Abstract
A 12-week study was conducted in eight dementia care communities involving 77 participants addressing the hypothesis that an intervention of increasing indoor exposure to daylight will reduce depression and other neuropsychiatric symptoms. At four communities, staff were enlisted to increase daylight exposure by taking participants to a perimeter room with daylight exposure for socialization in the morning (8:00–10:00 AM) each day. At the other four communities, a control group were taken to a similar sized area without daylight for socialization under typical electrical lighting conditions. Participants in the daylight intervention experienced an average decrease over the trial in the Neuropsychiatric Inventory Nursing Home Version (NPI-NH) scores (p=0.33) and the Cornell Scale for Depression in Dementia (CSDD) scores (p=0.025), while the control participants showed average but nonsignificant increases in both NPI-NH (p=0.33) and CSDD (p=0.13). Difference in outcome changes of the intervention group achieved statistical significance for CSDD (p=0.01) but not for NPI-NH (p=0.17). Our results suggest that increased exposure to daylight can reduce depression in people living with dementia.
Keywords: dementia, daylight, depression, memory care communities
Introduction
Depression is one of the most common conditions affecting people living with Alzheimer’s disease and related dementias (ADRD). Clinically significant depression occurs in about 20%–30% of people with ADRD.1 Treatment of depression in people living with ADRD can improve well-being, quality of life, and individual function, even in the presence of ongoing declines in memory and cognition. Exposure to sufficient daylight is a potentially effective non-drug treatment option for depression and other neuropsychiatric symptoms of dementia. Exposure to bright white light at the cornea (2,000–10,000 lux) typically administered in the morning has been shown to be an effective nonpharmacological treatment option for depressed adults with normal cognitive function.2 Light therapy has also been shown to be effective in treating sleep disturbances3 and for ameliorating behavioral problems4 in people living with ADRD. Riemersma-van der Lek et al5 showed that exposure throughout the day to bright white light from large numbers of ceiling-mounted fluorescent lamps (>1,000 lux at the cornea) could increase sleep quality and reduce cognitive decline in people living with ADRD.
However, reliable and well-designed studies examining the efficacy of light therapy in treating depression in people living with ADRD are extremely limited.6 Moreover, the conventional approach to administering the bright light stimulus, through light boxes containing arrays of bright light fixtures, is problematic for people with ADRD due to reported side effects of headache, eye strain, nausea, and agitation.7,8 Furthermore, many light therapy studies were performed prior to scientific understanding of the important role that specific wavelengths of light play in maintaining healthy human biological functions. Research has revealed that the human circadian system is maximally sensitive to short-wavelength (blue) light.9,10 This has led to more recent light therapy studies using “blue-enriched” electrical light sources tailored to the spectral response of the circadian system.11,12 The circadian system is the body’s internal biological clock that regulates most 24-hour behavioral and physiological rhythms, such as sleep/wake cycle, alertness level, hormone suppression/secretion, and core body temperature. A bright light stimulus in the morning is the most powerful timing cue to maintain entrainment with the astronomical 24-hour day.
In institutionalized settings, where light levels can be very low,13,14 lack of sufficient exposure to bright light is considered one of the primary contributors to circadian disruption. Circadian disruption, in turn, is associated with depression, sleep disruption, agitated behavior, and cognitive decline.15,16 In the present study, we hypothesized that exposure to sufficient daylight indoors can serve as an effective nonpharmacological treatment for reducing depression and other neuropsychiatric symptoms in people living with ADRD. Furthermore, it has the potential for fewer adverse effects and has less practical limitations than specialized electrical lighting devices.
Methods
The pilot study was a nonrandomized clustered trial, where communities were assigned to the daylight intervention or usual care control; all eligible and consenting participants within a community received the same intervention. All study procedures were approved by the University of Southern California (USC) Institutional Review Board (USC UPIRB #UP-16-00487). Participation was voluntary, and participants’ legally authorized representatives provided written informed consent and Health Insurance Portability and Accountability Act authorization. ClinicalTrials.gov Identifier: NCT03483896.
Protocol
The research was conducted at eight Silverado Senior Living dementia care communities in Los Angeles and Orange counties using a two-arm (daylight intervention and control) parallel intervention study design. Silverado Senior Living is a memory care/assisted living provider that delivers care for persons with Alzheimer’s and other memory-impairing diseases. The study was run over a period of 12 weeks at each community, with staggered start dates ranging from 1 to 3 days per community to enable the research team to visit each site in series to collect baseline and outcome measures.
Participants
Residents were recruited according to the trial inclusion criteria: 1) ADRD diagnosis, 2) no physical comorbidities that precluded participation in the daily group intervention, and 3) a Mini-Mental State Exam score of ≥10. A total of 83 residents who met these criteria were enrolled into the study. Of the 83 participants enrolled, one declined when asked for verbal assent, three passed away during the study, and two were unable to be scored at outcome for the Neuropsychiatric Inventory Nursing Home Version (NPI-NH) (due to absence of regular caregiver at the time of NPI-NH scoring visits).
Intervention group
At the four communities in the active light intervention arm, staff increased the daylight exposure of participants by taking them to the perimeter zone of a daylit room from 8:00 to 10:00 AM for socialization over a period of 12 weeks. The perimeter zone was defined to be the region of the room within 3 m from the windows. The intervention was administered each day (7 days/week) over the duration of the study. Randomization at the community level was not feasible in this pilot study because not all of the eight participating communities had a suitably sized perimeter zone space available for use during the study timeframe. Therefore, the four communities assigned to the intervention were those where a suitably sized perimeter zone space was available for use during the intervention period (8:00–10:00 AM). As all communities were operated by Silverado Senior Living, all had similar interior design and furnishing, caregiver staff training, and programmatic activities.
Control group
Participants at the other four communities received the usual care (control arm). During the period from 8:00–10:00 AM each day, the control group were taken to a similar sized area indoors (without daylight) for socialization under typical electrical lighting conditions.
Outcome measures
The Cornell Scale for Depression in Dementia (CSDD) was used to measure depression. The CSDD is designed for the assessment of depression in older people with dementia who can at least communicate basic needs. Scores >10 indicate a probable major depression. Scores >18 indicate a definite major depression. Scores <6 are associated with the absence of significant depressive symptoms.17 The care provider uses the CSDD to evaluate the mental state of higher functioning residents at regular intervals. Consequently, CSDD scores were obtained from the participants’ health records. The most recent CSDD score prior to the beginning of the study was used as the baseline score wherever feasible. In some cases, no CSDD was found, or the date of completion was more than 4 months prior to the start of the study. These participants were excluded from the analyses for CSDD, leading to a smaller sample size (n=36 daylight, n=28 control). Due to a delay in the scheduled start of the study, CSDD scores were completed by the care provider on an average of 7.5 weeks prior to the start of the study for the intervention group and 5.9 weeks prior to the start for the control group. However, the group difference in mean weeks prior to start of the study was not statistically significant (p=0.22). The CSDD at outcome was completed, on average, within 3 days of the end of the study.
The NPI-NH is designed for the assessment of people with dementia residing in extended care housing communities.18 The NPI-NH includes 10 behavioral areas (delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, and aberrant motor behavior) and two types of neurovegetative changes (sleep and nighttime behavior disorders, and appetite and eating disorders). A summary score is obtained by summing all 12 domain scores, where higher scores indicate greater neuropsychiatric symptomatology.
Light measurements
Lighting measurements were taken periodically during the study to evaluate the fidelity of the daylight intervention. Due to the difference in spectral sensitivity between the visual system and the circadian system, conventional photometric sensors are problematic for assessing human circadian lighting needs.19 To address this challenge, a digital charge coupled device spectrometer mounted on a mobile cart was used to evaluate the spectral composition of light by taking spot measurements of Spectral Power Distribution (SPD). The spectrometer lens was positioned on a vertical plane at a distance of 1.07 m (42 in.) from the floor to represent views from seated eye height. Use of a calibrated spectrometer for measurement of light exposures follows the consensus research recommendation to record light exposures in the most complete form, that is corneal SPDs.19 In each intervention space, SPD measurements were taken during the intervention at distances of 1, 3, and 5 m from windows and in four directions at each distance, beginning with a window-facing view and rotating in the clockwise direction by 90° increments. Each daylight intervention space was measured on five separate visits during the study, with visits spaced 2–3 weeks apart. Due to the uniform and steady-state lighting conditions of the control spaces, each control space was measured once; measurements were taken in four directions at a single location representative of typical eye-level lighting conditions in the space.
Analyses
Light measurements
Each SPD was analyzed to calculate melanopic illuminance (mLux) by applying the melanopic spectral efficiency function to the measured spectral irradiance following the methodology outlined in Lucas et al19 and Enezi et al.20 To calculate a proxy indicator of the overall light exposure in each daylight intervention space, the average of all measurements taken in each space under clear sky conditions at the 3 m distance in all four view directions was used. For the control, the average of all four view directions in each space was used. This proxy indicator is referred to as “mLuxAVG” in the following sections.
Outcome measures
Mean values of the trial variables, CSDD and NPI-NH, were analyzed by treatment group and by community at baseline and at the end of study. The change in each variable (end – baseline) was examined. Between- and within-group comparisons (daylight vs usual care) in CSDD and NPI-NH scores were analyzed by linear mixed effects models to reflect the clustered (ie, community level) assignment to interventions. A random effect at the community level was specified to model correlated outcomes among participants within communities. Intraclass (within community) correlations in the change in CSDD and NPI-NH were calculated using estimates of variability (mean squares) within and between communities from analysis of variance. The linear association of mLuxAVG measurements with changes in trial outcome measures was also analyzed with linear mixed effects models as above; rather than intervention group, the primary independent variable was each participant’s mLuxAVG measurement.
Results
While mean values of the CSDD and NPI-NH trial outcomes varied markedly between communities (Table 1), most of these community means (baseline, endpoint, and change) did not significantly differ between communities. However, significant differences between communities were observed in the CSDD mean values at baseline (p=0.0003) and endpoint (p=0.0001). Mean NPI-NH measures did not significantly differ across communities. Numbers of participants at communities ranged from 3 to 18. The mLuxAVG measures at each community reflected the daylight versus control interventions (Table 1).
Table 1.
Comparisons by community
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| N | 18 | 11 | 18 | 9 | 7 | 6 | 5 | 3 | |
| Treatment | Daylight | Control | Daylight | Control | Daylight | Control | Control | Daylight | |
| mLuxAVG | 230* | 20 | 100 | 56 | 75 | 57 | 49 | 120 | |
| Baseline | |||||||||
| NPI-NH | 18.4 (0, 96) | 22.7 (2, 54) | 11.5 (0, 66) | 11.6 (0, 46) | 20.6 (3, 43) | 9.7 (1, 29) | 18.6 (9, 32) | 18.3 (0, 31) | 0.64 |
| CSDD | 9.4 (0, 20) | 8.7 (2, 16) | 4.1 (0, 17) | 0.9 (0, 2) | 1.6 (0, 4) | 1.3 (0, 3) | 4.8 (0, 8) | 1.7 (0, 4) | 0.0003 |
| End | |||||||||
| NPI-NH | 20.3 (0, 93) | 26.8 (5, 62) | 10.7 (0, 39) | 13.6 (0, 44) | 6.4 (0, 18) | 6.3 (0, 22) | 29.2 (2, 59) | 14.7 (0, 30) | 0.08 |
| CSDD | 4.8 (0, 17) | 10.9 (2, 24) | 1.9 (0, 7) | 1.0 (0, 2) | 1.8 (0, 4) | 1.8 (0, 5) | 6.4 (0, 11) | 1.3 (0, 4) | 0.0001 |
| Change | |||||||||
| NPI-NH | 1.9 (−47, 42) | 4.1 (−9, 36) | −0.8 (−27, 20) | 2.0 (−5, 16) | −14.1 (−25, −3) | −3.3 (−28, 13) | 10.6 (−7, 41) | −3.7 (−17, 6) | 0.13 |
| CSDD | −4.6 (−15, 9) | 2.2 (−8, 15) | −2.6 (−10, 3) | 0.1 (−2, 2) | 0.2 (0, 1) | 0.2 (0, 1) | 1.6 (0, 3) | −0.3 (−1, 0) | 0.1 |
Notes: Numbers in table are mean (minimum, maximum); p-value from analysis of variance; participants with baseline CSDD >4 months before enrollment excluded;
median value.
Abbreviations: mLuxAVG, average melanopic illuminance at 3 m from windows; NPI-NH, Neuropsychiatric Inventory Nursing Home Version; CSDD, Cornell Scale for Depression in Dementia.
Eligible and enrolled participants who completed the study included 46 participants at daylight intervention communities and 31 participants at control intervention communities (Table 2). Baseline demographic variables did not differ between the groups. Overall, the sample had a mean age of 85.3 years with a SD of 7.0 years. Subjects had resided in their communities for an average of 1.0 (1.1) years. The great majority of the sample was white (92.2%), female (72.7%), with diagnoses of either Alzheimer’s disease (32.5%) or dementia not otherwise specified (41.7%). The baseline NPI-NH (n=77) and CSDD (n=66) means did not differ significantly between the control and intervention groups (Table 2).
Table 2.
Baseline and endpoint comparisons by treatment
| Daylight | Control | p-value between treatments | |
|---|---|---|---|
| N | 46 | 31 | |
| Female | 31 (67.4%) | 25 (80.6%) | 0.30 |
| Age (years) | 85.6 (1.0) | 84.7 (1.3) | 0.56 |
| White race | 42 (91.3%) | 29 (93.5%) | 1.00 |
| Clinical group | 0.69 | ||
| AD | 17 (37.0%) | 8 (25.8%) | |
| Frontotemporal dementia | 1 (2.2%) | 1 (3.2%) | |
| Lewy body dementia | 0 | 1 (3.2%) | |
| Vascular dementia | 2 (4.3%) | 1 (3.2%) | |
| Dementia NOS | 19 (41.3%) | 17 (54.8%) | |
| Mild cognitive impairment | 6 (13.0%) | 2 (6.5%) | |
| Not specified | 1 (2.2%) | 1 (3.2%) | |
| Years in community | 1.2 (0.2) | 0.6 (0.3) | 0.14 |
| Baseline outcomes | |||
| NPI-NH | 16.2 (3.2) | 16.1 (2.7) | 0.97 |
| CSDD | 4.2 (1.9) | 3.9 (1.9) | 0.93 |
| Endpoint outcomes | |||
| NPI-NH | 13.4 (4.2) | 19.1 (4.4) | 0.35 |
| CSDD | 2.7 (2.1) | 5.3 (2.0) | 0.39 |
| Change (endpoint minus baseline) outcomes | |||
| NPI-NH | −2.8 (2.9) | 3.1 (3.2) | 0.17 |
| p-value within group | 0.33 | 0.33 | |
| CSDD | −2.0 (0.9) | 1.5 (1.0) | 0.01 |
| p-value within group | 0.025 | 0.13 | |
| mLuxAVG | 159.3 (13.8) | 42.3 (3.1) | <0.0001 |
Notes: Numbers in table are mean (SEM); for age, years in community, and trial outcome measures, p-values between treatments are by mixed effects linear models, specifying a random effect for community to account for within-community clustering. Sample size for years in community (n=46 daylight, n=24 control); sample size for CSDD: (n=36 daylight, n=28 control), intraclass correlation (ICC) for within-community change in outcomes: ICC =0.065 for change in NPI-NH; ICC =0.03 for change in CSDD.
Abbreviations: AD, Alzheimer’s disease; mLuxAVG, average melanopic illuminance at 3 m from windows; NOS, not otherwise specified; NPI-NH, Neuropsychiatric Inventory Nursing Home Version; CSDD, Cornell Scale for Depression in Dementia; SEM, standard error of the mean.
The mLuxAVG measures at daylight communities were significantly higher than in control communities (p<0.0001, Table 2) confirming the effectiveness of the daylight intervention. Changes in NPI-NH and CSDD varied substantially between communities (Figures 1 and 2). Participants in the daylight intervention showed an average decrease over the trial in both NPI-NH and CSDD, while the control participants showed an average increase in both NPI-NH and CSDD (Table 2). The group differences in outcome changes achieved statistical significance for CSDD (p=0.01) but not for NPI-NH (p=0.17). Group differences in the CSDD change were also evident among nine participants with baseline CSDD >10 (Table 3, p=0.03 between groups). The mLuxAVG measures were significantly inversely correlated with the change in CSDD over the trial (Spearman r=−0.37, p=0.002), but not with change in NPI-NH (Table 3, Figure 3).
Figure 1.
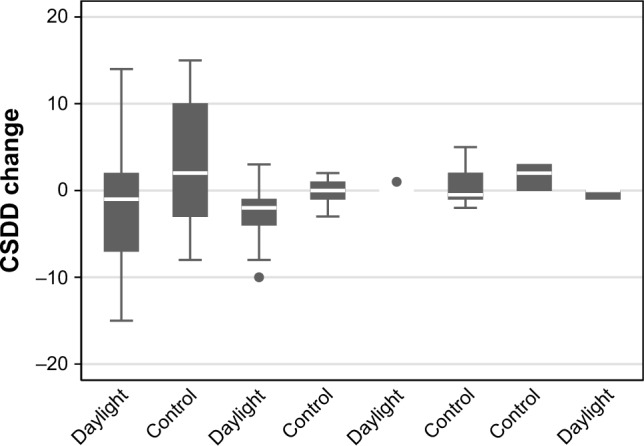
CSDD change by community.
Note: Intraclass correlation of Cornell change (within-community correlation) =0.03.
Abbreviation: CSDD, Cornell Scale for Depression in Dementia.
Figure 2.
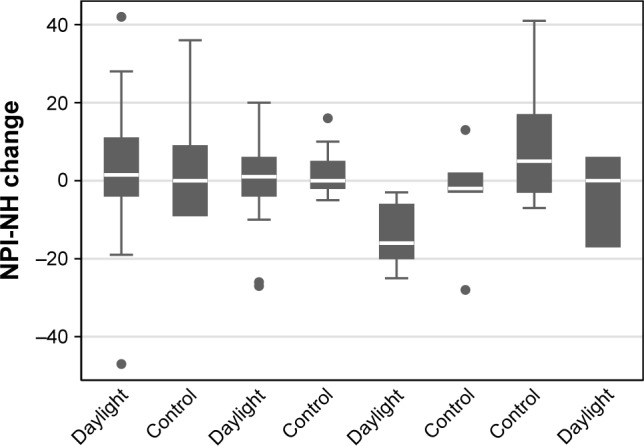
NPI-NH change by community.
Note: Intraclass correlation of NPI-NH change (within-community correlation) =0.065.
Abbreviation: NPI-NH, Neuropsychiatric Inventory Nursing Home Version.
Table 3.
Correlations among mLuxAVG and change in outcomes
| Spearman Correlation (p-value) | Beta coefficient (SE) [p-value] from mixed model | |
|---|---|---|
| NPI-NH | 0.002 (0.99) | 0.012 (0.026) [0.64] |
| CSDD | −0.37 (0.002) | −0.016 (0.01) [0.054] |
Notes: Sample size: n=77 in NPI-NH analysis; n=64 in CSDD analysis.
Abbreviations: NPI-NH, Neuropsychiatric Inventory Nursing Home Version; CSDD, Cornell Scale for Depression in Dementia; mLuxAVG, average melanopic illuminance at 3 m from windows; SE, standard error.
Figure 3.
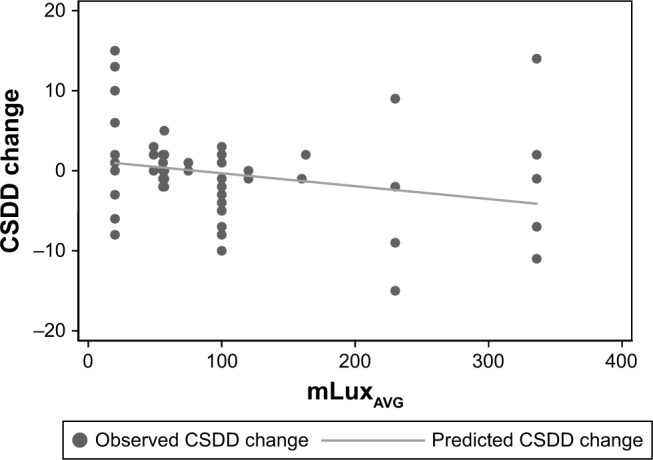
Scatter plot of changes in CSDD.
Abbreviations: CSDD, Cornell Scale for Depression in Dementia; mLuxAVG, average melanopic illuminance at 3 m from windows.
Discussion
Few, if any, studies of indoor daylight exposure have been conducted in dementia care communities. Our results indicate that an intervention that increases exposure to daylight in the morning (8:00–10:00 AM) may be used to reduce depression in people living with dementia. Overall, the mean mLuxAVG level measured in the daylight intervention spaces (159.3) was 3.8 times greater than the mean value for the control spaces (42.3) (Table 2). The mLuxAVG measures were found to be significantly inversely correlated with the change in CSDD over the trial (Spearman r=−0.37, p=0.002), suggesting that greater levels of melanopic illuminance are associated with a greater reduction in depression symptoms (Table 3). This finding has the potential to be clinically significant because it can inform the daily programming of dementia care communities, for example, by prioritizing the use of existing daylit spaces during the morning (ie, 8:00–10:00 AM) as a nonpharmacological treatment alternative for people living with dementia and depression.
Among the subset of participants with a probable major depression (CSDD >10) at the beginning of the trial (n=5 daylight, n=4 control), the daylight intervention was found to reduce the average CSDD score by over 11 points, from 16.8 at baseline to 5.6 at the end of the trial, which is below the threshold score of six used to indicate the absence of significant depressive symptoms (Table 4). In comparison, the average CSDD score for the control group with CSDD >10 increased slightly (from 12.7 to 13.5, Table 4). While this outcome should be interpreted with caution due to the small number of study participants (n=9 in the analysis), it suggests that increased light exposure has the potential to be an effective treatment for residents with probable major depression, producing a reduction in symptoms similar to the reduction that would be expected with an effective pharmacological treatment.
Table 4.
Treatment group comparisons on behavioral and depression change: baseline Cornell >10
| Daylight | Control | p-value between treatment | |
|---|---|---|---|
| NPI-NH | |||
| Baseline | 14.3 (12.0) | 34.7 (15.5) | 0.34 |
| End of study | 25.4 (9.3) | 39.0 (10.4) | 0.37 |
| Change | 10.6 (15.7) | 4.3 (19.7) | 0.81 |
| CSDD | |||
| Baseline | 16.8 (1.3) | 12.7 (1.5) | 0.09 |
| End of study | 5.6 (2.6) | 13.5 (2.9) | 0.09 |
| Change | −11.2 (2.7) | 0.7 (3.1) | 0.03 |
Notes: p-values from linear mixed effects models, with random effect for community. Numbers in table are model-estimated mean (SE) change in trial outcomes. N=9 in analysis (n=5 daylight, n=4 control).
Abbreviations: NPI-NH, Neuropsychiatric Inventory Nursing Home Version; CSDD, Cornell Scale for Depression in Dementia; SE, standard error.
There are currently no minimum requirements for light exposure in dementia care environments to ensure the effectiveness of the indoor environment as a nonpharmacological treatment option. Recommended practices such as Lighting and the Visual Environment for Seniors and the Low Vision Population21 and Lighting for Hospitals and Healthcare Communities22 have focused primarily on factors of safety and visual performance and have only recently begun to incorporate language addressing non-visual needs for light; yet, there remain no specific, measurable lighting requirements. Findings from this pilot study represent a first step toward the development of evidence-based lighting guidance to support the design and operation of dementia care environments that have the potential to reduce the need for pharmacological treatments for depression and other health conditions.
The lighting measurements recorded in the daylight intervention spaces should not be interpreted as a definitive “prescription” for effective indoor light stimulus. While our reported findings may be due to chance in light of multiple hypothesis testing in this pilot trial, the consistency of the mean changes in daylight versus control participants across the communities (Table 1) provides some assurance that these findings are real and estimates can be used for the design of a large informative clinical trial. Further studies are needed to build a body of evidence for the appropriate timing, duration, wavelength, and intensity of light exposure for adults with dementia, as well as to determine how patterns of light and dark should be orchestrated over 24-hour periods to suit the biological needs of each individual.
Acknowledgments
We are grateful to the administration, staff, and residents at the eight Silverado dementia care communities involved in this study. We also thank the Southern California Clinical and Translational Science Institute for providing biostatistical support as well as University of Southern California students Tian En Cheng, Yvonne Lau, Kyeongsuk Lee, and Tian Chen for their work in data collection. This work was supported by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science of the US National Institutes of Health and a gift from the National Investment Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tsuno N, Homma A. What is the association between depression and Alzheimer’s disease? Expert Rev Neurother. 2009;9:1667–1676. doi: 10.1586/ern.09.106. [DOI] [PubMed] [Google Scholar]
- 2.Golden RN, Gaynes BN, Ekstrom RD, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162:656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- 3.Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJW. Effect of morning bright light treatment for rest–activity disruption in institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatr. 2005;17:221–236. doi: 10.1017/S1041610205001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen-Mansfield J. Nonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critique. Am J Geriatr Psychiatry. 2001;9:361–381. [PubMed] [Google Scholar]
- 5.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 6.Forbes D, Morgan DG, Bangma J, Peacock S, Adamson J. Light therapy for managing sleep, behaviour, and mood disturbances in dementia [review] Cochrane Database of Syst Rev. 2009;4:CD003946. doi: 10.1002/14651858.CD003946.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Labbate LA, Lafer B, Thibault A, Sachs GS. Side effects induced by bright light treatment for seasonal affective disorder. J Clin Psychiatry. 1994;55:189–191. [PubMed] [Google Scholar]
- 8.Terman M, Terman JS. Bright light therapy: side effects and benefits across the symptom spectrum. J Clin Psyciatry. 1999;60:799–808. [PubMed] [Google Scholar]
- 9.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueiro MG, Plitnick BA, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527–1537. doi: 10.2147/CIA.S68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Figueiro MG, Eggleston G, Rea MS. Effects of light exposure on behavior of Alzheimer’s patients – a pilot study. Light and Human Health: EPRI/LRO 5th International Lighting Research Symposium. 2002. [Accessed September 5, 2018]. pp. 151–156. Available from: https://www.researchgate.net/publication/268428546_Effects_of_Light_Exposure_on_Behavior_of_Alzheimer%27s_Patients-A_Pilot_Study.
- 13.Shochat T, Martin J, Marler M, Ancoli-Israel S. Illumination levels in nursing home patients: effects on sleep and activity rhythms. J Sleep Res. 2000;9:373–379. doi: 10.1046/j.1365-2869.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 14.Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc. 2002;50:282–289. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day K, Carreon D, Stump D. The therapeutic design of environments for people with dementia: a review of the empirical research. Gerontologist. 2000;40:397–416. doi: 10.1093/geront/40.4.397. [DOI] [PubMed] [Google Scholar]
- 16.Zelinski EL, Deibel SH, McDonald RJ. The trouble with circadian clock dysfunction: multiple deleterious effects on the brain and body. Neurosci Biobehav Rev. 2014;40:80–101. doi: 10.1016/j.neubiorev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 18.Wood S, Cummings JL, Hsu MA, et al. The use of the neuropsychiatric inventory in nursing home residents, characterization and measurement. Am J Geriatr Psychiatry. 2000;8:75–83. doi: 10.1097/00019442-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Lucas RJ, Peirson S, Berson DM, et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014;37:1–9. doi: 10.1016/j.tins.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enezi JA, Revell V, Brown T, Wynne J, Schlangen L, Lucas R. A “melanopic” spectral efficiency function redicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J Biol Rhythms. 2011;26:314–323. doi: 10.1177/0748730411409719. [DOI] [PubMed] [Google Scholar]
- 21.Illuminating Engineering Society Lighting and the visual environment for seniors and the low vision population (IES RP-28-16) 2016. [Accessed January 4, 2018]. Available from: https://www.ies.org/store/recommended-practices-and-ansi-standards/lighting-and-the-visual-environment-for-seniors-and-the-low-vision-population.
- 22.Illuminating Engineering Society Lighting for hospitals and healthcare facilities (IES RP-29-16) 2016. [Accessed January 4, 2018]. Available from: https://www.ies.org/store/recommended-practices-and-ansi-standards/lighting-for-hospitals-and-healthcare-facilities.


