Abstract
Background
Disturbances of intestinal integrity, manifested by increased gastro-intestinal (GI) permeability, have been found in chronic obstructive pulmonary disease (COPD) patients during physical activity, often associated with intermittent hypoxic periods. Evidence about extrapulmonary organ disturbances, especially of the GI tract, during hospitalised acute exacerbation of COPD (AE-COPD) with hypoxaemic respiratory failure (RF) is lacking.
Objective
The aim was to assess changes in GI permeability in patients with AE-COPD and during recovery 4 weeks later.
Methods
All patients admitted to our hospital with AE-COPD accompanied by hypoxaemia at admission (PaO2 <8.7 kPa or O2 saturation <93%) were screened between October 2013 and February 2014. Patients with a history of GI or renal disease, chronic heart failure, or use of non-steroidal anti-inflammatory drugs in the 48 h before the test were excluded. GI permeability was assessed by evaluating urinary excretion ratios of the orally ingested sugars lactulose/L-rhamnose (L/R ratio), sucrose/L-rhamnose (Su/R ratio) and sucralose/erythritol (S/E ratio).
Results
Seventeen patients with severe to very severe COPD completed the study. L/R ratio (×103) at admission of AE-COPD was significantly higher than in the recovery condition (40.9 [29.4–49.6] vs. 27.3 [19.5–47.7], p = 0.039), indicating increased small intestinal permeability. There were no significant differences in the individual sugar levels in urine nor in the 0- to 5-h urinary S/E and Su/R ratios between the 2 visits.
Conclusion
This is the first study showing increased GI permeability during hospitalised AE-COPD accompanied by hypoxaemic RF. Therefore, GI integrity in COPD patients is an attractive target for future research and for the development of interventions to alleviate the consequences of AE-COPD.
Keywords: Hospital admission, Hypoxaemia, Lactulose/L-rhamnose ratio, Barrier function, Small intestine
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death worldwide, and particularly emergency department visits, hospitalisations and readmissions for acute exacerbations are drivers of high disease burden and societal costs [1]. Acute exacerbations of COPD (AE-COPD) are associated with increased airway and systemic inflammation [2]. During these acute episodes, gas exchange is often impaired, frequently resulting in acute respiratory failure (RF). Impaired gas exchange may be caused by increased airflow limitation, the rise in pulmonary vascular resistance, and increased mismatch of the ventilation/perfusion ratio [3, 4, 5, 6, 7, 8, 9, 10]. Although hypoxaemia is a frequent feature in patients admitted with severe AE-COPD and oxygen therapy is considered as a standard treatment in hospitalised AE-COPD, the extra-pulmonary systemic consequences of hypoxaemia are largely overlooked in clinical practice [8, 9, 10, 11, 12].
Gastro-intestinal (GI) disturbance as a potential extra-pulmonary systemic consequence of hospitalised AE-COPD accompanied by acute hypoxaemic RF has been scarcely studied in COPD patients. Former studies in this group of patients about GI permeability have only been performed in stable disease states [13]. Rutten et al. [13] have found a difference in intestinal permeability between patients suffering from COPD and healthy controls. In their study, the execution of activities of daily living (ADL) enhanced this difference in intestinal permeability. In patients suffering from chronic heart failure, the intestinal morphology, permeability and absorption are modified and disturbed [14, 15].
Mucosal epithelial integrity is essential to prevent the entry of potentially harmful luminal particles, e.g., bacteria and their products, into the systemic circulation by means of transcellular and paracellular pathways. Disturbed intestinal integrity might lead to increased permeability of the intestinal mucosal barrier, which is one of the mechanisms promoting bacterial translocation [16, 17]. Bacterial translocation might also contribute to the observed acute chronic flare-up of systemic inflammation, which has been reported in AE-COPD [2, 18].
Mechanistically, hypoxaemia might increase GI permeability. The enterocyte membranes, tight junctions, secreted mucus and immunologic factors form the intestinal barrier. The intestines are highly vascularized, and accordingly, the small gut villous epithelial cells are highly susceptible to oxygen shortage [14, 15]. This indicates that disturbances in the oxygen delivery to the intestinal mucosa could lead to disturbed intestinal integrity. Rutten et al. [13] proposed that redistribution of blood flow from the splanchnic bed to the skeletal muscle tissue and to vital organs may lead to local tissue hypoxia, contributing to enterocyte damage and increased GI permeability in COPD patients during ADL, as described above.
Moreover, it has been suggested that alterations in the GI tract might also be associated with systemic inflammation [19, 20]. The epithelial apical junction complexes, consisting of tight junctions and adherence junctions, are, besides hypoxaemia, sensitive to circulating pro-inflammatory cytokines, which are present in higher levels during AE-COPD [21]. Although systemic inflammation is an important component in AE-COPD, there is no evidence revealing a relationship with disturbances in the GI tract and systemic inflammation [13].
Based on the above-mentioned findings, disturbances of the intestinal integrity manifested by increased GI permeability might be present in patients with AE-COPD complicated by severe hypoxaemic RF. Accordingly, we hypothesized that patients admitted for AE-COPD accompanied by a hypoxaemic period show increased intestinal permeability.
Methods
Subject Inclusion
This prospective study was approved by the ethical committee of the Maastricht University Medical Centre (MUMC; METC 13-2-040) and conducted in the MUMC, The Netherlands, according to the Declaration of Helsinki (59th WMA General Assembly, Seoul, October 2008) and Good Clinical Practice guidelines.
All patients admitted to the MUMC with AE-COPD between October 2013 and February 2014 were screened according to the following inclusion criteria: clinical diagnosis of AE-COPD according to the GOLD statement, hypoxaemia at admission (PaO2 <8.7 kPa or O2 saturation <93%) and a minimum age of 40 years. Patients with a history of GI or renal disease, chronic heart failure and use of non-steroidal anti-inflammatory drugs in the 48 h before the test were excluded because these factors are known to influence GI permeability (inhibition of cyclooxygenases 1 and 2) [13, 22, 23, 24]. Patients who could not be tested within the first 72 h after hospital admission, mostly due to logistic reasons, were excluded. Nineteen patients gave written informed consent.
Study Design
The study consisted of 2 visits, the first shortly after admission to the hospital (visit 1) and the second visit during recovery, 4 weeks after the first test day (visit 2). Recovery was defined as regression of symptoms back to pre-existing levels, absence of additional oxygen or return to pre-existing long-term oxygen levels and normal vital signs. Because of uncertainty of recovery duration after AE-COPD, the follow-up visit was planned 4 weeks after the first visit (first test day) [25, 26, 27, 28]. During the second visit, regression of symptoms was assessed by the treating physician. In case of a readmission because of an AE-COPD, visit 2 would take place 4 weeks after discharge of this episode. The same study procedures took place at both visits. The study did not interfere with the standard medical care of the exacerbation.
Study Procedures
After overnight fast (24: 00–07: 00), a sugar solution (details see below) was orally ingested after collection of a baseline blood and urine sample. Before ingesting the sugar mixture, the saturation was measured using a pulse oximeter. Arterial blood gases were only collected for standard medical care. Until 1 h before the intake of the sugar solution, subjects were allowed to drink water ad libitum. Thereafter, subjects were allowed to drink a maximum of 500 mL water per hour. Medication intake was permitted. One hour after intake of the sugar solution, a standardized breakfast was served. Urine was collected during 5 h after drinking the sugar mixture. Patients were not allowed to eat after breakfast until the end of the test (4 h after breakfast).
Assessment of GI Permeability
The differential sugar absorption assays for gut permeability are based on orally ingested inert sugars that differentially cross the intestinal barrier based on their size [29]. Large sugars (oligosaccharides) typically cross the intestinal barrier paracellularly into the circulation in case of disturbed integrity, small sugars (monosaccharides) also cross the barrier in normal state via the transcellular route and are used to correct for, e.g., differences in gastric emptying, intestinal transit and renal function. Hence, if there is increased leakage due to barrier loss, especially the large sugars cross the intestinal barrier into the circulation, leading to a higher oligosaccharide/monosaccharide ratio. These sugars are rapidly cleared by the kidney and can be detected in the urine [16].
In this study, intestinal permeability was assessed using a validated multi-sugar test [24, 30]. The sugar mixture contained 1 g lactulose (Centrafarm BV, Etten-Leur, The Netherlands), 1 g sucralose (Brenntag AG, Mülheim, Germany), 0.5 g L-rhamnose (Danisco A/S, Copenhagen, Denmark), 1 g erythritol (Cargill Europe, Mechelen, Belgium) and 1 g sucrose (Van Gilse, Suiker Unie, Dinteloord, The Netherlands) and was dissolved in 100 mL tap water just before administration. After ingesting the sugar solution, the subjects were allowed to drink another 200 mL water. Urine sugar concentrations were determined by isocratic ion exchange high-pressure liquid chromatography (Model PU-1980 pump; Jasco, Easton, MD, USA) with mass spectrometry (Model LTQ XL; Thermo Fisher Scientific, Waltham, MA, USA) as described by van Wijck et al. [24, 30]. The ratio of lactulose/L-rhamnose (L/R) and sucralose/erythritol (S/E) reflects intestinal permeability status. Lactulose and L-rhamnose are often used as a marker for small intestinal permeability, since the microbiota in the colon degrades lactulose and rhamnose [20, 31]. Sucralose and erythritol can serve as a marker for whole-gut permeability, if collected over a 24-h period, since they endure colonic bacterial fermentation [13, 32, 33].
Outcomes
The primary outcome of this study was the change in GI permeability (L/R ratio, sucrose/L-rhamnose ratio [Su/R ratio] and S/E ratio) between admission and after recovery.
Data Collection
Medical history, most recent lung function data (including forced expiratory volume in 1 s [FEV1], forced vital capacity [FVC], FEV1/FVC ratio, residual volume, total lung capacity and diffusion capacity), comorbidities using the Charlson Comorbidity Index [34], medication, arterial blood gas, vital signs, physical examination at admission, full blood count, liver and renal function and inflammatory parameters (C-reactive protein [CRP] and white blood count) were assessed at admission. At both test days, vital signs, Borg score, medication and current symptoms were monitored.
Statistical Analysis
Data analyses were performed using SPSS for MAC version 21.0. Because of the small population, data were considered to be not normally distributed and analysed using non-parametric tests. Continuous data are presented as median and first and third quartiles (25–75% percentiles) and categorical data as percentages. Changes in L/R ratio between admission and recovery were calculated in absolute numbers and percentages. To compare characteristics which might be related to change in intestinal permeability between groups, the Wilcoxon signed-rank test was performed. A p value <0.05 was considered to be statistically significant.
The sample size was based on the outcome of a study in patients with chronic heart failure [15]. In this study, the permeability index (lactulose/mannitol ratio) was used as a marker of intestinal permeability. Performing a power calculation with the formula n = (α + β)2 × 2 × SD2/d2 and α = 0.05, β = 0.20, the difference (d) in lactulose/mannitol ratio = 0.35 and SD = 4.0, the amount of subjects needed was at least 16.3. Hence, 17 patients had to complete the study.
Results
Subject Selection
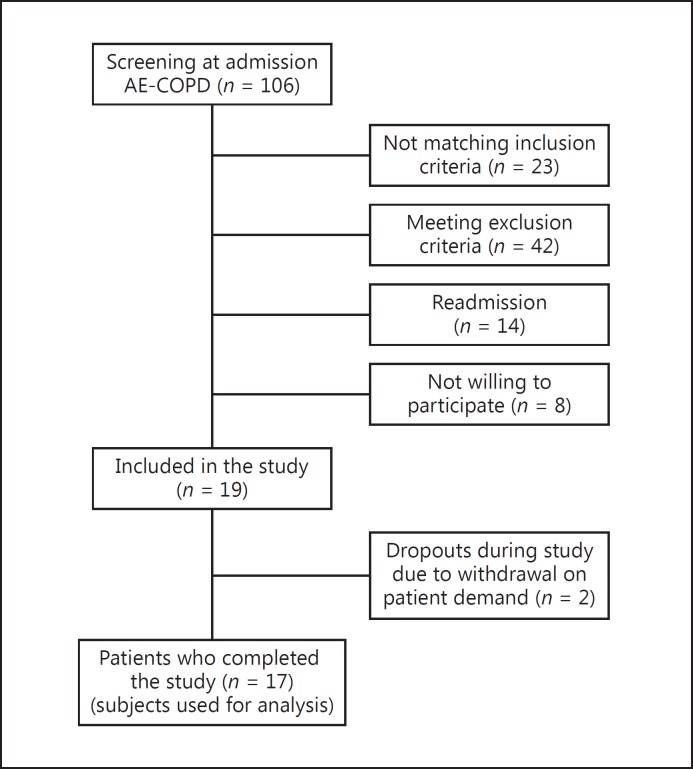
From October 2013 until February 2014, 106 patients admitted for an AE-COPD were screened (Fig. 1). Twenty-three patients did not meet to the inclusion criteria for the following reasons: absence of hypoxaemia (n = 18, 78.3%), not able to give informed consent (n = 4, 17.4%) and age <40 years (n = 1, 4.3%). Forty-two patients were excluded because of renal failure (n = 21, 50.0%), heart failure (n = 4, 9.5%), renal and heart failure (n = 2, 4.8%), acute GI complaints (n = 2, 4.8%), not being able to be tested within 72 h (n = 12, 28.6%) and urinary incontinence (n = 1, 2.4%). Fourteen had a readmission and had already been screened during an earlier admission. From the remaining 27 patients, 8 patients declined to participate. In total, 19 patients were included into the study. After the first visit, 2 patients withdrew from the study and did not participate in the second visit.
Fig. 1.
Flowchart of the subject selection. AE-COPD, acute exacerbation of chronic obstructive pulmonary disease.
Baseline Characteristics
Table 1 shows the baseline characteristics of the 17 study subjects who completed the study. In our study population, there were slightly more men than women, and the median age was 70 years. All patients were former or active smokers and had severe COPD, 2 patients in GOLD quadrant B and 15 patients in quadrant D. The median FEV1 in litres (L) was 1.04 (40% predicted) and the median diffusion capacity (in %predicted) was 45.85 (35.1–59.4). Five (30%) patients used long-term oxygen therapy, and 1 patient had chronic non-invasive ventilation. More than 82% had 2 or more comorbidities listed in the Charlson Comorbidity Index. All patients had had 1 or more hospital admissions for AE-COPD during the last year. Four patients had had more than 2 admissions for AE-COPD during the last year.
Table 1.
Basic characteristics of subjects who completed the study (n = 17)
| Gender, male/female | 10 (59)/7 (41) |
| Age, years | 70 (61.5–75.0) |
| Charlson Comorbidity Index score | 3 (2–3) |
| 1 | 3 (18) |
| 2 | 4 (24) |
| 3 | 8 (47) |
| 4 | 1 (6) |
| 5 | 1 (6) |
| COPD Gold Stage III/IV | 8 (47)/9 (53) |
| No. of AE-COPD during the last year | 3 (1–3) |
| Times hospitalized due to AE-COPD during the last year | 1 (1–2) |
| Smoking status, stopped/current smoker | 12 (70)/5 (30) |
| Smoking, pack-years | 39 (23.2–50.0) |
| BMI | 22.2 (20.56–25.1) |
| LTOT usage, no/yes | 12 (71)/5 (29) |
| Chronic NPPV | 1 (6) |
| FEV1, L | 1.04 (0.73–1.26) |
| FEV1, %pred | 40.0 (31.55–50.0) |
| FVC, L | 2.60 (1.90–3.47) |
| FVC, %pred | 92.4 (64.85–111.45) |
| FEV1/FVC, % | 39.62 (26.94–46.91) |
| FRC, L (n = 15) | 4.91 (4.50–5.69) |
| FRC, %pred (n = 15) | 163.0 (137.5–190.1) |
| RV, L (n = 15) | 3.83 (3.3–5.09) |
| RV, %pred (n = 15) | 173.2 (129.6–229.4) |
| TLC, L (n = 14) | 6.66 (5.64–8.11) |
| TLC, %pred (n = 14) | 128.2 (109.8–141.5) |
| TLCO, %pred (n = 13) | 45.85 (35.1–59.4) |
Data are presented as medians and 25–75% percentiles or n (%). AE-COPD, acute exacerbation of chronic obstructive pulmonary disease; BMI, body mass index; COPD, chronic obstructive pulmonary disease; LTOT, long-term oxygen treatment; NPPV, non-invasive positive pressure ventilation; FEV1, forced expiratory volume in 1 s; FVC, forced volume capacity; FEV1/FVC, Tiffeneau index; FRC, functional residual volume; RV, residual volume; TLC, total lung capacity; TLCO, diffusion capacity of carbon monoxide; %pred, percentage of predicted value.
At admission, all patients were hypoxaemic (initial SpO2 84% [80–89%]) at presentation to the emergency room (Table 2). All patients needed oxygen treatment at the emergency room. Seven patients were hypercapnic, and 2 patients required non-invasive ventilation because of acute respiratory acidosis. In 10 patients, antibiotic treatment was initiated, and 3 patients had a consolidation on chest X-ray. In 4 patients, the CRP level was higher than 50 mg/L. The median length of hospital stay was 6 days.
Table 2.
Clinical characteristics at admission for AE-COPD (n = 17)
| Initial SpO2, % | 84 (80–89) |
| Oxygen need at ER | 17 (100) |
| ABG at admission | |
| pO2, kPa | 7.4 (6.8–8.3) |
| pCO2, kPa | 5.7 (5.0–7.8) |
| pH | 7.39 (7.34–7.45) |
| HCO3–, mmol/L | 27.1 (23.9–32.6) |
| BE | 2.1 (0.2–5.3) |
| pCO2 ≥6.0, mmol/L | 7 (41) |
| SpO2 at admission after stabilizing, % | 88 (70–96) |
| Pneumonia and AE-COPD | 3 (18) |
| CRP >50 mg/L | 4 (24) |
| NPPV need | 2 (12) |
| Antibiotics started at admission | 10 (59) |
| LOS, days | 6.0 (3–9) |
Data are presented as medians and 25–75% percentiles or n (%). Hypoxia criteria were pO2 <8.7 kPa or SpO2 at admission ≤93%. AE-COPD, acute exacerbation of chronic obstructive pulmonary disease; SpO2, oxygen saturation; ABG, arterial blood gas; BE, base excess; CRP, C-reactive protein; NPPV, non-invasive positive pressure ventilation; LOS, length of hospital stay.
Clinical Parameters at Test Days
The clinical parameters at visit 1 and 2 are shown in Table 3. The oxygen saturation at visit 1 improved compared to the measurements at admission (SpO2 at admission, 88% [86–93%] vs. SpO2 at visit 1, 93% [91.5–95%], p = 0.005). Unfortunately, due to ethical considerations according to the approved protocol, we were not able to test patients directly at the time of admission when hypoxaemia was most prominent and, likely, the intestinal barrier damage was most severe. We did our best to schedule the tests as quickly as possible, which led to an interval time from admission until first visit of 52 h (28–69 h). After discharge, the second test day was scheduled. The median time between visit 1 and visit 2 was 34 days (28–90). Three patients had a readmission due to AE-COPD, and 1 patient had an AE-COPD but was not admitted during the follow-up. The second test day in these patients was postponed until the patient was in a stable condition. All patients were investigated according to the study protocol and were stable at the second visit.
Table 3.
Clinical parameters at test days (visit 1 and 2) (n = 17)
| Visit 1 | Visit 2 | p value* | |||||
|---|---|---|---|---|---|---|---|
| SpO2, % | 93 (91.5–95) | 95 (91.5–97) | 0.072 | ||||
| Pulse, /min | 80 (70.5–92) | 73 (67.5–90) | 0.343 | ||||
| RR, /min | 14 (13.5–19) | 14 (12–16.5) | 0.132 | ||||
| Systolic BP, mm Hg | 123 (118–137) | 132 (119–137.5) | 0.522 | ||||
| Diastolic BP, mm Hg | 69 (63–84.5) | 75 (67.5–78.5) | 0.579 | ||||
| Borg dyspnea score | 7 (4.5–7.5) | 3 (1.5–3.5) | 0.001 | ||||
| Oxygen amount, L/min | 1 (0–5) | 0.6 (0–4) | 0.116 | ||||
Data are presented as medians and 25–75% percentiles. SpO2, oxygen saturation; RR, respiratory rate; BP, blood pressure.
Wilcoxon signed-rank test.
The clinical parameters at visit 2 improved, including oxygen saturation (median SpO2 95% [91.5–97%]), and clinical symptoms were within a normal range. The Borg dyspnoea score at visit 2 improved significantly compared to visit 1 (3 [1–5] vs. 7 [2–8], p = 0.001). SpO2 was significantly improved at visit 2 compared to the situation at admission (median 95% vs. 88%, p = 0.006), and there was a trend to significant improvement of SpO2 at visit 2 if compared to visit 1 (p = 0.072).
GI Permeability
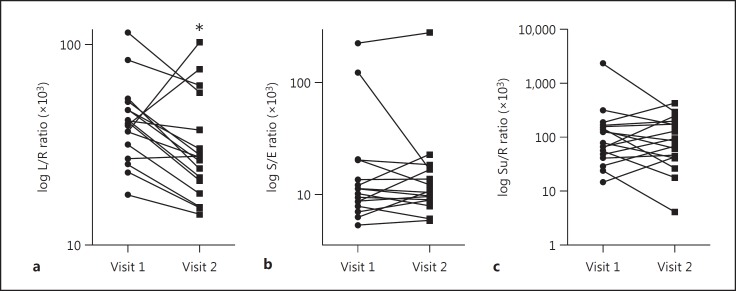
The 0- to 5-h urinary L/R ratio, reflecting small intestine permeability, was significantly increased at visit 1 (AE-COPD visit during hospital stay) compared to visit 2 (the stable condition at home) (Table 4). Figure 2a shows the individual values of the 0- to 5-h urinary L/R ratio and the change between both visits. The median absolute difference in the 0- to 5-h urinary L/R ratio (×103) in our study group was −13.67 (−57.15 to 66.48), and the relative change was −35.5% (−55.2 to 180.2%). In 3 patients, the L/R ratio was elevated at the stable condition (visit 2; Fig. 2a). The absolute delta of the 0- to 5-h urinary L/R ratio (×103) in these patients was, respectively, 0.73 (2.7%), 66.48 (180.2%) and 35.78 (90.0%). The first and second patient had hypercapnia at admission. The first patient had a readmission and the second visit was performed after 42 days. The second and third patient had a short hospital admission and no readmission, respectively. Their deviant L/R ratio change over time could not be explained based on other clinical parameters.
Table 4.
Gastro-intestinal permeability during AE-COPD compared to stable condition (n = 17)
| Visit 1 | Visit 2 | p value* | |
|---|---|---|---|
| Lactulose | 29.1 (16.9–43.8) | 20.3 (10.8–48.8) | 0.332 |
| L-Rhamnose | 795.8 (400.3–1,061.1) | 566.5 (383–1,679) | 0.586 |
| Erythritol | 10,454 (6,014–13,242) | 5,549 (3,768–14,491) | 0.266 |
| Sucrose | 50.4 (32.6–122.7) | 75.7 (22.2–122.3) | 0.687 |
| Sucralose | 104.5 (55.9–187.2) | 66.5 (27.4–222.3) | 0.227 |
| L/R ratio, ×103 | 40.9 (29.4–49.6) | 27.3 (19.5–47.7) | 0.039 |
| S/E ratio, ×103 | 11.2 (8.2–16.8) | 10.5 (8.8–16.7) (n = 16) | 1.00 |
| Su/R ratio, ×103 | 77.7 (44.0–159.7) | 86.2 (42.1–190.6) | 0.906 |
Data are presented as medians and 25–75% percentiles. The table shows the sugar urinary excretion levels after drinking a sugar solution measured during both visits (conditions). AE-COPD, acute exacerbation of chronic obstructive pulmonary disease; L/R ratio, ratio between lactose and L-rhamnose; S/E ratio, ratio between sucralose and erythritol; Su/R ratio, ratio between sucrose and rhamnose.
Wilcoxon signed-rank test.
Fig. 2.
Gastrointestinal permeability during AE-COPD (visit 1) compared to stable condition (visit 2) according to 0- to 5-h urinary L/R ratio (×103) (a), S/E ratio (×103) (b), and Su/R ratio (×103) (c). * p value < 0.05. AE-COPD, acute exacerbation of chronic obstructive pulmonary disease; L/R ratio, lactulose/L-rhamnose ratio; S/E ratio, sucralose/erythritol ratio; Su/R ratio, sucrose/L-rhamnose ratio.
The 0- to 5-h urinary S/E ratio, including the permeability of the proximal colon, was similar in both visits. Figure 2b shows the individual values and the trend of the 0- to 5-h urinary S/E ratio at the first and second visit. The median absolute difference in the 0- to 5-h urinary S/E ratio (×103) in our study group was −0.13 (−105.8 to 55.61), and the relative change was −1.6% (−86.4 to 92.6%). The anticipated decrease in the 0- to 5-h urinary S/E ratio between both visits was not observed in 8 patients (Fig. 2b). In 1 patient, the measurement of sucralose and erythritol concentrations in urine failed during the last visit.
Also the Su/R ratio, reflecting gastric/duodenal permeability, was not different between both visits. The individual values and the trend of the 0- to 5-h urinary Su/R ratio at the first and second visit are shown in Figure 2c. The median absolute difference in the 0- to 5-h urinary Su/R ratio (×103) in our study group was 4.43 (−2,051.27 to 231.23), and the relative change was 10.8% (−87.7 to 281.4%). In 8 patients, a negative trend (delta) in the 0- to 5-h urinary Su/R ratio was found.
There were no significant differences in the individual sugar levels in urine between the 2 visits (Table 4).
Discussion
To our knowledge, this is the first study showing increased small intestinal permeability, reflected by a significantly higher 0- to 5-h urinary L/R ratio, during hospitalised AE-COPD complicated by severe hypoxaemic RF compared to a clinically stable condition (4 weeks later). Increased intestinal permeability has already been described in various other extra-intestinal diseases and suggested to contribute to pathology. Sandek et al. [15] reported morphological and functional alterations of the gut in CHF patients, with an increased permeability index in both the small and large intestine. Pijls et al. [35] reported an increased colonic permeability in patients with compensated liver cirrhosis. In patients with burn injuries, in patients undergoing cardiopulmonary bypass surgery and in critically ill patients who developed multi-organ failure, intestinal permeability was shown to be increased [36, 37, 38, 39, 40]. Interestingly, also studies performed in healthy athletes suggest that strenuous exercise limits oxygen supply to the GI tract, which has a negative impact on GI barrier function [41, 42]. Oktedalen et al. [43] showed increased intestinal permeability after marathon running, and higher intestinal permeability was observed in runners with GI symptoms compared to asymptomatic runners [44].
The first study investigating GI permeability in COPD patients in a stable conditions has been published by Rutten et al. [13]. In that study, intestinal permeability was higher in patients suffering from COPD compared to healthy controls. In addition, the execution of an ADL test augmented the differences in intestinal permeability. Importantly, they found significantly higher plasma intestinal fatty acid-binding protein levels after performing this ADL test in stable COPD patients and not in healthy controls, suggesting the occurrence of enterocyte damage specifically in COPD patients [13]. Data from that study suggested splanchnic oxygen shortage to develop during physical activity in COPD patients and led us to conduct the present study in AE-COPD, in which hypoxaemia is an important manifestation.
Manifestations in the GI tract have not yet been investigated in patients hospitalised for AE-COPD suffering from hypoxaemia at the time of admission. Our results revealed an increased intestinal permeability in patients undergoing an AE-COPD compared to the same patients in a stable condition of COPD. In our pilot study, we observed a significant difference specifically for the urinary L/R ratio and not for the other ratios. This might be explained by the fact that the L/R ratio typically reflects permeability of the small intestine, which is more susceptible to ischaemic injury than the colon [45]. This is supported by the finding that the 0- to 5-h urinary S/E ratio, including the permeability of the proximal colon, was similar at both visits. Importantly, we tried to shorten the time window between the hypoxaemic event – overtly present in all patients at admission – and measurement of intestinal permeability as much as possible. For ethical reasons, we were, however, not able to perform the measurements directly at admission. This could imply that we did not capture the intestinal leakiness at its maximal level, as the recovery phase might have been already initiated at visit 1, as also objectified by improved hypoxaemia compared to hypoxaemia at admission. However, a tendency for worse SpO2 at visit 1 compared to the stable situation at visit 2 was observed.
Although our study was not designed to investigate the underlying mechanism of intestinal disturbances in AE-COPD, we discuss potential routes which might underlie the increased intestinal permeability observed in patients undergoing an AE-COPD with hypoxaemic RF. Severe hypoxaemia by itself might result in inadequate oxygen delivery to the intestinal mucosa causing tissue hypoxia, which contributes to disturbed intestinal mucosal barrier function. The oxygenation of the small intestinal villi is regulated by a countercurrent circulation in which oxygen diffuses through the mucosa to the tip of the villi where the oxygen tension is the lowest. In case of oxygen shortage, this leads to more pronounced hypoxia at the tips, which is associated with increased enterocyte damage and integrity loss [46, 47]. In addition, hypoxaemia is thought to evoke a redistribution of blood flow away from less critical organs, such as the intestine, to those vital to survival (e.g., heart, brain). A strong correlation was found between the level of hypoxaemia and the decrease in mesenteric blood flow in patients during bronchoscopy, a reflex sympathetic activation presumably prominent in this situation [48]. Such an increase in sympathetic activity has also been demonstrated in congestive heart failure patients and leads to a redistribution of blood flow away from the splanchnic circulation [49]. Diminished splanchnic perfusion strongly correlates with enterocyte damage and precedes increased intestinal permeability in exercising healthy athletes [42]. Similarly, in the present patient population, activation of the sympathetic nervous system by hypoxaemia could result in changes in bowel perfusion and subsequently contribute to the observed intestinal barrier loss. In stable COPD, it has been shown that an imbalance in auto-regulation of the cardiac rhythm leads to arrhythmias and that sympathetic activation is related to a higher rate of hospitalizations and mortality in COPD [50, 51].
Next to hypoxic damage, the intestinal barrier might be affected by inflammatory mediators, which are known to circulate during AE-COPD [52]. For instance, cytokines can lead to alterations in the structure of tight junctions that connect enterocytes, thereby resulting in enhanced paracellular permeability and barrier loss [18]. Some COPD patients have already increased levels of systemic inflammation markers in a stable state [53]. Systemic inflammation markers, mainly CRP levels, often increase during AE-COPD. In our patients, a quarter had a high CRP level (>50 mg/L). However, further subgroup analysis was not possible in our limited dataset, and correlation analysis between the urinary sugar ratio and parameters of inflammatory and gas exchange remained negative (data not shown). In summary, during AE-COPD, the intestinal barrier might be disturbed by hypoxic damage or inflammatory mediators, or a combination. However, future, more invasive studies need to investigate in more detail the underlying mechanisms for increased intestinal permeability in AE-COPD.
Intestinal disturbances can have several negative consequences. As mentioned above, inflammatory mediators in the circulation can affect the intestinal barrier. However, it has been shown that an increase in intestinal permeability also leads to an increase in bacterial translocation and systemic inflammation [19], provoking a vicious circle of inflammation. Next, hypoxic enterocyte damage and consequent loss of enterocyte mass may disturb the digestion and absorption of nutrients. Another possible clinical impact of the increased intestinal permeability due to hypoxia in AE-COPD is the decrease of effectiveness of oral medication. The uptake of medication may be disturbed leading to changes in pharmacokinetics and pharmacodynamics, which additionally may influence recovery from exacerbations.
Despite these findings, our study had some limitations. The number of patients that participated in this study was small; however, the power calculation indicated that we only needed 17 patients. In some of our patients with AE-COPD, consolidation on chest X-ray was found, which is not unusual in these patients. One may ask if this could have influenced our results. These patients did not differ in outcome parameters compared to patients without consolidation. These patients, but also some of the patients without consolidation, received antibiotics at admission. In this cohort, we did not find evidence that pneumonia itself could have led to GI disturbances. Some patients received antibiotics at admission. GI side effects occur during the use of antibiotics; however, in a recent placebo-controlled human study, it was shown that 7-day antibiotics use did not affect intestinal barrier function [54]. Blood gas analysis at the second test day would have supported our understanding of the patients' condition. However, in our setting, we used pulse oximetry to assess the patients' oxygenation, and it has been shown that pulse oximetry is an accurate measure in this setting [55]. Patients were considered stable after 4 weeks, but it might be possible that patients were not yet fully recovered. We did, however, thoroughly interview all patients about recovery of all exacerbation-related symptoms, and clinically, they were all fully recovered. Another possible limitation may be that we have not assessed cardiac status during exacerbation and after recovery as a contributing factor to impaired tissue oxygen delivery. However, patients did not show any clinical signs of acute heart failure.
In conclusion, this is the first study to demonstrate disturbances in intestinal permeability in patients with hospitalised AE-COPD compared to stable COPD after a 4-week recovery period. Although the hypoxaemic event itself seems a likely explanation, the underlying mechanisms of our observations remain to be elucidated. This disturbed GI integrity in COPD patients is an attractive target for future research but also the development of new interventions to alleviate the consequences of AE-COPD.
Financial Disclosure and Conflicts of Interest
None of the authors have any conflicts of interests.
References
- 1.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Eeden SF, Sin DD. Chronic obstructive pulmonary disease: a chronic systemic inflammatory disease. Respiration. 2008;75:224–238. doi: 10.1159/000111820. [DOI] [PubMed] [Google Scholar]
- 3.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, Committee GS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 4.Celli BR. MacNee W; ATS/ERS Task Force: Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 5.John BA, Wood SG, Hawkins DR. The pharmacokinetics and metabolism of sucralose in the mouse. Food Chem Toxicol. 2000;38((suppl 2)):S107–S110. doi: 10.1016/s0278-6915(00)00032-6. [DOI] [PubMed] [Google Scholar]
- 6.Wouters EF. Management of severe COPD. Lancet. 2004;364:883–895. doi: 10.1016/S0140-6736(04)16984-5. [DOI] [PubMed] [Google Scholar]
- 7.Gibson GJ. London. Hodder Arnold; 2009. Clinical Tests of Respiratory Function, ed 3. [Google Scholar]
- 8.Brill SE, Wedzicha JA. Oxygen therapy in acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1241–1252. doi: 10.2147/COPD.S41476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. doi: 10.2147/COPD.S10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim V, Benditt JO, Wise RA, Sharafkhaneh A. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:513–518. doi: 10.1513/pats.200708-124ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slenter RH, Sprooten RT, Kotz D, Wesseling G, Wouters EF, Rohde GG. Predictors of 1-year mortality at hospital admission for acute exacerbations of chronic obstructive pulmonary disease. Respiration. 2013;85:15–26. doi: 10.1159/000342036. [DOI] [PubMed] [Google Scholar]
- 12.Magnet FS, Schwarz SB, Callegari J, Criee CP, Storre JH, Windisch W. Long-term oxygen therapy: comparison of the German and British guidelines. Respiration. 2017;93:253–263. doi: 10.1159/000455879. [DOI] [PubMed] [Google Scholar]
- 13.Rutten EPA, Lenaerts K, Buurman WA, Wouters EFM. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest. 2014;145:245–252. doi: 10.1378/chest.13-0584. [DOI] [PubMed] [Google Scholar]
- 14.Krack A, Sharma R, Figulla HR, Anker SD. The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J. 2005;26:2368–2374. doi: 10.1093/eurheartj/ehi389. [DOI] [PubMed] [Google Scholar]
- 15.Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 17.Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149–154. doi: 10.1016/s0966-842x(00)88906-4. [DOI] [PubMed] [Google Scholar]
- 18.Turner JR. Intestinal mucosal barrier function in health and disease. Nature Rev. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 19.Hietbrink F, Besselink MG, Renooij W, de Smet MB, Draisma A, van der Hoeven H, et al. Systemic inflammation increases intestinal permeability during experimental human endotoxemia. Shock. 2009;32:374–378. doi: 10.1097/SHK.0b013e3181a2bcd6. [DOI] [PubMed] [Google Scholar]
- 20.Hietbrink F, Besselink MG, Renooij W, Leenen LP. Pitfalls in gastrointestinal permeability measurement in ICU patients. Intensive Care Med. 2007;33:2216. doi: 10.1007/s00134-007-0771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 22.Bours MJ, Bos HJ, Meddings JB, Brummer RJ, van den B, randt PA, Dagnelie PC. Effects of oral adenosine 5′-triphosphate and adenosine in enteric-coated capsules on indomethacin-induced permeability changes in the human small intestine: a randomized cross-over study. BMC Gastroenterol. 2007;7:23. doi: 10.1186/1471-230X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigthorsson G, Simpson RJ, Walley M, Anthony A, Foster R, Hotz-Behoftsitz C, et al. COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology. 2002;122:1913–1923. doi: 10.1053/gast.2002.33647. [DOI] [PubMed] [Google Scholar]
- 24.van Wijck K, Verlinden TJ, van Eijk HM, Dekker J, Buurman WA, Dejong CH, et al. Novel multi-sugar assay for site-specific gastrointestinal permeability analysis: a randomized controlled crossover trial. Clin Nutr. 2013;32:245–251. doi: 10.1016/j.clnu.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 26.Wedzicha JA. Mechanisms of chronic obstructive pulmonary disease exacerbations. Ann Am Thorac Soc. 2015;12((suppl 2)):S157–S159. doi: 10.1513/AnnalsATS.201507-427AW. [DOI] [PubMed] [Google Scholar]
- 27.Pavord ID, Jones PW, Burgel PR, Rabe KF. Exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11((Spec Iss)):21–30. doi: 10.2147/COPD.S85978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer S, Jones PW, Group GS. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax. 2003;58:589–593. doi: 10.1136/thorax.58.7.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rooyakkers DR, van Eijk HM, Deutz NE. Simple and sensitive multi-sugar-probe gut permeability test by high-performance liquid chromatography with fluorescence labelling. J Chromatogr A. 1996;730:99–105. doi: 10.1016/0021-9673(95)01113-7. [DOI] [PubMed] [Google Scholar]
- 30.van Wijck K, van Eijk HM, Buurman WA, Dejong CH, Lenaerts K. Novel analytical approach to a multi-sugar whole gut permeability assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:2794–2801. doi: 10.1016/j.jchromb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Fink MP. Clinical tests of gastrointestinal permeability that rely on the urinary recovery of enterally administered probes can yield invalid results in critically ill patients. Intensive Care Med. 2002;28:103–104. doi: 10.1007/s00134-001-1191-4. [DOI] [PubMed] [Google Scholar]
- 32.Farhadi A, Gundlapalli S, Shaikh M, Frantzides C, Harrell L, Kwasny MM, et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026–1033. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farhadi A, Keshavarzian A, Holmes EW, Fields J, Zhang L, Banan A. Gas chromatographic method for detection of urinary sucralose: application to the assessment of intestinal permeability. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:145–154. doi: 10.1016/s1570-0232(02)00787-0. [DOI] [PubMed] [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 35.Pijls KE, Koek GH, Elamin EE, de Vries H, Masclee AA, Jonkers DM. Large intestine permeability is increased in patients with compensated liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G147–G153. doi: 10.1152/ajpgi.00330.2013. [DOI] [PubMed] [Google Scholar]
- 36.Ohri SK, Somasundaram S, Koak Y, Macpherson A, Keogh BE, Taylor KM, et al. The effect of intestinal hypoperfusion on intestinal absorption and permeability during cardiopulmonary bypass. Gastroenterology. 1994;106:318–323. doi: 10.1016/0016-5085(94)90588-6. [DOI] [PubMed] [Google Scholar]
- 37.Riddington DW, Venkatesh B, Boivin CM, Bonser RS, Elliott TS, Marshall T, et al. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA. 1996;275:1007–1012. [PubMed] [Google Scholar]
- 38.Braun JP, Schroeder T, Buehner S, Dohmen P, Moshirzadeh M, Grosse J, et al. Splanchnic oxygen transport, hepatic function and gastrointestinal barrier after normothermic cardiopulmonary bypass. Acta Anaesthesiol Scand. 2004;48:697–703. doi: 10.1111/j.1399-6576.2004.00392.x. [DOI] [PubMed] [Google Scholar]
- 39.Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444–451. doi: 10.1164/ajrccm.158.2.9710092. [DOI] [PubMed] [Google Scholar]
- 40.Johnston JD, Harvey CJ, Menzies IS, Treacher DF. Gastrointestinal permeability and absorptive capacity in sepsis. Crit Care Med. 1996;24:1144–1149. doi: 10.1097/00003246-199607000-00013. [DOI] [PubMed] [Google Scholar]
- 41.de Oliveira EP, Burini RC, Jeukendrup A. Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sports Med. 2014;44(suppl 1):S79–S85. doi: 10.1007/s40279-014-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Wijck K, Lenaerts K, van Loon LJ, Peters WH, Buurman WA, Dejong CH. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS One. 2011;6:e22366. doi: 10.1371/journal.pone.0022366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oktedalen O, Lunde OC, Opstad PK, Aabakken L, Kvernebo K. Changes in the gastrointestinal mucosa after long-distance running. Scand J Gastroenterol. 1992;27:270–274. doi: 10.3109/00365529209000073. [DOI] [PubMed] [Google Scholar]
- 44.van Nieuwenhoven MA, Brouns F, Brummer RJ. Gastrointestinal profile of symptomatic athletes at rest and during physical exercise. Eur J Appl Physiol. 2004;91:429–434. doi: 10.1007/s00421-003-1007-z. [DOI] [PubMed] [Google Scholar]
- 45.Hundscheid IH, Grootjans J, Lenaerts K, Schellekens DH, Derikx JP, Boonen BT, et al. The human colon is more resistant to ischemia-reperfusion-induced tissue damage than the small intestine: an observational study. Ann Surg. 2015;262:304–311. doi: 10.1097/SLA.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 46.Takala J. Determinants of splanchnic blood flow. Br J Anaesth. 1996;77:50–58. doi: 10.1093/bja/77.1.50. [DOI] [PubMed] [Google Scholar]
- 47.Grootjans J, Lenaerts K, Derikx JP, Matthijsen RA, de Bruine AP, van Bijnen AA, et al. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176:2283–2291. doi: 10.2353/ajpath.2010.091069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nayci A, Atis S, Duce MN, Bayindir S, Tamer L, Ozturk C. Bronchoscopy is associated with decreased mesenteric arterial flow. Crit Care Med. 2008;36:2517–2522. doi: 10.1097/CCM.0b013e318183f35b. [DOI] [PubMed] [Google Scholar]
- 49.Parks DA, Jacobson ED. Physiology of the splanchnic circulation. Arch Intern Med. 1985;145:1278–1281. [PubMed] [Google Scholar]
- 50.Wang X, Jiang Z, Chen B, Zhou L, Kong Z, Zuo S, et al. Cardiac autonomic function in patients with acute exacerbation of chronic obstructive pulmonary disease with and without ventricular tachycardia. BMC Pulm Med. 2016;16:124. doi: 10.1186/s12890-016-0287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreas S, Haarmann H, Klarner S, Hasenfuss G, Raupach T. Increased sympathetic nerve activity in COPD is associated with morbidity and mortality. Lung. 2014;192:235–241. doi: 10.1007/s00408-013-9544-7. [DOI] [PubMed] [Google Scholar]
- 52.Bathoorn E, Kerstjens H, Postma D, Timens W, MacNee W. Airways inflammation and treatment during acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:217–229. doi: 10.2147/copd.s1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reijnders D, Goossens GH, Hermes GD, Neis EP, van der B, eek CM, Most J, et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metab. 2016;24:341. doi: 10.1016/j.cmet.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Barthelemy JC, Geyssant A, Riffat J, Antoniadis A, Berruyer J, Lacour JR. Accuracy of pulse oximetry during moderate exercise: a comparative study. Scand J Clin Lab Invest. 1990;50:533–539. doi: 10.1080/00365519009089168. [DOI] [PubMed] [Google Scholar]