partially supported by grants from the National Cancer Institute/National Institutes of Health (P50CA136411 and P50CA142509).
Footnotes
Authorship
Conception, design and writing: ZY, KHY. Research performance: ZY, LD, ZYX-M, GCM, AT, YL, KHY. Provision of study thought, materials, key reagents and technology: ZY, LD, ZYX-M, GCM, PJ, AT, CV, GB, JW, KD, AO, EDH, JH, MP, AJMF, MBM, JNW, MAP, JF, YL, YS, RZO, HK, LJM, YL, JC, KHY. Collection and assembly of data under approved IRB and Material Transfer Agreement: ZY, LD, ZYX-M, PJ, AT, CV, GB, JW, KD, AO, EDH, JH, MP, AJMF, MBM, JNW, MAP, JF, YL, YS, YL, KHY. Data analysis and interpretation: ZY, LD, ZYX-M, GCM, LJM, YL, JC, KHY. Manuscript editing: ZY, LD, ZYX-M, AT, LJM, YL, JC, KHY.
Final approval of manuscript: All authors.
Conflicts of interest disclosure
KHY receives research support from Roche Molecular System, Gilead Sciences Pharmaceutical, Seattle Genetics, Dai Sanyo Pharmaceutical, Adaptive Biotechnology, Incyte Pharmaceutical, and HTG Molecular Diagnostics.
References
- 1.Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. The Lancet Oncology. 2011 Oct;12(11):1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010 Sep 23;116(12):2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaidya R, Witzig TE. Prognostic factors for diffuse large B-cell lymphoma in the R(X)CHOP era. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014 Nov;25(11):2124–2133. doi: 10.1093/annonc/mdu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung R, Lai R, Wei P, Lee J, Hanson J, Belch AR, et al. Concordant but not discordant bone marrow involvement in diffuse large B-cell lymphoma predicts a poor clinical outcome independent of the International Prognostic Index. Blood. 2007 Aug 15;110(4):1278–1282. doi: 10.1182/blood-2007-01-070300. [DOI] [PubMed] [Google Scholar]
- 5.Campbell J, Seymour JF, Matthews J, Wolf M, Stone J, Juneja S. The prognostic impact of bone marrow involvement in patients with diffuse large cell lymphoma varies according to the degree of infiltration and presence of discordant marrow involvement. European journal of haematology. 2006 Jun;76(6):473–480. doi: 10.1111/j.1600-0609.2006.00644.x. [DOI] [PubMed] [Google Scholar]
- 6.Hodges GF, Lenhardt TM, Cotelingam JD. Bone marrow involvement in large-cell lymphoma. Prognostic implications of discordant disease. American journal of clinical pathology. 1994 Mar;101(3):305–311. doi: 10.1093/ajcp/101.3.305. [DOI] [PubMed] [Google Scholar]
- 7.Park MJ, Park SH, Park PW, Seo YH, Kim KH, Seo JY, et al. Prognostic impact of concordant and discordant bone marrow involvement and cell-of-origin in Korean patients with diffuse large B-cell lymphoma treated with R-CHOP. Journal of clinical pathology. 2015 Sep;68(9):733–738. doi: 10.1136/jclinpath-2014-202656. [DOI] [PubMed] [Google Scholar]
- 8.Song MK, Chung JS, Lee JJ, Yang DH, Kim IS, Shin DH, et al. High Ki-67 expression in involved bone marrow predicts worse clinical outcome in diffuse large B cell lymphoma patients treated with R-CHOP therapy. International journal of hematology. 2015 Feb;101(2):140–147. doi: 10.1007/s12185-014-1719-3. [DOI] [PubMed] [Google Scholar]
- 9.Shim H, Oh JI, Park SH, Jang S, Park CJ, Huh J, et al. Prognostic impact of concordant and discordant cytomorphology of bone marrow involvement in patients with diffuse, large, B-cell lymphoma treated with R-CHOP. Journal of clinical pathology. 2013 May;66(5):420–425. doi: 10.1136/jclinpath-2012-201158. [DOI] [PubMed] [Google Scholar]
- 10.Sehn LH, Scott DW, Chhanabhai M, Berry B, Ruskova A, Berkahn L, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Apr 10;29(11):1452–1457. doi: 10.1200/JCO.2010.33.3419. [DOI] [PubMed] [Google Scholar]
- 11.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. The New England journal of medicine. 1993 Sep 30;329(14):987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 12.Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 May 10;28(14):2373–238. doi: 10.1200/JCO.2009.26.2493. [DOI] [PubMed] [Google Scholar]
- 13.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000 Feb 3;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 14.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004 Jan 1;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 15.Choi WW, Weisenburger DD, Greiner TC, Piris MA, Banham AH, Delabie J, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009 Sep 1;15(17):5494–5502. doi: 10.1158/1078-0432.CCR-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, Gascoyne RD, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 Jan 10;29(2):200–207. doi: 10.1200/JCO.2010.30.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visco C, Li Y, Xu-Monette ZY, Miranda RN, Green TM, Li Y, et al. Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Leukemia. 2012 Sep;26(9):2103–2113. doi: 10.1038/leu.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iqbal J, Meyer PN, Smith LM, Johnson NA, Vose JM, Greiner TC, et al. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011 Dec 15;17(24):7785–7795. doi: 10.1158/1078-0432.CCR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012 Oct 1;30(28):3460–3467. doi: 10.1200/JCO.2011.41.4342. [DOI] [PubMed] [Google Scholar]
- 20.Hu S, Xu-Monette ZY, Tzankov A, Green T, Wu L, Balasubramanyam A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013 May 16;121(20):4021–4031. doi: 10.1182/blood-2012-10-460063. quiz 4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valera A, Lopez-Guillermo A, Cardesa-Salzmann T, Climent F, Gonzalez-Barca E, Mercadal S, et al. MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013 Oct;98(10):1554–1562. doi: 10.3324/haematol.2013.086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu-Monette ZY, Tu M, Jabbar KJ, Cao X, Tzankov A, Visco C, et al. Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget. 2015 Mar 20;6(8):5615–5633. doi: 10.18632/oncotarget.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu-Monette ZY, Wu L, Visco C, Tai YC, Tzankov A, Liu WM, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012 Nov 8;120(19):3986–3996. doi: 10.1182/blood-2012-05-433334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellas C, Garcia D, Vicente Y, Kilany L, Abraira V, Navarro B, et al. Immunohistochemical and molecular characteristics with prognostic significance in diffuse large B-cell lymphoma. PloS one. 2014;9(6):e98169. doi: 10.1371/journal.pone.0098169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 May 19;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007 Feb 10;25(5):579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 27.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer research. 1971 Nov;31(11):1860–1861. [PubMed] [Google Scholar]
- 28.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Sep 20;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson LE, Redman JR, Butler JJ, Osborne BM, Velasquez WS, McLaughlin P, et al. Discordant bone marrow involvement in diffuse large-cell lymphoma: a distinct clinical-pathologic entity associated with a continuous risk of relapse. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1991 Feb;9(2):236–242. doi: 10.1200/JCO.1991.9.2.236. [DOI] [PubMed] [Google Scholar]
- 30.Thiele J, Zirbes TK, Kvasnicka HM, Fischer R. Focal lymphoid aggregates (nodules) in bone marrow biopsies: differentiation between benign hyperplasia and malignant lymphoma--a practical guideline. Journal of clinical pathology. 1999 Apr;52(4):294–300. doi: 10.1136/jcp.52.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell JK, Matthews JP, Seymour JF, Wolf MM, Juneja SK Australasian Leukaemia Lymphoma G. Optimum trephine length in the assessment of bone marrow involvement in patients with diffuse large cell lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2003 Feb;14(2):273–276. doi: 10.1093/annonc/mdg055. [DOI] [PubMed] [Google Scholar]
- 32.Deng L, Xu-Monette ZY, Loghavi S, Manyam GC, Xia Y, Visco C, et al. Primary testicular diffuse large B-cell lymphoma displays distinct clinical and biological features for treatment failure in rituximab era: a report from the International PTL Consortium. Leukemia. 2016 Feb;30(2):361–372. doi: 10.1038/leu.2015.237. [DOI] [PubMed] [Google Scholar]
- 33.Hwang HS, Yoon DH, Suh C, Huh J. A new extranodal scoring system based on the prognostically relevant extranodal sites in diffuse large B-cell lymphoma, not otherwise specified treated with chemoimmunotherapy. Annals of hematology. 2016 Aug;95(8):1249–1258. doi: 10.1007/s00277-016-2689-8. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014 Feb 6;123(6):837–842. doi: 10.1182/blood-2013-09-524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chigrinova E, Mian M, Scandurra M, Greiner TC, Chan WC, Vose JM, et al. Diffuse large B-cell lymphoma with concordant bone marrow involvement has peculiar genomic profile and poor clinical outcome. Hematol Oncol. 2011 Mar;29(1):38–41. doi: 10.1002/hon.953. [DOI] [PubMed] [Google Scholar]
- 36.Juneja SK, Wolf MM, Cooper IA. Value of bilateral bone marrow biopsy specimens in non-Hodgkin's lymphoma. Journal of clinical pathology. 1990 Aug;43(8):630–632. doi: 10.1136/jcp.43.8.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011 Nov 23;147(5):992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Cheng L, Ruan Z. Tim-3 and Tim-4 as the potential targets for antitumor therapy. Hum Vaccin Immunother. 2015;11(10):2458–2462. doi: 10.1080/21645515.2015.1056953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kremer M, Spitzer M, Mandl-Weber S, Stecker K, Schmidt B, Hofler H, et al. Discordant bone marrow involvement in diffuse large B-cell lymphoma: comparative molecular analysis reveals a heterogeneous group of disorders. Laboratory investigation; a journal of technical methods and pathology. 2003 Jan;83(1):107–114. doi: 10.1097/01.lab.0000050762.61660.27. [DOI] [PubMed] [Google Scholar]
- 40.Kridel R, Mottok A, Farinha P, Ben-Neriah S, Ennishi D, Zheng Y, et al. Cell of origin of transformed follicular lymphoma. Blood. 2015 Oct 29;126(18):2118–2127. doi: 10.1182/blood-2015-06-649905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purroy N, Bergua J, Gallur L, Prieto J, Lopez LA, Sancho JM, et al. Long-term follow-up of dose-adjusted EPOCH plus rituximab (DA-EPOCH-R) in untreated patients with poor prognosis large B-cell lymphoma. A phase II study conducted by the Spanish PETHEMA Group. British journal of haematology. 2015 Apr;169(2):188–198. doi: 10.1111/bjh.13273. [DOI] [PubMed] [Google Scholar]
- 42.Oki Y, Westin JR, Vega F, Chuang H, Fowler N, Neelapu S, et al. Prospective phase II study of rituximab with alternating cycles of hyper-CVAD and high-dose methotrexate with cytarabine for young patients with high-risk diffuse large B-cell lymphoma. British journal of haematology. 2013 Dec;163(5):611–620. doi: 10.1111/bjh.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mato A, Feldman T, Zielonka T, Singavi A, Gadaletta G, Waksmundzki K, et al. Rituximab, cyclophosphamide-fractionated, vincristine, doxorubicin and dexamethasone alternating with rituximab, methotrexate and cytarabine overcomes risk features associated with inferior outcomes in treatment of newly diagnosed, high-risk diffuse large B-cell lymphoma. Leukemia & lymphoma. 2013 Dec;54(12):2606–2612. doi: 10.3109/10428194.2013.783909. [DOI] [PubMed] [Google Scholar]
- 44.Howlett C, Snedecor SJ, Landsburg DJ, Svoboda J, Chong EA, Schuster SJ, et al. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysis. British journal of haematology. 2015 Aug;170(4):504–514. doi: 10.1111/bjh.13463. [DOI] [PubMed] [Google Scholar]
- 45.Barta SK, Li H, Hochster HS, Hong F, Weller E, Gascoyne RD, et al. Randomized phase 3 study in low-grade lymphoma comparing maintenance anti-CD20 antibody with observation after induction therapy: A trial of the ECOG-ACRIN Cancer Research Group (E1496) Cancer. 2016 Oct;122(19):2996–3004. doi: 10.1002/cncr.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greil R, Obrtlikova P, Smolej L, Kozak T, Steurer M, Andel J, et al. Rituximab maintenance versus observation alone in patients with chronic lymphocytic leukaemia who respond to first-line or second-line rituximab-containing chemoimmunotherapy: final results of the AGMT CLL-8a Mabtenance randomised trial. The Lancet Haematology. 2016 Jul;3(7):e317–329. doi: 10.1016/S2352-3026(16)30045-X. [DOI] [PubMed] [Google Scholar]
- 47.Zahid MF, Khan N, Hashmi SK, Kizilbash SH, Barta SK. Central nervous system prophylaxis in diffuse large B-cell lymphoma. European journal of haematology. 2016 Aug;97(2):108–120. doi: 10.1111/ejh.12763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.