Abstract
We previously reported that sesame oil (SO) has anti-inflammatory, anti-atherosclerotic and lipid lowering properties in vivo. Our recent studies have shown that, an aqueous extract of sesame oil (SOAE) has also anti-inflammatory and anti-atherosclerotic properties but with no lipid lowering effects. The extent of reduction in atherosclerosis led us to identify components of SOAE and evaluate their anti-inflammatory properties in vitro. Liquid chromatography mass spectrometric method was used to detect and identify components of SOAE. Methoxyphenol derivatives, short and long chain carboxylic acids, dicarboxylic acids, hydroxy and oxo-carboxylic acids were detected. To our surprise, sesamol and its derivatives (lignans), were not present in the SOAE. Among the identified, a combination of methoxy phenol compounds were seleted and tested their ability to reduce LPS induced inflammatory gene expression. Monocyte derived macrophages/RAW 264.7 macrophages were pre-treated with these compounds for 2 hrs, followed by LPS stimulation for 24 hrs and pro-inflammatory gene expressions were analyzed. These methoxyphenol derivatives showed potent anti-inflammatory properties. In conclusion, the anti-inflammatory molecules associated with SO may contribute the anti-inflammatory and anti-atherosclerotic properties. Also, our results shed light for the development of SOAE based non-pharmacological therapeutics, nutritional supplements and health products for various inflammatory diseases in the future.
Keywords: inflammation, liquid chromatography, mass spectrometry, metabolomics, methoxyphenol derivatives
1. Introduction
Sesame oil (SO) is a well-known edible vegetable oil [1]. The scientific interest has been increasing on SO nutritional values due to its enormous health benefits. Others and we reported that SO has anti-inflammatory [2], anti-atherosclerotic [2,3] and reduce plasma lipids [2] properties in vivo, apart from the other health benefits such as, reduce high blood pressure [4–6], lowering hyperglycemia [7–8], improve plasma lipid profile [2–4, 6] and reduce oxidative stress [9–10]. Thus, many researchers have focused on characterization of SO composition to identify the responsible molecular network associated with SOs health benefits. Mostly, thin layer chromatography [1, 11–12], gas chromatography-mass spectrometry (GC-MS) [12–14] and liquid chromatography-mass spectrometry (LC-MS) [15–20] analytical procedures were used to characterize the composition of SO. The published data shows that SO contains predominantly mono and polyunsaturated fatty acids (70–80 %) [1, 12, 14, 16] and 2–3% of unsaponifiable matter such as, lignans (0.2 to 0.4 %) [1, 11–13, 15–18], tocopherols [1, 16, 19], phytosterols [1, 14, 20] and phenols [19–20]. A plethora of studies reported that the health benefits of SO are associated with sesame lignans such as, sesamol and sesamin [21–25], tocopherols [26–27] and phytosterols [28–29] due to their anti-oxidant properties. These lipophilic molecules (lignans, tocopherols and phytosterols) have been reported to be present in organic solvent (methanol, ethyl acetate, acetone, chloroform and hexane) extracts from SO or sesame seed (SS) or defatted SO [1, 11–16, 18–20]. To our surprise, our recent studies [30–31] have shown that the aqueous extract of SO (SOAE) has also shown anti-inflammatory and anti-atherosclerotic properties in vitro and in vivo without lowering cholesterol. These extraordinary findings have prompted us to identify molecules present in SOAE, and evaluate their efficacy against in vitro inflammation. This study highlights the identification of SOAE components by LC-high resolution (HR) MS and evaluation of a combination of specific molecules of SOAE anti-inflammatory properties in vitro.
2. Materials and methods
2.1. Chemicals and reagents
Analytical grade reference standard chemicals: 2,3 dihydroxy benzoic acid, 3,4-dihydroxy benzoic acid, p-hydroxyphenylacetic acid, 4-methoxy benzoic acid, alpha hydroxyisovaleric acid, azelaic acid, citramalic acid, coniferyl alcohol, p-coumaric acid, ferulic acid, glucose, gluconic acid, glutaric acid, linoleic acid, malic acid, oleic acid, oxalic acid, palmitic acid, quinic acid, sesamol, sinapic acid, suberic acid, succinic acid, stearic acid, syringic acid, vanillic acid, vanillyl alcohol, buffers: ammonium acetate (AmAc), ammonium hydroxide (AmOH), formic acid (HCOOH),), phorbol 12-myristate 13-acetate (PMA) and mobile phase solvents LC-MS grade were purchased from Sigma Aldrich (St. Louis, MO, USA). Sesamin and sesamolin were from Cayman chemicals (Ann Arbor, MI, USA). SO (Idhayam brand) was purchased from a local supplier and was stored in a cold room until use. SO components matched with an authentic samples obtained from the National Institute of Standards and Technology (Gaithersburg, MD). High purity water (≤ 18 MΩ) was obtained from Barnstead MegaPure Glass Stills (MP-3A, Thermo scientific, Waltham, MA, USA). All PCR primers and Trizol™ reagent were purchased from Invitrogen (Carlsbad, CA). All ELISA kits were purchased from R & D systems (Minneapolis, MN).
2.2. Sample preparation
SOAE was prepared by extracting SO with distilled water as described earlier [31] and the aqueous portion was separated by filtration and lyophilized. 10 mg of SOAE was weighed accurately and dissolved in 1 mL of 50 % methanol containing 0.1% HCOOH. A diluted sample of 200 μg/mL was prepared, centrifuged at 10,000 rpm for 15 min, and filtered through 0.22 μm syringe filter. Five microliters of the diluted sample solution was used for LC-HRMS/MS analysis.
2.3. Liquid chromatography and mass spectrometer conditions
Compounds were separated on Zorbax Eclipse Plus C18 (150 mm L× 4.6 mm ID, 5 μm PS) column using an Agilent 1200 series HPLC system (Agilent Technologies, CA, USA) consisted of G1379B degasser, G1311A quaternary pump, HTC PAL autosampler and G1316A column compartment. Binary mobile phase gradient program was employed to elute the components from the column, pump-A: acetonitrile and pump-B: water, both containing 0.1% HCOOH. The gradient program was as follows: 90% B: 0–7 min; 10% B: 7–22 min; 10% B, 22–37 min; 90% B: 37–37.1; 10% B: 37.1–42 min; 42–45 min, at the end of the each run, column was washed with a solvent composition consisted of, 50% isopropyl alcohol, 30% methanol, 20% water, 0.1% HCOOH (v/v). The column was operated at 40°C with a constant mobile phase flow rate at 750 μL/min.
Detection of analytes was performed using a quadrupole time-of-flight (QTOF) mass spectrometer (Agilent 6520B, Agilent Technologies CA, USA) equipped with a dual electrospray ionization (ESI) source coupled to HPLC. Mass spectral data were acquired over the mass range of 50 to 1700 m/z in both, positive (+) and negative (−) modes of ESI+/− separately. Mass spectrometer was tuned at 4GHz high resolution mode at low mass 1700 m/z range with a manufacturer calibration solution (Agilent, # G1969–85000, CA, USA). The reference mass solution was continuously infused through the second nebulizer to ensure better mass accuracy throughout the analysis. ESI source parameters were optimized and operated under the following conditions: values were similar in ESI+/− modes unless specified. Capillary voltage: 3.5 kV; nitrogen used as a drying and nebulizer gas and the values were set at 13 L/min and 55 psig respectively; the source temperature was set at 320°C, TOF parameters: Fragmentor and skimmer voltages were set at 100 V and 65 V respectively. For fragmentation studies, analysis was done in auto MS/MS mode, in which all the ions produced in full scan mode are fragmented by collision induced dissociation (CID). Nitrogen was used as a CID gas at optimized collision energy (CE) set at 20–22 volts. The MS and MS/MS data were collected and processed using MassHunter qualitative analysis software version B.07.00.
2.4. Compounds identification
The obtained LC-HRMS data from SOAE analysis was interpreted by considering multiple orthogonal properties of molecules as described in the literature for untargeted molecular identification [32–35]. The untargeted molecular identification workflow was carried out as follows: 1) an experimental monoisotopic masses obtained from SOAE analysis were entered at less than 10 ppm mass accuracy in publicly available accurate mass spectral libraries such as, Metlin (http://metlin.scripps.edu), Massbank (www.massbank.jp), Chemspider (www.chemspider.com), LIPIDMAPS (www.lipidmaps.org), HMDB (www.hmdb.ca), Pubchem (https://pubchem.ncbi.nlm.nih.gov) and KEGG (www.genome.jp) to get tentative molecules, 2) MS/MS fragmentation studies of precursor molecular ions, 3) conformation of the identified molecules with the authentic reference standards or with its structural analogues, analyzed in identical experimental conditions to compare their chromatographic and mass spectral profiles and 4) the literature survey on SO.
2.5. Confirmation of absence of lignans in SOAE
To conform that the SOAE is free from sesame lignans (sesamol, sesamin, sesamolin, sesaminol, sesamolinol) and its glucosides, tocopherols and phytosterols, LC-MS chromatograms were extracted at 30 ppm mass accuracy for corresponding ions of M+H, M+H-H2O, M+Na in ESI+ and M-H in ESI− mode, and their fragments reported in literature [36–37]. In addition to this, to evaluate the water-based extraction recovery of these liphophilic molecules from SO, sesamol (most polar molecule amongst the rest of the lignans) was chosen for the experiments at three different concentrations ranged from 0.5 to 50 ng/mL.
2.6. Extraction recovery and matrix effect
The extraction recovery (ER, %) of sesamol, ferulic acid, syringic acid, vanillic acid and coniferyl alcohol was performed at three different concentration levels, 0.5, 5 and 50 ng/mL in triplicates (n=3) as described previously [38]. Stock solution of mixture of compounds was prepared at 5 mg/5 mL in deionized (dI) warm water. Desired concentration of working solutions were prepared by dilution of stock solution with dI water and spiked appropriate volumes into 5 mL of SO leading to the required final concentrations. Spiked SO was extracted with warm dI water as described previously [31].
Matrix effect (ME, %) of the method was determined by comparing the response of molecules in a standard solution against the post extraction SO spiked samples as described previously [38]. ME was calculated at three different concentration levels 0.5, 5 and 50 ng/mL, in triplicates (n=3).
2.7. Cell culture
THP-1 monocytes (ATCC, Manassas, VA) were differentiated with 50ng/mL PMA for 72h in RPMI 1640 supplemented with 10% fetal bovine serum (Sigma, St Louis, MO), 2mM L-glutamine, and 1× penicillin-streptomycin antibiotic solution. Monocyte derived macrophages (MDMs) were used for the experiments. RAW 264.7 mouse macrophages (ATCC, Manassas, VA) were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (Sigma, St Louis, MO), 2mM L-glutamine, and 1× penicillin-streptomycin antibiotic solution. Cultures were maintained in a 5% CO2 atmosphere at 37°C and plated for experiments in 6-well plates at concentrations of 1×106 cells/well. Treatments of cells were performed in serum-free, medium after a 3h starvation period.
2.8. SOAE-8 individual plus their combinations
Among the identified SOAE molecules, few specific molecules were selected and evaluated for their anti-inflammatory properties individually as well as in combinations. In our first trial, we have selected the methoxyphenol derivatives (vanillyl alcohol, p-hydroxyphenylacetic acid, vanillic acid, coniferyl alcohol, p-coumaric acid, ferulic acid, sinapic acid, syringic acid) and designated as SOAE-8. SOAE-8 stock solutions (individual plus their mixtures) were prepared by dissolving 5 mg of each compound in one ml of CHCl3 and CH3OH (50:50, v/v), and were evaporated to complete dryness under the stream of nitrogen. The samples were resuspended in one ml of pyrogen free water, vigorously mixed, filter sterilized and used for cell culture experiments. SOAE-8 molecules were used in different combinations at various concentrations to test their ability in attenuation of LPS induced inflammation in MDMs/RAW 264.7 macrophages.
2.9. Inflammatory cytokine gene expression
MDMs/RAW 264.7 cells were plated in 6-well plates at a density of 1×106 cells per well. Cells were pre-incubated in serum-free RPMI medium for 3 hrs. They were pre-treated with SOAE-8 (50 and 250 μg/ml for MDMs, 5 and 25 μg/ml for RAW macrophages,) for 2 hrs, followed by addition of LPS (100 ng/ml for MDMs and 10 ng/ml for RAW). Cells were incubated for 24 hours. RNA was extracted using Trizol™ reagent. RNA quality and quantity was determined using Nanodrop (Thermoscientific, MA, USA). One μg of RNA was reverse transcribed into cDNA using the Superscript™ III First Strand Synthesis system (Invitrogen, Carlsbad, CA). Quantitative real time PCR was performed using iQTM5 iCycler Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA) with SYBR Green (Invitrogen, Carlsbad, CA). Human and mouse oligonucleotide primers for RT-PCR were purchased from Invitrogen (Carlsbad, CA). mRNA expression of inflammatory cytokines such as TNF-α, IL-1β, and IL-6 was analyzed in macrophages resulting in a 200 bp fragment each for sample. As a reference gene, GAPDH primers were utilized. PCR was performed with an initial step of denaturation at 50°C for 2 mins, 95°C for 10 mins followed by 40 cycles of 95°C for 20 sec and 60°C for 20 sec. Melt curves were established for the reactions. Normalized fold expression was calculated using 2-ΔΔCt method. The primers for the genes were represented in supplementary Table 2s. The entire reaction products were electrophoresed on 2% agarose gels. Gene expression experiments were carried out for more than three times.
2.10. ELISA
MDMs/RAW 264.7 were cultured in a 6-well plate. They were treated with SOAE 8 and LPS as described above. Medium was collected after 24 hours of incubation and centrifuged at 2000 rpm for 5min. The supernatant was used to determine TNFα, IL1β, IL-6 levels for MDMs and IL-6 for RAW cells. Fifty microliters of samples were analyzed using a sandwich ELISA kit (R and D, Minneapolis, MN) following suppliers protocol. The absorbance was measured at 450 nm (correction absorbance set at 540 nm) using a microplate reader (Bio-Rad, Hercules, CA). Concentration of cytokines was expressed in pg/ml.
2.11. Statistical analysis
Values were presented as mean ±SD, and statistical analyses were performed by using Student t test at significance of P<0.05.
3. Results
3.1. Compounds identified
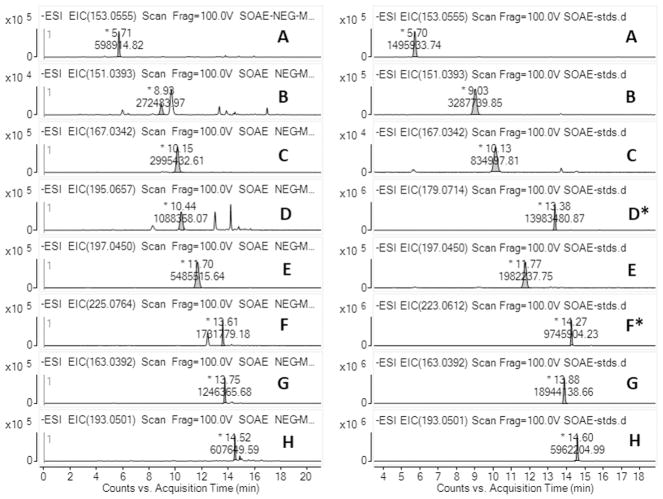
In this study, we have identified 28 molecules in SOAE samples. The molecules were moderate to polar in nature and many of them possessed a carboxylic acid functional group with aliphatic, hydroxy, oxo and aromatic sub chains. Few of them were dicarboxylic acids ranged from C6 to C9 carbon chain. The complete list of SOAE components were shown in Table. 1, and the relative percentage of each individual molecule were shown in supplementary Table. 1. The representative LC-ESI-MS extracted ion chromatograms (EICs) of few SOAE components along with their corresponding reference standards were shown in Fig. 1. EICs of other molecules along with their standards were provided as supplementary material (Fig. 1S and Fig. 2S). Sesame lignans such as sesamol, sesamin, sesamolinol and their corresponding glucosides were not identified in SOAE. The absence of lignans were shown as the representative LC-MS extracted ion chromatograms of corresponding lignan molecules in supplementary Fig. 3S(A to D).
Table 1.
The complete list of molecules identified in SOAE.
| SN* | Compound name | LC-Avg RT* | Ion | EM* | TM* | ME* |
|---|---|---|---|---|---|---|
| 1 | Gluconic acid | 1.97 | [M-H]− | 195.0516 | 195.0510 | 2 |
| 2 | Glucose | 2.01(2.01) | [M-H]− | 179.0565 | 179.0561 | 2 |
| 3 | Malic acid | 2.17(2.17) | [M-H]− | 133.0146 | 133.0142 | 2 |
| 4 | Hydroxy glutaric acid | 2.40 | [M-H]− | 147.0290 | 147.0299 | 6 |
| 5 | 2-methylmalic acid | 2.70 | [M-H]− | 147.0304 | 147.0299 | 2 |
| 6 | Glutaconic acid | 3.10(3.12) | [M-H]− | 129.0194 | 129.0193 | 0 |
| 7 | 2-hydroxy-4-oxopentanoate | 3.26 | [M-H]− | 131.0352 | 131.0350 | 1 |
| 8 | Erythrono-1,4-lactone | 3.40 | [M-H]− | 117.0202 | 117.0193 | 7 |
| 9 | Quinic acid | 3.65(3.63) | [M-H]− | 191.0567 | 191.0561 | 3 |
| 10 | Vanillyl alcohol | 5.74(5.71) | [M-H]− | 153.0555 | 153.0557 | 2 |
| 11 | L-α-hydroxyisovaleric acid | 5.93 | [M-H]− | 117.0554 | 117.0557 | 2 |
| 12 | p-hydroxyphenylacetic acid | 8.87(8.98) | [M-H]− | 151.0393 | 151.0401 | 5 |
| 13 | Pimelic acid | 9.63 | [M-H]− | 159.0675 | 159.0663 | 7 |
| 14 | Vanillic acid | 10.17(10.15) | [M-H]− | 167.0342 | 167.0354 | 4 |
| 15 | 5-hydroxyconiferyl alcohol | 10.45(*13.38) | [M-H]− | 195.0657 | 195.0663 | 2 |
| 16 | Syringic acid | 11.70(11.70) | [M-H]− | 197.0450 | 197.0455 | 2 |
| 17 | 4-hydroxy-(3′,4′-dihydroxyphenyl)-valeric acid | 12.47 | [M-H]− | 225.0773 | 225.0768 | 2 |
| 18 | Hydroxy caproic acid | 12.91 | [M-H]− | 131.0712 | 131.0714 | 1 |
| 19 | Hydroxy caproic acid isomer | 13.19 | [M-H]− | 131.0712 | 131.0714 | 1 |
| 20 | Dihydrosinapic acid | 13.67(*14.27) | [M-H]− | 225.0764 | 225.0768 | 1 |
| 21 | p-coumaric acid | 13.76(13.75) | [M-H]− | 163.0392 | 163.0401 | 5 |
| 22 | Suberic acid | 14.14(14.11) | [M-H]− | 173.0825 | 173.0819 | 3 |
| 23 | Ferulic acid | 14.54(14.52) | [M-H]− | 193.0501 | 193.0506 | 2 |
| 24 | Azelaic acid | 15.55(15.62) | [M-H]− | 187.0962 | 187.0976 | 7 |
| 25 | Hydroxy heptanoic acid | 15.98 | [M-H]− | 145.0872 | 145.0870 | 1 |
| 26 | Oxo nonannoic acid | 17.14(17.13) | [M-H]− | 171.1030 | 171.1027 | 1 |
| 27 | Traumatic acid | 17.99(18.20) | [M-H]− | 227.1293 | 227.1289 | 1 |
| 28 | Hydroxy-linoleic acid isomer | 23.36 | [M-H]− | 295.2285 | 295.2279 | 2 |
SN*-Serial Number, LC-Avg RT*-Liquid Chromatography – Average Retention Time, EM*-Experimental Mass, TM*-Theoretical Mass, ME*-Mass Error,
structural analogues RT.
Fig. 1.
LC-ESI-MS-EICs of SOAE-8 shown in left panel, and reference standards shown in right panel. A) vanillyl alcohol, B) p-hydroxyphenylacetic acid, C) vanillic acid, D) 5-hydroxyconiferyl alcohol (coniferyl alcohol), E) syringic acid, F) dihydrosinapic acid (sinapic acid), G) p-coumaric acid and H) ferulic acid.
3.2. Mass spectral libraries and MS/MS studies
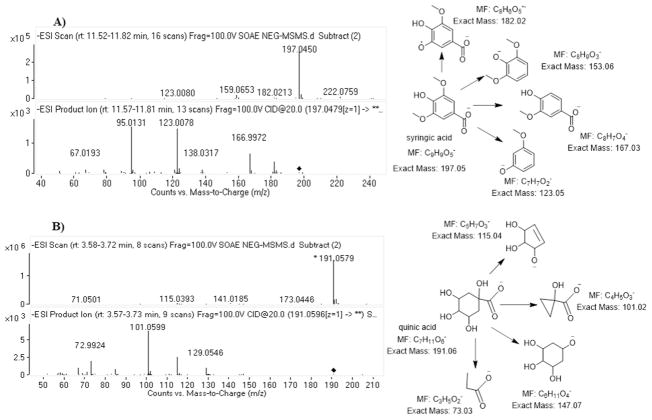
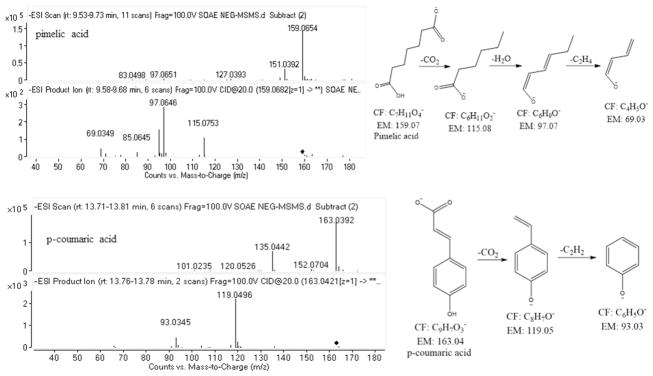
The mass spectral libraries have shown many numbers of possible molecules and their structural analogues upon the entries of monoisotopic masses obtained from SOAE analysis. Among them, the identification of appropriate molecule was done by performing collision induced dissociation (CID) tandem mass spectrometric (MS/MS) studies to further characterize their fragments towards the structural elucidation. The database search for the mass ion at m/z = 197.0460, resulted in 4 structural analogues: 1) syringic acid, 2) 3 hydroxy 4-methoxy mandelate, 3) dimethyl gallic acid and 4) 2 hydroxy 4, 5 dimethoxy benzoic acid. The MS/MS spectra of m/z = 197.0460 has shown ions at m/z = 182.02 (loss of CH3, 15 Da), m/z = 153.06 (loss of CO2, 44 Da), m/z = 167.03 (loss of OCH3, 31 Da) based on these fragment ions, compound 2 was ruled out, but the other three compounds (1, 3 & 4) likely to produce similar fragment ions. Hence, it is difficult to find out the exact molecule even by its MS/MS data. Later, based on the literature search on SO (20), the mass ion was tentatively hypothesized as syringic acid and was confirmed by matching with its reference standard analyzed in identical experimental conditions. The representative ESI-MS and MS/MS spectra of syringic acid and quinic acid were shown in Fig. 2. The mass ion at m/z = 159. 0675 also shown few structural analogues, up on MS/MS studies, based on the fragment ions at m/z = 115.08 (loss of CO2, 44 Da), at m/z = 97.07 (loss of H2O, 18 Da) and at m/z = 69.03 (loss of C2H4, 28 Da) confirmed the structure as pimelic acid. The mass ion at m/z = 163.0402 resulted in two compounds, 1) p-coumaric acid and 2) caffeic aldehyde. The MS/MS studies produced the fragment ions at m/z = 119.0496 (loss of CO2, 44 Da) which confirms the acid functional group of the molecule and ion at m/z = 93.0345 confirms the structure of phenoxide anion, thus p-coumaric acid structure was established. The ESI-HRMS/MS spectra along with the proposed fragmentation pattern of described molecules were shown in Fig. 3 and few other molecules were shown in supplementary Fig. 4S and 5S.
Fig. 2.
ESI-MS and MS/MS spectra with the proposed fragments of A) syringic acid and B) quinic acid.
Fig. 3.
ESI-MS/MS spectra along with the fragmentation pattern of pimelic acid and p-coumaric acid.
3.3. Extraction recovery and matrix effect
Among the tested compounds, sesamol has shown the lowest percentage (%) recoveries over the range of 16.3 to 26.6 % and the maximum % recoveries obtained for vanillic acid, which lie between 27.8 to 44.6 %. The rest of the tested molecules have shown % recoveries between 19.1 to 37.6 %. Matrix effect was observed to be lesser than 18 % for all the tested molecules. The extraction recovery and matrix effect values were presented in Table 2.
Table 2.
Extraction recovery and matrix effect values of few SOAE identified molecules along with sesamol.
| S.NO. | Compound | Extraction Recovery (%), ng/mL | Matrix Effect (%), ng/mL | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0.5 | 5 | 50 | 0.5 | 5 | 50 | ||
| 1 | Sesamol | 16.3(18.1) | 21.2(12.2) | 26.6(7.2) | 15.3(14.2) | 10.2(8.4) | 8.6(3.7) |
| 2 | Ferulic acid | 22.3(6.4) | 25.6(6.4) | 33.0(4.9) | 17.8 (6.3) | 12.6(6.7) | 11.0(5.4) |
| 3 | Syringic acid | 25.6(11.6) | 27.1(9.5) | 37.6(13.5) | 12.67(8.4) | 16.4(10.4) | 10.2(7.2) |
| 4 | Vanillic acid | 27.8(9.6) | 31.6(8.6) | 44.6(6.7) | 9.45(11.4) | 12.1(3.7) | 7.3(4.6) |
| 5 | Coniferyl alcohol | 19.1(15.3) | 23.0(10.4) | 27.2(5.5) | 17.0(17.5) | 12.5(9.4) | 15.1(9.4) |
Values in the parentheses are relative standard deviations (n=3).
3.4. SOAE 8 attenuates LPS-induced inflammatory cytokines in MDMs/RAW 264.7 cells
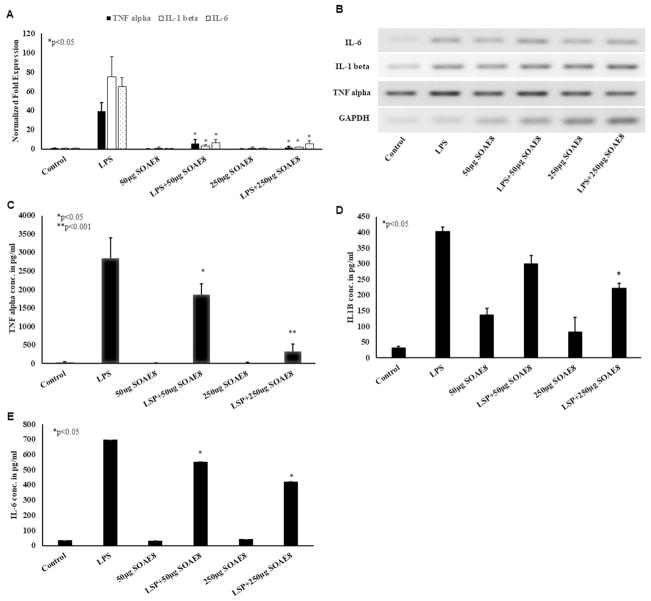
MDMs were treated with LPS (100 ng/ml) ± SOAE-8 (50 and 250 μg/ml) for 24 hours. LPS strongly induced mRNA levels of TNFα, IL-1β and IL-6 (Fig. 4A) in MDMs. SOAE8, components were able to attenuate the LPS induced inflammatory markers in MDMs in a dose dependent manner. PCR products (Fig. 4B) also corroborated with RT-PCR results. As shown in Fig. 4, a significant reduction in all pro-inflammatory cytokines was observed in presence of SOAE-8 as compared to control. The SOAE-8 alone did not induce any of the inflammatory cytokines by itself. Individual components of SOAE-8 have failed to attenuate inflammation at the concentrations used (data not shown). ELISA for TNFα, IL-1β and IL-6 (Fig. 4, C–E) also supported gene expression results. Pro-inflammatory cytokine secretion into the medium was increased when cells were treated with LPS alone. However, presence of SOAE-8 significantly inhibited LPS-induced inflammatory cytokine secretion from MDMs in a dose dependent manner.
Fig. 4.
SOAE-8 attenuates LPS-induced inflammatory cytokines in MDMs: MDMs were pre-treated with SOAE-8 (50 and 250μg/ml) followed by addition of 100ng/ml LPS. Cells were incubated for 24 hours following which RNA was isolated and real time PCR analysis was performed for TNFα, IL-1β, and IL-6(A) gene expressions, PCR products (B). (n>3; * P<0.05) and ELISA for TNF alpha (C), IL-1β (D) and IL-6 (E).
Similarly, RAW 264.7 cells were treated with LPS (10 ng/ml) ± SOAE-8 (5 and 25 μg/ml) for 24 hours. LPS strongly induced mRNA levels of IL-1α, IL-1β and IL-6 (Fig. 6S,A–C) in RAW cells. PCR products (Fig. 6SD) corroborated with RT-PCR results. However, in the presence of SOAE-8, the expression of these inflammatory markers was significantly inhibited in a concentration dependent manner, whereas the expression was vice versa in case of TNF-α in these cells (data not shown). ELISA for IL-6 (Fig. 6SE) also supported gene expression results.
4. Discussion
4.1. Method development and optimization of LC-HRMS
LC-MS based identification is a well-established analytical tool with various applications for the analysis of polar molecules [20, 31] and has advantages over GC-MS, in which GC-MS requires complex derivatization process for the analysis of polar molecules [39–40]. In an untargeted molecular identification (unknown molecular identification), the main hurdles are the mass accuracy and the identification of exact molecule from its structural analogues due to a poor chromatographic separation. For the last ten years, LC-Q-TOF-MS has been successfully employed for the analysis of untargeted molecular identification in various fields [33–35] due to its advantage of fast scanning speed, which could surpass the drawback of poor chromatographic resolution. Thus, continuous mass access correction and the fast scanning speed of QTOF-MS in full scan mode enabled us to acquire an unlimited mass data and allowed us to identify most accurate molecules present in SOAE, hence this methodology is well suitable for the current application.
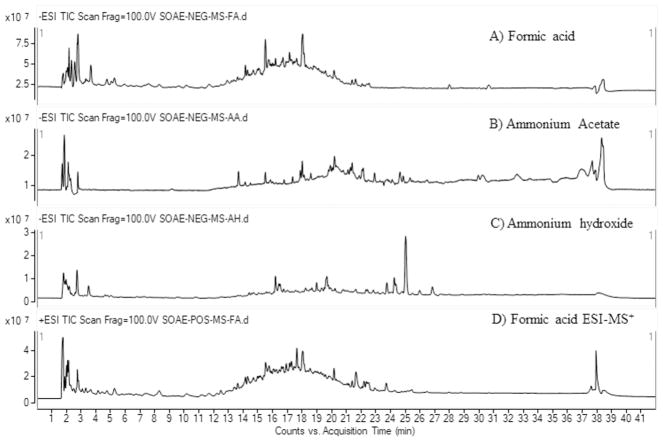
In addition to the detection, the chromatographic separation, in which the selection of mobile phase and the stationary phase play a vital role in an untargeted molecular screening [32–35, 42]. SOAE is a water based extract and hence it is expected to has polar components of SO. The hilic based HPLC column has been reported as best suitable column for the separation polar compounds [43–44], hence we have analyzed SOAE samples on HILIC (PerkinElmer, MA, USA) column, but it did not work well for the current application. Later, the separation of components tested on different types of reversed phase HPLC columns available in the laboratory. Brownlee C18 (PerkinElmer, MA, USA), Zorbax eclipse C18 (Agilent, CA, USA) and Waters Aquity UPLC CSH C18 (1.7 μm, 2.1 × 75 mm) were tested with various solvent compositions (methanol 10–90%: v/v in water or acetonitrile 10–90%: v/v in water) and with different buffers (HCOOH, AmAc and AmOH) to identify molecules present in SOAE. Zorbax eclipse C18 column was served well for most of the molecules identified in SOAE, especially for the retention of polar molecules, which were eluted between 1.8 – 5 min retention time (tR).
However, the fast scanning speed of Q-TOF mass spectrometer surpassed the poor resolution of few phenolic compounds on Zorbax C18 column. PerkinElmer Brownlee C18 column worked well for phenolic compounds separation, but with poor retention of polar molecules. The mobile phase gradient program was optimized by changing the solvent compositions against the time (min) on Zorbax C18 column. Finally, forty two minutes mobile phase gradient program, composed with acetonitrile and water was observed to be the optimum solvent system for SOAE components separation on Zorbax C18 column. The EICs of few molecules analyzed on different type of columns and mobile phases were shown in the supplementary Fig. 7S. Among the tested buffers, HCOOH was provided more number of molecules with optimal peak shape and the better detection sensitivities in ESI mass spectrometry. The representative LC-MS chromatographic profile of SOAE samples were analyzed in different types of buffers has shown in Fig. 5. Mass spectrometer source conditions were optimized by infusion of reference standards (vanillic acid, sesamol, tocopherol) via HPLC autosampler with different types of mobile phase buffers. One of the main drawbacks of ESI is the poor ionization of planar and non-polar molecules [36–37, 45]. Alpha tocopherol and few of the sesame lignan molecules are non-polar in nature, hence to improve the ionization of those molecules in ESI, SOAE samples were analyzed in three different types of mobile phase buffers, HCOOH, CH3COOH and AmOH. The addition of these volatile buffers improve the ionization process of non-polar molecules and thus, the molecules can be ionized more efficiently and detected as their quasimolecular ions, i.e. adducts of corresponding buffer ions, either ammoniated [M+NH4]+ or protonated [M+H]+ molecular ions in ESI+ mode, acetate [M+CH3COO]− or formate [M+HCOO]− or deprotonated [M-H]− molecular ions in ESI− mode. However, the planar molecules with a hydroxy functional group such as sesaminol, sesamolinol, pinoresinol, γ-tocopherol, sitosterol can form dehydrated molecular ions [M+H-H2O]+ at higher ESI source conditions [37]. The mobile phase optimized ESI-HRMS spectra of reference standards, sesamol and alpha-tocopherol were shown in supplementary Fig. 8S.
Fig. 5.
LC-ESI +/−-MS total ion chromatographic profile of SOAE obtained in different types of mobile phase buffers.
4.2. Mass spectral libraries and MS/MS studies
The current study is unknown molecular identification, hence the mass spectral libraries and MS/MS studies have played an important role in the identification of SOAE molecules. However the mass spectral libraries have shown numerous possible molecules upon the entries of few monoisotopic masses of SOAE. Later the appropriate molecules were identified based on their MS/MS fragment ions. Molecular ion at m/z = 133.0178 resulted in, diglycolic acid, 2,3-Dihydroxy-4-oxobutanoic acid and malic acid Among them, the identification of appropriate molecule was done by CID studies. Its MS/MS studies produced ions at m/z = 115.00 (loss of H2O, 18 Da), m/z = 71.01 (H2C=CH-COO−) and m/z = 59.01 (CH3COO−), based on these fragment ions, molecule was confirmed as malic acid. The mass ion at m/z = 167.0347 also shown a few structural analogues, 5-Methoxysalicylic acid, Dihydroxyphenylacetic acid, 3-Hydroxymandelic acid and vanillic acid. Its MS/MS experiments, produced fragment ions at m/z = 152.0099 (loss of CH3, 15 Da) and at m/z = 108.0214 (loss of CO2, 44 Da). Based on its fragments and the LC-retention time, the structure was confirmed as vanillic acid. Hydroxy fatty acids were confirmed by the observation of the loss of 18 Da (H2O) and the other fragment ions from its MS/MS studies. The mass ion at m/z = 131.0712 resulted in numerous molecules Leucinic acid, 2-Ethyl-2-Hydroxybutyric acid, butoxyacetic acid and hydroxy caproic acid. Its MS/MS experiments produced fragments at m/z = 85.03 (loss of H2O and C2H4, 46 Da) and m/z =69.00 (loss of CH4, 16). The ESI-HRMs and MS/MS spectra of SOAE molecules along with their corresponding standards spectra were shown in supplementary Figs. 9S (A to J).
4.3. Absence of lignans and presence of its precursors in SOAE
SO has been reported to have antioxidants such as, lignans, α–tocopherol and phytosterols [1, 11–19]. These liphophilic molecules were not likely to extract into water from SO, since SOAE is water-based extract. It was confirmed by spiked sesamol extraction experiments, in which sesamol has shown very low recoveries in the range of 16 – 26%. As mentioned in the results section, sesame lignans or its glucosides were not identified in SOAE. However, ferulic acid, 5-hydroxy coniferyl alcohol, p-hydroxyphenylacetic acid and other methoxyphenol derivative were identified and confirmed in SOAE. These methoxyphenol derivatives are involved in the biosynthesis of sesame lignans [46–47] and are polar in nature when compared to sesamol and other sesame lignan molecules. The SO spiked methoxyphenol derivatives have shown higher extraction recoveries when compared to sesamol, ranged from 20 – 45%. The reason could be methoxyphenol derivatives hold more number of polar functional groups such as, acid (−COOH) and hydroxyl (−OH) when compared to sesamol and other sesame lignans, which holds only one hydroxyl (−OH) group direcly attached to the benzene ring. These polar functional groups (−COOH) and (−OH) can form hydrogen bonding with water (H2O) molecules and could be extracted in to aqueous portion to more extent.
4.4. SOAE health benefits
Our results suggest that, apart from the lignans, tocopherols and phytosterols, SO has other health beneficial molecules which were polar in nature, which might contribute the oils potent anti-inflammatory and anti-atherosclerotic properties and hence SOAE could show anti-inflammatory and anti-atherosclerotic properties in both in vitro and in vivo (2, 30–31). LPS exerts its toxic effects by potently activating macrophages, and inducing the expression of pro-inflammatory cytokines [48–49]. Our current observations shown that SOAE-8 has effective anti-inflammatory action against LPS-induced inflammation in MDMs/RAW macrophages, similar to that of whole SOAE [31], however, the variation was observed in TNF-α expression with mouse cell line (data not shown). Further studies needed to evaluate this discrepancy. An acute phase immune response is known to be elicited by IL-6 through the release of CRP, which is known as an inflammatory biomarker of cardiovascular risk [50–51]. SOAE-8 could also significantly inhibited IL-6 expression (Fig. 4 & 6S) similar to that of whole SOAE [31] in MDMs/RAW 264.7 macrophages. Evidences suggest that, the anti-inflammatory effect of methoxy phenol compounds is due to their inhibitory action on either TNF alpha or TLR4 or NFkB signaling pathways (52–53). In our whole SOAE studies we have already shown that the NFkB mediated inflammatory mechanism was efficiently inhibited by SOAE (31). Thus, compounds of SOAE-8 might have the potential to regulate many inflammatory pathways and possess several beneficial effects. Further, evaluation of SOAE-8 mixture in animal models of atherosclerosis and other inflammatory diseases will provide valuable information on its efficacy in vivo. Our group is currently focusing on determining the effect of SOAE-8 in animal models of acute and chronic inflammation.
4.5. Selection of SOAE-8 components
SOAE is a complex mixture, so far we have identified 28 molecules. Among those, azelaic acid (54–55), quinic acid (56), malic acid and 2-methyl malic acid (57) and caproic acid (58) have been reported as anti-inflammatory molecules. In the current study, as our first trial, we have tested anti-inflammatory properties of eight methoxyphenol derivatives in vitro. The reason for selection of methoxyphenol derivatives is their action on myeloperoxidase (MPO) inhibition (59–60), since MPO has been reported as an important biomarker in atherosclerosis (61–63). In SOAE-8 mixture, except p-hydroxyphenylacetic acid and p-coumaric acid the remaining six molecules hold methoxyphenol group in common (The structures of SOAE-8 have shown in supplementary Fig. 10S). Recently the pathophysiological role of MPO in CVD has attained more attention in the development of MPO inhibitors for therapeutic use [64]. Hence, these SOAE components, particularly ferulic acid and its derivatives (structural analogues) which are known to be MPO inhibitors, might also be responsible for SOAEs beneficial effects.
5. Conclusions
The established untargeted LC-HRMS approach was successfully employed for identification SOAE components. Among the identified, in our first trial, a combination of few specific molecules was able to reduced LPS induced inflammation in MDMs/Raw 264.7 macrophages. Our results suggest that, apart from lignans, tocopherols and phytosterols, SO has other polar biological active molecules, hence SOAE could show anti-inflammatory properties in vitro as well as in vivo. So far 28 molecules were identified and confirmed in SOAE, however, few of the unidentified molecular weights of SOAE yet to be characterized using NMR and IR spectroscopic studies followed by successful isolation of molecules by preparative chromatography. The proposed approach can be used as a rapid screening for identification of polar to moderate polar molecules in different types of food commodities. This is the first unique study reporting that a combination of SOAE specific components has anti-inflammatory properties in vitro, which might shed light on nature of compounds and also suggests more powerful non-pharmacological agents due to their potential synergistic interaction among the components itself.
Supplementary Material
Highlights.
LC-HRMS/MS method was developed to identify sesame oil aqueous extract components.
Different types of mobile phase buffers were examined.
Evaluated anti-inflammatory properties of few specific molecules.
Methoxyphenol derivatives have shown anti-inflammatory properties in vitro.
Acknowledgments
This study was supported by National Institutes of Health Grant 5R01AT004106-05
Footnotes
Authors’ disclosure statement: No competing financial interests exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sun Hwang LS. Sesame oil. In: Shahidi F, editor. book: Bailey’s Industrial Oil and Fat Products. 6. John Wiley & Sons Inc; 2005. pp. 2–12. [Google Scholar]
- 2.Aluganti Narasimhulu C, Selvarajan K, Litvinov D, Parthasarathy S. Anti-Atherosclerotic and Anti-Inflammatory Actions of Sesame Oil. J Med Food. 2015;18:11–20. doi: 10.1089/jmf.2014.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaskaran S, Santanam N, Penumetcha M, Parthasarathy S. Inhibition of Atherosclerosis in Low-Density Lipoprotein Receptor-Negative Mice by Sesame Oil. J Med Food. 2006;9:487–490. doi: 10.1089/jmf.2006.9.487. [DOI] [PubMed] [Google Scholar]
- 4.Sankar D, Ramakrishna Rao M, Sambandam G, Pugalendi KV. A Pilot Study of Open Label Sesame Oil in Hypertensive Diabetics. J Med Food. 2006;9:408–412. doi: 10.1089/jmf.2006.9.408. [DOI] [PubMed] [Google Scholar]
- 5.Karatzi K, Stamatelopoulos K, Lykka M, Mantzouratou P, Sofia Skalidi S, Manios E, Georgiopoulos G, Zakopoulos N, Papamichael C, Sidossis LS. Acute and Long-Term Hemodynamic Effects of Sesame Oil Consumption in Hypertensive Men. J Clin Hypertens. 2012;14:630–636. doi: 10.1111/j.1751-7176.2012.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sankar D, Ramakrishna Rao M, Sambandam G, Pugalendi KV. Effect of Sesame Oil on Diuretics or β-blockers in the Modulation of Blood Pressure, Anthropometry, Lipid Profile, and Redox Status. Yale J Biol Med. 2006;79:19–26. [PMC free article] [PubMed] [Google Scholar]
- 7.Aslam F, Iqbal S, Nasir M, Anjum A, Swan P, Sweazea K. Evaluation of White Sesame Seed Oil on Glucose Control and Biomarkers of Hepatic, Cardiac, and Renal Functions in Male Sprague-Dawley Rats with Chemically Induced Diabetes. J Med Food. 2017;20:448–457. doi: 10.1089/jmf.2016.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devarajan S, Chatterjee B, Urata H, Zhang B, Ali A, Singh A, Ganapathy S. Blend of Sesame and Rice Bran Oils Lowers Hyperglycemia and Improves the Lipids. Am J Med. 2016;129:731–739. doi: 10.1016/j.amjmed.2016.02.044. [DOI] [PubMed] [Google Scholar]
- 9.Periasamy S, Yang SS, Chen SY, Chang CC, Liu MY. Prophylactic Sesame Oil Attenuates Sinusoidal Obstruction Syndrome by Inhibiting Matrix Metalloproteinase–9 and Oxidative Stress. J Parenter Enteral Nutr. 2013;37:529–537. doi: 10.1177/0148607112454299. [DOI] [PubMed] [Google Scholar]
- 10.Liu CT, Liu MY. Daily sesame oil supplementation attenuates local renin-angiotensin system via inhibiting MAPK activation and oxidative stress in cardiac hypertrophy. J Nutr Biochem. 2017;42:108–116. doi: 10.1016/j.jnutbio.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Kamal-lldln A, Yousif G, Appelqvist LA. Thin-Layer Chromatographic Separations of Seed Oil Unsaponifiables from Four Sesamum Species. JAOCS. 1991;68:844–847. [Google Scholar]
- 12.Reshma MV, Balachandran C, Arumughan C, Sunderasan A, Sukumaran D, Thomas S, Saritha SS. Extraction, separation and characterisation of sesame oil lignan for nutraceutical applications. Food Chem. 2010;120:1041–1046. [Google Scholar]
- 13.Mohamed HMA, Awatif II. The use of sesame oil unsaponifiable matter as a natural antioxidant. Food Chem. 1998;62:269–276. [Google Scholar]
- 14.Tir R, Dutta PC, Badjah-Hadj-Ahmed AY. Effect of the extraction solvent polarity on the sesame seeds oil composition. Eur J Lipid Sci Technol. 2012;114:1427–1438. [Google Scholar]
- 15.Ali A, Stefanie ML, Kamal-Eldin HA. Lignan contents in sesame seeds and products. Eur J Lipid Sci Technol. 2007;109:1022–1027. [Google Scholar]
- 16.Hemalatha S, Unissa G. Lignans and Tocopherols in Indian Sesame Cultivars. j am oil chem soc. 2014;81:467–470. [Google Scholar]
- 17.Wu L, Yu L, Ding X, Li P, Dai X, Chen X, Zhou H, Bai Y, Ding J. Magnetic solid-phase extraction based on graphene oxide for the determination of lignans in sesame oil. Food Chem. 2017;217:320–325. doi: 10.1016/j.foodchem.2016.08.109. [DOI] [PubMed] [Google Scholar]
- 18.Suna W, Xiao R. Determination of Sesamol in Sesame Oil by Anion Exchange Solid Phase Extraction Couple with HPLC. Anal Methods. 2014;6:6432–6436. [Google Scholar]
- 19.Jannata B, Oveisi MR, Sadeghi N, Hajimahmoodi M, Behzad M, Nahavandi B, Tehrani S, Sadeghi F, Oveisi M. Effect of Roasting Process on Total Phenolic Compounds and γ-tocopherol Contents of Iranian Sesame Seeds (Sesamum indicum) Iran J Pharm Res. 2013;12:751–758. [PMC free article] [PubMed] [Google Scholar]
- 20.Wu R, Ma F, Zhang L, Li P, Li G, Zhang Q, Zhang W, Wang X. Simultaneous determination of phenolic compounds in sesame oil using LC–MS/MS combined with magnetic carboxylated multi-walled carbon nanotubes. Food Chem. 2016;204:334–342. doi: 10.1016/j.foodchem.2016.02.086. [DOI] [PubMed] [Google Scholar]
- 21.Yashaswini PS, Sadashivaiah B, Ramaprasad TR, Sridevi AS. In vivo modulation of LPS induced leukotrienes generation and oxidative stress by sesame lignans. J Nutr Biochem. 2017;41:151–157. doi: 10.1016/j.jnutbio.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Ashakumary L, Rouyer I, Takahashi Y, Ide T, Fukuda N, Aoyama T, Hashimoto T, Mizugaki M, Sugano M. Sesamin, a sesame lignan, is a potent inducer of hepatic fatty acid oxidation in the rat. Metabolism. 1999;48:1303–1313. doi: 10.1016/s0026-0495(99)90272-x. [DOI] [PubMed] [Google Scholar]
- 23.Hirose N, Inoue T, Nishihara K, Sugano M, Akimoto K, Shimizu S, Yamada H. Inhibition of cholesterol absorption and synthesis in rats by sesamin. J Lipid Res. 1991;32:629–638. [PubMed] [Google Scholar]
- 24.Henriques Monteiro EM, Chibli LA, Yamamoto CH, Santana Pereira MC, Pinto Vilela FM, Rodarte MP, de Sousa OV. Antinociceptive and Anti-Inflammatory Activities of the Sesame Oil and Sesamin. Nutrients. 2014;6:1931–1944. doi: 10.3390/nu6051931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng FC, Jinn TR, Hou RCW, Tzen JTC. Neuroprotective Effects of Sesamin and Sesamolin on Gerbil Brain in Cerebral Ischemia. International Journal of Biomedical Science IJBS. 2006;2:284–288. [PMC free article] [PubMed] [Google Scholar]
- 26.Cooney RV, Custer LJ, Okinaka L, Franke AA. Effects of Dietary Sesame Seeds on Plasma Tocopherol Levels. Nutr Cancer. 2001;39:66–71. doi: 10.1207/S15327914nc391_9. [DOI] [PubMed] [Google Scholar]
- 27.Wichitsranoi J, Weerapreeyakul N, Boonsiri P, Settasatian C, Settasatian N, Komanasin N, Sirijaichingkul S, Teerajetgul Y, Rangkadilok N, Leelayuwat N. Antihypertensive and antioxidant effects of dietary black sesame meal in pre-hypertensive humans. Nutr J. 2011;10:82. doi: 10.1186/1475-2891-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogbe RJ, Ochalefu DO, Mafulul SG, Olaniru OB. A review on dietary phytosterols: Their occurrence, metabolism and health benefits. Asian J Plant Sci Res. 2015;5:10–21. [Google Scholar]
- 29.Ikeda I, Kawasaki A, Samezima K, Sugano M. Antihypercholesterolemic Activity of β-Sitostanol in Rabbits. J Nutr Sci Vitaminol. 1981;27:243–251. doi: 10.3177/jnsv.27.243. [DOI] [PubMed] [Google Scholar]
- 30.Aluganti Narasimhulu C, Selvarajan K, Young Burge K, Litvinov D, Sengupta B, Parthasarath S. Water-Soluble Components of Sesame Oil Reduce Inflammation and Atherosclerosis. J Med Food. 2016;19:1–9. doi: 10.1089/jmf.2015.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selvarajan K, Aluganti Narasimhulu C, Bapputty R, Parthasarathy S. Anti-Inflammatory and Antioxidant Activities of the Nonlipid (Aqueous) components of Sesame Oil: Potential Use in Atherosclerosis. J Med Food. 2015;18:393–402. doi: 10.1089/jmf.2014.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn WB, Erban A, Weber RJM, Creek DJ, Brown M, Breitling R, Hankemeier T, Goodacre R, Neumann S, Kopka J, Viant MR. Mass appeal: metabolite identification in mass spectrometry-focused untargeted metabolomics. Metabolomics. 2012;9:1–23. [Google Scholar]
- 33.Hoffmann T, Krug D, Huttel S, Muller R. Improving Natural Products Identification through Targeted LC-MS/MS in an Untargeted Secondary Metabolomics Workflow. Anal Chem. 2014;86:10780–10788. doi: 10.1021/ac502805w. [DOI] [PubMed] [Google Scholar]
- 34.Cajka T, Fiehn O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal Chem. 2016;88:524–545. doi: 10.1021/acs.analchem.5b04491. [DOI] [PubMed] [Google Scholar]
- 35.Sawada Y, Hirai MY. Integrated LC-MS/MS system for plant metabolomics. Comput Struct Biote. 2013;4:1–6. doi: 10.5936/csbj.201301011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eklund PC, Backman MJ, Kronberg LA, Smeds AI, Sjoholm RE. Identification of lignans by Liquid chromatography electrospray ionization ion trap mass spectrometry. J Mass Spectrom. 2008;43:97–107. doi: 10.1002/jms.1276. [DOI] [PubMed] [Google Scholar]
- 37.Struijs K, Vincken JP, Gruppen H. Comparison of atmospheric pressure chemical ionization and electrospray ionization mass spectrometry for the detection of lignans from sesame seeds. Rapid Commun Mass Spectrom. 2008;22:3615–3623. doi: 10.1002/rcm.3777. [DOI] [PubMed] [Google Scholar]
- 38.Niessen WMA, Manini P, Andreoli R. Matrix effect in quantitative pesticide analysis using liquid chromatography mass spectrometry. Mass Spectrom Rev. 2006;25:881–899. doi: 10.1002/mas.20097. [DOI] [PubMed] [Google Scholar]
- 39.Zhao L, Ni Y, Su M, Li H, Dong F, Chen W, Wei R, Zhang L, Guiraud SP, Martin FP, Rajani C, Xie G, Jia W. High Throughput and Quantitative Measurement of Microbial Metabolome by Gas Chromatography/Mass Spectrometry Using Automated Alkyl Chloroformate Derivatization. Anal Chem. 2017;89:5565–5577. doi: 10.1021/acs.analchem.7b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schummer C, Delhomme O, Appenzeller BMR, Robert NR, Wennig R, Millet M. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta. 2009;77:1473–1482. doi: 10.1016/j.talanta.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 41.Kostiainen R, Kauppila TJ. Effect of eluent on the ionization process in liquid chromatography–mass spectrometry. J Chromatogr A. 2009;1216:685–699. doi: 10.1016/j.chroma.2008.08.095. [DOI] [PubMed] [Google Scholar]
- 42.Hua Y, Jenke D. Increasing the Sensitivity of an LC–MS Method for Screening Material Extracts for Organic Extractables via Mobile Phase Optimization. J Chromatogr Sci. 2012;50:213–227. doi: 10.1093/chromsci/bmr049. [DOI] [PubMed] [Google Scholar]
- 43.Xu XM, Huang BF, Xu JJ, Cai ZX, Zhang J, Chen Q, Han JL. Fast and quantitative determination of saxitoxin and neosaxitoxin in urine by ultra-performance liquid chromatography-triple quadrupole mass spectrometry based on the cleanup of solid phase extraction with hydrophilic interaction mechanism. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1072:267–272. doi: 10.1016/j.jchromb.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 44.Novakova L, Havilkova L, Vickova H. Hydrophilic interaction chromatography of polar and ionizable compounds by UHPLC. TrAC. 2014;63:55–64. [Google Scholar]
- 45.Lee HR, Kochhar S, Shim SM. Comparison of Electrospray Ionization and Atmospheric Chemical Ionization Coupled with the Liquid Chromatography-Tandem Mass Spectrometry for the Analysis of Cholesteryl Esters. Int J Anal Chem. 2015;2015:1–6. doi: 10.1155/2015/650927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dar AA, Arumugam N. Lignans of sesame: Purification methods, biological activities and biosynthesis – A review. Bioorg Chem. 2013;50:1–10. doi: 10.1016/j.bioorg.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Chung Suh M, Kim MJ, Hur CG, Bae JM, Park YI, Chung CH, Kang CW, Ohlrogge JB. Comparative analysis of expressed sequence tags from Sesamum indicum and Arabidopsis thaliana developing seeds. Plant Mol Biol. 2003;52:1107–1123. doi: 10.1023/b:plan.0000004304.22770.e9. [DOI] [PubMed] [Google Scholar]
- 48.Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 49.Marsh CB, Wewers MD. The pathogenesis of sepsis. Factors that modulate the response to gram-negative bacterial infection. Clin Chest Med. 1996;17:183–197. doi: 10.1016/s0272-5231(05)70308-7. [DOI] [PubMed] [Google Scholar]
- 50.Majello B, Arcone R, Toniatti C, Ciliberto G. Constitutive and IL-6-induced nuclear factors that interact with the human C-reactive protein promoter. EMBO J. 1990;9:457–465. doi: 10.1002/j.1460-2075.1990.tb08131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houser KR, Johnson DK, Ishmael FT. Anti-inflammatory effects of methoxyphenolic compounds on human airway cells. Journal of Inflammation (London, England) 2012;9:6. doi: 10.1186/1476-9255-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Youn HS, Lee JK, Choi YJ, Saitoh SI, Miyake K, Hwang DH, Lee JY. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol. 2008;75:494–502. doi: 10.1016/j.bcp.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 54.Sieber MA, Hegel JKE. Azelaic Acid: Properties and Mode of Action. Skin Pharmacol Physiol. 2014;27:9–17. doi: 10.1159/000354888. [DOI] [PubMed] [Google Scholar]
- 55.Schulte BC, Wu W, Rosen T. Azelaic Acid: Evidence-based Update on Mechanism of Action and Clinical Application. J Drugs Dermatol. 2015;14:964–968. [PubMed] [Google Scholar]
- 56.Jang SA, Park DW, Kwon JE, Song HS, Park B, Jeon H, Sohn EH, Koo HJ, Kang SC. Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomedicine & Pharmacotherapy Biomedicine & Pharmacotherapy. 2017;96:563–571. doi: 10.1016/j.biopha.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 57.Tang X, Liu J, Dong W, et al. The Cardioprotective Effects of Citric Acid and L-Malic Acid on Myocardial Ischemia/Reperfusion Injury. Evid-Based Complement Altern Med. 2013;2013:1–11. doi: 10.1155/2013/820695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang WC, Tsai TH, Chuang LT, Li YY, Zouboulis CC, Tsai PJ. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: a comparative study with lauric acid. J Dermatol Sci. 2014;2014:232–40. doi: 10.1016/j.jdermsci.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Kato Y, Nagao A, Terao J, Osawa T. Inhibition of Myeloperoxidase-catalyzed Tyrosylation by Phenolic Antioxidants in vitro. Biosci Biotechnol Biochem. 2003;67:1136–1139. doi: 10.1271/bbb.67.1136. [DOI] [PubMed] [Google Scholar]
- 60.Desikan R, Chandrakala AN, Khan B, Rajagopalan S, Parthasarathy S. Myeloperoxidase (MPO): Do We Need Inhibitors? Advances in Biochemistry in Health and Disease-Mechanisms of Vascular Defects in Diabetes Mellitus, PA. 2017:535–571. [Google Scholar]
- 61.Schindhelm Rk, Van der Zwan LP, Teerlink T, Scheffer PG. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin Chem. 2009;55:1462–70. doi: 10.1373/clinchem.2009.126029. [DOI] [PubMed] [Google Scholar]
- 62.Wong ND, Gransar H, Narula J, Shaw L, Moon JH, Miranda-peats R, Rozanski A, Hayes SW, Thomson LE, Rriedman JD, Berman DS. Myeloperoxidase, subclinical atherosclerosis, and cardiovascular disease events. JACC Cardiovasc Imaging. 20091;2:093–9. doi: 10.1016/j.jcmg.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 63.Brown TM, Bittner V. Biomarkers of Atherosclerosis: Clinical Applications. Curr Cardiol Rep. 2008 Nov;10(6):497–504. doi: 10.1007/s11886-008-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu C, Desikan R, Ying Z, Gushchina L, Kampfrath T, Deiuliis J, Wang A, Xu X, Zhong J, Rao X, Sun Q, Maiseyeu A, Parthasarathy S, Rajagopalan S. Effects of a Novel Pharmacologic Inhibitor of Myeloperoxidase in a Mouse Atherosclerosis Model. PLoS One. 2012;7:e50767. doi: 10.1371/journal.pone.0050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.