Abstract
Tuberous sclerosis complex (TSC) is an autosomal dominant neurodevelopmental disorder and the quintessential disorder of mechanistic Target of Rapamycin Complex 1 (mTORC1) dysregulation. Loss of either causative gene, TSC1 or TSC2, leads to constitutive mTORC1 kinase activation and a pathologically anabolic state of macromolecular biosynthesis. Little is known about the organ-specific metabolic reprogramming that occurs in TSC-affected organs. Using a mouse model of TSC in which Tsc2 is disrupted in radial glial precursors and their neuronal and glial descendants, we performed an unbiased metabolomic analysis of hippocampi to identify Tsc2-dependent metabolic changes. Significant metabolic reprogramming was found in well-established pathways associated with mTORC1 activation, including redox homeostasis, glutamine/tricarboxylic acid cycle, pentose and nucleotide metabolism. Changes in two novel pathways were identified: transmethylation and polyamine metabolism. Changes in transmethylation included reduced methionine, cystathionine, S-adenosylmethionine (SAM—the major methyl donor), reduced SAM/S-adenosylhomocysteine ratio (cellular methylation potential), and elevated betaine, an alternative methyl donor. These changes were associated with alterations in SAM-dependent methylation pathways and expression of the enzymes methionine adenosyltransferase 2A and cystathionine beta synthase. We also found increased levels of the polyamine putrescine due to increased activity of ornithine decarboxylase, the rate-determining enzyme in polyamine synthesis. Treatment of Tsc2+/− mice with the ornithine decarboxylase inhibitor α-difluoromethylornithine, to reduce putrescine synthesis dose-dependently reduced hippocampal astrogliosis. These data establish roles for SAM-dependent methylation reactions and polyamine metabolism in TSC neuropathology. Importantly, both pathways are amenable to nutritional or pharmacologic therapy.
Introduction
Tuberous sclerosis complex (TSC) (OMIM 191100, 613254) is an autosomal dominant disorder causing neurologic morbidity and mortality most commonly due to refractory epilepsy, intellectual disability, autism, psychiatric disease (TSC-associated neuropsychiatric disease), and subependymal giant cell astrocytomas (SEGAs) (1). Substantial progress has been made in understanding and treating TSC since the identification of the causative genes, TSC1 and TSC2 (2,3), and their major role in regulating the eukaryotic kinase mTORC1 (mechanistic target of rapamycin complex 1) in response to growth signals. The mTORC1 kinase is a major regulator of anabolism/catabolism in response to not only growth factors but also nutrients (4–6). The protein products, hamartin (TSC1), tuberin (TSC2) and TBC1D7 form a TSC complex that inhibits the mTORC1 kinase under growth-restrictive conditions such as low oxygen tension or energy depletion [high adenosine monophosphate/adenosine triphosphate (ATP) ratio] (7). Inhibition is due to the GTPase activating domain of tuberin on RAS Homolog Enriched in Brain (RHEB) (8). Loss of either hamartin or tuberin in TSC results in constitutive mTORC1 activity leading to disease manifestations. Pathogenic loss of TSC complex activity can be either due to haploinsufficiency or loss of heterozygosity depending on the specific TSC lesion (9–12). Rapamycin is a bacterial metabolite that specifically inhibits mTORC1, and was used to identify the mTORC1 kinase (13). The discovery of the TSC complex as a major regulator of mTORC1 led to the successful use of rapamycin and its derivatives (rapalogs) in many preclinical mouse models of TSC (14–18). These successes were soon translated into clinical trials (19,20). Currently the Food and Drug Administration has approved everolimus (a rapalog) to treat SEGAs. SEGAs are non-malignant tumors that develop in 20% of TSC patients and are difficult to treat due to their location along the ventricles (21,22). Everolimus can shrink SEGAs, but treatment is indefinite and discontinuation of everolimus causes regrowth (19,23,24). Recently, a placebo-controlled double blind study showed that rapalogs are useful adjunctive therapy for refractory epilepsy associated with TSC (20). In spite of these successes, new TSC therapies are needed that could be combined with lower doses of rapalogs or used in their place. Toward this goal, research into mTORC1 physiology has uncovered a major role for nutrients and changes in intermediary metabolism that might also serve as therapeutic targets. In order to orchestrate anabolism, mTORC1 activation triggers a cascade of transcriptional, translational, and post-translational programs that activate requisite metabolic pathways. For example, protein synthesis of 5’-TOP messenger ribonucleic acid translation is augmented by inactivation of 4E-BP and activation of S6 Kinase to boost the ribosomal translational apparatus (25). MYC-activated glutaminolysis preferentially converts glutamine to alpha-ketoglutarate for tricarboxylic acid (TCA) anaplerosis (26). To support nucleic acid biosynthesis, the de novo purine and pyrimidine pathways are activated (27). The transcription factor SREBP stimulates lipid biosynthesis and the pentose phosphate shunt to generate NADPH reducing equivalents as well as ribose for nucleic acid biosynthesis (28).
Although substantial progress has been made in the identification of metabolic pathways associated with Tsc1 or Tsc2 loss and mTORC1 activation, little is known about organ-specific effects. Since brain pathology is such a prevalent and debilitating feature of TSC, we performed untargeted metabolomic profiling using a well-characterized brain-specific mouse model of TSC, Tsc2-RG [formerly Tsc2flox/ko; hGFAP-Cre: deletion of Tsc2 in radial glial progenitors starting at E12.5 (14)]. Our results identified several important pathways already shown to be affected by mTORC1 activity such as glutathione metabolism, glutaminolysis/TCA cycle, and the pentose phosphate pathway. Importantly, we identified changes in two novel pathways, transmethylation and polyamine metabolism, that are associated with the loss of Tsc2 in the mouse brain. Using the results of these studies, we pharmacologically targeted polyamine synthesis and demonstrated the importance of this pathway for disease pathogenesis. Implications for neuropathology and future treatment trials are discussed.
Results
Metabolomic profiling of Tsc2-RG mice
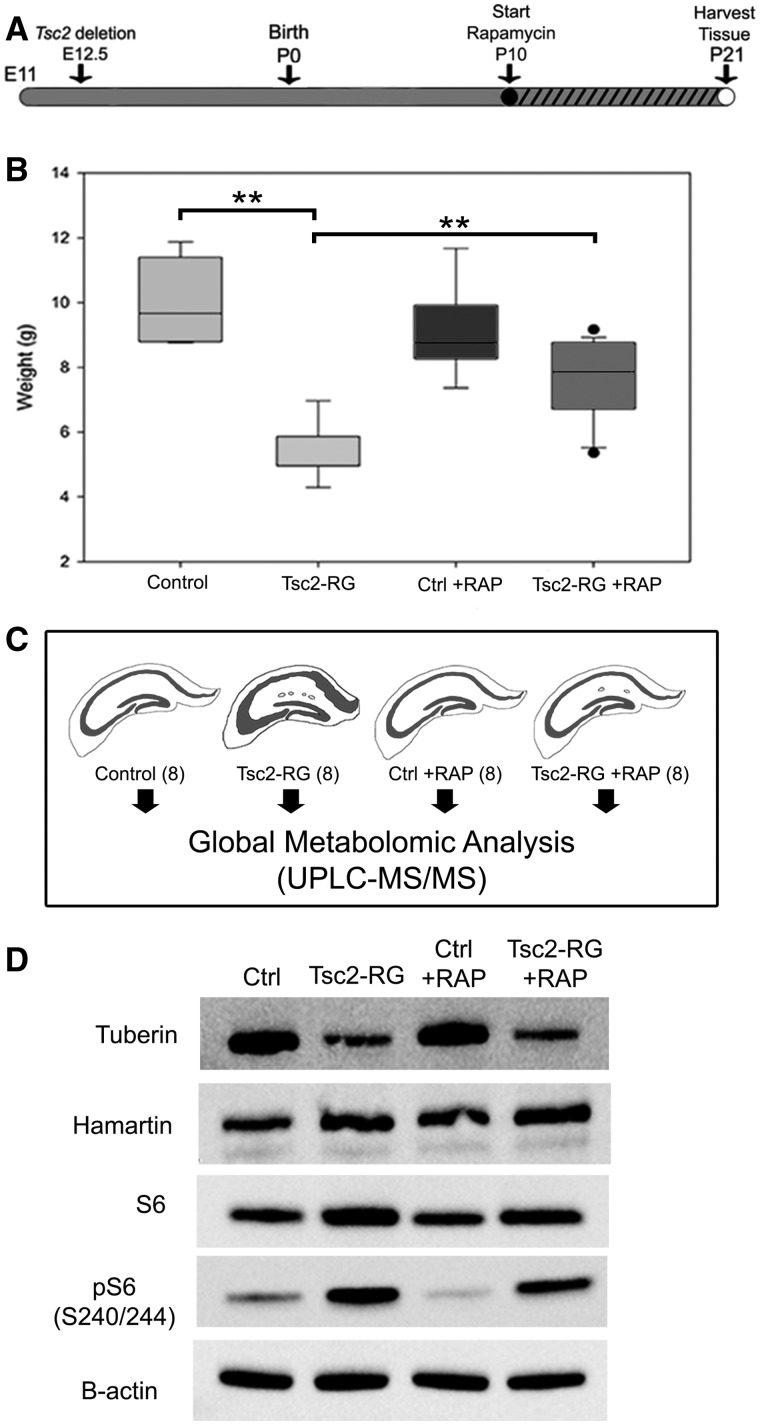
To identify the metabolic changes in the brains of Tsc2-RG mice, we isolated hippocampal lysates at postnatal (P) day 21 from eight control (Tsc2 +/flox), eight Tsc2-RG, eight control rapamycin treated and eight Tsc2-RG rapamycin-treated animals. Rapamycin treatment started at day P10 (when mutant animals begin to manifest a failure to thrive phenotype) (Fig. 1A). At P21 there was a significant weight difference between untreated control and mutant animals that was largely corrected by rapamycin treatment as previously reported (Fig. 1B) (14). Animals were sacrificed at P21, and hippocampi were isolated for metabolomic profiling (Fig. 1C). Immunoblot analyses of representative cortical samples showed decreased tuberin and control levels of hamartin in the untreated and rapamycin-treated Tsc2-RG mice as expected due to Cre-mediated deletion in radial glial cells starting at E12.5 (Fig. 1D). The level of phospho-S6 (S240/244) was increased in untreated Tsc2-RG mice, consistent with activated mTORC1. Rapamycin treatment of Tsc2-RG mice reduced phospho-S6 by 20% of untreated Tsc2-RG levels.
Figure 1.
Preparation and characterization of hippocampal samples for metabolomic profiling. (A) Time course of rapamycin treatment of control and Tsc2-RG mice. hGFAP-Cre-mediated disruption of the Tsc2 gene begins at embryonic day 12.5. Mice were treated with rapamycin by daily i.p. injection from P10 to P21 (denoted by hatched bar) and sacrificed for metabolomic profiling at P21. (B) Animal weights at P21. Note rapamycin treatment partially rescued impaired weight gain of Tsc2-RG mice. (C) Hippocampal tissue was isolated from eight animals per group and sent for metabolomic analysis. (D) Immunoblot analysis illustrating reduced tuberin protein and increased mTORC1 activity (pS6) in cortical lysates of Tsc2-RG mice compared with control lysates. Rapamycin treatment partially rescued mTORC1 activity in Tsc2-RG brains by P21. RAP, rapamycin. **P < 0.001 for pairwise comparisons.
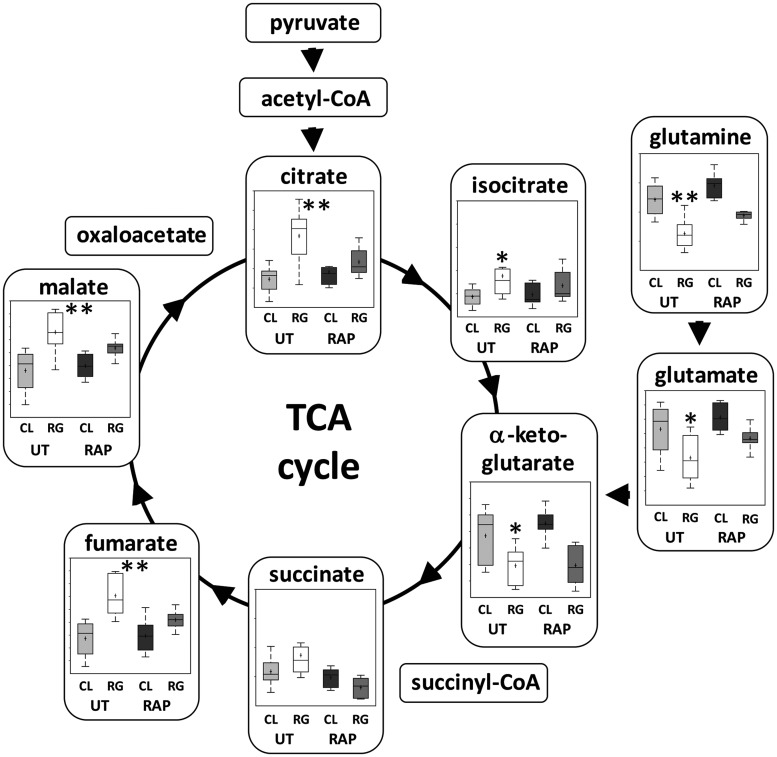
In total, 434 compounds were annotated (mass-normalized quantitative data can be found in the Supplementary Material). Principal component and hierarchical cluster analysis (Fig. 2A) demonstrated significant clustering by genotype. There were 103 biochemicals upregulated (P < 0.05) and 68 downregulated (P < 0.05) in the untreated Tsc2-RG hippocampi compared with untreated control (Fig. 2B). Metabolites represented a variety of molecular families including amino acids (Fig. 2C), carbohydrates, lipids, nucleotides, and energy compounds. Notably, rapamycin treatment returned 42/103 increased metabolites and 29/68 decreased metabolites back to control range (Supplementary Material, Fig. S1). Enrichment analysis identified several metabolic pathways significantly different in mutant brains compared with control. Noteworthy are redox (ascorbate, aldarate and glutathione), pentose, glutamate and TCA cycle metabolism, pathways that have been demonstrated to be affected by loss of TSC-mediated mTORC1 inhibition (Fig. 2C and D) (29–32). Particularly noteworthy are the changes in glutamine/glutamate/TCA cycle metabolism (Fig. 3). In proliferating cells, mTORC1 stimulates glutamine anaplerosis (glutaminolysis) to supply metabolites to the TCA cycle (33). Under mTORC1 activating conditions, glutamine is converted to glutamate, then alpha-ketoglutarate to supply the TCA cycle with carbon skeletons for many biosynthetic reactions (34). Glutamine, glutamate and alpha-ketoglutarate are all low in Tsc2-RG samples compared with controls, suggesting that even in low proliferative brain tissue there is an anaplerotic demand. Most of these compounds return to control levels with rapamycin treatment. Levels of other TCA cycle intermediates were also significantly altered in Tsc2-RG hippocampi. In particular, citrate, a precursor of lipid biosynthesis, was elevated in the mutant brains. This may suggest a defect in lipid biosynthesis with accumulation of citrate precursor. Interestingly, a defect in myelin biosynthesis is well documented in Tsc2-RG mice as well as several other brain-specific TSC mouse models (14,35,36).
Figure 2.
Metabolomic profile of hippocampi in untreated and rapamycin-treated control and Tsc2-RG mice. (A) Principal component analysis of the dataset attributed the most variability in metabolite expression to genotype (x-axis), with a weaker effect of treatment (y-axis). (B) Heat map of significantly (P < 0.05) altered metabolites between untreated control and Tsc2-RG mice. Metabolites are grouped by those upregulated in untreated Tsc2-RG and those downregulated in Tsc2-RG mice and are further organized by general biological function. The response of these metabolites to rapamycin (RAPA) treatment in control and Tsc2-RG mice is also indicated. Columns of signals represent data from individual subjects (eight per genotype/treatment group) with blue indicating downregulation and yellow upregulation. The intensity of the signal reflects the Z-score for that data point. Note that rapamycin treatment partially normalized levels of this set of metabolites in Tsc2-RG mice to those of untreated control animals, whereas rapamycin had minimal effect in control mice. (C) Schematic of altered amino acid synthesis in untreated Tsc2-RG versus control mice. Yellow and blue circles indicate amino acid derivatives upregulated and downregulated, respectively, in Tsc2-RG mice compared with controls. Thickness of the circle outline indicates the relative magnitude of change. (D) Pathway fold enrichment plot illustrating metabolic pathways significantly altered in Tsc2-RG mice (P-values indicated).
Figure 3.
Metabolomic profile of glutamine and TCA cycle metabolism in hippocampus of untreated and rapamycin-treated control and Tsc2-RG mice. (A) Box and whisker plots show levels of pathway metabolites in hippocampus of untreated (UT) and rapamycin (RAP) treated control (CL) and Tsc2-RG (RG) mice. Arrows indicate direction of metabolism. Acetyl-CoA, succinyl-CoA and oxaloacetate were not present in the dataset. Note the elevation of most TCA metabolites in untreated Tsc2-RG samples, with the exception of glutamine, glutamate and alpha-ketoglutarate, which are downregulated. Box plots: +, mean value; center line of box, median value; top line of box, limit of upper quartile; bottom line of box, limit of lower quartile; top of whisker, maximum of distribution; bottom of whisker, minimum of distribution; ordinate represents scaled intensity. *P < 0.05, **P < 0.001 for pairwise comparison of untreated control and Tsc2-RG values.
Transmethylation pathway metabolism is altered in Tsc2-RG hippocampi and cerebral cortex
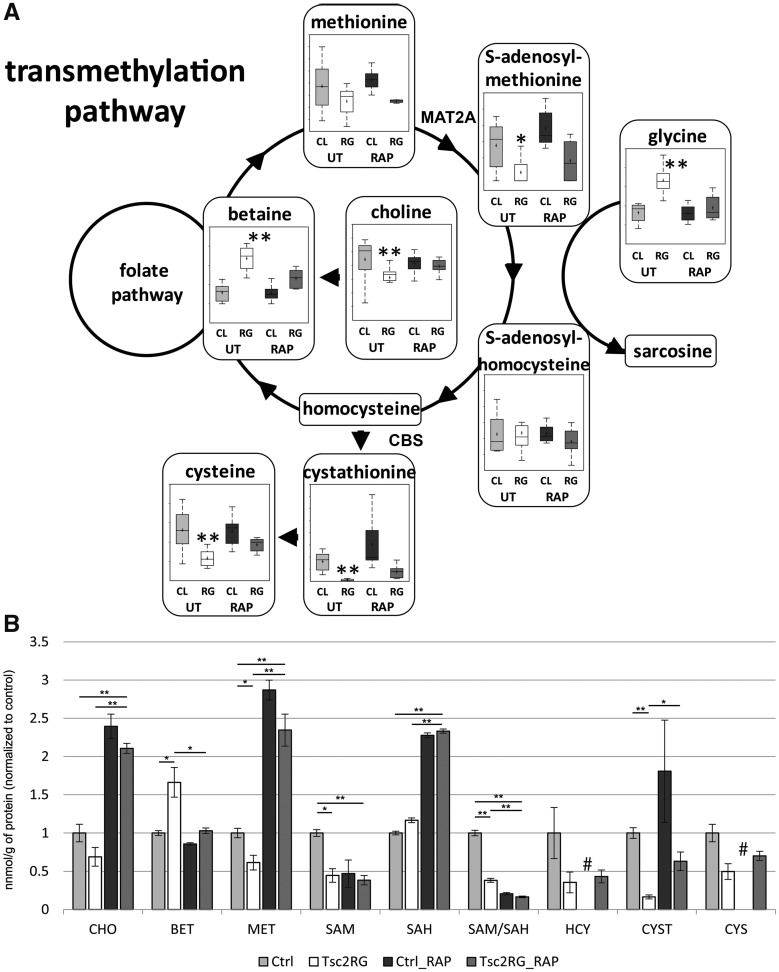
Pathway enrichment analysis revealed significant alterations in transmethylation intermediates (P < 0.05, Fig. 4), a pathway not previously associated with TSC or mTORC1 activation. This pathway is important for the recycling of methionine, the amino acid required for translation initiation and for the production of S-adenosylmethionine (SAM), the principal methyl donor in more than 200 enzymatic reactions, and once decarboxylated, the source of the aminopropyl donor for the synthesis of polyamines, spermidine and spermine (37). We observed a trend toward decreased methionine levels in hippocampal samples from Tsc2-RG mice. SAM, produced from methionine by methionine S-adenosyltransferase [MAT2A, (38)], was also reduced in hippocampus of Tsc2-RG mice. Cystathionine, a condensation of serine and homocysteine, and cysteine were reduced in mutant brains (Fig. 4A). These two compounds represent reduced flux into the transulfuration pathway. Betaine, an alternative methyl donor, was elevated in mutant hippocampus. Rapamycin treatment tended to partially reverse the changes found in the transmethylation pathway. To examine if these changes in the Tsc2-RG hippocampus were also present in the cerebral cortex, we performed targeted metabolomics of cortical lysates from the same animals from which hippocampal samples were isolated. Since this was a targeted analysis, samples could be deproteinized to add homocysteine to the analysis. Similar to what we observed in the hippocampus, methionine, SAM, and cystathionine were significantly reduced in Tsc2-RG cortices (Fig. 4B). A trend in reduction was also seen in choline, homocysteine and cysteine. Betaine was similarly elevated in the mutant cortex. Rapamycin treatment tended to reverse these trends, though SAM levels did not respond. These data demonstrate, by two distinct methodologies, that transmethylation and some components of the transulfuration pathway are altered in Tsc2-RG brains.
Figure 4.
Metabolomic profile of transmethylation and part of transulfuration pathways in untreated and rapamycin-treated control and Tsc2-RG mice. (A) Box and whisker plots show levels of pathway metabolites in hippocampus of untreated (UT) and rapamycin (RAP)-treated control (CL) and Tsc2-RG (RG) mice. Arrows indicate direction of metabolism. Sarcosine, homocysteine and folate pathway metabolites were not present in the dataset. Box plots: +, mean value; center line of box, median value; top line of box, limit of upper quartile; bottom line of box, limit of lower quartile; top of whisker, maximum of distribution; bottom of whisker, minimum of distribution. MAT2A, methionine S-adenosyltransferase 2A, CBS, cystathionine beta synthase. *P < 0.05, **P < 0.001 for pairwise comparison of untreated control and Tsc2-RG values. (B) Transmethylation pathway metabolites in cortical samples from control and Tsc2-RG mice as measured by LC-MS/MS. These values were normalized to untreated control values. CHO, choline; BET, betaine; MET, methionine; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; HCY, homocysteine; CYST, cystathionine; CYS, cysteine. Significant pairwise comparisons are indicated by * P < 0.05, **P < 0.01. # indicates absence of rapamycin-treated control samples from HCY and CYS metabolite datasets.
Dysregulated enzymes and SAM-dependent metabolites in Tsc2-RG brain
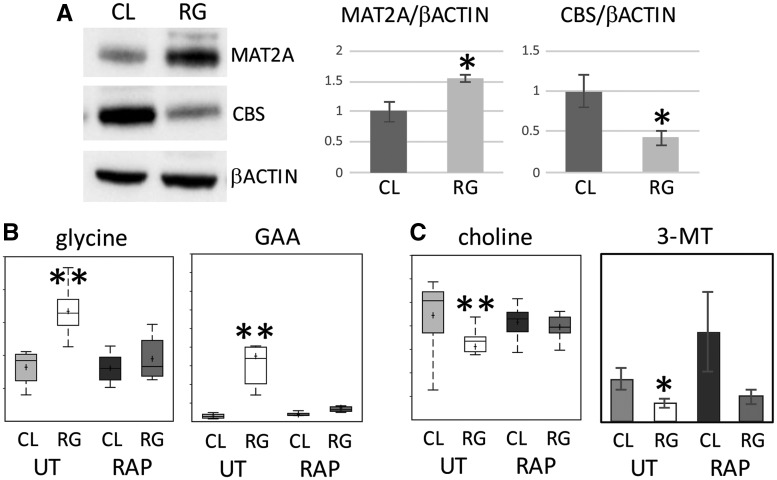
To further investigate the dysregulation of the transmethylation pathway in Tsc2-RG mice brains, we assessed the level of the enzyme MAT2A using immunoblotting. Since reduced levels of SAM and the SAM/ S-adenosylhomocysteine (SAH) ratio (a measure of the cellular methylation potential) are potent inducers of SAM-dependent methyl transferases, we reasoned that there should be an increase in MAT2A expression in the Tsc2-RG cortex (38). When compared with control samples, Tsc2-RG cortices showed about a 50% increase in MAT2A protein (Fig. 5A). We also observed about a 60% decrease in cystathionine beta synthase (CBS), required for conversion of homocysteine to cystathionine (39), in Tsc2-RG samples. Reduced CBS is consistent with the decreased level of cystathionine (Fig. 4A and B) and a presumptive retention of homocysteine in the transmethylation pathway. The reduction in SAM and SAM: SAH ratio would be expected to affect some SAM-dependent methyltransferase reactions. Consistent with this hypothesis, we observed an accumulation of guanidinoacetate (GAA) in Tsc2-RG hippocampus, likely the result of reduced SAM-dependent GAA N-methyltransferase (GAMT) function (Fig. 5B). GAMT utilizes SAM in the synthesis of creatine from GAA (40,41). Glycine accumulation may represent reduced activity of SAM-dependent glycine N-methyltransferase that converts glycine to sarcosine (42). We also observed decreases in some of the products of SAM-dependent methyltransferase reactions. In Tsc2-RG hippocampus and cortex there were reduced choline levels (Fig. 5C). Choline can be synthesized by the successive SAM-dependent methylation of phosphatidylethanolamine. Rapamycin treatment caused significant increases in choline levels in cortical samples. Catechol-O-methyltransferase (COMT) is involved in catecholamine metabolism. Based on the potential relevance of neurotransmitters to TSC neuropathology, we performed targeted analysis of neurotransmitters in control and Tsc2-RG cortical lysates. We observed a trend toward reduced homovanillic acid (HVA) (data not shown) and significantly reduced 3-methoxytyramine (3-MT) in Tsc2-RG cortical lysates (Fig. 5C) (43). Rapamycin returned Tsc2-RG levels back toward control. These data demonstrate functional consequences of altered transmethylation metabolism in Tsc2-RG mouse brains.
Figure 5.
Dysregulation of enzymes and SAM-dependent enzymatic reactions in Tsc2-RG mice. (A) Immunoblot showing increased expression of MAT2A and reduced expression of CBS proteins in cortices of Tsc2-RG (RG) mice compared with control. Immunoblot band intensities for MAT2A and CBS relative to β-actin are shown normalized to control (CL) values. (B) Accumulation of precursors of SAM-dependent enzymatic reactions in Tsc2-RG hippocampus. Box and whisker plots showing increased levels of glycine and GAA in Tsc2-RG hippocampus. (C) Decreased levels of products of SAM-dependent enzymatic reactions in Tsc2-RG brains. Box and whisker plot showing decreased levels of choline and histogram showing reduced 3-MT in Tsc2-RG hippocampus and cortex, respectively. UT, untreated; RAP, rapamycin treated. *P < 0.05, **P < 0.001 for pairwise comparison of untreated control and Tsc2-RG values.
Altered polyamine synthesis in Tsc2-RG mice
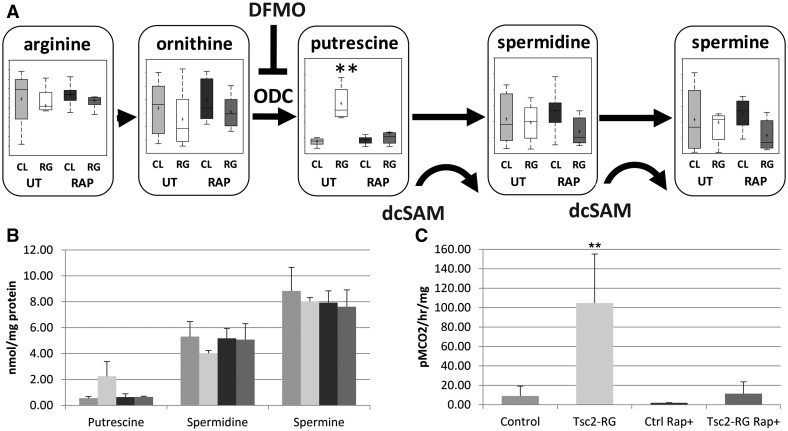
Metabolomic profiling detected significant changes in polyamine metabolism, most notably elevations of putrescine (Fig. 6A and B) and N-acetylputrescine (not shown). Polyamines are multifunctional polycations important for gene expression, translation initiation and elongation, cell proliferation, and autophagy, and their biosynthesis is upregulated in malignancies (44); these are processes that are also tightly regulated by mTORC1 (45). Putrescine is synthesized from ornithine by the rate-limiting enzyme, ornithine decarboxylase (ODC). Putrescine is sequentially converted to spermidine and spermine by spermidine and spermine synthetases, enzymes requiring decarboxylated-SAM as an aminopropyl donor (45). We observed an approximately 4-fold increase of putrescine in hippocampal samples from Tsc2-RG compared with control mice and putrescine levels returned to normal with rapamycin treatment (Fig. 6A). In contrast, spermidine and spermine levels remained unchanged in the mutants. To investigate whether altered polyamine synthesis also occurs in the cerebral cortex of Tsc2-RG mice, we performed high performance liquid chromatography (HPLC) on cortical lysates and observed increased putrescine levels, without altered spermine or spermidine concentrations (Fig. 6B). We next assessed ODC activity in Tsc2-RG cortical lysates, since ODC is the rate-limiting enzyme in putrescine synthesis. We observed an approximately 10-fold increase in ODC activity in Tsc2-RG cortical lysates compared with control samples, an effect attenuated by rapamycin treatment (Fig. 6C).
Figure 6.
Metabolomic profile of polyamine metabolism in untreated and rapamycin-treated control and Tsc2-RG mice. (A) Pathway diagram depicting dysregulation of polyamine synthesis, mainly the accumulation of putrescine, in Tsc2-RG hippocampus. Box and whisker plots show levels of pathway intermediate metabolites in hippocampus of untreated (UT) and rapamycin (RAP)-treated control (CL) and Tsc2-RG (RG) mice. dcSAM (decarboxylated SAM) acts as an aminopropyl donor in the conversion of putrescine to spermidine and spermidine to spermine. ODC, ornithine decarboxylase; DFMO, α-difluoromethylornithine; an irreversible ODC inhibitor. Box plots: +, mean value; center line of box, median value; top line of box, limit of upper quartile; bottom line of box, limit of lower quartile; top of whisker, maximum of distribution; bottom of whisker, minimum of distribution; **P < 0.001 for pairwise comparison of untreated control and Tsc2-RG values. (B) Elevated putrescine levels in cortical samples from Tsc2-RG mice by HPLC. Spermidine and spermine levels are unaffected. (C) Elevated ODC activity in cortical samples from Tsc2-RG mice.
Irreversible inhibition of ODC by α-Difluoromethylornithine attenuates astrogliosis in Tsc2+/− mice in a dose-dependent manner
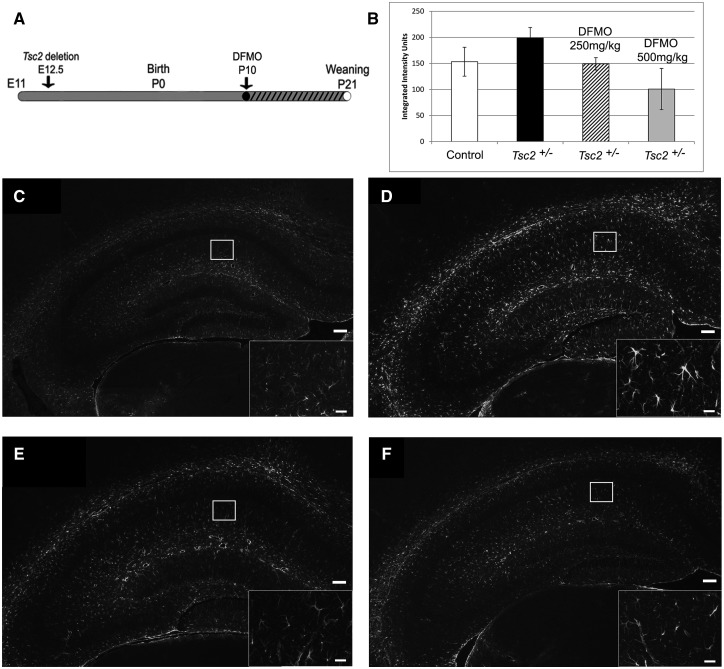
The increased ODC activity and putrescine levels in Tsc2-RG brains may be pathogenic and/or protective. To examine the consequences of ODC inhibition on TSC neuropathology, we used the irreversible and well-tolerated inhibitor α-difluoromethylornithine (DFMO), (eflornithine). DFMO is used for the treatment of African trypanosomiasis and has had limited success in treating malignancies overexpressing polyamines (46). Currently there are several clinical trials exploring the effect of DFMO on neuroblastoma (clinical trials.gov). For these experiments, we focused on the heterozygous Tsc2 KO (Tsc2+/−) rather than Tsc2-RG mice for several reasons. In contrast to Tsc2-RG mice, brain morphology of Tsc2+/− mice is grossly normal, with the exception of increased astrogliosis in the hippocampal CA1 region (47). Additionally, Tsc2 haploinsufficiency has been associated with synaptic plasticity and behavioral deficits in Tsc2+/− mice (48), indicating that Tsc2 heterozygosity may underlie some of the neuropsychiatric manifestations of TSC in humans. Indeed, failure to identify loss of heterozygosity in tubers supports the idea that TSC1 or TSC2 haploinsufficiency may be enough to contribute to some aspects of neuropathology (10,49). To determine if dysregulated polyamine synthesis contributes to the neuropathology associated with haploinsufficiency of Tsc2, we treated Tsc2+/− mice with DFMO from P10-P21 and examined the CA1 region for reduction of astrogliosis (Fig. 7A). There was a dose-dependent reduction of astrogliosis in the CA1 region of the hippocampus as determined by immunohistochemistry staining for glial fibrillary acidic protein (GFAP) (Fig. 7B–F). Consistent with these results, 250 mg/kg DFMO treatment of Tsc2+/− mice reduced cortical ODC activity (untreated Tsc2+/−: 4.8 ± 0.4 pmol CO2/h/mg protein vs. treated Tsc2+/−: 1.0 ± 0.4 pmol CO2/h/mg protein, P = 0.004) and putrescine levels (untreated Tsc2+/−: 0.21 ± 0.04 nmol/mg protein vs. treated Tsc2+/−: 0.11 ± 0.01 nmol/mg protein, P = 0.03), without altering spermidine or spermine levels. These data indicate that the neuropathology of TSC is, at least in part, dependent on polyamine flux.
Figure 7.
Inhibition of ODC with DFMO in Tsc2+/− mice decreases astrogliosis in the hippocampal CA1 region in a dose-dependent manner. (A) Time course of DFMO treatment of Tsc2+/− mice. Mice were treated with DFMO (250 or 500 mg/kg) by daily i.p. injection from P10 to P21 (denoted by hatched bar) and sacrificed for GFAP immunofluorescence (IF) at P21. (B) Quantification of hippocampal GFAP IF intensity from control mice and untreated and DFMO-treated Tsc2+/− mice. (C) Representative IF of control, (D) untreated Tsc2+/−, (E) 250 mg/kg DFMO-treated Tsc2+/− and (F) 500 mg/kg DFMO-treated Tsc2+/- hippocampi using anti-GFAP antibody to detect astrogliosis (scale bar = 100 µm). Astrocytes in the CA1 region (inset, scale bar = 20 µm). *P < 0.05.
Discussion
In this study, we present data profiling global metabolic changes associated with loss of TSC2 protein in the hippocampus of mice in which the Tsc2 gene is disrupted in radial glial precursor cells and their descendent neurons and glia. We identified several pathways shown previously to be affected by loss of Tsc1 or Tsc2 (and mTORC1 activation) in in vitro metabolomic studies. Noteworthy is the anabolic signature of increased glutaminolysis/TCA cycle metabolism, pentose phosphate pathway flux and nucleotide metabolism, in addition to changes in glutathione metabolism (29–32). Overlapping metabolic changes between brain tissue from our Tsc2-RG mouse model and cultured Tsc2−/−; p53−/− mouse embryonic fibroblasts underscore the global effects of Tsc2 disruption on metabolism in different cell types, both in vitro and in vivo.
Unique to our study is the identification of dysregulated transmethylation and polyamine metabolism in brains of Tsc2-deficient mice. The transmethylation pathway is critical for the recycling of the amino acid methionine and the generation of SAM, the major methyl donor in mammalian cells (50–52). When compared with control hippocampal samples, Tsc2-RG mice show reduced levels of many of the components of this pathway (Fig. 4A), possibly due to increased demand for methionine as a result of increased mTORC1-mediated protein synthesis. Targeted metabolomic measurements from cortical samples were similar to hippocampal data and also allowed the measurement of homocysteine which was also reduced in Tsc2-RG mice. Our data suggest a decreased flux through the transmethylation pathway. Betaine, synthesized from choline, also acts as a methyl donor (53) and is elevated in Tsc2-RG mice, potentially as a compensatory mechanism for the lack of methionine for SAM synthesis. Similarly, folate metabolism is linked to transmethylation (54), however folate metabolites were below the level of detection in our study. Previous work investigating protein translational changes in Tsc2-deficient neural stem cells suggests that several key enzymes in the transmethylation pathway may be differentially expressed (55). Chief among these is the gene encoding MAT2A, which catalyzes the formation of SAM from methionine and ATP. Our data indicate that MAT2A protein is increased in the brains of Tsc2-RG mice, likely to compensate for reduced SAM. We also observed a reduction in CBS in Tsc2-RG brains, which is consistent with lower levels of cystathionine. By reducing cystathionine production, homocysteine may be preferentially recycled to methionine in lieu of entering the transulfuration pathway. This decreased flux into the transulfuration pathway may affect downstream glutathione production.
Methylation is a crucial reaction in mammalian cells for the synthesis of many important small molecules (neurotransmitters, creatine, sarcosine, etc.) and the modification and regulation of macromolecules such as DNA, RNA and protein (56–58). We show some evidence of dysregulated methylation in Tsc2-RG mice that could contribute to TSC neuropathology. Among these, COMT converts 3, 4-dihydroxyphenylacetic acid and dopamine to HVA and 3-MT, respectively (43). We show that both HVA and 3-MT are decreased in Tsc2-RG brains, consistent with impaired SAM-dependent COMT activity. The possible contribution of altered catecholamine metabolism to tuberous sclerosis associated neuropsychiatric disorders bears further investigation. Another SAM-dependent enzyme, GAMT synthesizes creatine from GAA (40,41). We show an accumulation of GAA in Tsc2-RG brains, likely the result of reduced GAMT function. Increased GAA and glycine (also elevated in Tsc2-RG brains) may increase neuronal hyperexcitability and lower seizure threshold (59,60), a common feature of TSC (61,62). We have yet to analyze histones, DNA or RNA in Tsc2-RG brains to see if there are changes in methylation that may suggest alterations in epigenetic regulation of gene expression. Dysregulated methionine metabolism has been demonstrated in multiple sclerosis and is associated with altered histone methylation and mitochondrial energetics (63,64). It is intriguing to speculate that such potential methylation-based alterations in gene regulation could contribute to the variable expressivity of TSC.
We also observe increased levels of the polyamine putrescine in both hippocampal and cortical samples from Tsc2-RG mice. Putrescine is synthesized from ornithine by ODC and metabolized to spermidine by spermidine synthase (45). We show the increase in putrescine is likely due to elevated ODC activity. Odc is a transcriptional target of protooncogene Myc, the translation of which is regulated by mTORC1 (65–67). Consistent with this model, the increase in ODC activity we observe in Tsc2-RG cortical samples is attenuated by rapamycin, indicating an mTORC1-dependent effect. The regulation of ODC activity is further complicated however by its interaction with the inhibitory protein antizyme, the synthesis of which involves positive feedback mechanisms by polyamines and mTORC2 activity under conditions of amino acid starvation (68).
Our most exciting discovery is that inhibition of ODC and reduction of putrescine with DFMO dose-dependently reduces hippocampal astrogliosis in Tsc2+/− mice. These results underscore the utility of an unbiased metabolomic approach to identify novel biochemical pathways associated with TSC neuropathology. Tsc2+/− mice show increased astrogliosis in the hippocampus (47). Precisely how putrescine may contribute to this phenotype is unclear. Polyamines have been associated with cell growth and proliferation (45) which may contribute to the increased number of astrocytes observed in the Tsc2+/− hippocampus. Alternatively, the astrogliosis may be a non-cell autonomous response to increased putrescine in neurons. Interestingly, mice in which Tsc2 has been specifically deleted in postmitotic excitatory neurons of the developing forebrain exhibit cortical astrogliosis (69), arguing for a non-cell autonomous role. In any event, it will be important to determine if DFMO treatment also corrects the hippocampal L-LTP and learning and memory deficits of Tsc2+/− mice (48).
A systematic analysis of DFMO treatment on the neuropathology of Tsc2-RG mice will be necessary to realize the therapeutic potential of DFMO in TSC. Tsc2-RG mice exhibit many of the phenotypic hallmarks of human TSC patients such as enlarged neurons, neuronal heterotopia, myelination defects, astrocytosis and epilepsy (10,14). In multiple models of epilepsy, successive seizures increase both reactive astrogliosis and polyamine accumulation (70–76), suggesting a role for polyamines in epileptic progression. Interestingly, transgenic mice overexpressing Odc (with concomitant increase of putrescine in the brain) show grossly normal neurodevelopment with mild spatial learning deficits and an elevated seizure threshold (77,78). These data suggest that parsing out the effects of ODC/putrescine on different TSC phenotypes may not be straightforward. Nevertheless, our data raise the possibility that normalizing putrescine levels and more generally, increasing methionine/SAM levels and methylation potential through dietary and/or pharmacologic intervention may have therapeutic value for TSC patients.
Materials and Methods
Animals and drug treatment
All animal experiments were approved by the Emory University Institutional Animal Care and Use Committee, and were carried out in accordance with the Guide for the Humane Use and Care of Laboratory Animals. We generated and genotyped Tsc2-RG mice (Tsc2ko/flox; hGFAP-Cre) and controls (Tsc2+/flox) as previously described in (14). Rapamycyin (MP Biomedicals) was dissolved in 100% DMSO at 1.0 mg/ml for storage at −20°C. Before use, rapamycin was diluted in sterile PBS and administered i.p. at 0.1 mg/kg daily based on previous experiments (15). Treatment began at P10 and continued until P21. On P21 mice were cervically dislocated and the brains were quickly removed and dissected in ice cold, sterile PBS. Hippocampal brain tissue was isolated from eight untreated control mice, eight untreated Tsc2-RG mice, eight rapamycin-treated control mice and eight rapamycin-treated Tsc2-RG mice. Samples were quickly frozen with dry ice and 100% ethanol and shipped to Metabolon, Inc. on dry ice for metabolite analysis. An equal number of male and female mice contributed to each group, except for untreated control (two female and six male). For DFMO experiments, mice were treated intra-peritoneally with a single daily dose of either 250 mg/kg or 500 mg/kg DFMO diluted in sterile PBS, from P10 to P21. DFMO was a generous gift from Dr Patrick M. Woster, MUSC.
Untargeted hippocampal metabolomic profiling
Samples were sent to Metabolon for global metabolomic analysis as described in (79). Briefly, samples were homogenized and subjected to methanol extraction then split into aliquots for analysis by ultrahigh HPLC/mass spectrometry (UHPLC/MS) in the positive (two methods), negative or polar ion mode. Metabolites were then identified by automated comparison of ion features to a reference library of chemical standards followed by visual inspection for quality control as previously described in (80). For statistical analyses and data display, any missing values are assumed to be below the limits of detection; these values were imputed with the compound minimum (minimum value imputation). Statistical tests (two-way ANOVA) were performed in ArrayStudio (Omicsoft) or “R” to compare data between experimental groups; P < 0.05 is considered significant. An estimate of the false discovery rate (Q-value) is also calculated to take into account the multiple comparisons that normally occur in metabolomic-based studies, with Q < 0.05 used as an indication of high confidence in a result.
Targeted analysis of cortical transulfuration, methylation pathway and neurotransmitter metabolites
Concentrations of methionine, SAM, SAH, cystathionine, choline and betaine were analyzed by stable-isotope dilution liquid chromatography-electrospray ionization (ESI) tandem MS (LC-ESI-MS/MS) from brain tissue as previously described in (81). Total Hcy was determined by LC-ESI-MS/MS in brain tissue as previously decribed in (64). Neurotransmitters were measured with HPLC and electrochemical detection.
ODC activity and determination of intracellular polyamines
Tissue homogenates were prepared on ice using a tissue tearor in buffer containing 25 mM TrisCl, pH 7.5, 0.1 mM EDTA and 2.5 mM DTT. ODC activity was measured as the release of radiolabeled CO2 following incubation with [14C]-ornithine, as previously described in (82). Aliquots of these lysates were also used for acid extraction and precolumn dansylation followed by HPLC analysis to determine intracellular concentrations of the individual polyamine (83). Both ODC activity and polyamine concentration are presented relative to total cellular protein, which was measured by the method of Bradford using interpolation on a bovine serum albumin standard curve (84).
Protein analysis
Antibodies used for Western blot analysis were as follows: mouse anti-β-actin (1:2000, Sigma-Aldrich), rabbit anti-tuberin, rabbit anti-hamartin, total rabbit anti-S6, phosphorylated (S240/244) rabbit anti-S6 and rabbit anti-CBS (1:1000, Cell Signaling Technology, Bedford, MA), and rabbit anti-MAT2A (1:1000, Abcam, San Francisco, CA). Whole cell lysates were made from day P21 cerebral cortex or hippocampus that was quick-frozen in liquid nitrogen. Samples were homogenized in a dounce homogenizer with 10 volumes of RIPA buffer with protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma-Aldrich). Lysates were centrifuged at 4°C, sonicated and frozen until use. Protein concentrations were determined with a Pierce BCA reagent kit (ThermoFisher Scientific, Rockford, IL, USA). Equal amounts of protein were separated on a denaturing 4–12% gradient gel (Invitrogen) and transferred to PVDF membranes (Immobilon, Sigma-Aldrich). Secondary antibodies were horseradish peroxidase conjugated. Visualization was conducted with an ECL kit (Amersham, Piscataway, NJ, USA) and a Chemidoc Imaging System (Bio-Rad, Hercules, CA). Protein band intensities were quantified with ImageJ software and normalized to β-actin control bands.
Histology
P21 mice were deeply anesthetized before undergoing transcardiac perfusion with PBS followed by 4% paraformaldehyde (PFA). Mouse brains were post-fixed in PFA overnight and stored in 70% ethanol prior to embedding in paraffin. Paraffin blocks were sectioned at 5 µm and slide mounted. Immunofluorescence was performed as previously described in (14) using mouse anti-GFAP antibody (1:400, Sigma-Aldrich, St Louis, MO).Tissue images were examined using a Leica DM6000 and captured with a Qimaging RETIGA-2000RV digital camera. Digital images were then processed using Adobe Photoshop CS6 (San Jose, CA, USA).
Supplementary Material
Supplementary Material is available at HMG online.
Conflict of Interest statement. None declared.
Funding
This work was supported by the National Institutes of Health (R21NS104410 to M.J.G. and RO1CA204345 to R.A.C).
Supplementary Material
References
- 1. Crino P.B., Nathanson K.L., Henske E.P. (2006) The tuberous sclerosis complex. N. Engl. J. Med., 355, 1345–1356. [DOI] [PubMed] [Google Scholar]
- 2. Consortium. (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell, 75, 1305–1315. [DOI] [PubMed] [Google Scholar]
- 3. van Slegtenhorst M., de Hoogt R., Hermans C., Nellist M., Janssen B., Verhoef S., Lindhout D., van den Ouweland A., Halley D., Young J.. et al. (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science, 277, 805–808. [DOI] [PubMed] [Google Scholar]
- 4. Menon S., Dibble C., Talbott G., Hoxhaj G., Valvezan A., Takahashi H., Cantley L.C., Manning B.D. (2014) Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell, 156, 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabatini D.M. (2017) Twenty-five years of mTOR: uncovering the link from nutrients to growth. Proc. Natl. Acad. Sci. U. S. A., 114, 11818–11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang J., Manning B.D. (2008) The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem. J., 412, 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dibble C., Elis W., Menon S., Qin W., Klekota J., Asara J., Finan P., Kwiatkowski D., Murphy L., Manning B.D. (2012) TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol. Cell, 47, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yoshida S., Hong S., Suzuki T., Nada S., Mannan A., Wang J., Okada M., Guan K.L., Inoki K. (2011) Redox regulates mammalian target of rapamycin complex 1 (mTORC1) activity by modulating the TSC1/TSC2-Rheb GTPase pathway. J. Biol. Chem., 286, 32651–32660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peri S., Caretti E., Tricarico R., Devarajan K., Cheung M., Sementino E., Menges C., Nicolas E., Vanderveer L., Howard S. (2017) Haploinsufficiency in tumor predisposition syndromes: altered genomic transcription in morphologically normal cells heterozygous for VHL or TSC. Oncotarget, 8, 17628–17642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jozwiak J., Jozwiak S., Wlodarski P. (2008) Possible mechanisms of disease development in tuberous sclerosis. Lancet Oncol., 9, 73–79. [DOI] [PubMed] [Google Scholar]
- 11. Crino P., Aronica E., Baltuch G., Nathanson K.L. (2010) Biallelic TSC gene inactivation in tuberous sclerosis complex. Neurology, 74, 1716–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chan J.A., Zhang H., Roberts P.S., Jozwiak S., Wieslawa G., Lewin-Kowalik J., Kotulska K., Kwiatkowski D.J. (2004) Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J. Neuropathol. Exp. Neurol., 63, 1236–1242. [DOI] [PubMed] [Google Scholar]
- 13. Sabatini D., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. (1994) RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell, 78, 35–43. [DOI] [PubMed] [Google Scholar]
- 14. Way S., McKenna J. III, Mietzsch U., Reith R., Wu H., Gambello M. (2009) Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum. Mol. Genet., 18, 1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Way S., Rozas N., Wu H., Mckenna J.r., Reith R., Hashmi S., Dash P., Gambello M. (2012) The differential effects of prenatal and/or postnatal rapamycin on neurodevelopmental defects and cognition in a neuroglial mouse model of tuberous sclerosis complex. Hum. Mol. Genet., 21, 3226–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeng L., Xu L., Gutman D., Wong M. (2008) Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann. Neurol., 63, 444.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeng L., Rensing N., Zhang B., Gutmann D., Gambello M., Wong M. (2011) Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum. Mol. Genet., 20, 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reith R., McKenna J., Wu H., Hashmi S., Cho S., Dash P., Gambello M. (2013) Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mose model of tuberous sclerosis complex. Neurobiol. Dis., 51, 93–103. [DOI] [PubMed] [Google Scholar]
- 19. Franz D.N., Belousova E., Sparagana S., Bebin E., Frost M., Kuperman R., Witt O., Kohrman M., Flamini J., Wu J. (2014) Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol., 15, 1513–1520. [DOI] [PubMed] [Google Scholar]
- 20. French J.A., Lawson J.A., Yapici Z., Ikeda H., Polster T., Nabbout R., Curatolo P., de Vries P.J., Dlugos D.J., Berkowitz N. (2016) Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet, 388, 2153–2163. [DOI] [PubMed] [Google Scholar]
- 21. Sharma M., Ralte A., Arora R., Santosh V., Shankar S., Sarkar C. (2004) Subependymal giant cell astrocytoma: a clinicopathological study of 23 cases with special emphasis on proliferative markers and expression of p53 and retinoblastoma gene proteins. Pathology, 36, 139–144. [DOI] [PubMed] [Google Scholar]
- 22. Phi J., Park S., Chae J., Hong K., Park S., Kang J., Jun J., Cho B., Wang K., Kim S. (2008) Congenital subependymal giant cell astrocytoma: clinical considerations and expression of radial glial cell markers in giant cells. Childs Nerv. Syst., 24, 1499–1503. [DOI] [PubMed] [Google Scholar]
- 23. Curran M. (2012) Everolimus: in patients with subependymal gaint cell astrocytomas associated with tuberous sclerosis complex. Paediatr. Drugs, 14, 51–60. [DOI] [PubMed] [Google Scholar]
- 24. Franz D.N., Agricola K., Mays M., Tudor C., Care M.M., Holland-Bouley K., Berkowitz N., Miao S., Peyrard S., Krueger D. (2015) Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann. Neurol., 78, 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma X., Blenis J. (2009) Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell. Biol., 10, 307–318. [DOI] [PubMed] [Google Scholar]
- 26. Wise D., DeBerardinis R., Mancuso A., Sayed N., Zhang X., Pfeiffer H., Nissim I., Daikhin E., Yudkoff M., McMahon S.. et al. (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. U. S. A., 105, 18782–18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoxhaj G., Hughes-Hallett J., Timson R., Ilagan E., Yuan M., Asara J., Ben-Sahra I., Manning B.D. (2017) The mTORC1 signaling network senses changes in cellular purine nucleotide levels. Cell Rep., 21, 1331–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bakan I., Laplante M. (2012) Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol., 23, 226–234. [DOI] [PubMed] [Google Scholar]
- 29. Duvel K., Yecies J., Menon S., Raman P., Lipovsky A., Souza A., Triantafellow E., Ma Q., Gorski R., Cleaver S.. et al. (2010) Activation of metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell, 39, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Navarrete A., Armitage E.G., Musteanu M., García A., Mastrangelo A., Bujak R., López-Casas P.P., Hidalgo M., Barbas C. (2014) Metabolomic evaluation of Mitomycin C and rapamycin in a personalized treatment of pancreatic cancer. Pharmacol. Res. Perspect., 2, e00067.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parkhitko A., Priolo C., Coloff J., Yun J., Wu J., Mizumura K., Xu W., Malinowska I., Yu J., Kwiatkowski D. (2014) Autophagy-dependent metabolic reprogramming sensitizes TSC2-deficient cells to the antimetabolite 6-aminonicotinamide. Mol. Cancer Res., 12, 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ben-Sahra I., Hoxhaj G., Ricoult S., Asara J., Manning B. (2016) mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science, 351, 728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Csibi A., Blenis J. (2011) Appetite for destruction: the inhibition of glycolysis as a therapy for tuberous sclerosis complex-related tumors. BMC Biol., 9, 69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moncada S., Higgs E.A., Colombo S.L. (2012) Fulfilling the metabolic requirements for cell proliferation. Biochem. J., 446, 1–7. [DOI] [PubMed] [Google Scholar]
- 35. Wesseling H., Elgersma Y., Bahn S. (2017) A brain proteomic investigation of rapamycin effects in the Tsc1+/- mouse model. Mol. Autism, 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carson R., Kelm N., West K., Does M., Fu C., Weaver G., McBrier E., Parker B., Grier M., Ess K. (2015) Hypomyelination following deletion of Tsc2 in oligodendrocyte precursors. Ann. Clin. Transl. Neurol., 2, 1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Casero R., Marton L. (2007) Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov., 6, 373–390. [DOI] [PubMed] [Google Scholar]
- 38. Lu S., Mato J. (2008) S-Adenosylmethionine in cell growth, apoptosis and liver cancer. J. Gastroenterol. Hepatol., 23, S73–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kruger W. (2017) Cystathionine b-synthesis deficiency: of mice and men. Mol. Genet. Metab., 121, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Komoto J., Yamada T., Takata Y., Konishi K., Ogawa H., Gomi T., Fujioka M., Takusagawa F. (2004) Catalytic mechanism of guanidinoacetate methyltransferase: crystal structures of guanidinoacetate methyltransferase ternary complexes. Biochemistry, 43, 14385–14394. [DOI] [PubMed] [Google Scholar]
- 41. Brosnan J., da Silva R., Brosnan M. (2011) The metabolic burden of creatine synthesis. Amino Acids, 40, 1325–1331. [DOI] [PubMed] [Google Scholar]
- 42. Yen C.H., Lin Y.T., Chen H.L., Chen S.Y., Chen Y.M. (2013) The multi-functional roles of GNMT in toxicolgy and cancer. Toxicol. Appl. Pharmacol., 266, 67–75. [DOI] [PubMed] [Google Scholar]
- 43. Mannisto P., Kaakkola S. (1999) Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev., 51, 593–628. [PubMed] [Google Scholar]
- 44. Murray-Stewart T., Woster P., Casero R.J. (2016) Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J., 473, 2937–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miller-Fleming L., Olin-Sandoval V., Campbell K., Ralser M. (2015) Remaining mysteries of molecular biology: the role of polyamines in the cell. J. Mol. Biol., 427, 3389–3406. [DOI] [PubMed] [Google Scholar]
- 46. Nowotarski S.L., Woster P.M., Casero R.A. (2013) Polyamines and cancer: implications for chemoprevention and chemotherapy. Expert Rev. Mol. Med., 55, 7378–7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Uhlmann E.J., Apicelli A.J., Baldwin R.L., Burke S.P., Bajenaru M.L., Onda H., Kwiatkowski D., Gutmann D.H. (2002) Heterozygosity for the tuberous sclerosis complex (TSC) gene products results in increased astrocyte numbers and decreased p27-Kip1 expression in TSC2+/- cells. Oncogene, 21, 4050–4059. [DOI] [PubMed] [Google Scholar]
- 48. Ehninger D., Han S., Shilyansky C., Zhou Y., Li W., Kwiatkowski D., Ramesh V., Silva A. (2008) Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat. Med., 14, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jozwiak J., Jozwiak S. (2007) Giant cells: contradiction to two-hit model of tuber formation? Cell. Mol. Neurobiol., 27, 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huidobro C., Fernandez A., Fraga M. (2013) The role of genetics in the establishment and maintenance of the epigenome. Cell. Mol. Life. Sci, 70, 1543–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Obeid R. (2013) The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients, 5, 3481–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grillo M., Colombatto S. (2008) S-adenosylmethionine and its products. Amino Acids, 34, 187–193. [DOI] [PubMed] [Google Scholar]
- 53. Ueland P., Holm P., Hustad S. (2005) Betaine: a key modulator of one-carbon metabolism and homocysteine status. Clin. Chem. Lab. Med., 43, 1069–1075. [DOI] [PubMed] [Google Scholar]
- 54. Bottiglieri T. (2013) Folate, vitamin B12, and S-adenosylmethionine. Psychiatr. Clin. North Am., 36, 1–13. [DOI] [PubMed] [Google Scholar]
- 55. Grabole N., Zhang J., Aigner S., Ruderisch N., Costa V., Weber F., Theron M., Berntenis N., Spleiss O., Ebeling M. (2016) Genomic analysis of the molecular neuropathology of tuberous sclerosis using a human stem cell model. Genome Med., 8, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jang H.S., Shin W.J., Lee J.E., Do J.T. (2017) CpG and Non-CpG Methylation in Epigenetic Gene Regulation and Brain Function. Genes (Basel), 8, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roundtree I.A., Evans M.E., Pan T., He C. (2017) Dynamic RNA modifications in gene expression regulation. Cell, 169, 1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Blanc R.S., Richard S. (2017) Arginine methylation: the coming of age. Mol. Cell, 65, 8–24. [DOI] [PubMed] [Google Scholar]
- 59. Schulze A., Tran C., Levandovskiy V., Patel V., Cortez M. (2016) Systemic availability of guanidinoacetate affects GABAA receptor function and seizure threshold in GAMT deficient mice. Amino Acids, 48, 2041–2047. [DOI] [PubMed] [Google Scholar]
- 60. Leuzzi V., Mastrangelo M., Battini R., Cioni G. (2013) Inborn errors of creatine metabolism and epilepsy. Epilepsia, 54, 217–227. [DOI] [PubMed] [Google Scholar]
- 61. Curatolo P., Moavero R., de Vries P. (2015) Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol., 14, 733–745. [DOI] [PubMed] [Google Scholar]
- 62. Feliciano D., Lin T., Hartman N., Bartley C., Kubera C., Hsieh L., Lafourcade C., O'Keefe R., Bordey A. (2013) A circuitry and biochemical basis for tuberous sclerosis symptoms: from epilepsy to neurocognitive deficits. Int. J. Dev. Neurosci., 31, 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singhal N.K., Freeman E., Arning E., Wasek B., Clements R., Sheppard C., Blake P., Bottiglieri T., McDonough J. (2017) Dysregulation of methionine metabolism in multiple sclerosis. Neurochem. Int., 112, 1–4. [DOI] [PubMed] [Google Scholar]
- 64. Singhal N.K., Li S., Arning E., Alkhayer K., Clements R., Sarcyk Z., Dassanayake R.S., Brasch N.E., Freeman E.J., Bottiglieri T. (2015) Changes in methionine metabolism and histone H3 trimethylation are linked to mitochondrial defects in multiple sclerosis. J. Neurosci, 35, 15170–15186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. De Benedetti A., Harris A. (1999) eIF4E expression in tumors: its possible role in progression of malignancies. Int. J. Biochem. Cell Biol., 31, 59–72. [DOI] [PubMed] [Google Scholar]
- 66. Bello-Fernandez C., Packham G., Cleveland J. (1993) The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc. Natl. Acad. Sci. U. S. A., 90, 7804–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wagner A., Meyers C., Laimins L., Hay N. (1993) c-Myc induces the expression and activity of ornithine decarboxylase. Cell Growth Differ., 4, 879–883. [PubMed] [Google Scholar]
- 68. Ray R., Bavaria M., Johnson L. (2015) Interaction of polyamines and mTOR signaling in the synthesis of antizyme (AZ). Cell Signal., 27, 1850–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Crowell B., Lee G., Nikolaeva I., Dal Pozzo V., D'Arcangelo G. (2015) Complex neurological phenotype in mutant mice lacking Tsc2 in excitatory neurons of the developing forebrain. eNeuro, 2, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Robel S., Buckingham S., Boni J., Campbell S., Danbolt N., Riedemann T., Sutor B., Sontheimer H. (2015) Reactive astrogliosis causes the development of spontaneous seizures. J. Neurosci., 35, 3330–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Becker A. (2018) Animal models of acquired epilepsy: insights into mechanisms of human epileptogenesis. Neuropathol. Appl. Neurobiol., 44, 112–129. [DOI] [PubMed] [Google Scholar]
- 72. Hadera M., Eloqayli H., Jaradat S., Nehlig A., Sonnewald U. (2015) Astrocyte-neuronal interactions in epileptogenesis. J. Neurosci. Res., 93, 1157–1164. [DOI] [PubMed] [Google Scholar]
- 73. Gibbons M., Smeal R., Takahashi D., Vargas J., Wilcox K. (2013) Contributions of astrocytes to epileptogenesis following status epilepticus: opportunities for preventive therapy? Neurochem. Int., 63, 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Laschet J., Trottier S., Leviel V., Guibert B., Bansard J., Chauvel P., Bureau M. (1999) Heterogeneous distribution of polyamines in temporal lobe epilepsy. Epilepsy Res., 35, 161–172. [DOI] [PubMed] [Google Scholar]
- 75. Hayashi Y., Hattori Y., Moriwaki A., Lu Y., Hori Y. (1993) Increases in brain polyamine concentrations in chemical kindling and single convulsion induced by pentylenetetrazol in rats. Neurosci. Lett., 149, 63–66. [DOI] [PubMed] [Google Scholar]
- 76. Camon L., de Vera N., Martinez E. (2001) Polyamine metabolism and glutamate receptor agonists-mediated excitotoxicity in the rat brain. J. Neurosci. Res., 66, 1101–1111. [DOI] [PubMed] [Google Scholar]
- 77. Halmekyto M., Alhonen L., Wahlfors J., Sinervirta R., Eloranta T., Janne J. (1991) Characterization of a transgenic mouse line over-expressing the human ornithine decarboxylase gene. Biochem. J., 278, 895–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Halonen T., Sivenius J., Miettinen R., Halmekyto M., Kauppinen R., Sinervirta R., Alakuijala L., Alhonen L., MacDonald E., Janne J.. et al. (1993) Elevated seizure threshold and impaired spatial learning in transgenic mice with putrescine overproduction in the brain. Eur. J. Neurosci., 5, 1233–1239. [DOI] [PubMed] [Google Scholar]
- 79. Long T., Hicks M., Yu H.C., Biggs W.H., Kirkness E.F., Menni C., Zierer J., Small K.S., Mangino M., Messier H.. et al. (2017) Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat. Genet., 49, 568–578. [DOI] [PubMed] [Google Scholar]
- 80. Dehaven C.D., Evans A.M., Dai H., Lawton K.A. (2010) Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J. Cheminform., 2, 9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Inoue-Choi M., Nelson H.H., Robien K., Arning E., Bottiglieri T., Koh W.P., Yuan J.M. (2013) Plasma S-adenosylmethionine, DNMT polymorphisms, and peripheral blood LINE-1 methylation among healthy Chinese adults in Singapore. BMC Cancer, 13, 389.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Coleman C.S., Stanley B.A., Pegg A.E. (1993) Effect of mutations at active site residues on the activity of ornithine decarboxylase and its inhibition by active site-directed irreversible inhibitors. J. Biol. Chem., 268, 24572–24579. [PubMed] [Google Scholar]
- 83. Kabra P.M., Lee H.K., Lubich W.P., Marton L.J. (1986) Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reverse-phase liquid chromatography: imprived separation systems for polyamines in cerebrospinal fluid, urine and tissue. J. Chromatogr., 380, 19–32. [DOI] [PubMed] [Google Scholar]
- 84. Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248–254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.