Abstract
Precise drug delivery to tumors with low system toxicity is one of the most important and challenging tasks for pharmaceutical researchers. Despite progress in the field of nanotherapeutics, the use of artificially synthesized nanocarriers still faces several challenges, including rapid clearance from blood circulation and limited capability of overcoming multiple physiological barriers, which hamper the clinical application of nanoparticle-based therapies. Since leukocytes (including monocytes/macrophages, neutrophils, dendritic cells and lymphocytes) target tumors and can migrate across physiological barriers, leukocytes are increasing utilized as carriers to transfer nanoparticles to tumors. In this review we specifically focus on the molecular and cellular mechanisms of leukocytes that can be exploited as a vehicle to deliver nanoparticles to tumors and summarize the latest research on how leukocytes can be harnessed to improve therapeutic end-points. We also discuss the challenges and opportunities of this leukocyte-derived nanoparticle drug delivery system.
KEY WORDS: Leukocytes, Tumor, Biomimetic carrier, Nanoparticulate drug delivery systems, Nanotherapeutics, Cancer therapy
Graphical abstract
This review summarizes the biological features and recent preclinical investigations of leukocyte-derived nanoparticulate delivery vehicles with special focus on the cell-mediated approaches that have been developed to overcome the physiological barriers for drug delivery.
1. Introduction
Nanotechnology has made a great impact on medicine, especially on cancer therapy, over the past several decades1, 2, 3. Doxil®, the doxorubicin HCl liposome injection, is used to treat over 300,000 patients annually for Kaposi's sarcoma and ovarian cancer after failure or intolerance to prior systemic chemotherapy4, 5. The introduction of nanotechnology enables a spatiotemporally-specified drug release pattern in cancer therapy and thus improves the pharmacokinetics of drugs, reduces adverse-effects of traditional chemotherapeutics administration, and contributes to better compliance in patients6. Nanotechnology offers another chance for chemical and biological entities that were once excluded due to their toxicity, rapid clearance, and off-target deposition7.
Nanoparticulate drug delivery systems are mainly constructed of synthetic lipid or polymer-based macromolecules that either physically encapsulate or chemically conjugate the drugs8. Despite the enormous progress in the field of nanotherapeutics, the use of artificially synthesized nanocarriers still faces several challenges, including rapid clearance from blood circulation, off-target effects and ineffectively nanoparticle transfer in patients with advanced forms of cancer6, 9. Most nanoparticle formulations are administered systemically, and accumulate in the tumor mainly via enhanced permeability and retention (EPR) effects1, 10, which is generally thought to be the result of intra-tumor leaky vasculature and poor lymphatic drainage in the tumor region. However, data derived from clinical experiments suggest that the EPR effects in patients are limited11, 12, 13. Furthermore, nanoparticles will encounter multiple physiological barriers that influence their effectiveness, such as blood circulation, nanoparticle-protein interaction, extravasation into tumor tissue or the tumor microenvironment (TME), phagocytic sequestration and renal clearance13, 14. Therefore, new tactics are needed to improve the therapeutic performance of nanoparticles.
To overcome these obstacles and push the limits of nanoparticle performance, there has been a recent paradigm shift towards cell-based strategies in carrier design15. In contrast to the relatively simple components and structures of nanocarriers, cells have a wealth of tactics to avoid attack from the immune system16; furthermore, nanocarriers are able to cross impermeable biological barriers and target specific regions6. Owing to these attractive features, cell-based targeting tactics are very exciting for the field of drug delivery due to their high specificity and long-term persistence.
Using mammalian autologous or donor-matched cells as the drug carriers has been proposed as a potential approach to efficiently deliver therapeutics to target tissues, and has gained considerable attention from researchers9. Red blood cells (RBC) and leukocytes are the most thoroughly investigated cell types. Owing to a long lifetime of nearly 3–4 months in body, RBC membrane coating has emerged as a promising method to prolong the circulation time of nanoparticles in the body. However, this approach lacks the ability to specifically target tumors. Since then, leukocytes have attracted attention. They function as the “military forces” in the body, capturing and destroying foreign targets that have been recognized as “invaders”. Furthermore, the inherit homing ability of leukocytes to inflamed/tumor regions makes them promising carrier candidates for targeting delivery of chemotherapeutics and TME regulators17.
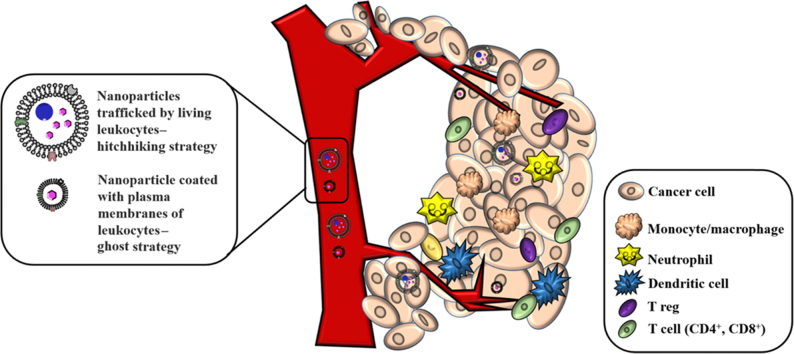
One applicable strategy of leukocyte-derived drug delivery is to take advantage of the biocompatibility and bio-functions of living leukocytes to extend the in vivo lifetime of drugs and to use leukocytes to target inflamed tissues for site-specific drug delivery. To this end, nanoparticles can be either incorporated or surface-immobilized on leukocytes in a “hitchhiking strategy” (Fig. 1). The other approach is to coat nanoparticles with leukocyte-derived membrane components, which is generally known as a “ghost-cell” strategy (Fig. 1). The “ghost cell” still preserves the intact membrane proteolipid components on the surface after an extraction and isolation process. The nanoparticles coated with plasma membranes18 or cell-derived extracellular vesicles19 can preserve the physicochemical properties of synthetic nanomaterials while acquiring complex cellular functions derived from leukocytes.
Figure 1.
The design schematic of leukocyte-dependent drug delivery and leukocyte infiltration into tumors. As depicted, nanoparticles can either be trafficked by living leukocytes, known as “hitchhiking strategy” or coated with plasma membranes of leukocytes, namely “ghost strategy”.
Here we review recent progress on leukocyte-derived nanoparticulate drug delivery systems. We start with an overview of features of leukocytes—monocytes/macrophages, neutrophils, dendritic cells and lymphocytes—that favor nanoparticle drug delivery, and also summarize recent applications that show how researchers design delivery platforms based on these features. At the end, we point out the challenges and opportunities of applications that use leukocytes in the construction of nanoparticulate drug delivery systems.
2. Cellular and molecular mechanisms involved in tumor targeting of leukocytes
In every step of tumor progression, leukocytes are recruited into the TME through leukocyte infiltration/extravasation20, and participate in the regulation of immune surveillance21. The infiltration is regulated by various chemokines and cytokines produced by tumor cells and other cells that occupy the TME8. Once leukocytes infiltrate into tumor tissues they establish an inflammatory microenvironment22, where leukocytes are engaged in a dynamic and extensive crosstalk with surrounding tumor cells23. Since tumor-infiltrating leukocytes are indispensable components in the progression of the tumor and TME, and each type of cell has its own set of unique characteristics24, a deep understanding of the roles of different types of leukocytes that are involved in immune surveillance would help us to develop novel targeted delivery strategies to kill tumor cells and regulate the TME. We summarize the basic properties of leukocytes in Table 1.
Table 1.
Properties of monocytes/macrophage, neutrophils, dendritic cells and lymphocytes.
| Cell type | Diameter (μm) | Lifespan | Amount per microliter in human blood |
|---|---|---|---|
| Monocytes/macrophage | ~25 | 10–20 h | 0–800 |
| Neutrophil | 10–12 | 3–4 days | 1800–7700 |
| Dendritic cell | 6–12 | days to weeks | 3000–17,000 |
| Lymphocyte | 6–12 | B cells: 4 days up to 5 weeks | 1000–4800 |
| T cells: lasting months to years |
2.1. Monocytes/macrophages
Macrophages, derived from monocytes, are vital regulators of the innate immune system8. The typical feature of macrophages is that they tend to migrate toward pathological regions, typically inflammation sites and tumors, along chemoattractant gradients20. Tumors and the surrounding stroma cells can secret chemoattractants, such as colony stimulating factor-1 (CSF-1) and chemokine ligand 2 (CCL2)25, 26, to recruit macrophages and monocytes to migrate to tumor tissue. Macrophages also tend to localize in hypoxic areas. Therefore, this tumor-tropic property of macrophages can be employed in the delivery of drug/diagnosis agents. Moreover, extracellular materials such as polysaccharides, complement, endotoxins and Fc-segment of immunoglobulins and low density lipoproteins can be recognized and internalized by macrophages, providing an opportunity to load nanoparticles into macrophages27. As a result, macrophages are attractive as carriers for therapeutic delivery.
Owing to intracellular degradation, loading bare drug directly into macrophages would probably lead to premature drug inactivation combined with uncontrolled drug release27, result in limited drug content in the desired sites. Incorporating drug into nanoparticles would be helpful to reduce the disintegration of drug inside cells. When macrophages are employed as delivery platform, they could be loaded first with the nanoparticulate drug delivery system ex vivo followed by re-infusion back into the host to distribute their contents to tissues where macrophages home.
Monocytes and macrophages can transform into tumor-associated macrophages (TAM) which can infiltrate into hypoxic areas of tumors15. Conventional nanoparticles only have limited penetration into the deep sites of tumors because of the elevated density of the extracellular matrix, high interstitial pressure, and the intra-tumoral discontinuous vasculature, which hinders the extravasation of nanoparticles through enhanced permeation-retention effects1, 28. Thus, the co-incubation of drug-encapsulated nanocarriers with TAMs may be a practical strategy to specifically carry anti-cancer agents to the deep areas of tumors.
2.2. Neutrophils
Neutrophils are the most abundant leukocytes and are generated from the bone marrow29. Daily production of neutrophils in humans is 2 × 1011 and they represent 50%–70% of white blood cells20. Importantly, neutrophils are the first arrivals to sites of inflammation via transmigration across blood vessel walls, and play a critical role in the host innate immune responses to infections or tissue damage20, 30. The recruitment and activation of neutrophils is mainly mediated by several adhesion molecules, such as LFA-1 and β1-integrin31, 32, which are highly expressed on the surface of both neutrophils and the endothelium.
During the inflammation, circulating neutrophils first recognize selectins expressed on the blood vessel epithelium, and then roll over and crawl along the blood vessels. This movement of neutrophils facilitates their contact with chemokines on the endothelium surface, which initiates neutrophil activation29. Once neutrophils are fully activated, they travel to the inflamed sites via cellular junctions and transcellular pathways33.
Once neutrophils have infiltrated into tumor sites they are termed “tumor-associated neutrophils” (TANs) and can differentiate into two divergent phenotypes, the N1 and N2 type, dependent upon certain specific tumor-derived factors and with opposite effects on tumor development20. Granulocyte colony-stimulating factor (G-CSF), transforming growth factor-β (TGF-β) and interferon-β (IFN-β) are the best-studied factors in this process. G-CSF and TGF-β promote tumor growth and metastasis by regulating transcription factors inhibitors that manipulate the immunosuppressive functions of neutrophils34, 35. IFN-β can negatively regulate the pro-tumorigenic phenotype of neutrophils36. Cytokine concentration and tumor physiology (such as hypoxia) may also influence the neutrophil polarization37.
2.3. Dendritic cells
Dendritic cells (DCs) are known to connect innate and adaptive immune responses6. They are the most potent antigen-presenting cells (APCs) of the mammalian immune system and can present foreign antigens associated with major histocompatibility complexes (MHCs) on their surfaces to interact with lymphocytes and natural killer cells, and thus shaping the immune system38. Dendritic cells are derived from hematopoietic bone marrow progenitor cells and reside in tissues that are frequent exposed to external environment, such as the skin, nose, lungs, stomach and intestines39, 40.
DCs can exist in either mature or immature states and have many different subtypes. Immature dendritic cells can constantly sample the surrounding environment for pathogens such as viruses and bacteria. Once recognizing disease, vaccine, or pathogen-associated danger signals, they are activated and migrate to nearby lymph nodes (LNs) where they encounter and stimulate naïve T cells to differentiate to cytotoxic T cells (CTLs)41. After that, CTLs can eliminate pathogens or infected cells. The mature state of DCs is characterized by the migration of DCs to LN to present antigens of pathogens or tumor to T cells. There are multiple DC subtypes presenting in blood or residing in tissues. The two main DCs are myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) which originate from myeloid (CD34+) precursors and CD14+ monocyte precursors respectively. pDCs are mostly resident in the bloodstream, spleen, LN, and diseased tissues42, while mDCs are presented in resident tissues as well as spleen and LNs43.
Current DC therapies mainly focus on the administration of pDCs due to their accessibility in the bloodstream compared to mDCs44, even though mDCs have demonstrated excellent antigen cross-presentation capabilities resulting in an increased CTL response. Intra-tumoral DCs can express indoleamine-pyrrole 2,3-dioxygenase (IDO) and programmed cell death-ligand 1 (PD-L1), contributing to immune suppression and survival of tumors45, 46.
Given the central role DCs play in initiating immune responses and surveillance, investigators speculate that DCs could serve as an ideal platform for boosting endogenous anti-tumor responses, and induce the effective eradication of tumors. Although DCs are potent antigen presenting cells, they are not usually present in adequate quantity to allow for a robust immune response. Thus, the clinical therapeutic DCs for vaccination are usually derived from peripheral blood mononuclear cells and ex vivo pulsed with antigens, activated, and transfused back to the patient's bloodstream. DC preparations required shorter culture times and are less variable than those of T cells, usually 5 days to produce immature DCs from monocytes and a 24–48 h period during which immature DCs are activated and disease antigens are loaded. A single DC formulation can be used for up to 5 doses due to the low cell dose requirement.
2.4. Lymphocytes
Lymphocytes constitute about 30% of the white blood cells of humans6. There are three main subtypes of lymphocytes, T cells (which function in cell-mediated, cytotoxic adaptive immunity), B cells (for humoral, antibody-secretive adaptive immunity) and natural killer cells (which function in cell-mediated, cytotoxic innate immunity). The potential of B cells and NK cells have been specifically reviewed in other literature7, 47; in this review we focus on T cells.
T cells can recognize specific “non-self” antigens through antigen presentation48. Once an invader is identified, T cells can generate specific responses that are tailored to maximally eliminate specific pathogen or pathogen-infected cells. In response to pathogen infection, some T cells, named T helper cells (Th), generate cytokines that can alter the immune response, while other T cells, called cytotoxic T cells (CTLs), produce toxic granules that contain perforin and lytic enzymes which induce the death of pathogen-infected cells. After being activated, T cells leave a lasting legacy of the antigens they have encountered, in the form of memory T cells (Tm). Throughout the lifetime, these memory cells will remember each specific pathogen encountered, and are able to mount a strong and rapid response if the same pathogen is detected again; this is known as acquired immunity.
There are three main functions of T lymphocytes: 1) directly destroy foreign invaders (e.g. bacteria); 2) activate nearby immune cells through cytokine secretion; 3) amplify the B cell response. The functions of T cell subpopulations varies. For example, CTLs can detect the existence of infected/mutated cells and attack them directly; Th can promote the activation and vitality of CTLs; and Tm can mount a fast immune response once they encounter foreign organisms that have invaded previously.
3. Leukocyte-mediated drug delivery
Leukocytes are intensely involved in inflammation disorders and cancers8. They circulate in bloodstream and are recruited to inflammation sites or TME, so they could be employed as carriers to deliver nanotherapeutics to inflammatory or tumorous sites. Herein, we mainly focus on nanoparticulate drug delivery systems derived from monocytes/macrophages, neutrophils, dendritic cells and lymphocytes as they are well established and studied (Table 2).
Table 2.
Some basic characters of leukocytes-derived drug delivery system.
| Cell source | Size | Assumed or proved key functional protein or peptide | Pathological model |
|---|---|---|---|
| Macrophage | 115.4 nm | α4β1 integrin49 | Lung metastasis of 4T1 murine breast cancer cells |
| Neutrophil | ~100 nm | Ligands response to IL-8 and CXCL1/KC50 | Regional inflammation after glioma resection |
| ~110 nm | L-selectin, LFA-1, β1 integrin, CXCR433 | Lung metastasis of 4T1 murine breast cancer cells | |
| T lymphocyte | ~7 μm | TCR, perforin, granzyme51 | Lung and bone marrow tumors |
3.1. Monocytes/macrophages
Macrophages are able to phagocytose substances through a variety of mechanisms; hence loading appropriately modified micro- and nanoparticles such as gold particles, drug crystals, bacteria, liposomes or emulsions can result in an appreciable level of drug content within a macrophage. A substantial amount of literature has been published with respect to monocyte/macrophage-mediated drug delivery27. The potential advantage of using macrophages for drug delivery is multifaceted. Drugs encapsulated inside macrophages may exhibit a prolonged lifespan since elimination via either renal excretion or liver metabolism is restricted, and these drugs are protected from immediate immune recognition, which can lead to rapid clearance by the mononuclear phagocyte system. In addition to an extended circulation time in body, macrophages enable the delayed release of drugs. One representative case is antiretroviral therapy52. HIV antiretroviral drugs were delivered via macrophages in a HIV infection model. A sustained drug release at a concentration capable of inhibiting HIV replication over 10 days was observed with no adverse effects reported.
While the drug carrying ability of macrophages has been proved in many studies, delivery of the drug to its target site and efficiently release of the cargo remains a challenge. Drug release through cell death is the most exploited tactic. In addition, drugs can be released by diffusion across the cell membrane53, upon the stimulation of the tumor microenvironment54 or through an exosome mechanism55. However, all the tactics would probably fail if macrophages cannot localize sufficiently to deliver an effective dose at the target site. Since most of the chemokine receptors are located on the plasma membrane of macrophages, which lead the movement of macrophages towards inflammation sites56, it would be promising to isolate the membranes alone and employ them as the drug carriers. Choi et al.57 constructed a nano-sized gold shell and allowed it being phagocytized by macrophages in vitro. This nanoparticle-incorporated macrophage demonstrated enhanced infiltration into tumor spheroid and induced tumor necrosis upon near infrared radiation.
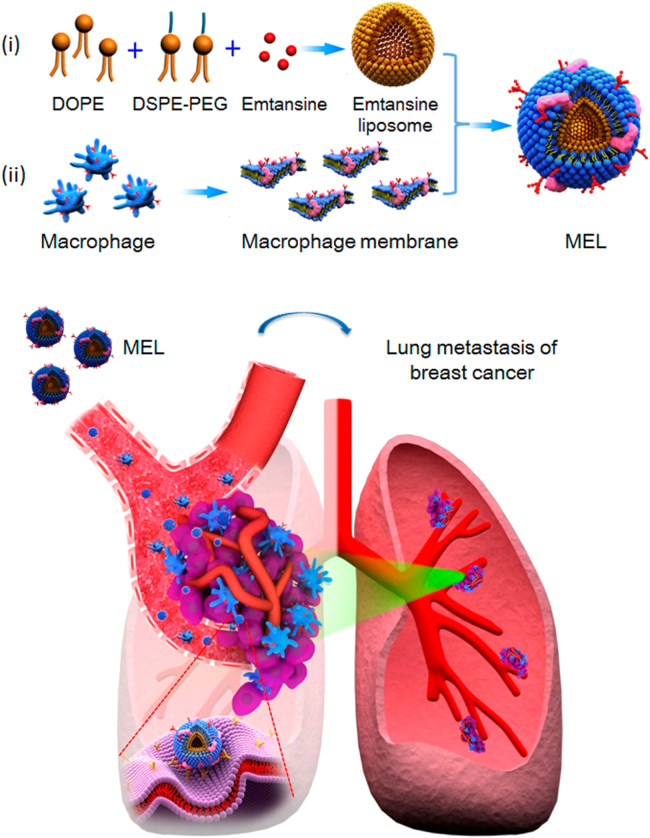
In another study, Cao et al.49 developed a macrophage membrane-decorated emtansine liposome (MEL) that can target lung metastatic sites of breast cancer (Fig. 2). MEL was fabricated by coating emtansine liposomes with a macrophage membrane to confer the bio-functions of macrophages. The macrophage membrane coating effectively enhanced cellular uptake in metastatic 4T1 breast cancer cells and had inhibitory effects on cell viability. In particular, specific targeting of MEL to metastatic foci in the lung was improved by coating emtansine liposomes with macrophage membrane, and lung metastasis of breast cancers was significantly suppressed.
Figure 2.
Emtansine-liposomes coated with macrophage membrane facilitates precise targeting to metastatic sites and improves the therapeutic efficacy against cancer metastasis. Reproduced with permission from ACS articles47. Copyright 2016, American Chemical Society.
Inorganic nanoparticles were also investigated. Xuan et al.58 developed macrophage cell membrane-enveloped mesoporous silica nanocapsules (MSNCs) through a top-down assembly as a biomimetic drug-delivery platform. Properties such as large surface area, high chemical and thermal stability, and easy functionalization of mesoporous silica nanoparticles (MSNs) enables the loading of various drugs at levels exceeding those of other common drug delivery systems, and thus have been used widely in controlled release and drug delivery systems. By employing a templating system, the prepared MSNCs greatly increase the loading capacity for doxorubicin after the template was removed. The MSNCs not only confer camouflage functions, but also endow the active targeting ability mediated by surface proteins on the MSNCs.
3.2. Neutrophils
There are several properties that make neutrophils potential carriers to deliver nanotherapeutics: 1) neutrophils can transmigrate to inflammatory sites very rapidly; 2) while the neutrophils' lifespan in circulation is short, the number of neutrophils constitutes nearly 60% of the circulating leukocytes and are readily available 3) the number of neutrophils can increase by hundred-fold or more in a short period as a respond to inflammation, and thus the targeting of neutrophils might improve the therapeutic efficacy20, 29.
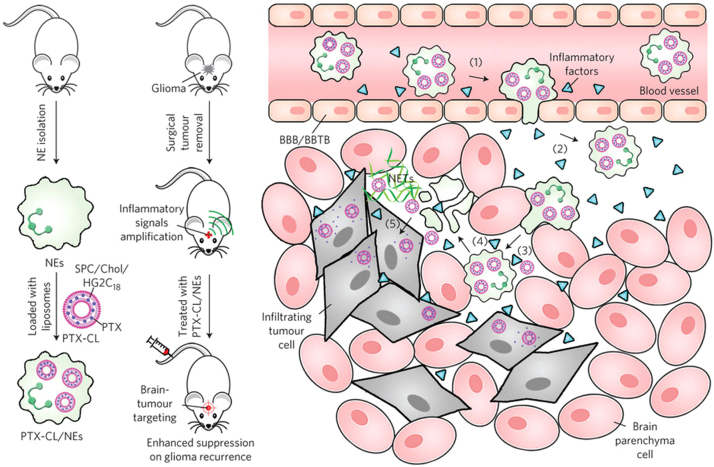
Inspired by the trans-endothelial migration ability of neutrophils to inflammation regions, the proof-of-concept investigation of such a theory has been further conducted. Xue et al.50 proposed that neutrophils were ideal carriers to deliver nanoparticulated chemotherapeutics aimed at suppressing glioblastoma recurrence after the tumor was surgically removed (Fig. 3). They took advantage of the inherent properties of neutrophils to be able to respond to chemokines released at sites of surgical tumor removal, which was a novel approach to locally recruit neutrophils. To maximize the carrying potential of therapeutic cargo, they synthesized a new type of glutamate-based cationic liposome (CL) and demonstrated efficient neutrophil loading and stable retention of paclitaxel (PTX). The PTX-CL-loaded neutrophils appeared to be unharmed by the loaded PTX and maintained their physiological functions; they exhibited a chemotactic response in response to inflammatory stimulation, and they actively generated superoxide and burst to release their PTX-CL cargo once inside the inflammatory region.
Figure 3.
The preparation and inflammation-driven therapeutic strategy of PTX-CL-loaded neutrophils (PTX-CL/NEs). Reproduced with permission from Nature Publishing Group articles48. Copyright 2017, Nature Publishing Group.
The potential of living neutrophil-based therapies to alleviate and eliminate tumors is widely studied; however, their clinical potential cannot be fully achieved without technologies to reproducibly manufacture high-quality cells, at large-scale and with low cost. Unlike traditional pharmaceutical manufacturing, the products in question are living organisms that can change with every process manipulation. In particular, the associated costs are a matter of ongoing discussion that will continue to evolve as products become more defined16.
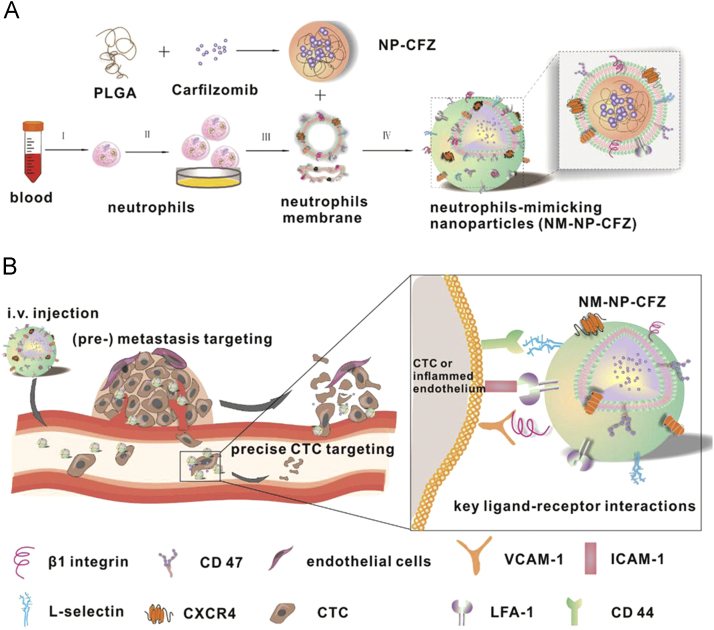
Through the proteomic analysis, it was discovered that most factors involved in the neutrophil migration are located on the plasma membrane. Since then, researchers attempt to coat nanoparticles with membrane of neutrophils to construct a biomimetic nanoplatform by endowing synthetic nanoparticles with both cell-mimicking potential and innate targeting abilities at the same time. Kang et al.33 designed neutrophil-mimicking nanoparticles by cloaking the surface of poly(D,L-lactic-coglycolic acid) nanoparticle (PLGA NP) with inflammatory neutrophil membrane (NM, Fig. 4). As a proof-of-principle, they hypothesized that the cocktail of abundant proteins of the neutrophil membrane would enable NM-NPs with neutrophils' property to continuously target circulating tumor cells in the circulation and home to their relevant distant colony. This work demonstrated that carfilzomib-loaded NM-NPs hold therapeutic potential for both preventing the de novo metastasis and inhibiting ongoing metastasis.
Figure 4.
Nanoparticles coated with neutrophil membranes to treat cancer metastasis. The schematic illustration of the preparation of carfilzomib-loaded PLGA NP coated with neutrophils membrane (NM-NP-CFZ) (A) and the molecular and molecular mechanism involved in the (pre-) metastasis and circulating tumor cell-targeting of NM-NP-CFZ (B). Reproduced with permission from ACS articles31. Copyright 2017, American Chemical Society.
Neutrophils are capable of crossing the blood vessel wall and transmigrating into tissues to rapidly respond to injury and infection. Therefore, neutrophils could be an excellent carrier to mediate nanoparticle transport across vessel barriers. Chu et al.59 demonstrated a strategy for activating neutrophil infiltration by direct priming of the tumor tissue using photosensitization. The activated neutrophil can capture the intravenously injected anti-CD11b mAb-linked NPs in blood circulation, which was later being trafficked to the tumor tissues.
3.3. Dendritic cells
Dendritic cell (DC)-based vaccination is an FDA-approved approach for harnessing the potential of a patient's own immune system to eliminate tumor cells in metastatic hormone-refractory cancer38. A phase III trial of a mixture of high concentration circulating DCs with monocytes resulted in positive outcomes, leading to approval of the first cellular immunotherapy (Provenge® from Dendreon) by the FDA16. Provenge® (Sipuleucel-T) increased the median survival by four months by educating APCs to recognize a specific marker on cancer cells such that targeted T cells were produced.
However, in recent preclinical and clinical studies, the efficacy of DC-based immunotherapy has been limited by insufficient antigen loading and maturation of DCs60. DCs display several receptors such as the DEC-205, C-type lectin receptor family, Fc receptor, mannose receptor, and complement receptor that allow internalization and presentation of antigens. Furthermore, antigens and adjuvants can be simultaneously delivered into a single DC, which enables full activation38. Based on these features, researchers can design and engineer nanoparticles that modulate the immunological functions of DCs, which include enhanced antigen uptake by phagocytosis, cellular activation, and efficient MHC class I- and class II-restricted antigen presentation.
Cell membrane-coated nanoparticles carrying an antigenic exterior can closely mimic the immunogenicity of the source cells. Kim et al.61 designed and synthesized immune complexes (antigen-antibody complexes) mimicking synthetic nanoparticulate vaccine for the immunomodulation of DCs and enhanced cancer immunotherapy. Antigen-antibody immune complexes were decorated on the shell of polymer NPs, containing immune-stimulatory vaccine adjuvants. After the in vitro stimulation with NP to induce an effective antigen processing and cross-presentation stimulation, stimulated DCs were then subcutaneously injected to induce T cell-based immune responses and enhanced antitumor immunity.
3.4. Lymphocyte
With the advancement of gene-editing technologies, autologous T cells are ex vivo engineered with chimeric antigen receptors (CAR) and reinfused back to patients for the cancer therapy, namely the CAR-T therapy48. The major limitation of these therapies is the rapid decline in cell viability and in the functions of the transplanted cells. To optimize these cell therapies, Stephan et al.51 have proposed a novel strategy to attach drug loaded nanoparticles to the surface of transplanted T cells. Through a two-step process, cytokine-loaded nanoparticles were first covalently linked with free thiol groups on the cell surface via maleimide-thiol coupling, and then the reactive residues of the nanoparticles were sheltered by PEGylation. A combination of two interleukins (IL-5 and IL-21) was incorporated into the nanoparticle. After intravenous administration of the modified T-cells, interleukins were allowed to be released continuously, regulating in an autocrine fashion their carrier cells, resulting in extensive T cell expansion and elimination of tumors.
Similar strategies were also investigated by the same group to deliver chemotherapeutic drug into lymphoma tumors in vivo62. SN-38, a potent topoisomerase I poison hampered by poor pharmacokinetics, was encapsulated into multilayer lipid nanocapsules which were attached to the surface of the reprogrammed T lymphocytes. The tissue-homing ability of lymphocytes specifically targets SN-38 nanocapsules into sites difficult to access from the circulation, and thus improve the therapeutic index of chemotherapeutic drugs with unfavorable pharmacokinetics. The potential of T lymphocytes in the treatment of cancer has been well described in other literature48. Autologous immune T cells are ex vivo modified with tumor-specific antigens and re-infused in patients for the treatment of malignant diseases. One of the limitations of these therapies is the requirement of using adjuvant and drugs simultaneously which generally target multiple cell populations leading to dose-limiting toxicities.
Jones et al.63 have demonstrated a system that achieves antigen-triggered release of CTL-attached nanoparticle drug cargos, which could be useful in cancer therapy. In their work, the killing components of cytotoxic lymphocytes, lytic granules and perforin, were employed as triggers to release therapeutic payloads from CTL-attached nanoparticles. As CTLs recognize antigen presented on the surface of target cells, the lipid-based nanoparticles that were chemically attached to the surface of CTLs are trafficked to the immunological synapse, where the T cell and target cell contact each other. Membrane pore-forming perforin subsequently released at the synapse leads to both disruption of the target cell membrane and the lipid membrane of drug carrier, leading to rapid cargo release. In an in vivo model of HIV infection, they demonstrated that HIV-specific CTLs carrying nanoparticles loaded with an immunotherapeutic agent (the IL-15), can specifically release this cytokine in tissues where infected cells are detected, resulting in strengthened elimination of infected cells as compared to HIV-specific CTLs with empty nanoparticles.
4. Conclusions and prospects
Leukocyte-derived drug delivery has great potential of becoming implemented in clinics for cancer treatment. As mentioned above, depending on the desired therapeutic effects, there are numerous strategies or approaches which can be selected. The leukocyte-derived carriers can find their best applications in improving pharmacokinetic profiles, and their inherent ability of homing to inflamed sites and tumor-specific recognition may alter the distribution of loaded drugs and make the therapy more precise.
However, although leukocyte-derived drug delivery systems have good prospects, they are still in their infancy and there are many technical barriers to overcome if such delivery systems are to succeed on a commercial scale. First of all, batch repeatability presents a big challenge. The epigenetic alterations of leukocytes during in vitro culture, purification and sterilization may lead to low batch-to-batch repeatability64. Secondly, the viability and function of leukocytes may vary depending on the source, such as ethnicity, age, gender, health conditions and so on, which hinder the development of allogeneic transfusion of leukocytes15. Moreover, detailed operating procedures and measurement techniques need to be created, validated and standardized, and the workforces already involved in the pharmaceutical industry have to be retrained to manage these new technologies16. In addition, while lymphocytes can target tumor cells more precisely, they are restricted by the sophisticated in vitro stimulation, maintenance of cell viability and the limited yield. In contrast, neutrophils and macrophages are more accessible but less precise in tumor-specific targeting20.
Drug delivery within living cells also raises concerns of immunogenicity and safety. Regarding the source of eukaryotic cell types, two approaches can generally be distinguished including the use of autologous and allogeneic cells. The advantage of autologous therapies is the minimal expected immunogenic responses, since the immune system of the host recognizes the engineered cells as “self”65. However, maintenance of autologous cells is usually difficult and regulatory issues hamper the development. Alternatively, when allogeneic cell types are used to engineer advanced drug delivery systems, concerns about immune responses of the host immune system are raised. From the viewpoint of clinical operation, repeated administration is complicated, due to the development of neutralizing antibodies or severe infusion reactions. In macrophage- and dendritic-based therapies, effectiveness relies on the ability to direct the cells toward pathological tissues. The main challenge still remains controlling these homing properties to ensure the efficacy of these therapies.
Cell membrane-coated nanoparticles exploit a top-down approach to faithfully transfer the entire cell exterior, including both lipids and membrane-associated proteins, onto synthetic nanoparticles. This new class of biomimetic nanoparticles has shown promising therapeutic potential. From a targeted-therapy perspective, they are capable of prolonging systemic circulation which is crucial for both passive and active targeting, while allowing for the use of synthetic biomaterials such as biocompatible polymers to carry therapeutic agents. To fulfill the biofunctions of the membrane, one of the key requirements is to keep the proteins on the plasma membrane structurally intact during the isolation process. Moreover, for cell-specific targeting, the lipid insertion approach provides these nanoparticles with desirable targeting ligands and controlled density without involving any chemical reactions that may potentially disrupt the protein makeup on the nanoparticle surfaces66. Alternatively, when coated with membranes derived from selected cells, these nanoparticles achieve cell-specific targeting ability through inherent homotypic or heterotypic adhesions. Meanwhile, cell membrane-coated nanoparticles carry an antigenic exterior closely mimicking that of the source cells, making them excellent antigen-presenting platforms. Effective immune targeting is made possible by tailoring the physicochemical properties of the synthetic cores. Since its initial discovery, the membrane-coating technique has been rapidly applied to nanostructures made with various materials including PLGA, gelatin, gold and silica. For future applications, these materials provide enormous engineering flexibility particularly useful to implement responsive mechanisms for more efficient and specific drug targeting.
In parallel, membranes derived from distinct leukocytes have been explored as coating materials, unleashing tremendous potential to harness novel drug targeting strategies. The use of cell membranes to coat nanoparticles has emerged as a robust and versatile approach for integrating natural and synthetic biomaterials to form functional nanostructures for effective drug targeting. Such functionalization of nanoparticles represents a feasible method for developing novel, nature-inspired nanotherapeutics with complex antigenic information and surface properties. Looking to the future, cell-mediated nanoparticle drug delivery systems will continue to inspire researchers to develop new nanotherapeutics for effective intervention. They are expected to lead to a new paradigm of thinking on design and applications in nanomedicine.
In addition to the function of transporting drugs inside the body, cell-based drug delivery could also be approached by creating “cell-factories”, a platform allowing the production of therapeutic compounds67. Several approaches in constructing genetic circuits with various triggers for inducing gene expression have been developed, but they are in essence a universal proof of concept. Dependent on the specific disease, the selected gene segment is placed downstream of a specific promoter element. Upon addition of the trigger, gene expression is induced and the therapeutic protein can be released in the bloodstream. This could lead to a significant improvement in current strategies, in which patients are dependent on injections of therapeutic proteins. In addition, by matching the downstream gene to different triggers, a more versatile and patient-friendly therapy could be generated.
There are still some limitations in cell-based drug delivery approaches, which have to be overcome before they can be introduced as standard practice in clinical treatment. However, researchers from the field of synthetic biology have shown proof-of-concept for an extend range of treatment strategies. It is no longer impossible to safely modify the patient's own cells and convert them into drug-producing cells that generate biological drugs at will and in a highly controlled fashion.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Nos. 81673019, 81690263 and 81373353), “Shu Guang” project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (15SG14).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong Eun Ji, Choi Dae Gun, Shim Min Suk. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm Sin B. 2016;6:297–307. doi: 10.1016/j.apsb.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang Lin, Gao Zhonggao, Huang Wei, Jin Mingji, Wang Qiming. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm Sin B. 2015;5:169–175. doi: 10.1016/j.apsb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barenholz Y. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan V.P., Jain R.K. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fliervoet L.A., Mastrobattista E. Drug delivery with living cells. Adv Drug Deliv Rev. 2016;106:63–72. doi: 10.1016/j.addr.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Rezvani K., Rouce R., Liu E., Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017;25:1769–1781. doi: 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell M.J., King M.R. Leukocytes as carriers for targeted cancer drug delivery. Expert Opin Drug Deliv. 2015;12:375–392. doi: 10.1517/17425247.2015.966684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batrakova E.V., Gendelman H.E., Kabanov A.V. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8:415–433. doi: 10.1517/17425247.2011.559457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. Pegylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhakar U., Maeda H., Jain R.K., Sevick-Muraca E.M., Zamboni W., Farokhzad O.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danhier F. To exploit the tumor microenvironment: since the epr effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244:108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm S., Tavares A.J., Dai Q., Ohta S., Audet J., Dvorak H.F. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014. [Google Scholar]
- 14.Nagarsheth N., Wicha M.S., Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559–572. doi: 10.1038/nri.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q., Cheng H., Peng H., Zhou H., Li P.Y., Langer R. Non-genetic engineering of cells for drug delivery and cell-based therapy. Adv Drug Deliv Rev. 2015;91:125–140. doi: 10.1016/j.addr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Dwarshuis N.J., Parratt K., Santiago-Miranda A., Roy K. Cells as advanced therapeutics: state-of-the-art, challenges, and opportunities in large scale biomanufacturing of high-quality cells for adoptive immunotherapies. Adv Drug Deliv Rev. 2017;114:222–239. doi: 10.1016/j.addr.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Turley S.J., Cremasco V., Astarita J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15:669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 18.Parodi A., Quattrocchi N., van de Ven A.L., Chiappini C., Evangelopoulos M., Martinez J.O. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang S.C., Kim O.Y., Yoon C.M., Choi D.-S., Roh T.-Y., Park J. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 20.Dong X., Chu D., Wang Z. Leukocyte-mediated delivery of nanotherapeutics in inflammatory and tumor sites. Theranostics. 2017;7:751–763. doi: 10.7150/thno.18069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30:668–681. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Allavena P., Sica A., Solinas G., Porta C., Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Croci D.O., Zacarías Fluck M.F., Rico M.J., Matar P., Rabinovich G.A., Scharovsky O.G. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol Immunother. 2007;56:1687–1700. doi: 10.1007/s00262-007-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiteside T.L. The role of immune cells in the tumor microenvironment. In: Dalgleish A.G., Haefner B., editors. Vol. 130. Springer; Boston, MA: 2006. pp. 103–124. (The link between inflammation and cancer. Cancer treatment and research, vol.). [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Shao Q., Sun J., Ma C., Gao W., Wang Q. Interactions between colon cancer cells and tumor-infiltrated macrophages depending on cancer cell-derived colony stimulating factor 1. Oncoimmunology. 2016;5:e1122157. doi: 10.1080/2162402X.2015.1122157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierigè F., Serafini S., Rossi L., Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60:286–295. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 28.Wang S., Huang P., Chen X. Hierarchical targeting strategy for enhanced tumor tissue accumulation/retention and cellular internalization. Adv Mater. 2016;28:7340–7364. doi: 10.1002/adma.201601498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffelt S.B., Wellenstein M.D., de Visser K.E. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 30.de Oliveira S., Rosowski E.E., Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16:378–391. doi: 10.1038/nri.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zenaro E., Pietronigro E., Della Bianca V., Piacentino G., Marongiu L., Budui S. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian P., Mitroulis I., Hajishengallis G., Chavakis T. Regulation of tissue infiltration by neutrophils: role of integrin α3β1 and other factors. Curr Opin Hematol. 2016;23:36–43. doi: 10.1097/MOH.0000000000000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang T., Zhu Q., Wei D., Feng J., Yao J., Jiang T. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11:1397–1411. doi: 10.1021/acsnano.6b06477. [DOI] [PubMed] [Google Scholar]
- 34.Eyles J.L., Hickey M.J., Norman M.U., Croker B.A., Roberts A.W., Drake S.F. A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood. 2008;112:5193–5201. doi: 10.1182/blood-2008-02-139535. [DOI] [PubMed] [Google Scholar]
- 35.Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G., Ling L. Polarization of tumor-associated neutrophil phenotype by TGF-β: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jablonska J., Leschner S., Westphal K., Lienenklaus S., Weiss S. Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Investig. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y., Huang X., Ding T.W., Gong Z. Enhanced angiogenesis, hypoxia and neutrophil recruitment during myc-induced liver tumorigenesis in zebrafish. Sci Rep. 2016;6:31952. doi: 10.1038/srep31952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulis L.E., Mandal S., Kreutz M., Figdor C.G. Dendritic cell-based nanovaccines for cancer immunotherapy. Curr Opin Immunol. 2013;25:389–395. doi: 10.1016/j.coi.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Galy A., Travis M., Cen D., Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. doi: 10.1016/1074-7613(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 40.Steinman R.M. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Granot T., Senda T., Carpenter D.J., Matsuoka N., Weiner J., Gordon C.L. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity. 2017;46:504–515. doi: 10.1016/j.immuni.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Worbs T., Hammerschmidt S.I., Förster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17:30–48. doi: 10.1038/nri.2016.116. [DOI] [PubMed] [Google Scholar]
- 43.Engblom C., Pfirschke C., Pittet M.J. The role of myeloid cells in cancer therapies. Nat Rev Cancer. 2016;16:447–462. doi: 10.1038/nrc.2016.54. [DOI] [PubMed] [Google Scholar]
- 44.Filley A.C., Dey M. Dendritic cell based vaccination strategy: an evolving paradigm. J Neurooncol. 2017;133:223–235. doi: 10.1007/s11060-017-2446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munn D.H., Mellor A.L. Ido in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schreiner B., Mitsdoerffer M., Kieseier B.C., Chen L., Hartung H.-P., Weller M. Interferon-β enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol. 2004;155:172–182. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Tsou P., Katayama H., Ostrin E.J., Hanash S.M. The emerging role of B cells in tumor immunity. Cancer Res. 2016;76:5597–5601. doi: 10.1158/0008-5472.CAN-16-0431. [DOI] [PubMed] [Google Scholar]
- 48.Fesnak A.D., June C.H., Levine B.L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao H., Dan Z., He X., Zhang Z., Yu H., Yin Q. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10:7738–7748. doi: 10.1021/acsnano.6b03148. [DOI] [PubMed] [Google Scholar]
- 50.Xue J., Zhao Z., Zhang L., Xue L., Shen S., Wen Y. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- 51.Stephan M.T., Moon J.J., Um S.H., Bershteyn A., Irvine D.J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16:1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nowacek A.S., Balkundi S., McMillan J., Roy U., Martinez-Skinner A., Mosley R.L. Analyses of nanoformulated antiretroviral drug charge, size, shape and content for uptake, drug release and antiviral activities in human monocyte-derived macrophages. J Control Release. 2011;150:204–211. doi: 10.1016/j.jconrel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu J., Wang D., Mei D., Zhang H., Wang Z., He B. Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J Control Release. 2015;204:11–19. doi: 10.1016/j.jconrel.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 54.He X., Cao H., Wang H., Tan T., Yu H., Zhang P. Inflammatory monocytes loading protease-sensitive nanoparticles enable lung metastasis targeting and intelligent drug release for anti-metastasis therapy. Nano Lett. 2017;17:5546–5554. doi: 10.1021/acs.nanolett.7b02330. [DOI] [PubMed] [Google Scholar]
- 55.Lee S., Kivimäe S., Dolor A., Szoka F.C. Macrophage-based cell therapies: the long and winding road. J Control Release. 2016;240:527–540. doi: 10.1016/j.jconrel.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen S.J., Crown S.E., Handel T.M. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 57.Choi M.R., Stanton-Maxey K.J., Stanley J.K., Levin C.S., Bardhan R., Akin D. A cellular trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759–3765. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 58.Xuan M., Shao J., Dai L., He Q., Li J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv Healthc Mater. 2015;4:1645–1652. doi: 10.1002/adhm.201500129. [DOI] [PubMed] [Google Scholar]
- 59.Chu D., Dong X., Zhao Q., Gu J., Wang Z. Photosensitization priming of tumor microenvironments improves delivery of nanotherapeutics via neutrophil infiltration. Adv Mater. 2017;29:1701021. doi: 10.1002/adma.201701021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bol K.F., Schreibelt G., Gerritsen W.R., de Vries I.J., Figdor C.G. Dendritic cell-based immunotherapy: state of the art and beyond. Clin Cancer Res. 2016;22:1897–1906. doi: 10.1158/1078-0432.CCR-15-1399. [DOI] [PubMed] [Google Scholar]
- 61.Kim S.Y., Phuengkham H., Noh Y.W., Lee H.G., Um S.H., Lim Y.T. Immune complexes mimicking synthetic vaccine nanoparticles for enhanced migration and cross-presentation of dendritic cells. Adv Funct Mater. 2016;26:8072–8082. [Google Scholar]
- 62.Huang B., Abraham W.D., Zheng Y., Bustamante López S.C., Luo S.S., Irvine D.J. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci Transl Med. 2015;7:291ra94. doi: 10.1126/scitranslmed.aaa5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones R.B., Mueller S., Kumari S., Vrbanac V., Genel S., Tager A.M. Antigen recognition-triggered drug delivery mediated by nanocapsule-functionalized cytotoxic T-cells. Biomaterials. 2017;117:44–53. doi: 10.1016/j.biomaterials.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bluestone J.A. Regulatory T-cell therapy: is it ready for the clinic? Nat Rev Immunol. 2005;5:343–349. doi: 10.1038/nri1574. [DOI] [PubMed] [Google Scholar]
- 65.Liu X., Li W., Fu X., Xu Y. The immunogenicity and immune tolerance of pluripotent stem cell derivatives. Front Immunol. 2017;8:645. doi: 10.3389/fimmu.2017.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuhn A., Haase M., Leptihn S. Assisted and unassisted protein insertion into liposomes. Biophys J. 2017;113:1187–1193. doi: 10.1016/j.bpj.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orelle C., Carlson E.D., Szal T., Florin T., Jewett M.C., Mankin A.S. Protein synthesis by ribosomes with tethered subunits. Nature. 2015;524:119–124. doi: 10.1038/nature14862. [DOI] [PubMed] [Google Scholar]