Abstract
AIM:
The present study aims to assess the therapeutic effect of the combination of topical ozone and steroid therapy in comparison to topical ozone alone versus topical steroid as a control in the management of atrophic - erosive oral lichen planus (OLP).
METHODS:
Sixty-six patients are having atrophic-erosive OLP were included in the study. They were randomly divided into three equal groups to be treated with topical corticosteroids alone (steroid group) as control, topical ozone alone (ozone group) or combination of topical steroids and ozone (combined group). Assessment of pain and sign scores was done before and after each treatment modality.
RESULTS:
The results revealed that the greatest significant percentage of change and subsequent improvement in pain and sign scores were recorded in the combined group.
CONCLUSION:
Reported data in this study using the combination of ozone and steroid therapy could provide a new promising safe and effective adjunct therapy for management of OLP.
Keywords: Steroids, Ozone, Combined, Oral lichen planus
Introduction
Lichen planus is a common chronic mucocutaneous disorder of uncertain cause. It has been reported that it involved about 0.5% to 2.2% of the examined populations worldwide [1].
Oral lichen planus (OLP) can be seen commonly in the fifth and sixth decades of life with a twice predominance in females as compared to males [2] [3]. The exact aetiology and pathogenesis of OLP are undetermined including a cell-mediated immune response associated with degeneration of the basal cell layer of the epithelium [4]. Six clinical forms of OLP lesions are recognised divided into two essential categories either white keratotic (plaque-like, papular, reticular) or white keratotic with red areas (bullous, erosive, atrophic) [5]. Keratotic lesions are asymptomatic with no need of treatment. Meanwhile, red sores are painful and require treatment, in addition to their potential and risk for malignant changes to squamous cell carcinoma mostly occurring in 0.4-2% of cases which should be taken into consideration [6]. OLP is generally observed bilaterally or symmetrically on the buccal mucosa, less regular on the tongue, labial mucosa and gingiva [7].
Multiple and various therapeutic approaches have been discussed in the management of OLP. As there is an alteration in disease activity, the use of a sole and definitive therapeutic modality is challenging. Existing treatment modalities are chiefly concerned with the improvement of the painful symptoms and mucosal ulcerations. The available therapies are still unable to cure the disease completely because of its refractory nature. Various available treatment options for management of OLP include topical and systemic agents. Intralesional and systemic corticosteroids are mainly utilised yet with frequently unsatisfactory outcomes [8].
Corticosteroids still considered the main treatment for symptomatic OLP; however, its prolonged use revealed several adverse effects including atrophy of mucosal tissues with subsequent discomfort, candida overgrowth, adrenal suppression, hypertension, gastrointestinal upset and hyperglycemia. Efficient treatment option with minimal adverse effects still appears to be essential regarding the development of resistance to topical steroids with its inconveniences in some patients [9].
Ozone therapy has gained a prominent consideration in the medical and dental fields due to its strong antimicrobial activity (against bacteria, viruses, yeasts and protozoa) and as a powerful oxidising agent. It is also capable of stimulating the blood circulation and the immune system with reported analgesic effect [10].
Dental applications of ozone included prevention and management of dental caries, teeth remineralisation, control of infection, disinfection of periodontal pockets, teeth bleaching, management of pain accompanying exposed roots and tooth sensitivity, TMJ disorders, endodontic treatment, biofilm removal, enhancement of healing, tissue regeneration and control of halitosis. Ozone therapy is an alternative non-medication therapy that has also been introduced as a treatment option in the management of OLP [11].
Accordingly, this study aimed to evaluate the therapeutic effect of the combination of topical ozone and steroid in comparison to topical ozone alone and topical steroids as a control in the management of atrophic-erosive OLP.
Patients and Methods
This randomised controlled clinical study included sixty-six patients according to the sample size calculation with an age range between 30-70 years.
Power analysis for the 3 study groups were conducted in G* power to determine the sufficient sample size using an alpha of 0.05, a power of 0.80, and large effect size (f = 0.40). Based on the assumptions above, the desired sample size was 66 patients.
Patients were recruited from the outpatient clinic of the Oral Medicine and Periodontology Department, Faculty of Dentistry, Cairo University and from the outpatient clinic of the Skin and Venereal Diseases Department, Faculty of Medicine, Cairo University.
The study protocol was approved by the Medical Ethical Committee of the National Research Centre (NRC) code no. 17 115. After the study procedures were explained before starting the treatment to the patients, they all signed an informed consent form stating their approval. This contemplates conducted on atrophic-erosive OLP patients affecting the tongue or buccal mucosa. Diagnosis of OLP Patients was based on the diagnostic steps criteria approved by the World Health Organization (WHO) [12].
Medical data were collected from the patients according to the Modified Cornel Medical Index questionnaire [13]. Smokers, pregnant or lactating ladies and patients under topical or systemic steroids during the last two months were excluded from the study. Patients using lichenoid reaction-inducing drugs, patients with positive hepatitis C virus (HCV) antibodies, those having systemic diseases that may contribute in the occurrence of OLP such as uncontrolled diabetes and hypertension were not allowed to participate in this study. Patients having amalgam filling adjacent lesions are also not included. All participants in the study groups underwent adequate oral hygiene performance measures with complete removal of plaque and calculus as they implement intraoral inflammation and intensify both extension and symptoms of OLP lesions. Patients were advised to evade accidental trauma on soft tissues using soft bristles toothbrush. Acidic, spicy, hard, hot food and beverages were avoided.
The included 66 patients were randomly assigned, by preoperative envelope drawing, to be treated in the different study groups. The patients were divided into three equal groups. The steroid group (n = 22) as a control in which patients were treated by topical steroid alone. The ozone group (n = 22) in which patients were treated with topical ozone alone. The combined group (n = 22) in which patients were treated with a combination of topical ozone and topical steroid therapy.
Topical steroid therapy involved use of commercially available ointment (triamcinolone acetonide 0.1%, Kenacort-A Orabase®, Turkey) repeated four times per day for four weeks. Topical ozone therapy was done by using an ozone generator. Ozone generator type N 1888A, China was used in the application procedures of gaseous ozone with an ozone rate of 500 mg/hour.
An ozone measuring device was used to confirm the ppm of ozone delivered and a flow meter was used to confirm the flow rate immediately before the start of the treatment. Ozone was applied on the lesions through special disposable glass cups that permitted adequate seal to avoid gas escape which ensured the safety of the machine for human use. No ozone could escape and therefore no ozone smell could be detected which allowed blinding. Ozone was applied intraoral with an intensity of 60% for 1 minute according to the manufacturer instructions in each session twice a week for four weeks. Combined topical ozone and steroids therapy involved both topical ozone application (twice weekly) followed by topical steroid use (four times daily) for four weeks with at least 2 hours interval between topical ozone and steroid application in the day of ozone session as previously mentioned. All the patients in the three groups were followed up weekly during the four weeks.
All cases in the three groups were assessed using the sign scoring scale of Thongprasom et al., 1992 [14] as follows: 5 (white striae with an erosive area > 1 cm2), 4 (white striae with an erosive area < 1 cm2), 3 (white striae with an atrophic area > 1 cm2), 2 (white striae with an atrophic area < 1 cm2), 1 (mild white striae only), and 0 (no lesions, normal mucosa).
Pain assessment for all cases of the study groups was done using grade of pain scale before, during and after different treatments according to Garnick et al., 1998 [15], as follows: grade 0 (no symptoms), grade 1 (mild discomfort and capable of eating), grade 2 (moderate discomfort but still capable of eating), grade 3 (severe discomfort and unable to eat), grade 4 (tolerated pain and unable to eat).
Data were analysed by using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics included mean, median, and standard deviation (SD) values. The paired t-test was used to compare sign scores at baseline and after the end of the treatment within the same group. Friedman test was used to evaluate the difference in the sign and pain scores of lesions throughout the study. The percentage of change was also calculated. Comparison of the percentage of change in the study groups was performed using one-way analysis of variance (ANOVA) test, followed by Tukey’s post hoc test for multiple comparison. Values of p < 0.05 was considered statistically significant.
Results
A total of 66 patients (39 female and 27 males) having atrophic-erosive OLP having an age range from 30-70 years (mean 54.67 ± 4.63 years) were included in the study.
In this contemplate topical ozone application alone and the combination of topical steroid and ozone did not cause any unwanted tissue reactions or complications. It was found that 3 cases in the steroid-treated group complained from oral candidiasis.
No statistically significant difference was shown between mean age values (p = 0.14) and gender distributions (p = 1) in the three groups as presented in Table 1).
Table 1.
Mean age values and gender distributions in the three study groups
| Age | Gender | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Steroid | Ozone | Combined | Steroid | Ozone | Combined | |||||
| Male | Female | Male | Female | Male | Female | |||||
| Mean ± SD | 56.2 ± 5.5 | 53.4 ± 4.2 | 54.4 ± 4.2 | Number (%) | 9 (40.9%) | 13 (60.1%) | 9 (40.9%) | 13 (60.1%) | 9 (40.9%) | 13 (60.1%) |
| F value | 2.028 | X2 | 0 | |||||||
| P (ANOVA) | 0.140 ns | P value (chi square) | 1 ns | |||||||
Significance level p < 0.05; *= significant; ns = non-significant
Within each group, sign scores decreased after treatment. Paired t test revealed that this difference was statistically significant in steroid (p = 0.016), ozone (p = 0.0038) and combined group (p = 0.0004), (Table 2, Figure 1).
Table 2.
Comparison of sign scores expressed as mean ± SD at baseline and after the end of treatment within the same group (paired t-test)
| Groups | Before treatment | After treatment | t value | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Sign score | Pain score | Sign score | Pain score | Sign score | Pain score | Sign score | Pain score | |
| Steroid | 4.6 ±0.55 | 3.6 ±0.55 | 3.6 ±0.55 | 0.8 ±0.45 | -0.4 | -0.14 | 0.016* | 0.0001* |
| Ozone | 4.2 ±1.1 | 3.4 ±0.89 | 3 ±1 | 2.8 ±0.45 | -0.6 | -2.45 | 0.0038* | 0.07 ns |
| Combined | 4.2 ±0.84 | 3.2 ±0.84 | 1.6 ±0.44 | 0.6 ±0.15 | -10.6 | -10.6 | 0.0004* | 0.0004* |
Significance level p<0.05;
=significant; ns=non-significant.
Figure 1.
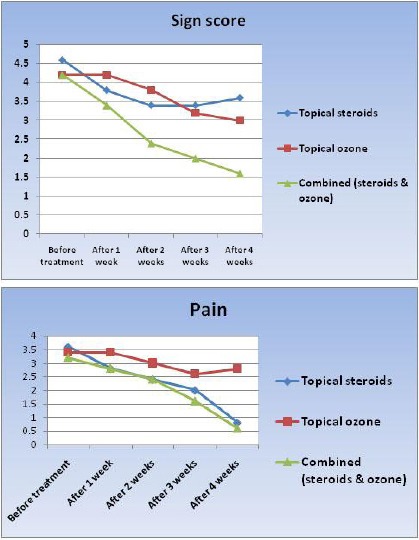
Sign score and pain during the treatment
Within each group, pain scores decreased after treatment. Paired t-test revealed that this difference was statistically significant in steroid (p = 0.0001), and combined group (p = 0.0004). However, the difference between baseline and after treatment values of pain score in ozone group was not statistically significant (p = 0.07), (Table 2, Figure 1).
Comparing the sign and pain scores at baseline and throughout the study at 1, 2, 3 and 4 weeks within the same group using Friedman test it was revealed that a significant difference in each of the 3 groups was obtained (Table 3).
Table 3.
Comparison of sign and pain scores t baseline and after 1, 2, 3 and 4 weeks within the same group (Friedman test)
| Sign score | Pain score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After 1 w | After 2 w | After 3 w | After 4 w | Baseline | After 1 w | After 2 w | After 3 w | After 4 w | ||
| Steroid | Mean ± SD | 4.6 ± 0.55 | 3.8 ± 0.84 | 3.4 ± 0.89 | 3.4 ± 0.89 | 3.6 ± 0.55 | 3.6 ± 0.55 | 2.8 ± 0.45 | 2.4 ± 0.89 | 2 ± 0.71 | 0.8 ± 0.45 |
| Median | 5 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 2 | 1 | |
| Min | 4 | 3 | 2 | 2 | 3 | 3 | 2 | 1 | 1 | 0 | |
| Max | 5 | 5 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 1 | |
| Mean rank | 4.9 | 3.1 | 2.2 | 2.2 | 2.6 | 4.7 | 3.7 | 3.1 | 2.5 | 1 | |
| P value | Chi square = 96.38, P < 0.0001* | Chi square = 106.6, P < 0.0001* | |||||||||
| Ozone | Mean ± SD | 4.2 ± 1.1 | 4.2 ± 1.1 | 3.8 ± 0.84 | 3.2 ± 1.1 | 3 ± 1.0 | 3.4 ± 0.89 | 3.4 ± 0.89 | 3 ± 0.71 | 2.6 ± 0.55 | 2.8 ± 0.45 |
| Median | 5 | 5 | 4 | 4 | 3 | 4 | 4 | 3 | 3 | 3 | |
| Min | 3 | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Max | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | |
| Mean rank | 4.20 | 4.20 | 3.20 | 1.80 | 1.60 | 3.8 | 3.8 | 2.9 | 2.1 | 2.4 | |
| P value | Chi square = 99.79, p < 0.0001* | Chi square = 61.5, p < 0.0001* | |||||||||
| Combine | Mean ± SD | 4.2 ± 0.84 | 3.4 ± 0.55 | 2.4 ± 0.55 | 2 ± 1.0 | 1.6 ± 0.44 | 3.2 ± 0.84 | 2.8 ± 0.45 | 2.4 ± 0.89 | 1.6 ± 0.55 | 0.6 ± 0.15 |
| Median | 4 | 3 | 2 | 2 | 2 | 3 | 3 | 3 | 2 | 1 | |
| Min | 3 | 3 | 2 | 1 | 0 | 2 | 2 | 1 | 1 | 0 | |
| Max | 5 | 4 | 3 | 3 | 3 | 4 | 3 | 3 | 2 | 1 | |
| Mean rank | 4.9 | 4.1 | 2.5 | 2 | 1.5 | 4.6 | 4 | 3.3 | 2 | 1.1 | |
| P value | Chi square = 112.18, p < 0.0001* | Chi square = 110.13, p < 0.0001* | |||||||||
Significance level p<0.05;
=significant; ns=non-significant.
Regarding the sign scores, the greatest percentage of change was noted in the combined group, whereas the least percent of change was recorded in the steroid group. ANOVA test revealed that the difference was statistically significant (p<0.0001). Tukey’s post hoc test revealed a significant difference between every 2 groups (Table 4).
Table 4.
Comparison between groups regarding sign and pain scores percentage of change after treatment (ANOVA test)
| Groups | % of the change in Sign score | % of the change in Pain score |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Steroid | -22.00c ± 2.74 | -78.33a ± 12.64 |
| Ozone | -29.33b ± 8.94 | -15.00b ± 4.69 |
| Combined | -65.00a ± 21.45 | -83.33a ± 15.59 |
| F value | 92.78 | 327.536 |
| p-value | <0.0001* | <0.0001* |
Tukey’s post hoc test: means sharing the same superscript letter are not significantly different
Significance level p<0.05;
=significant; ns=non-significant.
Regarding the pain scores, the greatest percentage of change was also noted in the combined group, whereas the least percentage of change was recorded in the ozone group. ANOVA test revealed that the difference was statistically significant (p<0.0001). Tukey’s post hoc test revealed no significant difference between combined, ozone and steroid groups (Table 4).
Improvement of OLP lesion following combined ozone and steroid therapy reported healing in the area treated as shown in Figure 2.
Figure 2.
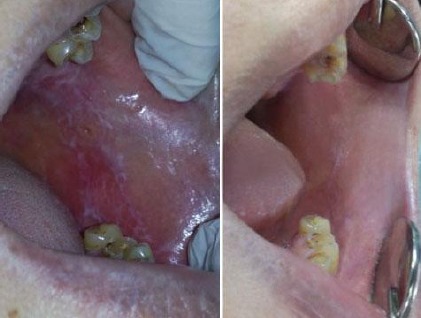
Improvement of OLP lesion following combined ozone and steroid therapy
Discussion
Complete curative management of OLP has not yet accomplished because of the chronic and refractory nature of the disease [16].
The inflammatory and immunologically mediated characters of OLP recommended the use of corticosteroids; thus topical, intralesional and systemic steroids are utilised. Corticosteroids are accepted as a palliative and relieving therapy rather than a therapeutic agent in the management of OLP [17] [18].
In addition to corticosteroids, various interventions have been presented for management of OLP including immunosuppressants (e.g., cyclosporine and tacrolimus), topical or systemic retinoids, and oral metronidazole. Also, various herbal extracts and laser therapy are among the different modalities that have been introduced in the management of OLP. All remedies have been applied in an attempt to improve OLP lesion and associated symptoms such as pain and burning sensation [19] [20].
Topical steroid is considered the first-line effective treatment option for erosive-atrophic OLP with promising outcomes as regarding pain and soreness relief. Several patients encountered various adverse effects with this treatment modality including candidal overgrowth and mucosal atrophy as previously documented [21] [22] [23].
Thus, various randomised clinical trials suggested diverse treatment options in addition to topical steroids as a combination or as a substitute for steroids [24].
New non-medication treatment modalities are suggested including ozone. Ozone has been utilised effectively for the treatment of different disorders for over 100 years. Its special properties incorporate immuno-stimulant, pain relieving, antihypnotic, detoxicating, antimicrobial, bio-energetic and biosynthetic activities with powerful wound healing properties. Ozone is capable of interacting with blood constituents (erythrocytes, platelets, leukocytes, and endothelial cells) and induces oxygen metabolism, cell energy and immuno-modulatory changes. Ozone can enhance the antioxidant defence system and stimulate the microcirculation in tissues [23].
The findings of this study revealed that the sign scores decreased after treatment within the 3 groups where this decrease was statistically significant. This is by the results demonstrated by Kazancioglu and Erisen, 2015 [21].
The outcomes of this study also showed that pain scores decreased after treatment within each group. This difference was statistically significant both in the steroid and combined groups. However, this difference was not statistically significant in the ozone group. This is against the findings previously conducted. This may be due to the difference in the ozone generator used and follow up periods between the studies [21].
Also, the results of this contemplate showed that topical ozone application prevented the candidal overgrowth in ozone and combined groups. This was in line with a study conducted by Arita et al., 2015 who concluded that the use of ozonated water might be useful in oral candidal treatment due to the strong and effective antifungal properties of ozone [24].
The bactericidal, fungicidal, and virucidal properties of ozone may be explained on the basis of its powerful oxidising ability with the creation of free radicals and direct destruction of almost all pathogenic microorganisms. Adding to that, ozone favours tissue healing and increases blood perfusion. It can improve blood flow and immunological reaction. Ozone affects both the cellular and humoral immune responses, oxidises poisons making their discharge simpler, empowers the creation of immunocompetent cells and immunoglobulins, enhances phagocytosis capacity of macrophages, which closures inflammation and fasten tissue healing. Besides, ozone improves the oxygen conveying limit of blood causing better metabolism of inflamed tissues cells and more usage of energy using actuation of aerobic pathways of metabolism. What’s more, oxidant action of ozone helps protein production and upgrades cell ribosomes and mitochondria. Thus, cell action and recovery possibilities will be enhanced with the improvement of the tissue healing process. This might explains the study outcomes reporting the greatest percentage of change which was noted in the combined group in both sign and pain scores [24].
Considering the various beneficial effects obtained from the reported data in this study using the combined ozone and steroid therapy a new promising adjunct therapy might be presented for management of OLP. However, with the limitations of this study many more long-term studies are needed to substantiate the use of this combination.
Accordingly, it could be concluded that topical ozone can be combined with topical steroid therapy as a new more effective and safe treatment modality for symptomatic OLP.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Scully C, Carrozzo M. Oral mucosal disease:lichen planus. Br J Oral Maxillofac Surg. 2008;46(1):15–21. doi: 10.1016/j.bjoms.2007.07.199. https://doi.org/10.1016/j.bjoms.2007.07.199 PMid:17822813. [DOI] [PubMed] [Google Scholar]
- 2.Kini R, Nagaranta DV, Saha A. Therapeutic management of Oral Lichen Planus:a review for the clinicians. World J Dent. 2011;2(3):249–253. https://doi.org/10.5005/jp-journals-10015-1091. [Google Scholar]
- 3.Basma Mostafa, Enji Ahmed. Prevalence of oral lichen planus among a sample of the Egyptian population. J Clin Exp Dent. 2015;7(1):e7–12. doi: 10.4317/jced.51875. https://doi.org/10.4317/jced.51875 PMid:25810846 PMCid:PMC4368022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Ghosh S, Gupta S. Interventions for the management of oral lichen planus:a review of the conventional and novel therapies. Oral Diseases. 2017;23(8):1029–1042. doi: 10.1111/odi.12634. https://doi.org/10.1111/odi.12634 PMid:28055124. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Saimbi CS, Koirala B. Erosive oral lichen planus and its management:A case series. Journal of the Nepal Medical Association. 2008;47(170):86–90. PMid:18709038. [PubMed] [Google Scholar]
- 6.Dissemond J. Oral lichen planus:an overview. Dermatol Treat. 2004;15(3):136–140. doi: 10.1080/09546630410030720. https://doi.org/10.1080/09546630410030720 PMid:15204144. [DOI] [PubMed] [Google Scholar]
- 7.Aghahosseini F, Arbabi-Kalati F, Fashtami LA, Fateh M, Djavid GE. Treatment of oral lichen planus with photodynamic therapy mediated methylene blue:a case report. Medicina Oral, Patología Oral y Cirugía Bucal. 2006;11(2):126–9. [PubMed] [Google Scholar]
- 8.Little J, Falace D, Miller C. Dental management of medically compromised patient. 7th ed. Philadelphia: 2007. pp. 236–245. [Google Scholar]
- 9.Tiwari S, Avinash A, Katiyar S, Iyer AA, Jain S. Dental applications of ozone therapy:A review of literature. The Saudi Journal for Dental Research. 2017;8(1-2):105–11. https://doi.org/10.1016/j.sjdr.2016.06.005. [Google Scholar]
- 10.Mandhare M. Miracle of ozone therapy as an alternative medicine. Int J Pharm Chem Biol Sci. 2012;2(1):63–71. [Google Scholar]
- 11.Ozdemir H, Toker H, Balci H, Ozer H. Effect of ozone therapy on autogenous bone graft healing in calvarial defects:a histologic and histometric study in rats. J Periodontal Res. 2013;48(6):722–726. doi: 10.1111/jre.12060. https://doi.org/10.1111/jre.12060. [DOI] [PubMed] [Google Scholar]
- 12.Brightman VJ. Rational procedure for diagnosis and medical risk assessment. In: Lynch MS, Brightman VJ, Greenberg MS, editors. Bercket's Oral Medicine. 9th ed. Philadelphia: Lippincott Co; 2003. pp. 729–763. [Google Scholar]
- 13.Norton JC, Powell BJ, Penick EC, Sauers CA. Screening alcoholics for medical problems with the Cornell Medical Index. J Stud Alcohol. 1977;38(11):2193–2196. doi: 10.15288/jsa.1977.38.2193. https://doi.org/10.15288/jsa.1977.38.2193 PMid:592838. [DOI] [PubMed] [Google Scholar]
- 14.Thongprasom K, Luangjarmekorn L, Sererat T, Taweesap W. Relative efficacy of fluocinolone acetonide compared with triamcinolone acetonide in treatment of oral lichen planus. J Oral Pathol Med. 1992;21(10):456–458. doi: 10.1111/j.1600-0714.1992.tb00974.x. https://doi.org/10.1111/j.1600-0714.1992.tb00974.x PMid:1460584. [DOI] [PubMed] [Google Scholar]
- 15.Garnick JJ, Singh B, Winkley G. Effectiveness of a medicament containing silicon dioxide, aloe, and allantoin on aphthous stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(5):550–556. doi: 10.1016/s1079-2104(98)90344-4. https://doi.org/10.1016/S1079-2104(98)90344-4. [DOI] [PubMed] [Google Scholar]
- 16.Thongprasom K, Dhanuthai K. Steroid in the treatment of lichen planus:a review. J Oral Sci. 2008;50(4):377–385. doi: 10.2334/josnusd.50.377. https://doi.org/10.2334/josnusd.50.377 PMid:19106464. [DOI] [PubMed] [Google Scholar]
- 17.Amirchaghmaghi M, Delavarian Z, Iranshahi M, Shakeri MT, Mosannen Mozafari P, Mohammadpour AH, Farazi F, Iranshahy M. A randomized placebo-controlled double blind clinical trial of quercetin for treatment of oral lichen planus. J Dent Res Dent Clin Dent Prospects. 2015;9(1):23–28. doi: 10.15171/joddd.2015.005. https://doi.org/10.15171/joddd.2015.005 PMid:25973150 PMCid:PMC4417489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jajarm HH, Falaki F, Sanatkhani M, Ahmadzadeh M, Ahrari F, Shafaee H. A comparative study of toluidine blue mediated photodynamic therapy versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus:a randomized clinical controlled trial. Lasers Med Sci. 2015;30(5):1475–1480. doi: 10.1007/s10103-014-1694-1. https://doi.org/10.1007/s10103-014-1694-1 PMid:25487185. [DOI] [PubMed] [Google Scholar]
- 19.Torti DC, Jorizzo JL, McCarty MA. Oral lichen planus:a case series with emphasis on therapy. Arch Dermatol. 2007;143(4):511–515. doi: 10.1001/archderm.143.4.511. https://doi.org/10.1001/archderm.143.4.511 PMid:17438185. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Moles MA. The use of topical steroids in oral pathology. Med Oral Patol Oral Cir Bucal. 2010;15(6):e827–e831. https://doi.org/10.4317/medoral.15.ne827 PMid:20526258. [PubMed] [Google Scholar]
- 21.Kazancioglu HO, Erisen M. Comparison of low-level laser therapy versus ozone therapy in the treatment of oral lichen planus. Ann Dermatol. 2015;27(5):485–491. doi: 10.5021/ad.2015.27.5.485. https://doi.org/10.5021/ad.2015.27.5.485 PMid:26512161 PMCid:PMC4622881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khatri I, Moger G, Kumar NA. Evaluation of effect of topical ozone therapy on salivary Candidal carriage in oral candidiasis. Indian Journal of Dental Research. 2015;26(2):158. doi: 10.4103/0970-9290.159146. https://doi.org/10.4103/0970-9290.159146 PMid:26096109. [DOI] [PubMed] [Google Scholar]
- 23.Kia SJ, Shirazian S, Mansourian A, Khodadadi FL, Ashnagar S. Comparative efficacy of topical curcumin and triamcinolone for oral lichen planus:a randomized, controlled clinical trial. J Dent. 2015;12(11):789–796. [PMC free article] [PubMed] [Google Scholar]
- 24.Arita M, Nagayoshi M, Fukuizumi T, Okinaga T, Masumi S, Morikawa M, Kakinoki Y, Nishihara T. Microbicidal efficacy of ozonated water against Candida albicans adhering to acrylic denture plates. Oral Microbiol Immunol. 2005;20(4):206e10. doi: 10.1111/j.1399-302X.2005.00213.x. [DOI] [PubMed] [Google Scholar]


