Chronic granulomatous disease (CGD) is a primary immunodeficiency, characterized by recurrent severe infections and inflammatory complications, caused by genetic defects that result in defects in the function of the nicotinamide adenine dinucleotide phosphate (NADPH) complex. These defects result in an inability to produce the phagocyte respiratory burst and an inability to produce superoxide and reactive oxygen species. Often diagnosed in the first few years of life, a mutation in any of the 5 phagocyte oxidase (phox) genes that comprise the NADPH complex—including gp91phox or cytochrome b-245 β polypeptide (CYBB), p22phox or cytochrome b-245 α polypeptide (CYBA), p47phox or neutrophil cytosolic factor 1 (NCF1), p67phox or neutrophil cytosolic factor 2 (NCF2), and p40phox or neutrophil cytosolic factor 4 (NCF4)—lead to CGD [1–3]. The most common form in European and North American patients with CGD is caused by defects in the X-linked CYBB gene (see Rider et al, this supplement). The only current cure for CGD is hematopoietic stem cell therapy [4–7] (see Connelly et al, this supplement).
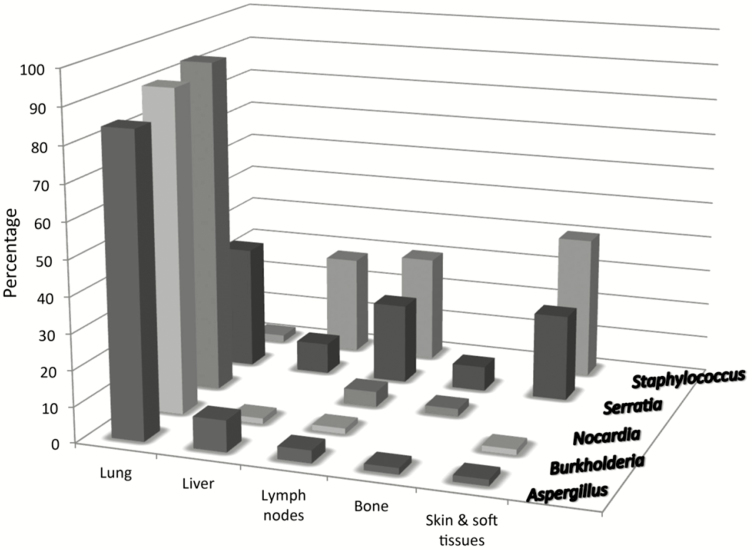
Death has largely been a result of infection and is related directly to the amount of residual superoxide production, which is determined by the genetic defect [8]. Overall, patients with CGD are susceptible to a narrow spectrum of organisms. These bacteria include Staphylococcus aureus, Burkholderia cepacia complex, Serratia marcescens, Nocardia spp, and, less commonly, Granulibacter bethesdensis and Chromobacterium violaceum [9–12] (Figure 1). Salmonella spp and Mycobacterium tuberculosis are also major pathogens to which patients with CGD are susceptible not often seen in the United States. Patients with CGD who live in countries where Bacille Calmette-Guérin (BCG) vaccine is given can get BCGitis, but they rarely have disseminated disease, as opposed to patients with another type of primary immunodeficiency [13, 14]. The most common causes of invasive fungal infections (IFIs) in patients with CGD are Aspergillus spp (most commonly Aspergillus fumigatus) and other species such as Aspergillus niger, Aspergillus nidulans, and Aspergillus tanneri [15, 16]. These patients also develop infections with dematiaceous (dark-walled) molds such as Neosartorya, Phaeoacremonium, Paecilomyces, and Phellinus spp, which can be associated with a high mortality rate in patients with CGD [17–19]. Mulch pneumonitis is an especially important entity in patients with CGD of which to be aware, because it is a medical emergency treated with high-dose corticosteroids in addition to antifungal and antibacterial agents [20, 21].
Figure 1.
Frequencies of organisms isolated according to site of infection in a retrospective series of 268 patients with chronic granulomatous disease seen at the National Institutes of Health [9].
The current standard of care largely depends on good antimicrobial and antifungal prophylaxis. However, great progress is being made in understanding the treatment of bacterial infections, which in some cases, such as liver abscess, includes administration of a steroid in conjunction with antimicrobial therapy [22, 23].
The diagnosis of pneumonia can be difficult; sputum culture and radiographs are often not useful for patients with CGD, because they might not produce a normal inflammatory response that leads to sputum or radiographic infiltrates. Computed tomography remains the mainstay for diagnosing pulmonary infection in patients with CGD. Direct biopsy, easily performed with a transthoracic needle, provides samples with a high diagnostic yield of infection in patients with CGD, especially with the new sequence-based diagnostic methods, which can increase the yield when the culture results are negative. Every effort should be made to obtain a microbiologic diagnosis, because successful targeted therapy helps preserve long-term organ function [9].
PNEUMONIA AND LUNG ABSCESSES
In a large series of European patients with CGD, 66% had lung involvement [24]; in the United States, pulmonary disease has been reported to be even more common. Data from a US registry of 368 patients with CGD revealed that 79% of the subjects had a history of pneumonia, and a large single-center report from the National Institutes of Health found that 87% of 268 patients with CGD had a history of lung infection [9, 25]. The most common pulmonary disease encountered in patients with CGD is pneumonia. In the European study, pneumonia was the etiology of 547 of 634 cases of pulmonary involvement in patients with CGD [24]. Lung abscesses, although potentially severe, are relatively uncommon and were reported for only 24 of the 429 patients in the European study. Pneumonia and pulmonary abscesses collectively are likely to be the greatest cause of death in patients with CGD; they caused 18 of the 84 deaths in the European cohort and represented the major cause of death in both the multicenter American study and the National Institutes of Health study [9, 25]. Furthermore, complications of recurrent pulmonary infections and inflammation, such as fibrosis, honeycomb lung, pleural thickening, and pulmonary hypertension, can emerge as chronic complications in patients with CGD [26, 27].
The causative pathogen in the majority of pulmonary infections in patients with CGD is not identified. Prophylactic antibiotics might inhibit the isolation of bacteria from these patients [28]. Thus, in many cases, treatment must be tailored to cover the most likely infectious agents. Aspergillus spp are among the pathogens most frequently isolated from the respiratory tract in patients with CGD [24]. Bacterial pathogens, including B cepacia complex organisms, S aureus, Nocardia spp, and Serratia spp, are also common pulmonary pathogens in patients with CGD [9, 29, 30]. Less common respiratory pathogens in patients with CGD include Actinomyces spp and M tuberculosis [31–33]. Important to note is that noninfectious inflammatory pulmonary disease can be a significant feature of CGD; in a European study [34], it was reported in 28% of adult subjects (see also Henrickson et al, this supplement).
LIVER ABSCESSES
Liver abscesses are a frequent complication in patients with CGD and impart significant morbidity. However, the most common signs and symptoms of this complication, fever and elevated erythrocyte sedimentation rate, are nonspecific and require clinicians to maintain a high level of vigilance [35]. Although liver abscesses that affect the general population are typically polymicrobial in nature, S aureus is by far the most commonly isolated pathogen and primary driver of this complication in patients with CGD [9, 24]. Less common pathogens associated with liver abscesses in patients with CGD include Aspergillus spp and Serratia spp. Although antibacterial prophylaxis effectively limits infections and enhances the survival rate of patients with CGD, liver abscesses caused by S aureus still occur frequently; approximately one-third of patients with CGD develop a liver abscess, and almost half of them experience recurrence [9, 24, 35]. Liver involvement in patients with CGD is a noted concern, because portal hypertension, splenomegaly, portal venopathy, and nodular regenerative hyperplasia can ensue [36]. In turn, splenomegaly can cause thrombocytopenia, a decline in platelet count that has been reported to be a poor prognostic indicator in patients with CGD [37].
The management of liver abscesses in patients with CGD is complicated by both the nature of the primary causative pathogen and inflammatory features and impaired wound healing that characterize the immune disorder itself. Staphylococcal liver abscesses in patients with CGD are typically difficult to drain percutaneously, which has led to the frequent use of resection [29]. Although resection is often effective in resolving the acute issue, it does not seem to reduce the incidence of liver abscess recurrence in patients with CGD, and the surgery can be complicated in this population [35]. For this reason, numerous nonsurgical approaches have been explored. The most promising of these approaches involves combination therapy with corticosteroids and antibiotics [22]. IFN-γ also can be a useful adjuvant for the treatment of liver abscess in some patients with CGD; in 1 study, it was reported to resolve liver abscesses in 2 adult brothers with autosomal recessive CGD [38]. It is unclear if IFN-γ prophylaxis prevents this complication. A large double-blind placebo-controlled study of IFN-γ prophylaxis in patients with CGD did not find a significant reduction in the incidence of liver abscesses [39]. Although infrequent, liver abscesses that are caused by pathogens other than S aureus are sometimes seen in these patients and can be managed differently. For example, streptococcal liver abscesses can be drained more easily and do not require surgery or corticosteroid therapy [40].
OTHER TYPES OF ORGAN INFECTION
Although not nearly as common as lung involvement, brain abscesses also occur in patients with CGD and are associated with a high mortality rate. The multicenter European study reported that brain abscesses occurred in 7% of the subjects and was the cause of death in approximately 5% of the patients who died [24]. In this same study, 11% of the patients with CGD had eye involvement. Although chronic inflammatory disease of the gastrointestinal tract is frequent in patients with CGD (see Henrickson et al, this supplement), gastrointestinal infections are not.
After pulmonary infections (which affects 57%–95% of patients with CGD), the most common locations for infection are the skin/soft tissue (43%–80%) and the lymph nodes (35%–72%) [41]. Although pneumonia has been reported to be the most common infection in patients with either the X-linked recessive or autosomal recessive form of CGD, suppurative adenitis and subcutaneous abscesses can be the first symptoms to cause clinicians to suspect CGD [42]. Subcutaneous abscesses are the most common form of abscess in patients with CGD and typically are caused by S aureus, but they can be caused also by Serratia and Klebsiella spp [25]. In terms of location, subcutaneous abscesses are frequently perianal. Cellulitis is relatively rare but, when diagnosed, tends to result from infection with a rare pathogen (eg, C violaceum, S marcescens, Nocardia spp) [25]. Less common causes of soft-tissue infection include Paecilomyces spp and other fungi or certain yeasts.
Suppurative or necrotizing lymphadenitis is also a major cause of morbidity in patients with CGD and can affect at least 50% of these patients [25]. S aureus is the most commonly identified bacterial etiology; however, in many cases, the causative organism is not identified. In the United States, suppurative lymphadenitis seems to be more common than subcutaneous abscesses [11, 25]. Individuals with the autosomal recessive forms of CGD have lower rates of suppurative lymphadenitis than those with the X-linked form, which suggests that residual oxidase activity, which is more common in patients with autosomal recessive disease, might protect against this complication. In addition to antimicrobial therapy, lymphadenitis often requires excisional surgery [37]. Relapsing necrotizing lymphadenitis can be caused by G bethesdensis [11, 12], a bacterium that causes a chronic disease that commonly affects the lymphatics and viscera. This fastidious multidrug-resistant organism highlights the need for precise antimicrobial therapy and microbiological diagnosis; it also might be responsible for a proportion of “culture-negative” cases of nonrelapsing lymphadenitis.
Suppurative lymphadenitis also can result from region-specific medical practices such as BCG vaccination. In Turkey, for instance, the rates of local and disseminated lymphadenitis are higher, which, in part, might be a result of Mycobacterium bovis infection [43]. A higher prevalence of lymphadenitis has been found in Iran [32] and Germany also [44]. Although patients with CGD are predisposed to lymphadenitis after receiving a BCG vaccination, their disease tends to be more localized than that in individuals with severe combined immunodeficiency or IFN-γ–interleukin 12 pathway defects [45]. Although it often results in severe infection, B cepacia infections occur at a lower rate in Europe than in the United States [24].
Osteomyelitis in patients with CGD commonly affects the extremities, skull, and chest wall. In the United States, S marcescens and Aspergillus spp were the most common bacteria isolated [25], whereas in the United Kingdom, S aureus and Aspergillus spp were isolated more commonly [42]. Osteomyelitis caused by invasive Aspergillus spp usually results from primary pulmonary disease [41].
Sepsis, with or without pneumonia, is a major cause of death in patients with CGD. The over rates of sepsis are higher in patients with X-linked CGD than in those with the autosomal recessive form [25]. Salmonella spp are the organisms isolated most commonly from the blood of patients with CGD in North America. Despite this fact, fatal sepsis is usually caused by pathogens such as Aspergillus spp, Burkholderia spp, Burkholderia spp, or, less commonly, Candida spp [25]. Infections with G bethesdensis, C violaceum, or Francisella philomiragia, all of which can present as sepsis, are pathognomonic for CGD [46]. Therefore, identification of the causative organism is important for tailoring antimicrobial therapy effectively and suggesting the diagnosis of CGD.
In addition to the infections listed earlier, patients with CGD are predisposed to impetigo, sinusitis, otitis media, septic arthritis, urinary tract infection/pyelonephritis, gingivitis, and gastroenteritis [25, 47]. Frequent and chronic infections are not without consequence; 75% of patients in the British cohort were diagnosed with failure to thrive [42].
As discussed elsewhere in this supplement (see Slack et al), the current standard of care for bacterial infection prophylaxis in patients with CGD is trimethoprim-sulfamethoxazole or, for those who cannot tolerate it, cefdinir; itraconazole or another antifungal azole is used for fungal infection prophylaxis [48, 49]. IFN-γ is also used, in conjunction with antimicrobial and antifungal agents, to prevent infection and, rarely, as an adjunctive therapy for active infection [39].
INVASIVE FUNGAL INFECTION
A number of IFIs occur in patients with CGD; historically, rates of 20% to 40% lifetime risk of IFI were seen in the era before routine antifungal prophylaxis, a practice which has reduced the risk for IFI by several-fold. The most common IFI is Aspergillus pneumonia [25], which begins insidiously, with relatively subtle findings on physical examination and chest radiography. Many patients are afebrile and can lack localizing signs, such as chest pain or cough. Some authors suggest screening with annual computed tomography scans, a very sensitive but not very specific radiographic test for IFI [50]. IFI should certainly be suspected in any patient with CGD who develops focal findings or pulmonary infiltrates, even in those who are receiving antifungal prophylaxis [51].
IFIs also include osteomyelitis and cerebral and gastrointestinal infections, along with pulmonary disease [27, 51–53]. Localized skin or lymph node infections can occur also, particularly from some of the less common environmental fungal species. Case reports of infection caused by Fusarium, Phellinus, and Penicillium spp and mucormycosis have all been reported [54–61]. Many of these fungi are more likely to have intrinsic resistance to some of the first-line therapeutic agents such as voriconazole or the echinocandins. Among the Aspergillus spp, A nidulans infection seems to occur more frequently in patients with CGD than in other patient groups and is more aggressive [30, 51].
A formal diagnosis of IFI in patients with CGD often relies on biopsy specimens for histology, culture, and nucleic acid amplification tests [16, 62, 63]. Because of the difficulty in obtaining a definitive diagnosis of IFI in patients with CGD, clinicians should have a low threshold for suspecting IFI and obtaining appropriate imaging and, when necessary, biopsy specimens.
The treatment of IFI is often empiric unless the organism is identified definitively by culture or nucleic acid amplification. Voriconazole has become popular as a treatment for infection caused by Aspergillus spp because of its broad spectrum of activity, relative tolerability, and the existence of both parenteral and oral formulations. Therapeutic drug monitoring improves its efficacy and lowers the risk of adverse effects, which can include altered liver function, prolonged QTc, hyperfluorosis, and phototoxicity [64, 65]. The pharmacokinetics of voriconazole differ in children and adults; dosing should be optimized, and adverse effects should be monitored closely. Posaconazole liquid requires certain dietary modifications to optimize its absorption (it should be taken with fatty foods or an acidic beverage), and therapeutic drug monitoring should be considered [19, 66]. The newer tablet formulation of posaconazole offers substantially increased bioavailability (for those who can swallow pills). The echinocandins have some activity against Aspergillus spp and can be considered if the triazoles cannot be tolerated or if antifungal resistance is present. Beyond antifungal therapy, immune reconstitution can be important in controlling and clearing IFI [67, 68]. Amphotericin B remains an option for treating severe disease or IFI caused by an organism that demonstrates acquired or intrinsic resistance to triazoles. The liposomal formation is typically best tolerated in terms of systemic adverse effects and renal toxicity. In refractory cases, granulocyte transfusions and hematopoietic stem cell transplant have been used successfully along with appropriate antifungal therapy [53, 58, 69].
Because antibiotics have reduced the risk of bacterial infections, Aspergillus infections became more relatively frequent [50, 59, 60]. For antifungal prophylaxis, oral azoles are preferred, and itraconazole historically has been the agent of choice. However, modern triazoles, such as voriconazole and posaconazole, are rather more potent and bioavailable than itraconazole, and many experts use voriconazole or posaconazole as the primary agent for fungal prophylaxis. However, it is important to monitor for skin toxicity (and even possible skin carcinogenicity) and fluoride accumulation in patients taking long-term voriconazole [70, 71]. The posaconazole liquid formulation is poorly bioavailable unless taken with fat-containing meals or acidic beverages. The new tablet formulation of posaconazole is highly bioavailable and likely has additional activity against non-fumigatus Aspergillus spp and the dematiaceous molds. Posaconazole does not carry the same skin and fluoride toxicities, so it might be better in the long term. A direct comparison of the clinical efficacies of antifungal agents in preventing IFI in patients with CGD is lacking as no prospective randomized trials have been published [72, 73]. The addition of IFN-γ to the prophylactic regimen does seem to confer additional protective benefit in terms of preventing fungal disease specifically [74].
Notes
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. Funding for this study was provided in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Supplement sponsorship. This article appears as part of the supplement “Chronic Granulomatous Disease,” sponsored by Horizon Pharma USA, Inc.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Matute JD, Arias AA, Wright NA et al. . A new genetic subgroup of chronic granulomatous disease with autosomal recessive mutations in p40 phox and selective defects in neutrophil NADPH oxidase activity. Blood 2009; 114:3309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roos D, Kuhns DB, Maddalena A et al. . Hematologically important mutations: the autosomal recessive forms of chronic granulomatous disease (second update). Blood Cells Mol Dis 2010; 44:291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roos D, Kuhns DB, Maddalena A et al. . Hematologically important mutations: X-linked chronic granulomatous disease (third update). Blood Cells Mol Dis 2010; 45:246–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horwitz ME, Barrett AJ, Brown MR et al. . Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoietic allograft. N Engl J Med 2001; 344:881–8. [DOI] [PubMed] [Google Scholar]
- 5. Seger RA, Gungor T, Belohradsky BH et al. . Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985–2000. Blood 2002; 100:4344–50. [DOI] [PubMed] [Google Scholar]
- 6. Güngör T, Teira P, Slatter M et al. ; Inborn Errors Working Party of the European Society for Blood and Marrow Transplantation Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet 2014; 383:436–48. [DOI] [PubMed] [Google Scholar]
- 7. Parta M, Hilligoss D, Kelly C et al. . Haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide in a patient with chronic granulomatous disease and active infection: a first report. J Clin Immunol 2015; 35:675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuhns DB, Alvord WG, Heller T et al. . Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med 2010; 363:2600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marciano BE, Spalding C, Fitzgerald A et al. . Common severe infections in chronic granulomatous disease. Clin Infect Dis 2015; 60:1176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meher-Homji Z, Mangalore RP, Johnson PDR, Chua KYL. Chromobacterium violaceum infection in chronic granulomatous disease: a case report and review of the literature. JMM Case Rep 2017; 4:e005084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greenberg DE, Ding L, Zelazny AM et al. . A novel bacterium associated with lymphadenitis in a patient with chronic granulomatous disease. PLoS Pathog 2006; 2:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greenberg DE, Shoffner AR, Zelazny AM et al. . Recurrent Granulibacter bethesdensis infections and chronic granulomatous disease. Emerg Infect Dis 2010; 16:1341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Movahedi Z, Norouzi S, Mamishi S, Rezaei N. BCGiosis as a presenting feature of a child with chronic granulomatous disease. Braz J Infect Dis 2011; 15:83–6. [DOI] [PubMed] [Google Scholar]
- 14. Norouzi S, Aghamohammadi A, Mamishi S et al. . Bacillus Calmette-Guérin (BCG) complications associated with primary immunodeficiency diseases. J Infect 2012; 64:543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sugui JA, Peterson SW, Clark LP et al. . Aspergillus tanneri sp. nov., a new pathogen that causes invasive disease refractory to antifungal therapy. J Clin Microbiol 2012; 50:3309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blumental S, Mouy R, Mahlaoui N et al. . Invasive mold infections in chronic granulomatous disease: a 25-year retrospective survey. Clin Infect Dis 2011; 53:e159–69. [DOI] [PubMed] [Google Scholar]
- 17. Haidar ZA, Malshe A, McKenna D. Chronic granulomatous disease carrier with recurrent poor obstetric outcome. Obstet Gynecol 2014; 123:484–6. [DOI] [PubMed] [Google Scholar]
- 18. Vinh DC, Shea YR, Sugui JA et al. . Invasive aspergillosis due to Neosartorya udagawae. Clin Infect Dis 2009; 49:102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Segal BH, Barnhart LA, Anderson VL et al. . Posaconazole as salvage therapy in patients with chronic granulomatous disease and invasive filamentous fungal infection. Clin Infect Dis 2005; 40:1684–8. [DOI] [PubMed] [Google Scholar]
- 20. Siddiqui S, Anderson VL, Hilligoss DM et al. . Fulminant mulch pneumonitis: an emergency presentation of chronic granulomatous disease. Clin Infect Dis 2007; 45:673–81. [DOI] [PubMed] [Google Scholar]
- 21. Ameratunga R, Woon ST, Vyas J, Roberts S. Fulminant mulch pneumonitis in undiagnosed chronic granulomatous disease: a medical emergency. Clin Pediatr (Phila) 2010; 49:1143–6. [DOI] [PubMed] [Google Scholar]
- 22. Leiding JW, Freeman AF, Marciano BE et al. . Corticosteroid therapy for liver abscess in chronic granulomatous disease. Clin Infect Dis 2012; 54:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Straughan DM, McLoughlin KC, Mullinax JE et al. . The changing paradigm of management of liver abscesses in chronic granulomatous disease. Clin Infect Dis 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Berg JM, van Koppen E, Ahlin A et al. . Chronic granulomatous disease: the European experience. PLoS One 2009; 4:e5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Winkelstein JA, Marino MC, Johnston RB Jr et al. . Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000; 79:155–69. [DOI] [PubMed] [Google Scholar]
- 26. Godoy MC, Vos PM, Cooperberg PL et al. . Chest radiographic and CT manifestations of chronic granulomatous disease in adults. AJR Am J Roentgenol 2008; 191:1570–5. [DOI] [PubMed] [Google Scholar]
- 27. Khanna G, Kao SC, Kirby P, Sato Y. Imaging of chronic granulomatous disease in children. Radiographics 2005; 25:1183–95. [DOI] [PubMed] [Google Scholar]
- 28. Mahdaviani SA, Mohajerani SA, Rezaei N et al. . Pulmonary manifestations of chronic granulomatous disease. Expert Rev Clin Immunol 2013; 9:153–60. [DOI] [PubMed] [Google Scholar]
- 29. Holland SM. Chronic granulomatous disease. Hematol Oncol Clin North Am 2013; 27:89–99, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johnston RB., Jr Clinical aspects of chronic granulomatous disease. Curr Opin Hematol 2001; 8:17–22. [DOI] [PubMed] [Google Scholar]
- 31. Reichenbach J, Lopatin U, Mahlaoui N et al. . Actinomyces in chronic granulomatous disease: an emerging and unanticipated pathogen. Clin Infect Dis 2009; 49:1703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fattahi F, Badalzadeh M, Sedighipour L et al. . Inheritance pattern and clinical aspects of 93 Iranian patients with chronic granulomatous disease. J Clin Immunol 2011; 31:792–801. [DOI] [PubMed] [Google Scholar]
- 33. Conti F, Lugo-Reyes SO, Blancas Galicia L et al. . Mycobacterial disease in patients with chronic granulomatous disease: a retrospective analysis of 71 cases. J Allergy Clin Immunol 2016; 138:241–248.e3. [DOI] [PubMed] [Google Scholar]
- 34. Salvator H, Mahlaoui N, Catherinot E et al. . Pulmonary manifestations in adult patients with chronic granulomatous disease. Eur Respir J 2015; 45:1613–23. [DOI] [PubMed] [Google Scholar]
- 35. Lublin M, Bartlett DL, Danforth DN et al. . Hepatic abscess in patients with chronic granulomatous disease. Ann Surg 2002; 235:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hussain N, Feld JJ, Kleiner DE et al. . Hepatic abnormalities in patients with chronic granulomatous disease. Hepatology 2007; 45:675–83. [DOI] [PubMed] [Google Scholar]
- 37. Feld JJ, Hussain N, Wright EC et al. . Hepatic involvement and portal hypertension predict mortality in chronic granulomatous disease. Gastroenterology 2008; 134:1917–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Conte D, Fraquelli M, Capsoni F et al. . Effectiveness of IFN-gamma for liver abscesses in chronic granulomatous disease. J Interferon Cytokine Res 1999; 19:705–10. [DOI] [PubMed] [Google Scholar]
- 39. International Chronic Granulomatous Disease Cooperative Study Group. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. The International Chronic Granulomatous Disease Cooperative Study Group. N Engl J Med 1991;324:509–16. [DOI] [PubMed] [Google Scholar]
- 40. Falcone EL, Hanses S, Stock F et al. . Streptococcal infections in patients with chronic granulomatous disease: case report and review of the literature. J Clin Immunol 2012; 32:649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ben-Ari J, Wolach O, Gavrieli R, Wolach B. Infections associated with chronic granulomatous disease: linking genetics to phenotypic expression. Expert Rev Anti Infect Ther 2012; 10:881–94. [DOI] [PubMed] [Google Scholar]
- 42. Jones LB, McGrogan P, Flood TJ et al. . Special article: chronic granulomatous disease in the United Kingdom and Ireland: a comprehensive national patient-based registry. Clin Exp Immunol 2008; 152:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Köker MY, Camcıoğlu Y, van Leeuwen K et al. . Clinical, functional, and genetic characterization of chronic granulomatous disease in 89 Turkish patients. J Allergy Clin Immunol 2013; 132:1156–63.e5. [DOI] [PubMed] [Google Scholar]
- 44. Liese J, Kloos S, Jendrossek V et al. . Long-term follow-up and outcome of 39 patients with chronic granulomatous disease. J Pediatr 2000; 137:687–93. [DOI] [PubMed] [Google Scholar]
- 45. Lee PP, Chan KW, Jiang L et al. . Susceptibility to mycobacterial infections in children with X-linked chronic granulomatous disease: a review of 17 patients living in a region endemic for tuberculosis. Pediatr Infect Dis J 2008; 27:224–30. [DOI] [PubMed] [Google Scholar]
- 46. Leiding JW, Holland SM. Chronic granulomatous disease. In: Pagon RA, Adam MP, Ardinger HH, et al, eds. GeneReviews. Seattle: GeneReviews; 1993. [PubMed] [Google Scholar]
- 47. Martire B, Rondelli R, Soresina A et al. ; IPINET Clinical features, long-term follow-up and outcome of a large cohort of patients with Chronic Granulomatous Disease: an Italian multicenter study. Clin Immunol 2008; 126:155–64. [DOI] [PubMed] [Google Scholar]
- 48. Margolis DM, Melnick DA, Alling DW, Gallin JI. Trimethoprim-sulfamethoxazole prophylaxis in the management of chronic granulomatous disease. J Infect Dis 1990; 162:723–6. [DOI] [PubMed] [Google Scholar]
- 49. Gallin JI, Alling DW, Malech HL et al. . Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med 2003; 348:2416–22. [DOI] [PubMed] [Google Scholar]
- 50. Bondioni MP, Lougaris V, Di Gaetano G et al. . Early identification of lung fungal infections in chronic granulomatous disease (CGD) using Multidetector Computer Tomography. J Clin Immunol 2017; 37:36–41. [DOI] [PubMed] [Google Scholar]
- 51. Segal BH, DeCarlo ES, Kwon-Chung KJ et al. . Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore) 1998; 77:345–54. [DOI] [PubMed] [Google Scholar]
- 52. Patiroglu T, Unal E, Yikilmaz A et al. . Atypical presentation of chronic granulomatous disease in an adolescent boy with frontal lobe located Aspergillus abscess mimicking intracranial tumor. Childs Nerv Syst 2010; 26:149–54. [DOI] [PubMed] [Google Scholar]
- 53. Bielorai B, Toren A, Wolach B et al. . Successful treatment of invasive aspergillosis in chronic granulomatous disease by granulocyte transfusions followed by peripheral blood stem cell transplantation. Bone Marrow Transplant 2000; 26:1025–8. [DOI] [PubMed] [Google Scholar]
- 54. Bassiri-Jahromi S, Doostkam A. Fungal infection and increased mortality in patients with chronic granulomatous disease. J Mycol Med 2012; 22:52–7. [DOI] [PubMed] [Google Scholar]
- 55. Al-Otaibi AM, Al-Shahrani DA, Al-Idrissi EM, Al-Abdely HM. Invasive mucormycosis in chronic granulomatous disease. Saudi Med J 2016; 37:567–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shigemura T, Nakazawa Y, Amano Y et al. . Subcutaneous abscess due to the basidiomycete Phellinus mori in a patient with chronic granulomatous disease. Infection 2015; 43:371–5. [DOI] [PubMed] [Google Scholar]
- 57. Ramesh M, Resnick E, Hui Y et al. . Phellinus tropicalis abscesses in a patient with chronic granulomatous disease. J Clin Immunol 2014; 34:130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Haidar G, Zerbe CS, Cheng M et al. . Phellinus species: an emerging cause of refractory fungal infections in patients with X-linked chronic granulomatous disease. Mycoses 2017; 60:155–60. [DOI] [PubMed] [Google Scholar]
- 59. Lyratzopoulos G, Ellis M, Nerringer R, Denning DW. Invasive infection due to penicillium species other than P. marneffei. J Infect 2002; 45:184–95. [DOI] [PubMed] [Google Scholar]
- 60. Santos PE, Piontelli E, Shea YR et al. . Penicillium piceum infection: diagnosis and successful treatment in chronic granulomatous disease. Med Mycol 2006; 44:749–53. [DOI] [PubMed] [Google Scholar]
- 61. Galluzzo ML, Hernandez C, Davila MT et al. . Clinical and histopathological features and a unique spectrum of organisms significantly associated with chronic granulomatous disease osteomyelitis during childhood. Clin Infect Dis 2008; 46:745–9. [DOI] [PubMed] [Google Scholar]
- 62. Falcone EL, Holland SM. Invasive fungal infection in chronic granulomatous disease: insights into pathogenesis and management. Curr Opin Infect Dis 2012; 25:658–69. [DOI] [PubMed] [Google Scholar]
- 63. Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev 2011; 24:247–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Job KM, Olson J, Stockmann C et al. . Pharmacodynamic studies of voriconazole: informing the clinical management of invasive fungal infections. Expert Rev Anti Infect Ther 2016; 14:731–46. [DOI] [PubMed] [Google Scholar]
- 65. Boast A, Curtis N, Cranswick N, Gwee A. Voriconazole dosing and therapeutic drug monitoring in children: experience from a paediatric tertiary care centre. J Antimicrob Chemother 2016; 71:2031–6. [DOI] [PubMed] [Google Scholar]
- 66. Dekkers BGJ, Bakker M, van der Elst KCM et al. . Therapeutic drug monitoring of posaconazole: an update. Curr Fungal Infect Rep 2016; 10:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsumura N, Akasu Y, Yamane H et al. . Aspergillus osteomyelitis in a child who has p67-phox-deficient chronic granulomatous disease. Kurume Med J 1999; 46:87–90. [DOI] [PubMed] [Google Scholar]
- 68. Ma HR, Mu SC, Yang YH et al. . Therapeutic effect of interferon-gamma for prevention of severe infection in X-linked chronic granulomatous disease. J Formos Med Assoc 2003; 102:189–92. [PubMed] [Google Scholar]
- 69. Kang EM, Marciano BE, DeRavin S et al. . Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J Allergy Clin Immunol 2011; 127:1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Miller DD, Cowen EW, Nguyen JC et al. . Melanoma associated with long-term voriconazole therapy: a new manifestation of chronic photosensitivity. Arch Dermatol 2010; 146:300–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Thompson GR 3rd, Bays D, Cohen SH, Pappagianis D. Fluoride excess in coccidioidomycosis patients receiving long-term antifungal therapy: an assessment of currently available triazoles. Antimicrob Agents Chemother 2012; 56:563–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Beauté J, Obenga G, Le Mignot L et al. ; French PID Study Group CEREDIH Epidemiology and outcome of invasive fungal diseases in patients with chronic granulomatous disease: a multicenter study in France. Pediatr Infect Dis J 2011; 30:57–62. [DOI] [PubMed] [Google Scholar]
- 73. Loffredo L, Perri L, Zicari AM et al. . Chronic granulomatous disease as an SOS call for multicenter cooperative effort to prevent infections: a meta-analysis of the treatments. Ann Allergy Asthma Immunol 2016; 117:285–9. [DOI] [PubMed] [Google Scholar]
- 74. Marciano BE, Wesley R, De Carlo ES et al. . Long-term interferon-gamma therapy for patients with chronic granulomatous disease. Clin Infect Dis 2004; 39:692–9. [DOI] [PubMed] [Google Scholar]