Abstract
Purpose
Carfilzomib has been approved for use in relapsed and refractory multiple myeloma (RRMM). Cardiac toxicities have been reported with the use of carfilzomib. We aimed to determine the overall incidence and risk of cardiac toxicities in RRMM patients treated with carfilzomib using a meta-analysis.
Methods
We searched several databases for relevant articles. Prospective trials evaluating carfilzomib in RRMM patients with adequate data on cardiac toxicities were included for analysis. Pooled incidence, Peto ORs, and 95% CIs were calculated according to the heterogeneity of selected studies.
Results
A total of 2,607 RRMM patients from eight prospective trials were included. The pooled incidence of all-grade congestive heart failure (CHF) and ischemic heart disease (IHD) related to carfilzomib in RRMM patients was 5.5% (95% CI: 4.3%–6.9%) and 2.7% (95% CI: 1.1%–6.7%), respectively. In addition, the use of carfilzomib significantly increased all-grade (Peto OR 2.33, 95% CI: 1.56–3.48, p<0.001) and high-grade (Peto OR 3.22, 95% CI: 1.84–5.61, p<0.001) CHF when compared to controls, whereas there was no significantly increased risk of developing all-grade (Peto OR 1.31, 95% CI: 0.79–2.18, p=0.30) and high-grade (Peto OR 1.41, 95% CI: 0.73–2.72, p=0.31) IHD in RRMM patients receiving carfilzomib.
Conclusion
The use of carfilzomib in RRMM patients significantly increases the risk of developing CHF but not IHD. Clinicians should be cautious about the risk of CHF associated with carfilzomib to maximize the benefits and minimize the toxicities.
Keywords: carfilzomib, cardiac toxicities, clinical trials, meta-analysis, congestive heart failure, ischemic heart disease
Introduction
The ubiquitin–proteasome system (UPS) plays a vital role in protein degradation involved in many cell events, including cell cycle progression, apoptosis, drug resistance, angiogenesis, and cell differentiation.1–5 In addition, many studies have also found that UPS plays a critical role in tumor cell survival;6,7 thus, targeting the UPS is a novel treatment strategy for anticancer therapy.5 Two major steps are involved in the UPS process: the first step is called the ubiquitination that involves the covalent attachment of ubiquitin(s) to its protein substrate and the second step involves the degradation of the substrate by 20S proteasome.8 Increased 26S proteasome levels and proteasome activity have been detected in several different kinds of malignancies.9–11 In addition, high proteasome activity has been found to maintain cancer cell survival because it might aid in protecting against apoptosis pathways and ridding the cell of damaged proteins.9 As a result, targeting the proteasome pathway could provide the possibility of developing new opportunities in cancer therapy.
In the past decades, two proteasome inhibitors (PIs), bortezomib and carfilzomib, have been approved for use in relapsed and refractory multiple myeloma (RRMM) due to their overall survival benefits.12,13 However, the UPS has also been involved in maintaining the normal functioning of cardiac myocytes. Thus, inhibition of proteasome function might lead to cardiac insufficiency. Indeed, cardiac toxicities related to PIs have been reported in several published studies. In their meta-analysis, Xiao et al found that the pooled incidence of all-grade and severe cardiotoxicity related to bortezomib in cancer patients was 3.8% and 2.3%, respectively, although there was no significantly increased risk of cardiotoxicity with bortezomib.14 Carfilzomib is a highly selective PI that selectively and irreversibly inhibits the chymotrypsin-like activity of the 20S proteasome. In addition, carfilzomib is structurally and mechanistically distinct from bortezomib. Therefore, the cardiac toxicities associated with carfilzomib might be different. However, to our best knowledge, there has been no systematic review to comprehensively assess the cardiotoxicity related to carfilzomib. Therefore, we performed this research to determine the potential cardiotoxicity risk in RRMM patients treated with carfilzomib.
Methods
Data source
Several databases including PubMed, Web of Science, and Cochrane Library were searched for relevant trials. The search key words were carfilzomib, Kyprolis, PR-171, clinical trials, and multiple myeloma (MM). In addition, relevant articles in the reference lists of recent meta-analyses that investigated targeted agents in RRMM patients were also searched. To avoid duplication, only the most complete, recent articles were considered for analysis. All results were input into Endnote X8 reference software (Thomson Reuters, Toronto, ON, Canada) for duplication exclusion and further reference management.
Clinical end point
Cardiac toxicities were defined by the National Cancer Institute’s Common Terminology Criteria for Adverse Events during a clinical trial as a result of exposure to an experimental drug, which had been widely used in cancer clinical trials.15 The following adverse outcomes were considered as cardiotoxic events: left ventricular ejection fraction decline or dysfunction, congestive heart failure (CHF) (not specified), and ischemic heart disease (IHD).
Study selection
The primary objective of the present study was to evaluate the association between carfilzomib treatment and treatment-related cardiac toxicities in RRMM patients; therefore, only prospective Phase II and Phase III trials evaluating carfilzomib in RRMM patients with adequate data on cardiotoxicity were incorporated in the analysis. Phase I trials were omitted due to multiple dose levels and limited sample sizes. Clinical trials that met the following criteria were included: prospective Phase II and Phase III clinical trials in RRMM patients treated with carfilzomib and available data regarding the incidence and causes of cardiac toxicities.
Data extraction
Two investigators independently conducted data abstraction, and any discrepancy between the reviewers was resolved by consensus. The following information was extracted from each study: first author’s name, year of publication, phases of trials, number of enrolled subjects, treatment arms, number of patients in the treatment and control groups, median age, median progression-free survival, adverse outcomes of interest (cardiotoxicity), and dosage of carfilzomib.
Statistical analysis
The primary summary measures were incidence, Peto ORs, and corresponding 95% CIs. All statistical analyses were performed using Open MetaAnalyst software version 4.16.12 (Tufts University). For the calculation of incidence, the number of patients with cardiac toxicities in the carfilzomib group and the total number of patients receiving carfilzomib were extracted; the proportion of patients with cardiac toxicities and 95% CIs were derived for each study. To calculate Peto ORs, patients assigned to carfilzomib were compared only with those assigned to the control treatment in the same trial. We used the Peto method to calculate ORs and 95% CIs because this method provided the best CI coverage when dealing with low event rates.16 Between-study heterogeneity was estimated using the χ2-based Q statistic.17 Heterogeneity was considered statistically significant when Pheterogeneity was <0.1. The presence of publication bias was evaluated using the Begg’s and Egger’s tests.18,19 A statistical test with a p-value <0.05 was considered significant.
Results
Search results
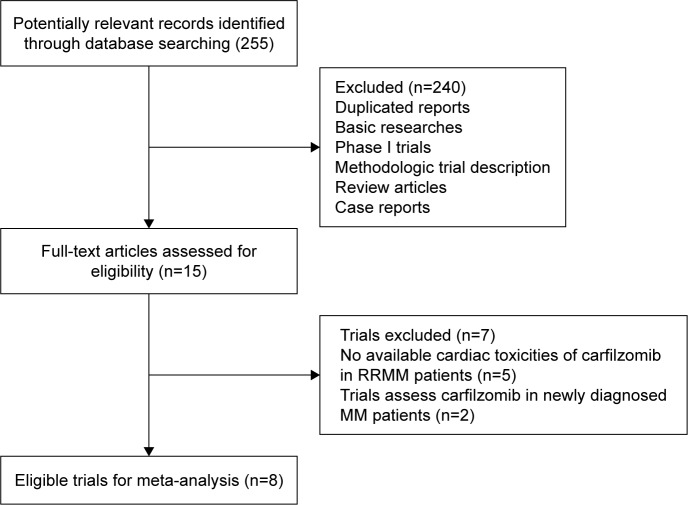
We performed the systematic review and meta-analysis in accordance with the PRISMA statement.20 Our initial search yielded 255 potentially relevant reports. After excluding review articles, Phase I studies, case reports, meta-analyses, and observation studies, a total of eight prospective clinical trials, included three Phase III21–23 and five Phase II trials,24–28 were included (Table 1). In total, 2,607 RRMM patients were pooled for analysis. A flowchart showing the study selection process is shown in Figure 1. The baseline characteristics of patients and included studies are shown in Table 1.
Table 1.
Baseline characteristics of included trials
| Study | Phase | Total | Treatment regimens | Median age (years) | Median PFS (months) | Median duration of carfilzomib |
|---|---|---|---|---|---|---|
| Siegel et al27 | II | 266 | Carfilzomib 20/27 mg/m2 | 63 | 15.6 | 3.0 |
| Vij et al26 | II | 164 | Carfilzomib 20 mg/m2 | 65 | NR | 7.0 |
| Jagannath et al28 | II | 46 | Carfilzomib 20 mg/m2 | 63.5 | 3.5 | NR |
| Badros et al25 | II | 50 | Carfilzomib 20 mg/m2 followed by 27 mg/m2 | 64 | NR | NR |
| Korde et al24 | II | 45 | Carfilzomib 20/36 mg/m2+lenalidomide+dexamethasone | 60 | NR | 16 |
| Dimopoulos et al22 | III | 929 | Carfilzomib 20 mg/m2+dexamethasone | 65 | 18.7 | 9.3 |
| Bortezomib+dexamethasone | 65 | 9.4 | 6.3 | |||
| Hajek et al21 | III | 315 | Carfilzomib 20 mg/m2 | 63 | 3.7 | 3.8 |
| Low-dose corticosteroids | 66 | 3.3 | 2.5 | |||
| Stewart et al23 | III | 792 | Carfilzomib 20 mg/m2+lenalidomide+dexamethasone | 64 | 26.3 | 20.5 |
| Lenalidomide+dexamethasone | 65 | 17.6 | 13.3 |
Abbreviations: PFS, progression-free survival; NR, not reported.
Figure 1.
Flowchart of trial selection process in the meta-analysis.
Abbreviations: RRMM, relapsed and refractory multiple myeloma; MM, multiple myeloma.
Overall incidence of cardiotoxicity
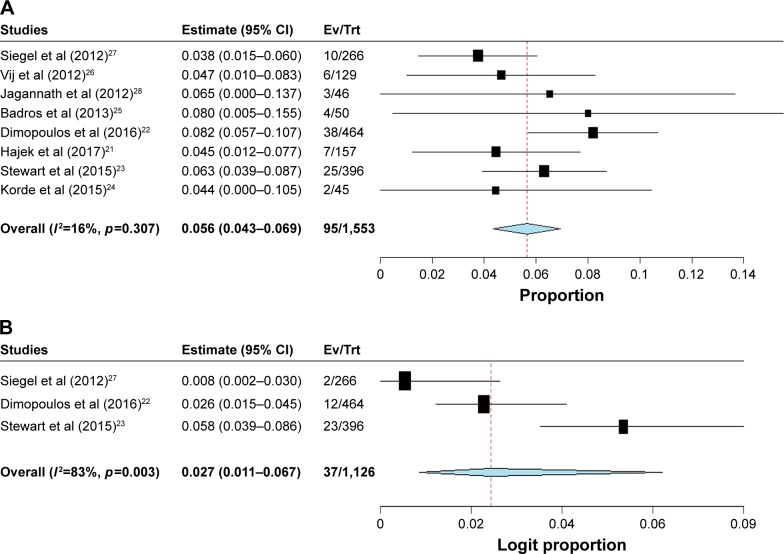
For the incidence analysis, we considered arms receiving either carfilzomib monotherapy or carfilzomib-based combination. A total of 2,607 MM patients were available for analysis. The reported all-grade CHF incidence ranged between 3.8% and 8.2%. The pooled incidence of all-grade CHF in RRMM patients was 5.6% (95% CI: 4.3%–6.9%; Figure 2A). There was significant heterogeneity among included trials; thus, we performed the meta-analysis using a random effects model. As for all-grade IHD, a total of 1,126 patients from three trials were included. All-grade IHD incidence ranged between 0.8% and 5.8%. The overall incidence of all-grade IHD in RRMM patients treated with carfilzomib was 2.7% (95% CI: 1.1%–6.7%; Figure 2B).
Figure 2.
Forest plot for meta-analysis of incidence of (A) all-grade and (B) high-grade CHF in RRMM patients receiving carfilzomib.
Abbreviations: CHF, congestive heart failure; Ev, events; RRMM, relapsed and refractory multiple myeloma; Trt, treatment.
Relative risk of cardiotoxicity
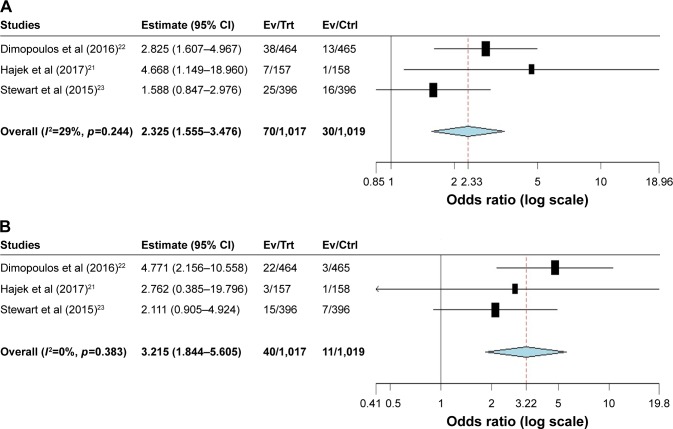
Three randomized studies were available to calculate the Peto OR of all-grade and high-grade CHF in RRMM patients assigned to carfilzomib versus controls in the same trial. The present study showed that the summary Peto OR of all-grade and high-grade CHF in carfilzomib versus controls arms was 2.33 (95% CI: 1.56–3.48, p<0.001; Figure 3A) and 3.22 (95% CI: 1.84–5.61, p<0.001; Figure 3B), respectively, suggesting a nearly three times higher risk of developing CHF associated with carfilzomib compared with controls. The test for heterogeneity was not significant (I2=29%, p=0.24 and I2=0%, p=0.38, respectively).
Figure 3.
Relative risk of carfilzomib-associated (A) all-grade and (B) high-grade CHF in RRMM patients receiving carfilzomib.
Abbreviations: CHF, congestive heart failure; Ctrl, control; Ev, events; RRMM, relapsed and refractory multiple myeloma; Trt, treatment.
Two randomized trials were available to calculate the Peto OR of all-grade and high-grade IHD. The present study showed that the summary Peto OR of all-grade and high-grade IHD in experimental versus control arms was nonsignificant (all-grade: Peto OR 1.31, 95% CI: 0.79–2.18, p=0.30; high-grade: Peto OR 1.41, 95% CI: 0.73–2.72, p=0.31). The test for heterogeneity was not found to be significant (I2=30%, p=0.25 and I2=0%, p=0.70, respectively).
Discussion
MM is a clonal neoplastic proliferation of plasma cells in the bone marrow associated with the production of monoclonal immunoglobulins in the blood or urine. Although great advances have been achieved by the introduction of novel drugs, a majority of MM patients eventually relapse, and the prognosis of these patients who are refractory to immunomodulatory drugs and/or bortezomib is very poor, highlighting the need for additional agents. In the past years, carfilzomib has been approved for use in heavily pretreated MM patients due to its survival benefits. However, the risk of cardiac toxicities is a serious concern with the increased application of carfilzomib in the treatment of RRMM patients.
As far as we know, our study is the first meta-analysis to specially assess the risk of cardiac toxicities in RRMM patients treated with carfilzomib. With available evidence from published clinical trials, the present research included 2,607 MM patients from eight prospective clinical trials. Our pooled results suggested that the incidence of all-grade CHF and IHD related to carfilzomib was 3.8% (95% CI: 2.6%–5.6%) and 2.3% (95% CI: 1.6%–3.5%), respectively. In addition, we found that the use of carfilzomib was associated with a significantly increased risk of developing all-grade (Peto OR 2.33, p<0.001) and high-grade (Peto OR 3.22, p<0.001) CHF when compared with controls, whereas there was no significantly increased risk of developing all-grade (Peto OR 1.31, p=0.30) and high-grade (Peto OR 1.41, p=0.31) IHD in RRMM patients receiving carfilzomib. However, only two prospective randomized controlled trials were included to investigate the risk of IHD associated with carfilzomib; thus, the power to investigate the risk was small. Nevertheless, as carfilzomib is used extensively in the routine treatment of RRMM patients, it is important for physicians to recognize the risk of CHF including cardiac ischemia during the administration of carfilzomib therapy, especially for patients with preexisting coronary cardiac disease. Further studies are recommended to determine the risk factors and underlying mechanisms for risk reduction. In addition, the risk of cardiac toxicities might be varied according to the length of carfilzomib treatments. Data on the length of treatment are reported in three randomized controlled trials. The result indicates that the relative risk of cardiac toxicities is not significantly associated with the duration of carfilzomib treatment. The highest risk of cardiac toxicities is observed in the trials conducted by Hajek et al,21 but the treatment duration of carfilzomib is shorter than the other trials (Table 1).
As proteasome plays a critical role in the myocardium, particularly in the context of cardiac stress, an elevated risk of cardiovascular toxicity has been observed with other PIs, including bortezomib. Because carfilzomib is a more potent, irreversible PI, its use could pose a greater cardiovascular risk when compared to bortezomib. A direct comparison of cardiovascular toxicities between carfilzomib and bortezomib is available in the ENDEAVOR trial.22 In that trial in the setting of relapsed/refractory disease, cardiac event of any grade was reported in 12% of patients in the carfilzomib arm compared with 4% in the bortezomib arm.
Currently, the detailed mechanism of cardiotoxicity associated with carfilzomib is still unknown. Several different theories regarding the pathogenesis of cardiotoxicity have been postulated. It has been found that reduced proteasome activity might increase apoptosis in smooth muscle cells, leading to atherosclerotic plaque instability.29,30 This could increase the propensity of the atherosclerotic plaque to rupture, which may lead to ischemic complications. In addition, in an in vitro study, bortezomib has been found to cause significant structural abnormalities in the mitochondria of the cardiomyocytes, which could result in reduced cardiac contractility.31 As a result, PIs treatment might be associated with significant left ventricular contractile dysfunction.
According to carfilzomib prescribing information, close monitoring is recommended for patients with signs or symptoms of cardiac events including heart failure, myocardial infarction, IHD, and hypertension. If patients experience a grade 3 or 4 cardiac event, carfilzomib should be held until resolution to grade 1, and a dose reduction may be necessary if therapy is continued. Patients with a cardiac history or advanced age (≥75 years) should be closely evaluated prior to and while receiving carfilzomib.
Several limitations are needed to be considered in this analysis. First, our study is a meta-analysis of published data but not a meta-analysis of individual patient data. Thus, patient variables could not be incorporated into the analysis. Second, studies included in our analysis are conducted at various institutions by distinct investigators and may have potential bias in reported incidences or specifications of cardiac toxicities. Finally, cardiac toxicities are not the primary end point in all included trials, and the process of attribution of cardiac toxicities’ causality might be a potential source of bias.
Conclusion
The present research shows that the use of carfilzomib in RRMM patients significantly increases the risk of developing CHF but not IHD in comparison with controls. It is important for physicians and patients to appropriately recognize the risks as well as the benefits associated with carfilzomib treatment with regular monitoring of cardiac toxicities during the treatment.
Footnotes
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101(6):2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 2.Lu G, Punj V, Chaudhary PM. Proteasome inhibitor Bortezomib induces cell cycle arrest and apoptosis in cell lines derived from Ewing’s sarcoma family of tumors and synergizes with TRAIL. Cancer Biol Ther. 2008;7(4):603–608. doi: 10.4161/cbt.7.4.5564. [DOI] [PubMed] [Google Scholar]
- 3.Galimberti S, Canestraro M, Pacini S, et al. PS-341 (Bortezomib) inhibits proliferation and induces apoptosis of megakaryoblastic MO7-e cells. Leuk Res. 2008;32(1):103–112. doi: 10.1016/j.leukres.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Baiz D, Pozzato G, Dapas B, et al. Bortezomib arrests the proliferation of hepatocellular carcinoma cells HepG2 and JHH6 by differentially affecting E2F1, p21 and p27 levels. Biochimie. 2009;91(3):373–382. doi: 10.1016/j.biochi.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 5.Paramore A, Frantz S. Bortezomib. Nat Rev Drug Discov. 2003;2(8):611–612. doi: 10.1038/nrd1159. [DOI] [PubMed] [Google Scholar]
- 6.Strous GJ, Govers R. The ubiquitin-proteasome system and endocytosis. J Cell Sci. 1999;112(Pt 10):1417–1423. doi: 10.1242/jcs.112.10.1417. [DOI] [PubMed] [Google Scholar]
- 7.Ruckrich T, Kraus M, Gogel J, et al. Characterization of the ubiquitin-proteasome system in bortezomib-adapted cells. Leukemia. 2009;23(6):1098–1105. doi: 10.1038/leu.2009.8. [DOI] [PubMed] [Google Scholar]
- 8.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82(2):373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 9.Arlt A, Bauer I, Schafmayer C, et al. Increased proteasome subunit protein expression and proteasome activity in colon cancer relate to an enhanced activation of nuclear factor E2-related factor 2 (Nrf2) Oncogene. 2009;28(45):3983–3996. doi: 10.1038/onc.2009.264. [DOI] [PubMed] [Google Scholar]
- 10.Kumatori A, Tanaka K, Inamura N, et al. Abnormally high expression of proteasomes in human leukemic cells. Proc Natl Acad Sci U S A. 1990;87(18):7071–7075. doi: 10.1073/pnas.87.18.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Madura K. Increased proteasome activity, ubiquitin-conjugating enzymes, and eEF1A translation factor detected in breast cancer tissue. Cancer Res. 2005;65(13):5599–5606. doi: 10.1158/0008-5472.CAN-05-0201. [DOI] [PubMed] [Google Scholar]
- 12.VELCADE (bortezomib) for injection [prescribing information] Cambridge, MA: Millennium Pharmaceutical, Inc; 2003. [Acceessed August 1, 2013]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021602s015lbl.pdf. [Google Scholar]
- 13.Herndon TM, Deisseroth A, Kaminskas E, et al. U.S. Food and Drug Administration approval: carfilzomib for the treatment of multiple myeloma. Clin Cancer Res. 2013;19(17):4559–4563. doi: 10.1158/1078-0432.CCR-13-0755. [DOI] [PubMed] [Google Scholar]
- 14.Xiao Y, Yin J, Wei J, Shang Z. Incidence and risk of cardiotoxicity associated with bortezomib in the treatment of cancer: a systematic review and meta-analysis. PLoS One. 2014;9(1):e87671. doi: 10.1371/journal.pone.0087671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCI Cancer Therapy Evaluation Program. CTC v 2.0 and common terminology criteria for adverse events criteria V3.0 (CTCAE) 2006. [Accessed April 10, 2018]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40.
- 16.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 17.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–137. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27(5):335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajek R, Masszi T, Petrucci MT, et al. A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS) Leukemia. 2017;31(1):107–114. doi: 10.1038/leu.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimopoulos MA, Moreau P, Palumbo A, et al. ENDEAVOR Investigators Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 23.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. ASPIRE Investigators Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 24.Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1(6):746–754. doi: 10.1001/jamaoncol.2015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badros AZ, Vij R, Martin T, et al. Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia. 2013;27(8):1707–1714. doi: 10.1038/leu.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vij R, Wang M, Kaufman JL, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119(24):5661–5670. doi: 10.1182/blood-2012-03-414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jagannath S, Vij R, Stewart AK, et al. An open-label single-arm pilot phase II study (PX-171-003-A0) of low-dose, single-agent carfilzomib in patients with relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2012;12(5):310–318. doi: 10.1016/j.clml.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Jancso G, Cserepes B, Gasz B, et al. Effect of acetylsalicylic acid on nuclear factor-kappaB activation and on late preconditioning against infarction in the myocardium. J Cardiovasc Pharmacol. 2005;46(3):295–301. doi: 10.1097/01.fjc.0000175240.64444.68. [DOI] [PubMed] [Google Scholar]
- 30.Versari D, Herrmann J, Gossl M, et al. Dysregulation of the ubiquitin-proteasome system in human carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(9):2132–2139. doi: 10.1161/01.ATV.0000232501.08576.73. [DOI] [PubMed] [Google Scholar]
- 31.Nowis D, Maczewski M, Mackiewicz U, et al. Cardiotoxicity of the anticancer therapeutic agent bortezomib. Am J Pathol. 2010;176(6):2658–2668. doi: 10.2353/ajpath.2010.090690. [DOI] [PMC free article] [PubMed] [Google Scholar]