Abstract
Health care systems primarily focus on patients after they present with disease, not before. The emerging field of precision health encourages disease prevention and earlier detection by monitoring health and disease based on an individual’s risk. Active participation in health care can be encouraged with continuous health-monitoring devices, providing a higher-resolution picture of human health and disease. However, the development of monitoring technologies must prioritize the collection of actionable data and long-term user engagement.
INTRODUCTION
Disease often begins with a period of subclinical decline before progressing to symptoms that lead a person to seek medical care. The U.S. health care system has traditionally focused on reacting to disease rather than prevention, treating people after they become patients. Although disease treatment has evolved with precision medicine to look beyond clinical symptoms and incorporate individual molecular variability (1), the ballooning cost of treating chronic and late-stage diseases has led to a renewed focus on disease prevention or “pre-patient” care. Preventive health care seeks to augment reactive medicine by preempting disease through preventive measures and early detection. Although not a new concept (2), modern day technological advances have created a unique opportunity to finesse preventive medicine with personalized data. Building on precision medicine, precision health, an active practice of personalized health, can change the custom of society so that the individual is empowered to prevent their own disease.
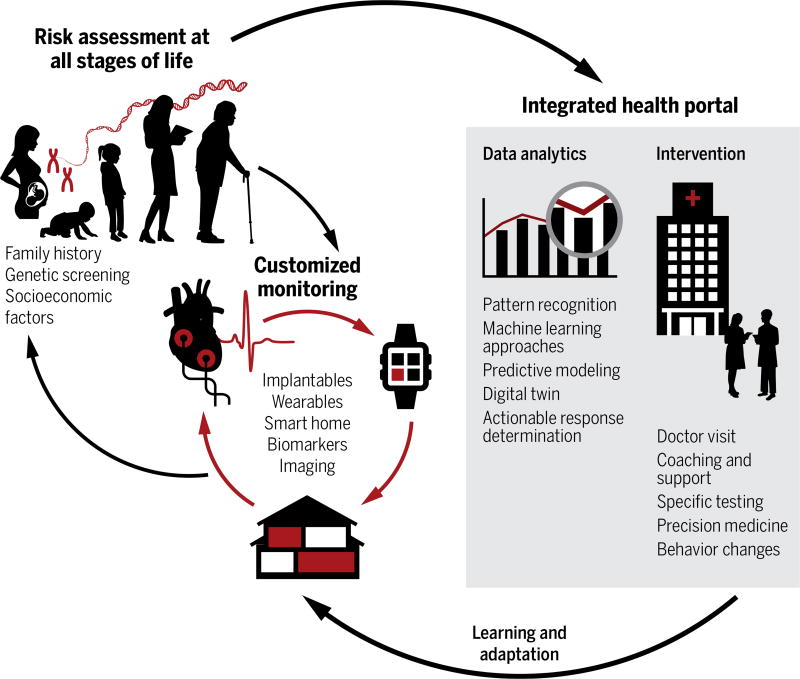
Precision health draws on the experience of another field in which prevention is paramount: aircraft engine health. Modern jet engines are constantly surveilled by hundreds of sensors to prevent engine failure. Meanwhile, the average American adult visits a health care provider fewer than four times a year (3). Engine repairs are forecasted by “digital twins” (4)—ultrahigh fidelity individual simulations that feed a physics-based engine model with terabytes of real-world sensor data. However, in the current paradigm of human health care, early disease detection is based on yearly screenings that coarsely target individuals at risk based on age and family history only. The foundation of precision health will one day rest on a similar ability to model human health and use real-world data to predict disease, revolutionizing our conception of health care (Fig. 1).
Fig. 1. Precision health care overview.
Many different components of health care can contribute to precision health. A person can be genotyped at or before birth to help determine their unique disease risk. This information allows customization of the health-monitoring devices that a person wears or uses in their home. Wearable and household monitors can measure environmental and physiological parameters and transmit data to the integrated health portal (shaded rectangle). The portal can assist with health decision-making by analyzing data to suggest an actionable response to the appropriate type of health care provider. Simple, low-risk interventions, such as diet or weight change, can be presented directly to the patient along with education and lifestyle guidance. Other interventions may require the involvement of family or caretakers. More complex disease and therapy management involves the physician, hospital, and outpatient centers. Customized monitoring and intervals of surveillance can be adjusted appropriately on a continual basis. Ultimately, precision health aims to engage patients in maintenance of their own health to prevent a return to the hospital.
In this paradigm, an individual is assessed for disease risk based on their genetic profile and family history—beginning even before birth (5). On the basis of their personalized risk profile, they are prescribed continuous health monitors to gather health data ranging from environmental and behavioral (pollution and physical activity) to physiological and biochemical (blood pressure and inflammatory biomarkers). Rather than relying on snapshots from infrequent office visits, these devices, worn on the body and present in the home, will aggregate and tailor longitudinal data to generate a real-time movie of an individual’s health. In turn, their health surveillance dynamically adapts to address changes in environment and physiology. Data are integrated and analyzed within a health portal and delivered as actionable biofeedback and guidance, allowing the individual to actively participate in their own health care.
The many disciplines of precision health cannot all be addressed within the scope of this article. Areas such as data transmission and security, quality control, and integration into existing health system networks have been addressed in-depth elsewhere (6–8). Here, we focus on the following aspects (Fig. 1): (i) modeling disease prediction, (ii) developing sensors for continuous monitoring of both basic physiologic signs and more complex biochemistry, and (iii) delivering information and guidance through the health portal. Finally, we discuss how the maturation of precision health is challenged by (iv) the human aspect of behavior. A jet engine cannot reject its sensors, whereas long-term human engagement in health monitoring may prove challenging.
MODELING DISEASE PREDICTION
Disease monitoring and risk prediction are two sides of the same coin: Although current screenings may overlook uncommon diseases, it would be inefficient and burdensome to comprehensively monitor the entire population. Disease risk has traditionally been evaluated on the basis of patient age and family history and more recently through genetic screening. Genome-wide association studies have allowed for more in-depth exploration of the genotypic landscape, advancing beyond monogenic disorders to complex diseases, such as diabetes and heart disease (9). However, many commonly identified genetic variants have only modest effects on disease. Thus, the “exposome,” the nongenetic exposures that affect human health and disease, must not be ignored. The exposome is vast and includes environmental factors, such as diet, pollution, and stress, as well as biological factors, such as hormones, inflammation, and the gut microbiome (10, 11). The exposome modulates the context of the genome, and both must be considered to keep one’s disease risk profile updated.
This wealth of genomic, microbiomic, and other -omic data must be translated into clinically actionable models of risk assessment (12). An early iteration of such a system is seen in M. Snyder’s integrative Personal Omics Profile, which combines several -omic analyses into a single framework, to compare health versus disease states and assess risk (13). This type of model requires a comprehensive understanding of the mechanisms of human disease (14), if we are to ever have a digital twin for disease prediction. Even if the data collected does not lead to intervention on disease, the information can still be used to update one’s disease risk profile or trajectory. To this end, the Project Baseline (www.projectbaseline.com, Verily Inc.) is one example of a study that is collecting data from thousands of “healthy” participants, some of whom will eventually transition to a state of illness, to lay the foundation for modeling human health and disease (15).
CONTINUOUSLY MONITORING THE HUMAN “MACHINE”
Precision health seeks to make health care contact more accessible by integrating monitoring and diagnostics into everyday life. Wearable health sensors have exploded in popularity over the past few years, forecasted to grow from a market of $50 million in 2013 to more than $650 million in 2020 (16). Although there will be costs associated with the implementation of wearables, remote patient monitoring technologies have been estimated to save our health care system nearly $200 billion over the next 25 years (17). Furthermore, implementation costs typically decrease substantially over time with advancements in development, scaling, and integration. Here, we provide an overview of emerging wearable technologies (Fig. 2A). Many standalone devices integrate with smartphones to facilitate data connection and processing, and developments and challenges within the field of mobile health have been thoroughly described by Steinhubl et al. (7) in a recent review.
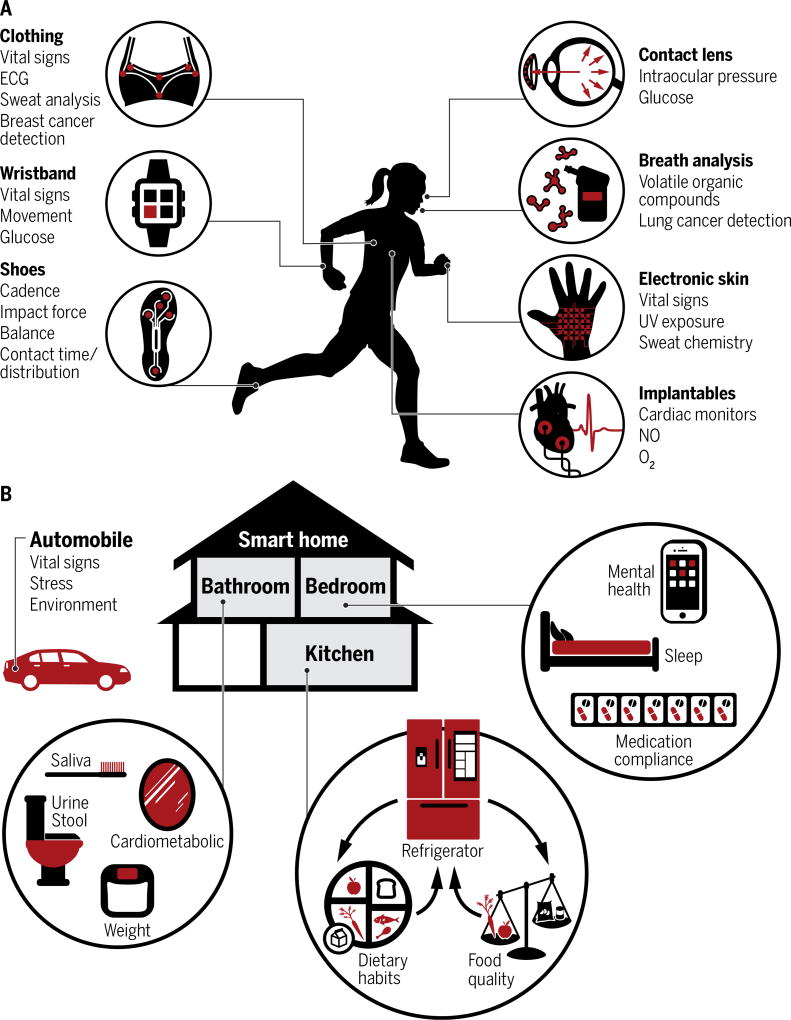
Fig. 2. Monitoring on the body and in the home.
A selection of wearable, implantable, and home devices are shown to demonstrate the variety of physiological and molecular parameters that can be measured using these devices. (A) Physiological monitoring has become ubiquitous but rarely provides the specificity needed for diagnostics. Monitoring of complex molecular parameters, such as biomarkers, is required for tracking specific disease states, but there are much fewer devices available with this capability. (B) Devices in the home or automobile are capable of passively monitoring biological fluids, human behavior, and physiological signs. These measurements range from dietary habits to urine and stool analysis. Passive monitoring approaches permit high-frequency monitoring without requiring a change in user behavior. UV, ultraviolet; ECG, electrocardiography.
One in 10 American adults already engage in health monitoring with activity trackers, such as wrist-worn Fitbits and Apple Watches (18). Miniaturized devices can measure simple parameters, such as heart rate or pulse oximetry, as well as more advanced measurements, such as electrocardiography (ECG). For example, instead of a bulky Holter ECG monitor with multiple leads, a patient can now wear a single ECG patch to detect arrhythmias (19). More comprehensive coverage of the body can be achieved by integrating sensors into clothing by weaving them into electronic textiles (20). Motion artifact can be addressed by sensors that maintain robust contact with the skin, such as the “epidermal electronics” designed by Kim et al. (21, 22). These flexible electronics are delivered on thin “temporary tattoo” membranes mimicking the mechanical properties of the skin (21) and measure parameters ranging from vital signs to environmental factors, such as ultraviolet exposure (22). Other emerging technologies integrate diagnostics to detect diseases, such as cancer. For instance, circadian temperature changes in the breast have been shown to correlate with breast cancer, and a clinical trial is under way to study a bra with integrated thermal sensors for breast cancer screening (23). Our own laboratory is developing a photoacoustics-based “smart bra” that uses the endogenous photoacoustic signal from hemoglobin to track increased vascularity and detect early breast cancer (24).
PASSIVE MONITORING AND THE SMART HOME: THE ENGINE THAT NEVER IDLES
Many factors will affect compliance with the use of continuous health-monitoring devices, such as bulk, comfort, and ease of use. Wearable devices are easily ignored and are useless if not actually worn. Implantable monitors (25, 26) circumvent this problem, but even minimally invasive devices may be met with resistance. Fortunately, noninvasive high-frequency health monitoring can be achieved passively during routine daily activities, overcoming the obstacle of actively modifying human behavior (Fig. 2B).
In a potential “smart home,” one could sleep on bed sheets that monitor cardiopulmonary function (27), look into a mirror that measures one’s vital signs using radar, use a toothbrush that performs biochemical analysis of one’s saliva (28, 29), and then have a smart toilet automatically analyze one’s urine and stool for markers of disease. During the daily commute, sensors in the car can monitor stress levels and drowsiness through driving behavior, discourage driving if alcohol is detected in the breath, or detect outdoor pollution levels. Patterns of smartphone use reflecting depression or anxiety, such as a decrease in texting frequency, can alert the user to proactively address mental health through integrated self-care tools (30).
These proposed sensors are not as farfetched as they may seem. Contactless sensors and cameras have been demonstrated in the Wize Mirror, a mirror that uses visual data to track weight gain and signs of stress, multispectral cameras to analyze skin tissue for cholesterol levels, and gas sensors to analyze the breath for alcohol or tobacco (31). Urine contains biomarkers, such as metabolites, proteins, exosomes, and nucleic acids, that provide rich information on diseases ranging from urinary tract infections to bladder and prostate cancer. Similarly, stool provides diagnostic information for diseases, including inflammatory bowel disease and colorectal cancer, representing potential alternatives to colonoscopy. The commercial TOTO “Intelligence Toilet” collects urine in a reservoir and tests for glucose levels to monitor diabetes (32). Our laboratory is also developing a smart toilet to sample both urine and stool to detect various biomarkers, including tumor DNA in stool for early detection of colorectal cancer.
CHALLENGES IN MOLECULAR MONITORING: UNDERSTANDING THE HUMAN MACHINE
The devices discussed thus far monitor parameters ranging from basic vital signs to sophisticated biomarkers. Passive measurement techniques are more conducive to capturing physiological parameters, which can inform about trends in general health, but are rarely diagnostic. However, molecular monitoring allows us to track specific disease states and perhaps even to identify disease before the appearance of symptoms. For example, circulating tumor cells or DNA in the blood can provide integral information about the underlying tumor and help guide cancer treatment. Although continuous blood sampling may be impractical, some biomarkers can be noninvasively measured from other biological materials, including saliva, urine, and stool, as well as sweat, interstitial fluid, tears, menstrual fluid, and even breath. Many technologies for real-time tissue and biofluid monitoring have been discussed in a comprehensive review (33).
Yet oftentimes, the challenge is not in how to monitor a biomarker, but in knowing which biomarker to monitor and understanding how it correlates with disease prognostication. For example, the pursuit of noninvasive glucose monitoring has been hindered by the challenge of accurately correlating peripheral glucose measurements with blood glucose. A staggering variety of strategies have been investigated to reduce the burden of repeated finger pricks for glycemic monitoring, ranging from enzymatic sampling of interstitial fluid to measuring tear glucose levels through a contact lens (34). Although these are promising routes for noninvasive monitoring, the correlation between interstitial and tear glucose with blood glucose is imprecise and suffers from a physiologic lag time, impairing their clinical utility (35). These profound technological advancements are outpacing the development of an accurate biological model. Compared to the “glass box” of the jet engine whose physics can be modeled in a digital twin, the biology of many human diseases remains relatively opaque.
One area of biomarker analysis is circumventing the problem of defining the exact biology of the disease. Volatile organic compounds (VOCs) are easily sampled from breath, as well as from blood, skin, urine, and feces, to diagnose disease, such as respiratory and urinary tract infections (36). These diagnoses have primarily relied on identifying specific gas-phase disease markers, but for most diseases, it is challenging to identify a truly specific set of biomarkers, assuming one even exists. Recently, VOC analysis has turned toward “electronic noses” or chemical sensors that analyze patterns of unidentified VOC biomarkers. These sensors have been used to distinguish the “smellprints” of diseases, including lung and ovarian cancer (37). Thus, in a successful diagnostic technology, (i) data from continuous monitoring technologies will help to improve understanding of disease and allow more intelligent selection of a biomarker; (ii) a biochemical marker is likely to be more disease-specific than a physiologic marker; and (iii) in the absence of a specific biomarker for a given disease, machine learning/ artificial intelligence may be able to recognize highly specific biochemical patterns.
SIZING DOWN BIG DATA FOR THE INTEGRATED HEALTH PORTAL
Data analytics and pattern recognition should be similarly applied to parse the large, complex data sets from these continuous monitoring devices. “Big data” has used machine learning to create predictive analytics platforms, such as GE and Intel’s Predix, for health care. Precision health data and analytics would use data ecosystems, such as SMART on FHIR (substitutable medical applications and reusable technologies on fast health care interoperability resources), to assimilate with existing health information systems (8), creating a health portal to decipher data trends and present actionable items (Fig. 1). The health portal could perform analysis on diet, genetics, and cardiometabolic health to keep a user informed of their risk for heart disease and encourage diet and exercise or detect a pattern of weight loss and rising stool biomarker levels to alert the patient and physician to screen for colorectal cancer. The portal’s back-end analytics would ideally provide clinical decision support and minimize the decision burden on health care providers. Although low-risk lifestyle modifications may be appropriate to present directly to the patient, it is critically important that the health care team, including physicians, remains involved to confirm diagnoses and higher-level decisions. Improvements in analytics to minimize false positives will help to alleviate “alert fatigue” (becoming desensitized from too many alerts), but there must be a conscious effort to present information selectively and intelligently to prevent subpar utilization.
MAINTAINING PRECISION HEALTH
Precision health promises more data for better diagnostics, but worrisome health information could hypothetically increase anxiety when there is no solution offered alongside. However, recent studies have demonstrated no significant anxiety, depression, or test-related distress from informing patients of their risk of developing Alzheimer’s disease (38), and other projects seek to study the psychosocial impact of receiving genetic information in infancy “without regard for actionability” (39). Many are additionally concerned about the life-long ramifications of identifying their disease risk. Genetic discrimination from employers and health insurance has been prohibited by federal law since 2008 with the Genetic Information Nondiscrimination Act, or GINA, but 80% of Americans were still unaware of GINA in 2014 (40). GINA’s regulation of risk classification does not currently extend to other domains, such as life insurance, but as genomic information permeates modern society, legislation will likely pursue more comprehensive nondiscrimination. Policy for data security and privacy has also been investigated. HIPAA (Health Insurance Portability and Accountability Act) regulations protect health information under certain entities, such as health care providers; however, non- HIPAA entities, such as medical device companies, have no obligation to enforce secure communications. A recent 2016 governmental report found that there is not yet a widely adopted standard for health data security in the private sector (41), but these investigations and advisory committees will lay the groundwork to keep policy in pace with technology.
Precision health is also based on the active practice of personalized health. However, despite the popularity of wearables and enthusiasm about the “quantified self,” one-third of U.S. consumers used their wearable activity tracker for no more than 6 months before discontinuing use (18). Thus, the “behavioral economics” of health monitoring must be addressed (42) and should focus on removing barriers rather than changing behavior. Adoption can be most successfully facilitated by emphasizing passive and unobtrusive monitoring (home devices or epidermal electronics) (18). Devices can also use “hooks” and “nudges” to emphasize immediate and future benefits, such as through goal formation or gamification of health activities. For example, financial incentives for participation in a health promotion program have been shown to result in an increase in fitness activity and decrease in hospital admissions (43).
The same considerations of adoption and maintenance must be taken on the physician’s side. Many of these new and intriguing technologies will likely be rapidly adopted while the evidence base for their benefit is still growing. The effect of refined disease risk prediction and monitoring must be followed with well-designed clinical trials to determine whether there is a true benefit in outcomes, but the data can be used regardless of the outcome to further our understanding of disease, as with Project Baseline. Precision health must be just that and ought to be applied precisely: Not all diseases will benefit from the same degree of monitoring, and not all patients will be monitored the same for a given disease based on their risk.
The future direction of precision health and integrated diagnostics as detailed here may someday become the future of health care. Such a future will likely take several decades to realize, but it is a long-term goal worthy of pursuit. Several major challenges must be tackled, including accurately predicting disease risk, discovering biomarkers of health as well as early disease, developing back-end data analytics to accurately distinguish true from false positives, and understanding the behavioral economics of engagement with precision health. However, even if only a subset of the strategies discussed here are eventually fully realized, their potential to affect human health and health care economics warrants further study and increased research investment.
Footnotes
Competing interests: S.S.G. is co-founder and holds stock options for CellSight Technologies and Endra Inc. S.S.G. is on the Scientific Advisory Board, receives grants, or is a consultant for Grail Inc., MagArray Inc., ImaginAb, GE Medical, Philips Medical, Pliant, PureTech, Biogen, Vor Biopharma, Click Diagnostics, Stage 0, Verily, Infinitus, Vave, Nine Point Medical, Reflexion, Visualsonics, Bracco Inc., and CytomX Therapeutics Inc.
References
- 1.Collins FS, Varmus H. A new initiative on precision medicine. N. Engl. J. Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terris M. Evolution of public health and preventive medicine in the United States. Am. J. Public Health. 1975;65:161–169. doi: 10.2105/ajph.65.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summary Health Statistics: National Health Interview Survey. 2014 [Google Scholar]
- 4.Tuegel EJ, Ingraffea AR, Eason TG, Spottswood SM. Reengineering aircraft structural life prediction using a digital twin. Int. J. Aerosp. Eng. 2011;2011:154798. [Google Scholar]
- 5.Vermeesch JR, Voet T, Devriendt K. Prenatal and pre-implantation genetic diagnosis. Nat. Rev. Genet. 2016;17:643–656. doi: 10.1038/nrg.2016.97. [DOI] [PubMed] [Google Scholar]
- 6.Al Ameen M, Liu J, Kwak K. Security and privacy issues in wireless sensor networks for healthcare applications. J. Med. Syst. 2012;36:93–101. doi: 10.1007/s10916-010-9449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinhubl SR, Muse ED, Topol EJ. The emerging field of mobile health. Sci. Transl. Med. 2015;7:283rv3. doi: 10.1126/scitranslmed.aaa3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alterovitz G, Warner J, Zhang P, Chen Y, Ullman-Cullere M, Kreda D, Kohane IS. SMART on FHIR Genomics: Facilitating standardized clinicogenomic apps. J. Am. Med. Inform. Assoc. 2015;22:1173–1178. doi: 10.1093/jamia/ocv045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manolio TA. Bringing genome-wide association findings into clinical use. Nat. Rev. Genet. 2013;14:549–558. doi: 10.1038/nrg3523. [DOI] [PubMed] [Google Scholar]
- 10.Stingone JA, Buck Louis GM, Nakayama SF, Vermeulen RCH, Kwok RK, Cui Y, Balshaw DM, Teitelbaum SL. Toward greater implementation of the exposome research paradigm within environmental epidemiology. Annu. Rev. Public Health. 2017;38:315–327. doi: 10.1146/annurev-publhealth-082516-012750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes E, Li JV, Marchesi JR, Nicholson JK. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012;16:559–564. doi: 10.1016/j.cmet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Bromberg Y. Building a genome analysis pipeline to predict disease risk and prevent disease. J. Mol. Biol. 2013;425:3993–4005. doi: 10.1016/j.jmb.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Snyder M. Promise of personalized omics to precision medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013;5:73–82. doi: 10.1002/wsbm.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hood L, Friend SH. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. 2011;8:184–187. doi: 10.1038/nrclinonc.2010.227. [DOI] [PubMed] [Google Scholar]
- 15.Barr A. Google’s new moonshot project: The human body. The Wall Street Journal. 2014 Jul 27; [Google Scholar]
- 16.Motion sensors set to capture global wearable sensor market. Transparency Market Research. 2015 [Google Scholar]
- 17.Litan RE. Vital signs via broadband: Remote health monitoring transmits savings, enhances lives. Better Health Care Together. 2008 [Google Scholar]
- 18.Ledger D, McCaffrey D. Inside Wearables: How the Science of Human Behavior Change Offers the Secret to Long-term Engagement. Endevour Partners. 2014 [Google Scholar]
- 19.Barrett PM, Komatireddy R, Haaser S, Topol S, Sheard J, Encinas J, Fought AJ, Topol EJ. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am. J. Med. 2014;127:95.e11–95.e17. doi: 10.1016/j.amjmed.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuhisa N, Kaltenbrunner M, Yokota T, Jinno H, Kuribara K, Sekitani T, Someya T. Printable elastic conductors with a high conductivity for electronic textile applications. Nat. Commun. 2015;6:7461. doi: 10.1038/ncomms8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D-H, Lu N, Ma R, Kim Y-S, Kim R-H, Wang S, Wu J, Won SM, Tao H, Islam A, Yu KJ, Kim T-i, Chowdhury R, Ying M, Xu L, Li M, Chung H-J, Keum H, McCormick M, Liu P, Zhang Y-W, Omenetto FG, Huang Y, Coleman T, Rogers JA. Epidermal electronics. Science. 2011;333:838–843. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- 22.MC10 Incorporated. L’Oreal debuts first-ever stretchable electronic UV monitor at the 2016 consumer electronics show. 2016 www.prnewswire.com/news-releases/loreal-debuts-first-ever-stretchable-electronic-uv-monitor-at-the-2016-consumer-electronics-show-300200110.html.
- 23.Cyrcadia Health, Circadian thermal sensing to detect breast disease (ClinicalTrials.gov, 2015) https://clinicaltrials.gov/ct2/show/NCT02511301?term=NCT02511301&rank=1.
- 24.Eisenbrey JR, Merton DA, Marshall A, Liu J-B, Fox TB, Sridharan A, Forsberg F. Comparison of photoacoustically derived hemoglobin and oxygenation measurements with contrast-enhanced ultrasound estimated vascularity and immunohistochemical staining in a breast cancer model. Ultrason. Imaging. 2015;37:42–52. doi: 10.1177/0161734614527435. [DOI] [PubMed] [Google Scholar]
- 25.Montero-Baker MF, Au-Yeung KY, Wisniewski NA, Gamsey S, Morelli-Alvarez L, Mills JL, Sr, Campos M, Helton KL. The First-in-Man “Si Se Puede” study for the use of micro-oxygen sensors (MOXYs) to determine dynamic relative oxygen indices in the feet of patients with limb-threatening ischemia during endovascular therapy. J. Vasc. Surg. 2015;61:1501–1510. e1. doi: 10.1016/j.jvs.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 26.Iverson NM, Barone PW, Shandell M, Trudel LJ, Sen S, Sen F, Ivanov V, Atolia E, Farias E, McNicholas TP, Reuel N, Parry NMA, Wogan GN, Strano MS. In vivo biosensing via tissue localizable near infrared fluorescent single walled carbon nanotubes. Nat. Nanotechnol. 2013;8:873–880. doi: 10.1038/nnano.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishijima M. Cardiopulmonary monitoring by textile electrodes without subject-awareness of being monitored. Med. Biol. Eng. Comput. 1997;35:685–690. doi: 10.1007/BF02510978. [DOI] [PubMed] [Google Scholar]
- 28.Wong DT, Segal A, Wong DT. Salivary diagnostics: enhancing disease detection and making medicine better. Eur. J. Dent. Educ. 2008;12:22–29. doi: 10.1111/j.1600-0579.2007.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong D. Saliva Liquid Biopsy for Cancer Detection, paper presented at the American Association for the Advancement of Science 2016 Annual Meeting. Washington, DC: Feb 11 to 15, 2016. [Google Scholar]
- 30.Matheson R. Mental-health monitoring goes mobile. MIT News. 2014 Jul 16; http://news.mit.edu/2014/mental-health-monitoring-goes-mobile-0716)
- 31.Colantonio S, Coppini G, Germanese D, Giorgi D, Magrini M, Marraccini P, Martinelli M, Morales MA, Pascali MA, Raccichini G, Righi M, Salvetti O. A smart mirror to promote a healthy lifestyle. Biosyst. Eng. 2015;138:33–43. [Google Scholar]
- 32.Johnson J. Intelligent toilets, smart couches and the house of the future. Financial Post. 2012 Jun 6; http://business.financialpost.com/uncategorized/intelligent-toilets-smart-couches-and-the-house-of-the-future.
- 33.Rogers ML, Boutelle MG. Real-time clinical monitoring of biomolecules. Annu. Rev. Anal. Chem. 2013;6:427–453. doi: 10.1146/annurev.anchem.111808.073648. [DOI] [PubMed] [Google Scholar]
- 34.Yao H, Liao Y, Lingley AR, Afanasiev A, Lähdesmäki I, Otis BP, Parviz BA. A contact lens with integrated telecommunication circuit and sensors for wireless and continuous tear glucose monitoring. J. Micromech. Microeng. 2012;22:075007. [Google Scholar]
- 35.Lane JD, Krumholz DM, Sack RA, Morris C. Tear glucose dynamics in diabetes mellitus. Curr. Eye Res. 2006;31:895–901. doi: 10.1080/02713680600976552. [DOI] [PubMed] [Google Scholar]
- 36.Turner APF, Magan N. Electronic noses and disease diagnostics. Nat. Rev. Microbiol. 2004;2:161–166. doi: 10.1038/nrmicro823. [DOI] [PubMed] [Google Scholar]
- 37.Kahn N, Lavie O, Paz M, Segev Y, Haick H. Dynamic nanoparticle-based flexible sensors: Diagnosis of ovarian carcinoma from exhaled breath. Nano Lett. 2015:7023–7028. doi: 10.1021/acs.nanolett.5b03052. [DOI] [PubMed] [Google Scholar]
- 38.Green RC, Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Brown T, Eckert SL, Butson M, Sadovnick AD, Quaid KA, Chen C, Cook-Deegan R, Farrer LA REVEAL Study Group. Disclosure of APOE genotype for risk of Alzheimer’s disease. N. Engl. J. Med. 2009;361:245–254. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frankel LA, Pereira S, McGuire AL. Potential psychosocial risks of sequencing newborns. Pediatrics. 2016;137(suppl. 1):S24–S29. doi: 10.1542/peds.2015-3731F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green RC, Lautenbach D, McGuire AL. GINA, genetic discrimination, and genomic medicine. N. Engl. J. Med. 2015;372:397–399. doi: 10.1056/NEJMp1404776. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services. Examining oversight of the privacy and security of health data collected by entities not regulated by HIPAA. U.S. Department of Health and Human Services; 2016. [Google Scholar]
- 42.Rice T. The behavioral economics of health and health care. Annu. Rev. Public Health. 2013;34:431–447. doi: 10.1146/annurev-publhealth-031912-114353. [DOI] [PubMed] [Google Scholar]
- 43.Patel D, Lambert EV, da Silva R, Greyling M, Kolbe-Alexander T, Noach A, Conradie J, Nossel C, Borresen J, Gaziano T. Participation in fitness-related activities of an incentive-based health promotion program and hospital costs: A retrospective longitudinal study. Am. J. Health Promot. 2011;25:341–348. doi: 10.4278/ajhp.100603-QUAN-172. [DOI] [PubMed] [Google Scholar]