Abstract
Objectives
To evaluate 1) the prognostic implication of baseline global longitudinal strain (GLS) and 2) the added value of GLS beyond the circulating cardiac biomarkers for risk stratification in patients with light chain (AL) amyloid undergoing autologous hematopoietic cell transplant (HCT).
Background
Autologous HCT is a first line therapy for prolonging survival in patients with AL amyloidosis. Cardiac involvement is the most important determinant of survival. However, patients with advanced cardiac involvement have often been excluded from HCT due to high risk of transplant related mortality and poor overall survival. Whether GLS can provide additional risk stratification and predict survival after HCT in this high risk population remains unclear.
Methods
82 patients with newly diagnosed AL amyloidosis who underwent upfront HCT between January 2007 to April 2014 were included in the study. Clinical, echo and serum cardiac biomarker data were collected at baseline and 12 months following HCT. GLS measurements were performed using a vendor independent offline system. The median follow-up time for survivors was 58 months.
Results
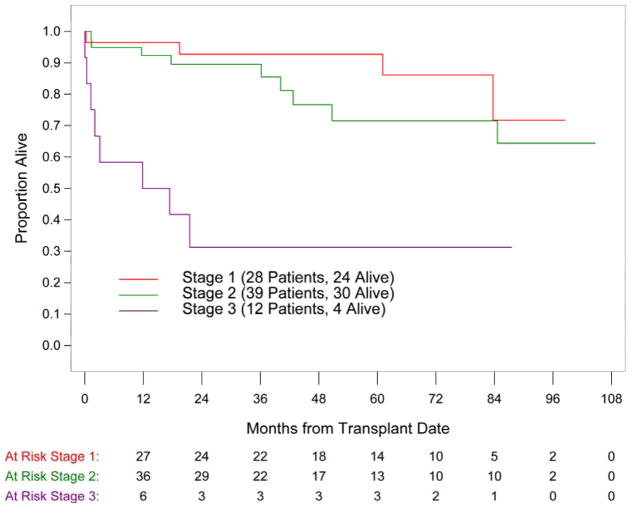
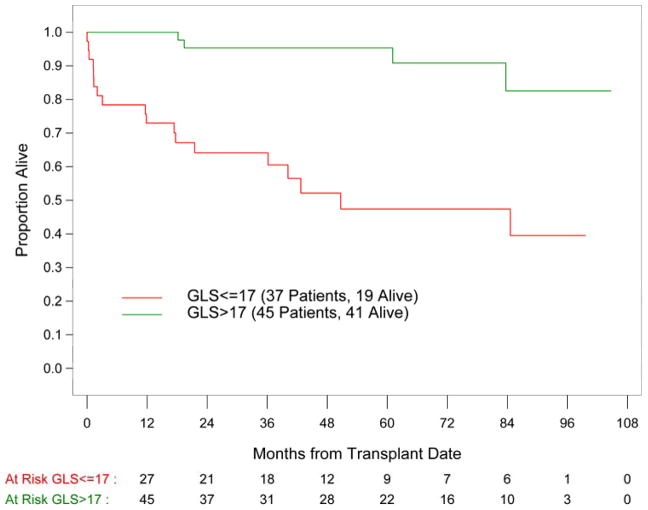
64% of the patients were in biomarker based Mayo stage II or III. GLS, BNP, troponin and mitral E/A ratio were identified as the strongest predictors of survival (p<0.0001). Other predictors included sex, creatinine, free light chain, wall thickness and EF. The Mayo stage was significantly associated with outcome with 5 year survival of 93%, 72% and 31% in stage I, II and III patients, respectively. A GLS of 17% was identified as the value which best discriminated survivors from non-survivors and the application of this cutoff value provided further mortality risk stratification within each Mayo stage.
Conclusions
GLS is a strong predictor of survival in patients with AL amyloidosis undergoing HCT, potentially providing incremental value over the serum cardiac biomarkers for risk stratification. GLS should be considered as a standard parameter along with the serum cardiac biomarkers when evaluating eligibility for HCT or other investigational therapies.
Keywords: AL amyloidosis, autologous hematopoietic cell transplant, global longitudinal strain, serum cardiac biomarkers, survival
Introduction
Primary or systemic light chain (AL) amyloidosis is a rare but potentially fatal plasma cell dyscrasia characterized by tissue deposition of amyloid fibrils derived from monoclonal light chains leading to progressive organ failure. (1, 2) Treatment has primarily targeted the pathologic plasma cells to terminate monoclonal light chain production. First line therapy with high dose chemotherapy followed by autologous hematopoietic cell transplant (HCT) has resulted in complete hematologic remission (CR) and improved 5 year survival. (3–7) Cardiac involvement occurs in 50% of cases and is the most important determinant of survival. (2, 8) Troponin and NT-proBNP are sensitive and reproducible prognostic markers in AL amyloidosis. A prognostically validated Mayo staging system based on these biomarkers is commonly used for risk stratification and prediction of overall survival (OS) in newly diagnosed patients undergoing frontline therapy including hematopoietic cell transplant. (9–11). Patients are classified as Stage I, II or III based on whether NT-pro BNP and troponin levels are both normal, are increased for only one, or are both elevated, respectively. Survival decreases with each higher stage as it correlates with the severity of underlying cardiac involvement. Patients with advanced cardiac amyloidosis classified as Mayo Stage III (elevated troponin and N-terminal pro-b type brain natriuretic peptide (NT-proBNP)) are often not considered for HCT due to high risk of transplant related mortality and poor overall survival.(12)
Echocardiography provides diagnostic and prognostic information in patients with AL amyloidosis suspected of having cardiac involvement. (13–18) Multiple echocardiographic parameters are predictive of outcome. Recently myocardial strain by 2D speckle tracking echocardiography has emerged as a highly useful tool in the evaluation of patients with cardiac amyloidosis. (19–22) Global longitudinal strain (GLS) has shown to be a strong and independent predictor of outcome in patients with cardiac amyloidosis.(23–28) However whether GLS is a useful marker for prognostication of survival in patients undergoing HCT independent of troponin and BNP, remains unknown.
The objectives of this study were to investigate 1) the prognostic implication of baseline (pretreatment) GLS and 2) the added value of GLS beyond the circulating cardiac biomarkers for risk stratification in AL amyloid patients undergoing HCT.
METHODS
Study Population
Eighty-two patients with newly diagnosed biopsy-proven AL amyloidosis who received upfront treatment with HCT at Memorial Sloan Kettering Cancer Center between January 2007 to April 2014 were included in this study. Patients with more than 2 major organs involved, NYHA class III or IV heart failure or critical cardiac arrhythmias resulting in unstable hemodynamics were not eligible for HCT. Patients who received chemotherapy prior to HCT were excluded in order to eliminate any effect of prior potentially cardiotoxic exposure on the baseline echocardiogram and post HCT outcome. Hence, all baseline echocardiograms were obtained prior to any treatment. Clinical, laboratory, echocardiographic and treatment data were extracted from a prospectively maintained database of an ongoing IRB protocol which prospectively collects baseline characteristics and outcomes of patients with systemic light-chain (AL) amyloidosis. One of the objectives of the protocol is to assess the prognostic value of cardiac characteristics such as laboratory and echocardiographic data in patients with AL amyloidosis receiving various types of treatment. Though the testing was performed as part of routine care, the data were prospectively collected with the intent of addressing the questions examined in the current study.
Patients were assigned a cardiac stage (Mayo I, II, III) based on the cardiac biomarkers brain natriuretic peptide (BNP) and troponin. (9, 29) Conversion between BNP and NT-proBNP was as follows: log BNP = 0.28+0.66 × log NT-ProBNP; 86 pg/ml was identified as the appropriate cutoff. Stage I included patients with a BNP <86 ng/ml and TnI <0.10 ng/ml, stage II included patients with either BNP ≥ 86 ng/ml or TnI ≥ 0.10 ng/ml, and stage III included patients with both BNP ≥ 86 ng/ml and TnI ≥ 0.10 ng/ml.
Echocardiography
Conventional 2D and Doppler echocardiography was performed using commercially available standard ultrasound scanners (Vivid E9, General Electric Medical Systems and iE33, Philips Medical Systems), according to the standardized American Society Echocardiography protocol. (30) Left ventricular ejection fraction (LVEF) was calculated using the modified Simpson’s method. Mitral inflow velocity pattern was recorded from the apical 4-chamber view with the pulsed-wave Doppler sample volume positioned at the tips of the leaflets during diastole. Peak early filling (E-wave) and late diastolic filling (A-wave) velocities were measured and their ratio (mitral E/A) derived. Doppler tissue imaging of the mitral annulus was performed with measurement of the early (e′) diastolic velocity at the lateral annulus. The studies were performed following a strain protocol. Images from the apical four-chamber, two-chamber and three-chamber views were acquired sequentially to minimize heart rate variability. Three complete cardiac cycles per loop was recorded for each view to ensure that at least one complete cycle without any truncation is available for analysis. All images were acquired in breath-hold to avoid any breathing artifact and minimize image translation. High quality ECG trace was obtained to allow proper gating of the images. Settings were optimized with utmost attention paid to the image quality and resolution of the endocardial border. The depth and the sector angle were adjusted to include the LV but minimizing the sector size to achieve a higher frame rate which was maintained between 40 and 90 frames/sec. All echo images were digitally archived in a Digital Imaging and Communcations in Medicine format on the echo information management system and retrieved offline for GLS analysis.
Myocardial Strain Measurement
GLS measurements were performed using vendor independent offline 2D Cardiac Performance Analysis software, version 1.1.3 (Tom Tec Imaging Systems, Munich, Germany). The endocardial border was traced in end-diastole in the 3 apical views, which allowed the software to track myocardial movement throughout the cardiac cycle. After careful inspection, manual correction was performed if the myocardial tracking was suboptimal. Each view was divided into 6 segments, for a total of 18 segments representing the entire left ventricle. Longitudinal strain curves were generated for each segment. GLS was calculated as the average value of the peak negative systolic strain values for all the segments within the 3 standard apical views. The negative nature of systolic strain or contraction can lead to confusion when describing increases or decreases in strain as lower arithmetic value implies more vigorous contraction. For example, GLS of −20% implies better LV systolic contraction but is lower in value than GLS of −14%. To avoid confusion, the normally negative GLS numbers are manually converted to positive numbers as recommended in the 2015 ASE guideline on Cardiac Chamber Quantification (31), such that the arithmatic value and the amplitude of contraction are concordant, allowing clearer communication of strain changes as an increase or decrease in the absolute value. GLS was measured by two well -trained operators (SP, JJ). All echocardiographic measurements were made with the operator blinded to the clinical and outcome data.
Reproducibility
Inter-observer and intra-observer variability was assessed using the intra-class correlation coefficient (ICC). Bland-Altman plots were constructed by plotting the average of the two readings on the x-axis versus the difference between the two readings on the y-axis. The mean and standard deviation of the differences were calculated. Because of the small sample size, the t-distribution was used as the reference distribution to calculate the 95% limits of agreement. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using either SAS software version 9.2 (SAS Institute, Cary, NC) or R software version 2.13.1 (R Core Development Team, Vienna, Austria). The inter-observer variability was calculated by comparing the original GLS calculation with that calculated by a blinded second observer in 20 randomly selected patients. Intra-observer variability was calculated by repeated measurements in 20 patients by the primary reviewer 3 weeks after the initial measurement.
Statistical Analysis
Prospective follow-up began on the date of hematopoietic cell transplant. Overall survival was the primary outcome. No patient was lost to follow-up during the study. Comparisons between groups were made using Fisher’s exact test for categorical variables and Wilcoxon Rank Sum test for continuous variables. The Spearman rank correlation coefficient was used to estimate the correlation between continuous variables. Differences between echocardiographic parameters and biomarkers before and after transplant were evaluated using Wilcoxon Signed Rank test. Overall survival and survival stratified by categorical variables was estimated using the Kaplan-Meier method. The associations of baseline characteristics on overall survival were examined using the logrank test for categorical variables and Cox proportional hazards regression for continuous variables. There were too few events for a multivariate survival analysis. The cutpoint for GLS to predict overall survival was chosen using the maximally selected log-rank statistic (i.e., the cutpoint chosen best separates outcomes according to the maximum test statistic).
RESULTS
Baseline patient characteristics
Demographics and clinical features of 82 patients are summarized in Table I. Sixty-four percent of the patients had elevated troponin and/or BNP with 15% classified as Mayo stage III (elevated troponin and BNP). Baseline standard 2D echocardiographic parameters as well as GLS are shown in Table II. The mean LVEF was 65% with only 3 patients having LVEF <50%. Though the mean GLS was 17% which is the lower limit of normal, 20% of the patients (16/82) had GLS less than 12%, consistent with significant left ventricular (LV) dysfunction which has been associated with an adverse prognosis in other clinical settings (32). The Spearman correlation coefficient of GLS with BNP was r= −0.46 (P<0.0001), and with EF was r= 0.50 (p<0.0001). GLS was not significantly correlated with TnI (−0.17; p = 0.15).
Table I.
Demographic and Clinical Characteristics; N = 82
| Variable | |
|---|---|
| Median Age, years (range) | 59 (38–73) |
| Male Sex (%) | 46 (56.1%) |
| Body Mass Index (kg/m2) | 27.5 ±6.8 |
| Systolic Blood Pressure (mm Hg) | 120 ± 21 |
| Diastolic Blood Pressure (mm Hg) | 75 ± 12 |
| Heart Rate (beats/min) | 81 ± 12 |
| FLC diff (mg/L) | 52.5 ±105.7 |
| Troponin I (μg/mL) | 0.04 ± 0.06 |
| BNP (pg/mL) | 252 ±307 |
| Creatinine (mg/dL) | 1.4 ± 1.0 |
| Mayo Stage | |
| I | 28 (35.4%) |
| II | 39 (49.4%) |
| III | 12 (15.2%) |
| CR at 1 year | 48 (58.5%) |
| Atrial fibrillation | 5(6.1%) |
| Hypertension | 28 (34.1%) |
| Diabetes Mellitus | 5 (6%) |
| Hyperlipidemia | 36 (43.9%) |
| Coronary artery disease | 2 (2.4 %) |
Values are mean ± SD or n(%) unless otherwise noted
Table II.
Echocardiographic Characteristics
| Variable | N | |
|---|---|---|
| GLS % | 82 | 17 ± 4.7 |
| Ejection Fraction % | 82 | 65 ± 8 |
| IVS, cm | 82 | 1.2 ± 0.3 |
| Mitral E (cm/sec) | 80 | 79 ± 22 |
| Mitral A (cm/sec) | 78 | 74 ± 22 |
| E/A Ratio | 78 | 1.2 ±0.8 |
| Lateral E/e′ | 74 | 11 ± 7 |
| Deceleration Time (sec) | 81 | 0.19 ± 0.05 |
e′ = tissue Doppler at the lateral annulus; values are mean ± SD
Pretreatment predictors of survival
Out of 82 patients, 21 patients (25%) died. The median follow-up time for survivors was 58 months. Global longitudinal strain as well as cardiac biomarkers and mitral E/A ratio were identified as the strongest predictors of survival in the univariate analysis (p<0.0001; Table III). Other variables including patient sex, difference in the involved and uninvolved free light chain (dFLC), and echo parameters of LV morphology and function were also significantly associated with survival. Mayo cardiac stage was significantly associated with survival with 5 year estimates of 93%, 72% and 31% in cardiac stage I, II and III patients, respectively (Figure 1). A GLS of 17% was identified as the value which best discriminated survivors from non-survivors. For the entire study population, patients with a baseline GLS of > 17% had a 5 year survival of 95% as compared to 47% in patients with GLS ≤ 17% (Figure 2). Among the 12 patients with Mayo stage III disease, 9 patients (75%) had GLS ≤17% and 3 patients had GLS > 17%. By 5-year follow up, only those 3 patients with GLS > 17% were alive, suggesting additional value of GLS for prognostication of this high risk subgroup. Similarly for patients in Mayo stage I and II, GLS ≤ 17% was associated with worse prognosis than GLS > 17%. (Table IV) Multivariate analysis was not possible given the limited number of deaths.
Table III.
Univariate Analysis for Predictors of Survival
| Variable | Category | Hazard Ratio (95% CI) | 5 Year Survival (95% CI) | p-value |
|---|---|---|---|---|
| Age years | Per 1 year increase | 0.97 (0.92–1.02) | 0.23 | |
| Sex | Female | 89% (72%–96%) | 0.009 | |
| Male | 60% (41%–75%) | |||
| Mayo Stage | I | 93% (74%–98%) | <0.0001 | |
| II | 72% (50%–85%) | |||
| III | 31% (8%–58) | |||
| FLC diff (mg/ml) | Per 10 unit increase | 1.03 (1.01–1.05) | 0.02 | |
| Troponin (μg/mL) | Per 0.1 unit increase | 3.06 (1.80–5.21) | <0.0001 | |
| BNP (pg/mL) | Per 100 unit increase | 1.23 (1.12–1.35) | <0.0001 | |
| Creatinine (mg/dL) | Per unit increase | 1.36 (1.05–1.76) | 0.02 | |
| GLS % | Per unit increase | 0.82 (0.74–0.90) | <0.0001 | |
| EF % | Per unit increase | 0.94 (0.88–0.99) | 0.03 | |
| IVS (cm) | Per 0.1 unit increase | 1.29 (1.12–1.49) | 0.0003 | |
| Mitral E (cm/sec) | Per 10 unit increase | 1.12 (0.95–1.33) | 0.17 | |
| Mitral A (cm/sec) | Per 10 unit increase | 0.70 (0.56–.87) | 0.002 | |
| E/A Ratio | Per unit increase | 2.70 (1.81–4.02) | <0.0001 | |
| Lateral E/e′ | Per 10 unit increase | 2.43 (1.45–4.08) | 0.0008 | |
| Deceleration Time (sec) | Per 0.1 unit increase | 0.50 (0.21–1.18) | 0.11 |
Hazard ratio estimates are presented for continuous variables per unit increases and 5 year survival estimates are provided for categorical variables.
Figure 1.
Overall survival in patients with AL amyloidosis represented by Kaplan-Meier survival curves according to the biomarker based Mayo stage; p= 0.0001
Figure 2.
Overall survival in patients with AL amyloidosis represented by Kaplan-Meier survival curves according to GLS cutoff value of 17%; p<0.0001
Table IV.
Survival according to Mayo stage stratified by GLS
| Stage I | Stage II | Stage III | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GLS | N | 5 yr OS | P val* | N | 5 yr OS | P val* | N | 5 yr OS | P val* |
| ≤17% | 5 | 80% | 0.41 | 23 | 55% | 0.01 | 9 | 0% | 0.01 |
| >17% | 23 | 95% | 16 | 100% | 3 | 100% | |||
OS = overall survival;
p value of comparison between GLS ≤ 17% vs. GLS >17%.
Indices of cardiac structure and function following HCT
At one year following HCT, 48 (59%) patients were in complete hematologic remission (CR). Fifty patients out of the total population (61%) had a one year post HCT echo available. Among the patients in Mayo stage II and III, there was no significant change in GLS, IVS, mitral E/A ratio or lateral E/e′ in the CR or non-CR group (Table V). EF decreased slightly in both groups. BNP or troponin also showed no change in the CR and the non-CR groups.
Table V.
Echocardiographic indices and biomarkers based on hematologic response at 1 year among patients in cardiac stage II and III
| Parameter | CR | Non-CR | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Baseline | N | 1 year | N | Baseline | N | 1 year | |
| GLS, % | 27 | 16.4 ± 5.2 | 22 | 17.2 ± 4.5 | 16 | 15.6 ± 3.6 | 12 | 14.8 ± 4.2 |
| EF, % | 27 | 66 ± 7 | 21 | 60 ± 8 * | 16 | 64 ± 9 | 13 | 59 ± 11 * |
| IVS, cm | 27 | 1.3 ± 0.3 | 21 | 1.3 ± 0.3 | 16 | 1.3 ± 0.3 | 13 | 1.4 ± 0.2 |
| Mitral E/A | 25 | 1.1 ± 0.4 | 20 | 1.2 ± 0.6 | 15 | 1.1 ± 0.5 | 11 | 1.3 ± 1.4 |
| Lateral E/e′ | 24 | 12.3 ± 6.7 | 21 | 12.2 ± 5.8 | 14 | 13.5 ± 6.5 | 13 | 16.3 ± 7.1 |
| BNP | 27 | 293 ± 273 | 22 | 291 ± 392 | 16 | 286 ± 183 | 13 | 189 ± 209 |
| Troponin | 27 | 0.04 ± 0.06 | 21 | 0.04 ± 0.09 | 16 | 0.04 ± 0.06 | 13 | 0.14 ± 0.47 |
p<0.05 baseline vs. 1 year
Intra-observer and Inter-observer Variability
For GLS, the ICC for inter-observer agreement was 0.883 (95% confidence interval (CI): 0.732, 0.952) and the ICC for intra-observer agreement was 0.923 (95% CI: 0.820, 0.969), reflecting substantial agreement for measurement of GLS.
DISCUSSION
This is the first study to demonstrate the prognostic value of GLS for risk stratification among patients with AL amyloidosis undergoing HCT. Not only is GLS strongly prognostic, these data indicate that GLS provides additional information beyond the well- validated cardiac biomarker staging for survival among patients treated with HCT.
High dose chemotherapy coupled with hematopoietic cell transplant is one of the most effective treatments in AL amyloidosis. Elimination of pathologic plasma cells and suppression of amyloidogenic immunoglobulin light chain production, prevents further amyloid deposition and organ damage. Many centers have excluded advanced cardiac involvement as determined by cardiac biomarker levels (Mayo stage III disease) from HCT as well as from clinical trials given their overall poor prognosis. Our data suggests that functional assessment with GLS improves risk stratification of patients within each biomarker stage. Although GLS directly correlates with Mayo Stage, 25% of the patients in Mayo stage III had GLS > 17% and all were alive after 5 years of follow-up. While this observation is based on a small number of patients, it suggests that the cardiac phenotype and prognosis are not uniform among patients classified within the same cardiac biomarker based stage. The additional risk stratification provided by GLS may be useful in selecting appropriate patients for HCT and other investigational therapies.
This study confirms existing published data demonstrating the superiority of GLS as a predictor of survival when compared to left ventricular ejection fraction in patients with cardiac amyloid.(20, 22–24) It is well recognized that GLS, a measure of longitudinal LV function, is more sensitive and accurate than LVEF in the assessment of myocardial contractility, particularly in cardiomyopathic conditions.(33, 34) LVEF is often normal despite the presence of myocardial dysfunction in the presence of the low end-diastolic volume and small cavity size seen in patients with amyloid cardiomyopathy. Longitudinal LV mechanics which are governed primarily by the subendocardial layer, are the most sensitive and vulnerable to myocardial disease. This likely explains why GLS remains a strong prognostic predictor despite normal EF in the current study population. Other investigators have also showed the incremental value of GLS for the assessment of outcome as compared to clinical, echocardiographic and serum biomarkers in patients with AL amyloidosis.(23, 27, 35) The variability in GLS cutoff values for the discrimination of survivors from nonsurvivors that have been reported in the literature are likely attributable to differences in the study population, duration of follow-up, treatment regimens, and platforms used for GLS analysis. Cardiac MRI late gadolinium enhancement has also been shown to provide incremental prognostic value over the serum biomarkers in patients with cardiac amyloidosis. (22, 35–37) Ternacle et al (22) demonstrated a strong negative association between segmental LV longitudinal strain and amyloid burden measured by histopathology and late gadolinium enhancement. The study showed higher amyloid load with lower longitudinal strain from LV base to apex, providing further insight on the pathophysiology of the amyloid deposition and its contribution to myocardial contractile dysfunction.
The lack of improvement in cardiac structure and function among patients with and without CR one year post HCT is disappointing. However, it is possible that the small sample size limits the detection of such differences. Similarly, a recently published paper by Salinaro et al (27) also failed to show improvement in wall thickness, GLS, EF or diastolic function among patients who achieved CR. They did demonstrate mild improvement in the basal strain segments among patients in the CR group only. Although normalization of the precursor protein is a major predictor of survival in patients with AL amyloidosis with studies demonstrating organ response in association with hematologic response, it may be of limited benefit to patients with persistent or progression of cardiac dysfunction as suggested by these findings. It is possible that longer follow-up beyond 1 year is needed in order to detect any improvement of echocardiographic parameters after HCT. Larger studies with longer follow-up are needed to better define the long-term effect of hematologic remission on cardiac function. In addition, there are ongoing studies to explore the effect of novel therapy that directly targets amyloid deposits on organ function response.(38)
STUDY LIMITATIONS
This study is limited due to its small population size and single center design. 2D GLS is not standardized and the cutoff identified in the current study may not be applicable when using another system other than the one used in the current study (Tom Tec Imaging System) or studying AL amyloid patients in different clinical settings. GLS of 17%, the lower limit of normal, being identified as the cutoff to discriminate survivors from non-survivors, reflects the heterogeneity of the patient population with regard to the extent of cardiac involvement. But it was nevertheless predictive among patients within Mayo stage II and III. Given the small sample size, subgroup analysis for each Mayo Stage to determine the optimal GLS cutoff was not possible. Larger studies are required to validate the optimal GLS cutoff for risk stratification. Classification of the Mayo stage system may not be exactly identical to the standard definition as BNP was used instead of NT-proBNP though both forms of the biomarker have been prognostically validated. This study used the original staging system with cardiac biomarkers only and not the revised system (39)which also includes a light chain based parameter since the goal of the study is to investigate the added value of GLS to the serum biomarkers for risk stratification.
CONCLUSION
GLS is a strong predictor of survival in patients with AL amyloidosis undergoing HCT, potentially roviding incremental value over the serum cardiac biomarkers for risk stratification. GLS should be considered as a standard parameter along with the serum cardiac biomarkers when evaluating eligibility for HCT or other investigational therapies.
Abbreviations
- BNP
brain natriuretic peptide
- CR
complete hematologic remission
- dFLC
difference in the involved and uninvolved free light chain
- GLS
global longitudinal strain
- HCT
hematopoietic cell transplant
- IVS
interventricular septum
- LVEF
left ventricular ejection fraction
- NT-proBNP
N-terminal pro-b type brain natriuretic peptide
- OS
overall survival
- TnI
troponin I
Footnotes
No relationships with industry.
References
- 1.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337(13):898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 2.Roberts WC, Waller BF. Cardiac amyloidosis causing cardiac dysfunction: analysis of 54 necropsy patients. Am J Cardiol. 1983;52(1):137–46. doi: 10.1016/0002-9149(83)90084-x. [DOI] [PubMed] [Google Scholar]
- 3.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar SK, Leung N, et al. Effect of hematologic response on outcome of patients undergoing transplantation for primary amyloidosis: importance of achieving a complete response. Haematologica. 2007;92(10):1415–8. doi: 10.3324/haematol.11413. [DOI] [PubMed] [Google Scholar]
- 4.Cibeira MT, Sanchorawala V, Seldin DC, Quillen K, Berk JL, Dember LM, et al. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood. 2011;118(16):4346–52. doi: 10.1182/blood-2011-01-330738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen AD, Comenzo RL. Systemic light-chain amyloidosis: advances in diagnosis, prognosis, and therapy. Hematology Am Soc Hematol Educ Program. 2010;2010:287–94. doi: 10.1182/asheducation-2010.1.287. [DOI] [PubMed] [Google Scholar]
- 6.D’Souza A, Dispenzieri A, Wirk B, Zhang MJ, Huang J, Gertz MA, et al. Improved Outcomes After Autologous Hematopoietic Cell Transplantation for Light Chain Amyloidosis: A Center for International Blood and Marrow Transplant Research Study. J Clin Oncol. 2015;33(32):3741–9. doi: 10.1200/JCO.2015.62.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comenzo RL, Gertz MA. Autologous stem cell transplantation for primary systemic amyloidosis. Blood. 2002;99(12):4276–82. doi: 10.1182/blood.v99.12.4276. [DOI] [PubMed] [Google Scholar]
- 8.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112(13):2047–60. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 9.Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22(18):3751–7. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, et al. Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2004;104(6):1881–7. doi: 10.1182/blood-2004-01-0390. [DOI] [PubMed] [Google Scholar]
- 11.Palladini G, Dispenzieri A, Gertz MA, Kumar S, Wechalekar A, Hawkins PN, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541–9. doi: 10.1200/JCO.2011.37.7614. [DOI] [PubMed] [Google Scholar]
- 12.Gertz MA, Lacy MQ, Dispenzieri A, Kumar SK, Dingli D, Leung N, et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant. 2013;48(4):557–61. doi: 10.1038/bmt.2012.170. [DOI] [PubMed] [Google Scholar]
- 13.Apridonidze T, Steingart RM, Comenzo RL, Hoffman J, Goldsmith Y, Bella JN, et al. Clinical and echocardiographic correlates of elevated troponin in amyloid light-chain cardiac amyloidosis. Am J Cardiol. 2012;110(8):1180–4. doi: 10.1016/j.amjcard.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 14.Klein AL, Hatle LK, Burstow DJ, Seward JB, Kyle RA, Bailey KR, et al. Doppler characterization of left ventricular diastolic function in cardiac amyloidosis. J Am Coll Cardiol. 1989;13(5):1017–26. doi: 10.1016/0735-1097(89)90254-4. [DOI] [PubMed] [Google Scholar]
- 15.Cueto-Garcia L, Reeder GS, Kyle RA, Wood DL, Seward JB, Naessens J, et al. Echocardiographic findings in systemic amyloidosis: spectrum of cardiac involvement and relation to survival. J Am Coll Cardiol. 1985;6(4):737–43. doi: 10.1016/s0735-1097(85)80475-7. [DOI] [PubMed] [Google Scholar]
- 16.Austin BA, Duffy B, Tan C, Rodriguez ER, Starling RC, Desai MY. Comparison of functional status, electrocardiographic, and echocardiographic parameters to mortality in endomyocardial-biopsy proven cardiac amyloidosis. Am J Cardiol. 2009;103(10):1429–33. doi: 10.1016/j.amjcard.2009.01.361. [DOI] [PubMed] [Google Scholar]
- 17.Kado Y, Obokata M, Nagata Y, Ishizu T, Addetia K, Aonuma K, et al. Cumulative Burden of Myocardial Dysfunction in Cardiac Amyloidosis Assessed Using Four-Chamber Cardiac Strain. J Am Soc Echocardiogr. 2016;29(11):1092–9. e2. doi: 10.1016/j.echo.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Ochs MM, Riffel J, Kristen AV, Hegenbart U, Schonland S, Hardt SE, et al. Anterior Aortic Plane Systolic Excursion: A Novel Indicator of Transplant-Free Survival in Systemic Light-Chain Amyloidosis. J Am Soc Echocardiogr. 2016;29(12):1188–96. doi: 10.1016/j.echo.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Bellavia D, Abraham TP, Pellikka PA, Al-Zahrani GB, Dispenzieri A, Oh JK, et al. Detection of left ventricular systolic dysfunction in cardiac amyloidosis with strain rate echocardiography. J Am Soc Echocardiogr. 2007;20(10):1194–202. doi: 10.1016/j.echo.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Bellavia D, Pellikka PA, Abraham TP, Al-Zahrani GB, Dispenzieri A, Oh JK, et al. Evidence of impaired left ventricular systolic function by Doppler myocardial imaging in patients with systemic amyloidosis and no evidence of cardiac involvement by standard two-dimensional and Doppler echocardiography. Am J Cardiol. 2008;101(7):1039–45. doi: 10.1016/j.amjcard.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Phelan D, Collier P, Thavendiranathan P, Popovic ZB, Hanna M, Plana JC, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442–8. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 22.Ternacle J, Bodez D, Guellich A, Audureau E, Rappeneau S, Lim P, et al. Causes and Consequences of Longitudinal LV Dysfunction Assessed by 2D Strain Echocardiography in Cardiac Amyloidosis. JACC Cardiovasc Imaging. 2016;9(2):126–38. doi: 10.1016/j.jcmg.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Buss SJ, Emami M, Mereles D, Korosoglou G, Kristen AV, Voss A, et al. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol. 2012;60(12):1067–76. doi: 10.1016/j.jacc.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 24.Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging. 2010;3(4):333–42. doi: 10.1016/j.jcmg.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Krishnasamy R, Isbel NM, Hawley CM, Pascoe EM, Burrage M, Leano R, et al. Left Ventricular Global Longitudinal Strain (GLS) Is a Superior Predictor of All-Cause and Cardiovascular Mortality When Compared to Ejection Fraction in Advanced Chronic Kidney Disease. PLoS One. 2015;10(5):e0127044. doi: 10.1371/journal.pone.0127044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengelov M, Jorgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, et al. Global Longitudinal Strain Is a Superior Predictor of All-Cause Mortality in Heart Failure With Reduced Ejection Fraction. JACC Cardiovasc Imaging. 2015;8(12):1351–9. doi: 10.1016/j.jcmg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Salinaro F, Meier-Ewert HK, Miller EJ, Pandey S, Sanchorawala V, Berk JL, et al. Longitudinal systolic strain, cardiac function improvement, and survival following treatment of light-chain (AL) cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2016 doi: 10.1093/ehjci/jew298. [DOI] [PubMed] [Google Scholar]
- 28.Barros-Gomes S, Williams B, Nhola LF, Grogan M, Maalouf JF, Dispenzieri A, et al. Prognosis of Light Chain Amyloidosis With Preserved LVEF: Added Value of 2D Speckle-Tracking Echocardiography to the Current Prognostic Staging System. JACC Cardiovasc Imaging. 2016 doi: 10.1016/j.jcmg.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Dispenzieri A, Merlini G, Comenzo RL. Amyloidosis 2008 BMT Tandem Meetings (February 13–17, San Diego) Biol Blood Marrow Transplant. 2008;14(Supplement 1):6–11. doi: 10.1016/j.bbmt.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24(3):277–313. doi: 10.1016/j.echo.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2(5):356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 33.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63(25 Pt A):2751–68. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 34.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37(15):1196–207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boynton SJ, Geske JB, Dispenzieri A, Syed IS, Hanson TJ, Grogan M, et al. LGE Provides Incremental Prognostic Information Over Serum Biomarkers in AL Cardiac Amyloidosis. JACC Cardiovasc Imaging. 2016;9(6):680–6. doi: 10.1016/j.jcmg.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Syed IS, Glockner JF, Feng D, Araoz PA, Martinez MW, Edwards WD, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3(2):155–64. doi: 10.1016/j.jcmg.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 37.Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation. 2015;132(16):1570–9. doi: 10.1161/CIRCULATIONAHA.115.016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gertz MA, Landau H, Comenzo RL, Seldin D, Weiss B, Zonder J, et al. First-in-Human Phase I/II Study of NEOD001 in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction. J Clin Oncol. 2016;34(10):1097–103. doi: 10.1200/JCO.2015.63.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30(9):989–95. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]