Abstract
Maternal diabetes induces neural tube defects by suppressing neurogenesis in the developing neuroepithelium. Our recent study further revealed that high glucose inhibited embryonic stem cell differentiation into neural lineage cells. However, the mechanism whereby high glucose suppresses neural differentiation is unclear. To investigate whether high glucose-induced oxidative stress and endoplasmic reticulum (ER) stress lead to the inhibition of neural differentiation, the effect of high glucose on neural stem cell (the C17.2 cell line) differentiation was examined. Neural stem cells were cultured in normal glucose (5 mM) or high glucose (25 mM) differentiation medium for 3, 5, and 7 days. High glucose suppressed neural stem cell differentiation by significantly decreasing the expression of the neuron marker Tuj1 and the glial cell marker GFAP and the numbers of Tuj1+ and GFAP+ cells. The antioxidant enzyme superoxide dismutase mimetic Tempol reversed high glucose-decreased Tuj1 and GFAP expression and restored the numbers of neurons and glial cells differentiated from neural stem cells. Hydrogen peroxide treatment imitated the inhibitory effect of high glucose on neural stem cell differentiation. Both high glucose and hydrogen peroxide triggered ER stress, whereas Tempol blocked high glucose-induced ER stress. The ER stress inhibitor, 4-phenylbutyrate, abolished the inhibition of high glucose or hydrogen peroxide on neural stem cell differentiation. Thus, oxidative stress and its resultant ER stress mediate the inhibitory effect of high glucose on neural stem cell differentiation.
Keywords: : neural stem cell, differentiation, neuron, glial cells, high glucose, oxidative stress, endoplasmic reticulum stress
Introduction
High glucose of pregestational maternal diabetes mellitus affects embryonic development by inducing structural birth defects, including neural tube defects (NTDs) and congenital heart defects [1–4]. Studies have demonstrated that either delayed or advanced neurogenesis (neuron differentiation) from the neural stem cells-contained neuroepithelium results in NTDs [5,6]. Maternal diabetes delays neurogenesis in the developing neuroepithelium leading to NTD formation [7]. Our recent study using an in vitro model further demonstrated that high glucose suppressed embryonic stem cell differentiation into neural lineage cells [8]. However, the mechanism underlying high glucose-suppressed neural lineage differentiation from stem cells is still unknown.
During murine neurulation from embryonic day 8.0 (E8.0) to E10.0, the first neuron is differentiated from neural stem cells in the developing neuroepithelium [7]. High glucose specifically affects the ontogeny of neuron during neurulation [7,9]. Thus, the in vitro process of neural stem cell differentiation into neural lineage would closely reflect the in vivo condition. The multipotent C17.2 neural stem cells are newborn mouse cerebellar progenitor cells [10]. When C17.2 cells are transferred into the mouse cerebellum, they are integrated into the cerebellum and differentiated into neurons or glial cells in a manner appropriate to their site of engraftment [10]. Further evidence supports the notion that the C17.2 stem cell line is a suitable model in studying the effect of high glucose on neural differentiation. High glucose mimics maternal diabetes in vivo in inducing oxidative stress and endoplasmic reticulum (ER) stress in C17.2 cells [11]. High glucose of maternal diabetes induces an intracellular redox imbalance involving the overproduction of reactive oxygen species (ROS) and a low antioxidant enzyme capacity [8,12,13]. Oxidative stress mediates the detrimental effects of high glucose on the developing embryo and cultured cells [4,8,14–17]. However, the contribution of oxidative stress to high glucose-suppressed neural differentiation has not yet been evaluated.
The primary function of ER is to correctly fold the newly synthesized proteins into proper three-dimensional structures. Under cellular stress conditions, ER function is perturbed, and unfolded proteins are accumulated leading to the activation of unfolded protein responses (UPRs) and ER stress [18]. Our previous studies have revealed that the major UPR pathways, the inositol-requiring enzyme 1α (IRE1α) pathway and the protein kinase RNA–like ER kinase (PERK), are activated, and ER stress is manifested in the neuroepithelium of embryos exposed to maternal diabetes and high glucose-treated neural stem cells [11,16,17]. Further studies have determined the causal relationship between oxidative stress and ER stress in mediating the adverse effect of high glucose [19]. Thus, ER stress may play an important role in high glucose's inhibition on neural stem cell differentiation.
In the present study, we investigated the effect of high glucose on neural stem cell differentiation and evaluated the contribution of oxidative stress and ER stress to this process. Our study revealed that high glucose suppressed neural stem cell differentiation into neurons and glial cells through oxidative stress and oxidative stress-induced ER stress.
Materials and Methods
Cell lines and culture medium
The C17.2 cell line from the European Collection of Cell Culture is mouse-derived multipotent neural stem cells isolated from the cerebellum and immortalized by the avian myelocytomatosis viral-related myc oncogene transfection [10]. C17.2 cells were maintained in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. All cells were cultured at 37°C in a humidified atmosphere of 5% CO2.
Neural stem cell differentiation
Neural differentiation of C17.2 cells was described previously [19]. Briefly, 1 day after seeding, cells were changed to the differentiation medium (DMEM: F12 medium; Thermo Scientific) supplemented with N2 supplement (Thermo Scientific) with nerve growth factors (10 ng/mL; Cell signaling) and brain-derived neurotrophic factors (10 ng/mL; Cell signaling). The differentiation medium was changed every 3 days. C17.2 cells were treated with normal glucose (5 mM glucose) or high glucose (25 mM glucose). Cells were harvested at different differentiation days for specific analyses. To mimic high glucose-induced oxidative stress, H2O2 (Sigma) was added into the culture medium at final concentration of 5 and 10 μM. Tempol (4-Hydroxy-TEMPO) is a synthetic compound that mimics the antioxidant capability of superoxide dismutase (SOD) enzyme to reduce oxidative stress. Tempol (100 μM) was added into the cells to inhibit high glucose-induced oxidative stress. 4-phenylbutyric acid (4-PBA), which is currently used to treat human urea cycle disorders, is a chemical chaperone that prevents misfolded protein aggregation and alleviates ER stress. In present study, 4-PBA (2 mM) was used to treat cells.
RNA extraction and real-time quantitative PCR
Total RNA was isolated from cells using the TRIzol reagent (Ambion) and reverse transcribed using the QuantiTect Reverse Trancription Kit (Qiagen). Real-time quantitative PCR (RT-qPCR) for GFAP, Tuj1, and β-actin was performed using the Maxima SYBR Green/ROX qPCR Master Mix assay (Thermo Scientific). RT-qPCR and subsequent calculations were conducted on the StepOnePlus™ Real-Time PCR System (Applied Biosystem). Primer sequences used in RT-qPCR were as follows: Tuj1 F, GAATGACCTGGTGTCCGAGT, Tuj1 R, CAGAGCCAAGTGGACTCACA, GFAP F, CACGAACGAGTCCCTAGAGC, GFAP R, TCACATCACCACGTCCTTGT, β-actin F, GTGACGTTGACATCCGTAAAGA, β-actin R, GCCGGACTCATCGTACTCC.
Western blotting analysis
Equal amount of proteins from different experimental groups was separated by sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gel electrophoresis and transferred onto the Immobilon-P membranes (Millipore). Membranes were incubated in 5% nonfat milk for 45 min at room temperature and then incubated for 18 h at 4°C with the following primary antibodies in 5% nonfat milk: the Tuj1 antibody and the GFAP antibody. Membranes were then exposed to the goat anti-rabbit or anti-mouse secondary antibodies. To confirm that equivalent amounts of protein were loaded among samples, membranes were stripped and probed with the mouse antibody against β-actin. Signals were detected using the SuperSignal West Femto Maximum Sensitivity Substrate Kit (Thermo Scientific). All experiments were repeated three times using independently prepared cell lysates. The following antibodies were used in the western blot: Tuj1 (1:1,000) and β-actin (1:5,000) from Abcam and GFAP, BiP, CHOP, p-PERK, PERK, p-IRE1α, IRE1α from the Cell Signaling Technology at dilution of 1:1,000.
Immunostaining
Cells were seeded on four-well chamber slides (Thermo Scientific) at a density of 3 × 105/well. After differentiation, cells were fixed with 4% paraformaldehyde (PFA) for 10 min at room temperature, washed twice with PBS, blocked with 10% normal donkey serum (Sigma-Aldrich) for 1 h and incubated with primary antibodies at 4°C overnight, and followed by Alexa Fluor 594-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) for 1 h at room temperature. Cell nuclei were counterstained by 4′,6-diamidino-2-phenylindole (DAPI; Life Technologies). The number of positively stained cells was quantified by the ImageJ software (NIH, Rockville, MD).
Flow cytometry analysis
One million cells per experimental group were harvested and processed by the Fixation/Permeabilization Kit (BD bioscience). Then, cells were incubated with APC mouse anti-GFAP (BD bioscience) and anti-Tuj1 (Cell signaling) at room temperature for 20 minutes in dark and followed by the incubation with the secondary Alexa Fluor 488 donkey anti-mouse IgG antibody (Jackson ImmunoResearch Laboratories). Cells were washed twice using the staining wash buffer (BD bioscience). APC and FITC Mouse IgG1, K isotype control (BD bioscience) were used to exclude false positive events during fluorescence-activated cell sorting analysis. Samples were analyzed within 1 h. All experiments were performed three times.
TUNEL assay
The TUNEL assay was performed using the ApopTag Fluorescein Direct In Situ Apoptosis Detection Kit (Millipore) as previously described [2,7,9,16,17,20]. Briefly, C17.2 cells cultured in differentiation medium containing NG or HG for 7 days were fixed with 4% PFA in PBS and incubated with TUNEL reaction agents. After TUNEL staining, cells were counterstained with DAPI. TUNEL-positive cells from five random microscopic fields (∼200 cells) were counted. The percentage of TUNEL-positive cells versus DAPI labeled total cells was calculated.
Cell counting and measurement of neurite outgrowth
Cells were counted from the randomly chosen microscopic fields across the culture areas. Tuj1- or GFAP-immunostained and DAPI-counterstained cells were counted in at least five random areas of each culture wells. The ratios of Tuj1+/DAPI cells or GFAP+/DAPI cells were calculated. The group differences were analyzed, and P < 0.05 was considered significant. The data are presented as the mean ± standard error (SE) from three independent experiments.
Cells on the polylysine-coated 4-chamber slides were fixed with PFA and immunostained with the Tuj1 antibody, as described above. The Tuj1+ cells were photographed using the Nikon N1 microscope. The length of primary neurite was defined as the distance from the soma to the tip of the longest branch, and the measurement of neurite length was performed using the ImageJ software (NIH-http//rsb.Info.nih.gov/ij/).
Statistics
Data are presented as mean ± standard error (SE). Each set of experiments was repeated independently at least three times. Statistical differences were evaluated by one-way ANOVA for Figures 1 and 4 using the Sigma Stat 3.5 software or by two-way ANOVA for Figures 2, 3, and 5–7 using SPSS software. For one-way ANOVA, a Tukey test was used to estimate the significance of the data, with P < 0.05 indicative of statistical significance. In the control group (5 mM glucose group), we calculated the ratio of the Tuj1 or GFAP expression levels versus beta-actin levels (the internal control). The mean value and standard deviation were computed from the three values of the ratios generated in three independent repeated experiments. In the experimental groups, the mean value and standard deviation from three repeated experiments were calculated with the same way as described for the control group. After that, the values in all groups were normalized to the control (the 5 mM glucose group); therefore, the value in the control group was set to 1 with the error bars.
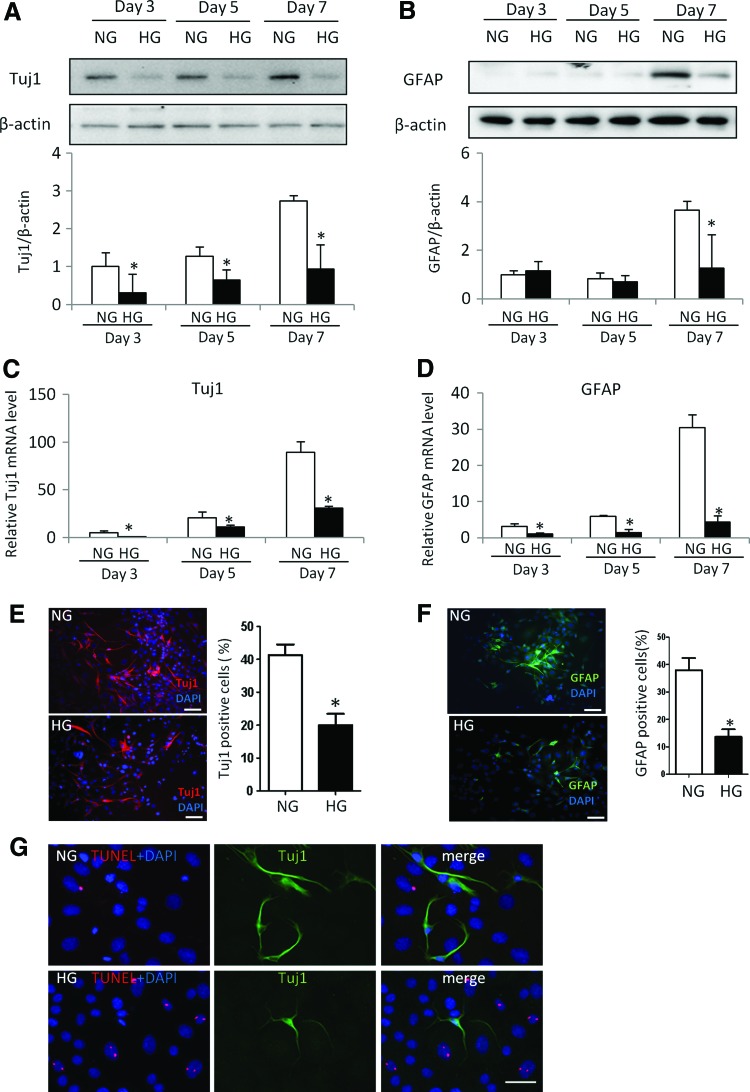
FIG. 1.
High glucose inhibits neural stem cell differentiation. C17.2 cells were treated by NG (5 mM) or HG (25 mM) in neural differentiation medium for 3, 5, and 7 days. Protein levels of Tuj1 (A) and GFAP (B) determined by immunoblotting. The quantification of the data was shown in the bar graph. mRNA level of Tuj1 (C) and GFAP (D) determined by RT-qPCR. Immunostaining of Tuj1 (E) or GFAP (F) in cells treated by normal glucose or high glucose and quantification for numbers of Tuj1 or GFAP positive cells. C17.2 cells cultured in differentiation medium for 7 days and dual-labeled with TUNEL and Tuj1 (G). Bars = 100 μm for (E, F); 50 μm for (G). Experiments were repeated three times (n = 3). Values are the mean ± SE from three separate experiments. *Indicates significant differences (P < 0.05) compared to the normal glucose (5 mM) group. HG, high glucose; NG, normal glucose; RT-qPCR, real-time quantitative PCR. Color images available online at www.liebertpub.com/scd
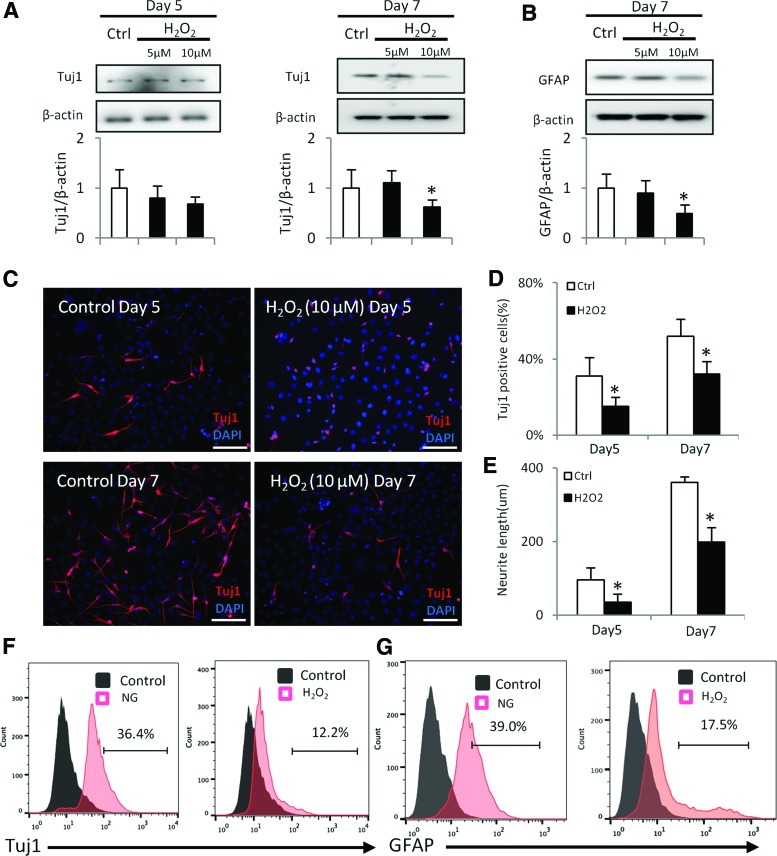
FIG. 4.
H2O2 inhibits neural differentiation. H2O2 was added into differentiating C17.2 cells for 5 or 7 days. Protein levels of Tuj1 (A) and GFAP (B) determined by immunoblotting. The quantification of the data was shown in the bar graph below. (C) Immunostaining of Tuj1 in the NG and NG plus H2O2 groups. Bars = 100 μm. (D) Quantification of Tuj1 positive cell number. (E) Quantification of the neurite length of neurons. Flow cytometry analysis on Tuj1+ and GFAP+ cells (F, G). Experiments were repeated three times (n = 3). Values are the mean ± SE from three separate experiments. *Indicates significant differences (P < 0.05) compared to the normal glucose (5 mM) groups. Color images available online at www.liebertpub.com/scd
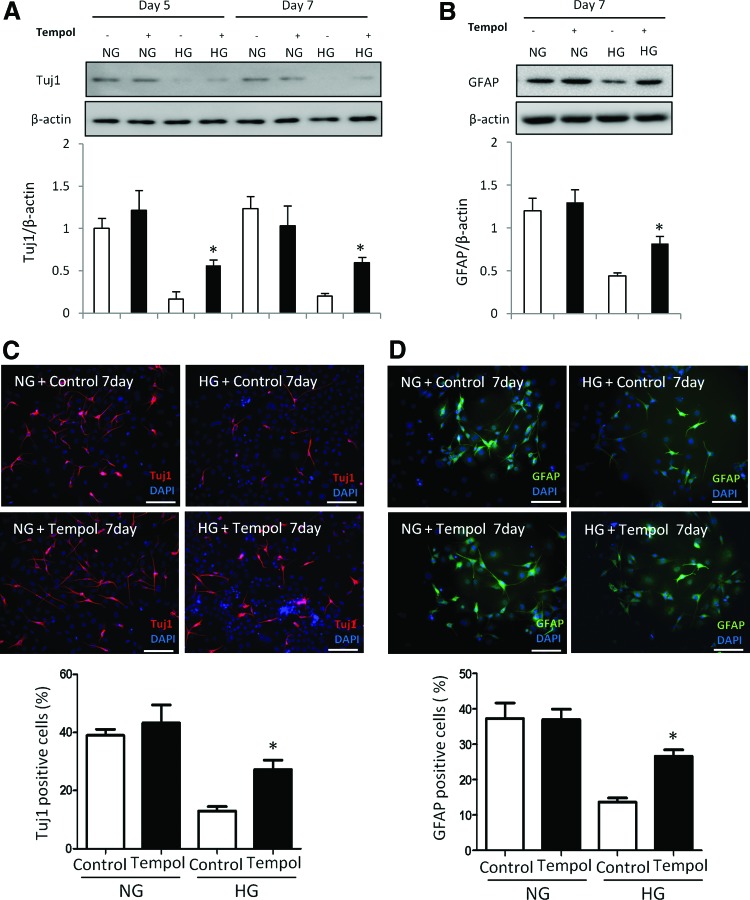
FIG. 2.
Oxidative stress mediates the inhibitory effect of high glucose on neural differentiation. An antioxidant reverses high glucose-inhibited neural differentiation. C17.2 cells were differentiated at NG (5 mM) or HG (25 mM), with or without Tempol (100 μM) for 5 and 7 days. Tempol stock solution (100 mM) was prepared by dissolving in water. During cell differentiation, Tempol was added to the differentiating C17.2 cells at a final concentration of 100 μM. Same volume of vehicle was added into the controls. Protein levels of Tuj1 (A) and GFAP (B) determined by immunoblotting. The quantification of the data was shown in the bar graph. Immunostaining of Tuj1 (C) or GFAP (D) in the NG, NG plus Tempol, HG, HG plus Tempol groups and quantification for numbers of Tuj1 or GFAP positive cells. Bars = 100 μm. All experiments were repeated three times (n = 3). Values are the mean ± SE from three separate experiments. *Indicates significant differences (P < 0.05) compared to the normal glucose (5 mM) group. Color images available online at www.liebertpub.com/scd
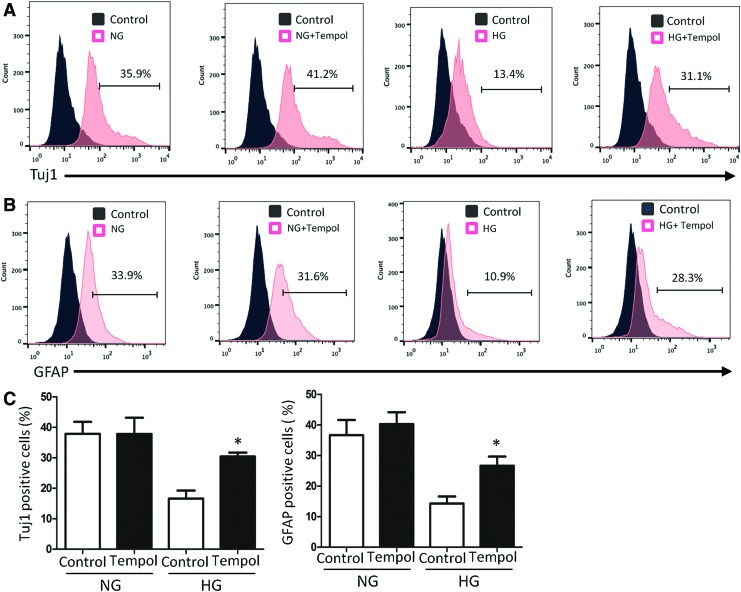
FIG. 3.
FACS analysis indicates Tempol blockage on high glucose-induced neural cell differentiation. Tuj1 frequency (A) and GFAP frequency (B) in the NG, NG plus Tempol, HG, and HG plus Tempol groups were analyzed with FACS. (C) Quantification of Tuj1 frequency and GFAP frequency for each group. The data are presented as mean ± SE from three independent experiments. *Indicates significant differences (P < 0.05) compared to the control group (NG+Tempol). FACS, fluorescence-activated cell sorting. Color images available online at www.liebertpub.com/scd
FIG. 5.
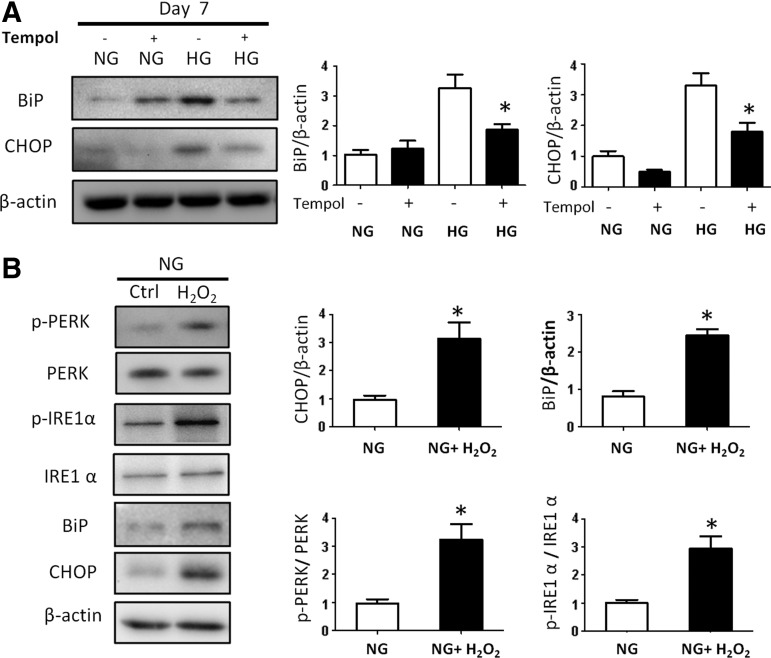
High glucose induces ER stress through oxidative stress. (A) Protein levels and quantification data of BiP and CHOP in the NG (5 mM), NG with Tempol, HG (25 mM), and HG with Tempol groups. (B) Protein levels and quantification data of BiP, CHOP, p-PERK, PERK, p-IRE1α, and IRE1α in the NG and NG plus H2O2 groups. Experiments were repeated three times (n = 3). Values are the mean ± SE from three separate experiments. *Indicates significant differences (P < 0.05) compared to the normal glucose (5 mM) groups.
FIG. 6.
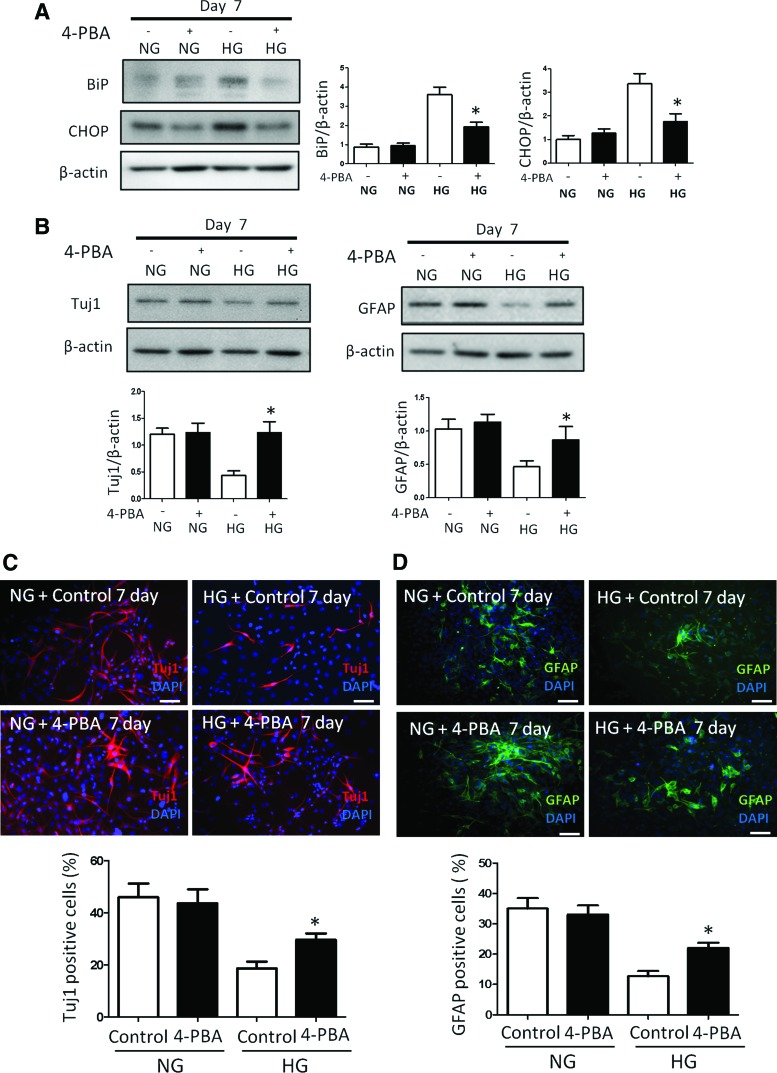
Blockage of ER stress by 4-PBA restores neural differentiation suppressed by high glucose. (A) High glucose increases the levels of ER stress marker BiP and CHOP during C17.2 differentiation. (B) 4-PBA alleviates ER stress and, subsequently, blocks high glucose-inhibited neural cell differentiation. Immunostaining of Tuj1+ neurons (C) and GFAP+ glia cells (D) in the NG, NG plus 4-PBA, HG, and HG plus 4-PBA groups. Bars = 100 μm. All experiments were repeated three times (n = 3). Values are the mean ± SE from three separate experiments. *Indicates significant differences (P < 0.05) compared to the normal glucose (5 mM) group. 4-PBA, 4-phenylbutyric acid; ER, endoplasmic reticulum. Color images available online at www.liebertpub.com/scd
FIG. 7.
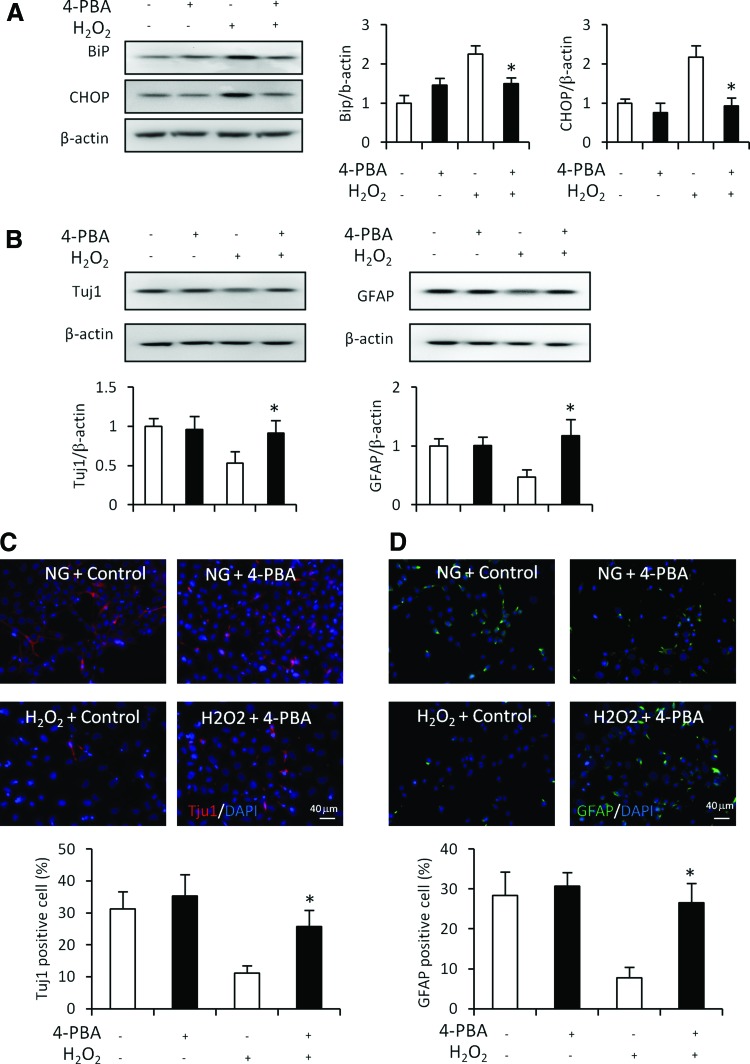
Blockage of ER stress by 4-PBA restores neural differentiation suppressed by hydrogen peroxide. Protein levels of BiP, CHOP (A) and Tuj1 (B) in the control group (normal glucose, 5 mM), 4-PBA group, H2O2 group (H2O2, 10 μM), and 4-PBA plus H2O2 groups. The quantification of the data was shown in the bar graph. Immunostaining of Tuj1 (C) or GFAP (D) in C17.2 cell treated with 4-PBA or H2O2 and quantification for numbers of Tuj1 or GFAP positive cells. Bars = 40 μm. All experiments were repeated three times (n = 3). Values are the mean ± SE from three separate experiments. *Indicates significant differences (P < 0.05) compared to the control (5 mM) group. Color images available online at www.liebertpub.com/scd
Results
High glucose inhibits neural stem cell differentiation
C17.2 cell can differentiate into either neuron or glial cells. Under normal glucose conditions, the expression of the neural marker Tuj1 and the glial cell marker GFAP was robustly detected at day 3 and day 7 (Fig. 1A, B) of differentiation. The comparison of Tuj1 or GFAP protein levels in cells cultured in normal glucose (NG) medium and high glucose (HG) medium indicated that high glucose significantly inhibited both Tuj1 and GFAP expression (Fig. 1A, B). Consistently, mRNA levels of Tuj1 and GFAP gradually increased during the course of neural differentiation under normal glucose conditions, whereas high glucose significantly inhibited Tuj1 and GFAP mRNA expression (Fig. 1C, D). Furthermore, high glucose decreased the numbers of Tuj1 positive neurons and GFAP positive glial cells (Fig. 1E, F). Collectively, high glucose suppresses the differentiation of neural stem cells into neurons and glial cells.
C17.2 cells cultured in NG or HG differentiation medium for 7 days were dual-labeled with TUNEL and Tuj1 immunostaining, which showed that HG triggered cell apoptosis and that apoptosis occurred in the nondifferentiated cells, and no apoptosis was observed in differentiated Tuj1+ cells (Fig. 1G), suggesting that neural progenitor cells are more sensitive to HG-induced oxidative stress. Because Tuj1+ and GFAP+ cells were counted as percentages against total cell numbers at time of measurement, it appears that apoptosis in nondifferentiated cells does not contribute to the impaired neural lineage differentiation.
An antioxidant blocks the inhibitory effect of high glucose on neural stem cell differentiation
Our previous studies have demonstrated that maternal diabetes in vivo and high glucose in vitro trigger a sustained generation of ROS, leading to intracellular oxidative stress that contributes to the adverse effects of high glucose on neural development [3,4,9,14,21,22]. To explore whether high glucose-induced oxidative stress mediates the inhibitory effect of high glucose on neural differentiation, C17.2 cells were treated with the SOD mimetic Tempol. Tempol did not affect Tuj1 and GFAP expression under normal glucose conditions (Fig. 2A, B). However, Tempol treatment partially restored high glucose-suppressed Tuj1 and GFAP protein levels (Fig. 2A, B). Moreover, the numbers of Tuj1 positive neurons and GFAP positive glial cells were restored to the levels of those in the normal glucose conditions when Tempol was added into the high glucose group (Fig. 2C, D). Thus, these findings indicate that high glucose inhibits neural differentiation through oxidative stress.
To further analyze the effect of Tempol on neural stem cell differentiation, flow cytometry analysis was used to determine the number of differentiated cells after treatment with high glucose and Tempol. As expected, the frequencies of both Tuj1 positive and GFAP positive cells were decreased by high glucose (Fig. 3A–C). Tempol treatment reversed the reduction of both Tuj1 positive and GFAP positive cell numbers under high glucose conditions (Fig. 3A–C). Together, these data suggested that high glucose mediates suppression of neural differentiation through oxidative stress.
Hydrogen peroxide inhibits neural stem cell differentiation
To test whether ROS inhibits neural differentiation, C17.2 cells were treated with H2O2. Although H2O2 at a low concentration (5 μM) did not affect Tuj1 and GFAP expression (Fig. 4A, B), 10 μM H2O2 treatment significantly decreased the protein levels of Tuj1 and GFAP in Day 7 of differentiation (Fig. 4A, B).
Immunostaining revealed that the Tuj1-positive cell number in the H2O2 group was significantly lower than that in control group (Fig. 4C, D). In addition, the neurite length in the H2O2 group was significantly shorter than that in the control group (Fig. 4E). Flow cytometry analysis further revealed that both Tuj1+ and GFAP+ cell numbers were significantly reduced after H2O2 treatment (Fig. 4F, G). Thus, H2O2 mimics the inhibitory effect of high glucose on neural stem cell differentiation.
Oxidative stress-induced ER stress suppresses neural stem cell differentiation
Increased ROS in embryos exposed to maternal diabetes causes an accumulation of misfolded proteins in the ER lumen leading to ER stress [3,4]. To investigate whether ER stress affects the process of neural differentiation, several ER stress markers were evaluated. High glucose significantly increased the ER stress markers, CHOP and BiP (Fig. 5A). Tempol inhibited high glucose-induced CHOP and BiP expression (Fig. 5A). H2O2 mimicked high glucose in inducing CHOP and BiP expression and activated the two UPR pathways by inducing phosphorylation of IRE1α and PERK (Fig. 5B).
The ER stress inhibitor 4-PBA reduced high glucose-induced CHOP and BiP expression (Fig. 6A). Tuj1 expression suppressed by high glucose was restored by 4-PBA (Fig. 6B). 4-PBA treatment under high glucose conditions resulted in comparable numbers of Tuj1+ and GFAP+ cells than those in normal glucose conditions (Fig. 6C, D). Collectively, these data may suggest that oxidative stress-induced ER stress mediates the inhibitory effect of high glucose on neural stem cell differentiation.
To determine whether oxidative stress suppresses neural differentiation through ER stress, the ROS, hydrogen peroxide, was used to induce oxidative stress. Hydrogen peroxide increased the expression of ER stress markers CHOP and BiP and suppressed the expression of Tuj1 and GFAP during differentiation (Fig. 7). The ER stress inhibitor 4-PBA treatment abrogated hydrogen peroxide-suppressed Tuj1 and GFAP expression (Fig. 7). Furthermore, the decreased numbers of Tuj1+ and GFAP+ cells by hydrogen peroxide were restored by 4-PBA treatment (Fig. 7C, D).
Discussion
In the present study, we revealed that high glucose suppressed neural stem cell differentiation into neurons and glial cells. This finding is consistent with previous in vivo demonstration that maternal diabetes inhibits neural stem cell differentiation in the developing neuroepithelium during neurulation leading to the failure of neural tube closure [9,17,19]. Our data demonstrated that high glucose exerts an inhibitory effect on neural stem cell differentiation.
Derived from the external germinal layer of a neonatal mouse cerebellum and immortalized through retroviral transduction with v-myc, C17.2 cells retain the characteristic of multipotent neural stem cells and can be used to mimic the developing neuroepithelium [10]. In vivo, C17.2 cells can differentiate into neuron and astrocytes [19]. Consisting with the preexisting data, we confirmed that C17.2 cells could differentiate into neuron or glial cell in the medium containing 5 mM or 25 mM glucose, which equals to the normal blood glucose level in nondiabetics and the high blood glucose level in diabetic patients, respectively. The present study demonstrated that high glucose inhibits neural stem cell differentiation into neurons and glial cells through oxidative stress.
Studies have revealed that maternal diabetes in vivo or high glucose in vitro induces oxidative stress in neural stem cells [9,11,14,17,19,21–24]. High glucose increases the production of ROS and impairs endogenous antioxidant enzymes, leading to oxidative stress [12]. Increased glucose flux disrupts mitochondrial function by impairing the mitochondrion integrity and, thus, increases mitochondrial ROS. It is reasoned that high levels of ROS cause stem cell dysfunction, whereas low basal levels of ROS may be beneficial for stem cells in maintaining their pluripotency and differentiation potentials [25]. In mesenchymal stem cells (MSCs), excess ROS or treatment with exogenous H2O2 impairs their differentiation into osteogenic lineage [26]. In agreement with the findings in MSC osteogenic differentiation [26], the present study found that the SOD mimetic, Tempol, reversed the inhibitory effect of high glucose on neural stem cell differentiation. H2O2 mimicked high glucose in suppressing neural stem cell differentiation. Chou et al. [27] also provided evidence in supporting the hypothesis that oxidative stress suppresses neural differentiation. Chou's study demonstrated that arsenite-induced oxidative stress arrested neuronal differentiation in mouse neuroblastoma N2a cells [27].
ER stress triggers the UPR pathways. Persistent UPR activation during prolonged ER stress results in cellular dysfunction, whereas transient UPR activation can resolve ER stress and restore cellular homeostasis. Kawada et al. demonstrated that the ER stress inducer tunicamycin causes aberrant neuronal differentiation from neural stem cells [28]. Our previous studies have demonstrated that maternal diabetes in vivo or high glucose in vitro induces prolonged ER stress and persistent UPR activation in neural stem cells [11]. The present study reveals that during neural stem cell differentiation, both high glucose and H2O2 induce ER stress markers, BiP and CHOP, leading to UPR activation manifested by increased phosphorylation of IRE1α and PERK. The ER stress inhibitor, 4-PBA, blocks the inhibitory effect of high glucose and H2O2 on neural stem cell differentiation. These evidences suggest that high glucose- or H2O2-induced ER stress and UPR are prolonged or excessive and, thus, exert adverse effects on neural differentiation. Indeed, ER stress-induced UPR stimulates endodermal differentiation but suppresses the other two germ layer commitment in ESC-derived embryoid bodies [29], supporting our findings that UPR suppresses neural differentiation.
Previous studies have established the causal link between high glucose-induced oxidative stress and ER stress [11]. The present study showed that the SOD mimetic Tempol blocked high glucose-induced ER stress and UPR. In addition, H2O2 triggered ER stress and UPR. This evidence further reinforces the causal role of oxidative stress in ER stress and UPR induction under high glucose conditions. The oxidative stress-ER stress-UPR pathway mediates the inhibitory effect of high glucose on neural stem cell differentiation.
The findings in the present studies well corroborate with our previous in vivo studies. Neurons and glial cells are two major cell types in the central neural system. Previous studies have shown that delayed [5], advanced [6], and inhibited [30] differentiation of neural stem cells in the developing neuroepithelium leads to NTDs. High glucose of maternal diabetes in vivo delays the expression of Tuj1 positive neurons in the developing neuroepithelium [7]. In the present study, we found that in vitro neural stem cell differentiation into neurons and glial cells was significantly impaired by high glucose.
In summary, our data indicate that oxidative stress and ER stress are responsible for high glucose inhibition on neural stem cell differentiation. High glucose induces severe ER stress and persistent UPR through oxidative stress. Antioxidants and ER stress inhibitors are effective in reversing impaired neural stem cell differentiation under high glucose conditions.
Acknowledgments
This research is supported by NIH R01DK083243, R01DK101972, R01HL131737, R01HL134368, R01HL139060, and R01DK103024.
Author Disclosure Statement
None of the authors have a conflict of interest.
References
- 1.Dong D, Reece EA, Lin X, Wu Y, AriasVillela N. and Yang P. (2016). New development of the yolk sac theory in diabetic embryopathy: molecular mechanism and link to structural birth defects. Am J Obstet Gynecol 214:192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabbay-Benziv R, Reece EA, Wang F. and Yang P. (2015). Birth defects in pregestational diabetes: defect range, glycemic threshold and pathogenesis. World J Diabetes 6:481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang F, Reece EA. and Yang P. (2015). Advances in revealing the molecular targets downstream of oxidative stress-induced proapoptotic kinase signaling in diabetic embryopathy. Am J Obstet Gynecol 213:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang P, Reece EA, Wang F. and Gabbay-Benziv R. (2015). Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. Am J Obstet Gynecol 212:569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lupu F, Alves A, Anderson K, Doye V. and Lacy E. (2008). Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Dev Cell 14:831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishibashi M, Ang SL, Shiota K, Nakanishi S, Kageyama R. and Guillemot F. (1995). Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev 9:3136–3148 [DOI] [PubMed] [Google Scholar]
- 7.Xu C, Li X, Wang F, Weng H. and Yang P. (2013). Trehalose prevents neural tube defects by correcting maternal diabetes-suppressed autophagy and neurogenesis. Am J Physiol Endocrinol Metab 305:E667–E678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Wu Y. and Yang P. (2016). High glucose-induced oxidative stress represses sirtuin deacetylase expression and increases histone acetylation leading to neural tube defects. J Neurochem 137:371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang P, Li X, Xu C, Eckert RL, Reece EA, Zielke HR. and Wang F. (2013). Maternal hyperglycemia activates an ASK1-FoxO3a-caspase 8 pathway that leads to embryonic neural tube defects. Sci Signal 6:ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA. and Cepko CL. (1992). Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 68:33–51 [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Wu Y, Gu H, Reece EA, Fang S, Gabbay-Benziv R, Aberdeen G. and Yang P. (2015). Ask1 gene deletion blocks maternal diabetes-induced endoplasmic reticulum stress in the developing embryo by disrupting the unfolded protein response signalosome. Diabetes 64:973–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong J, Xu C, Gabbay-Benziv R, Lin X. and Yang P. (2016). Superoxide dismutase 2 overexpression alleviates maternal diabetes-induced neural tube defects, restores mitochondrial function and suppresses cellular stress in diabetic embryopathy. Free Radic Biol Med 96:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong D, Reece EA. and Yang P. (2016). The Nrf2 activator vinylsulfone reduces high glucose-induced neural tube defects by suppressing cellular stress and apoptosis. Reprod Sci 23:993–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong D, Yu J, Wu Y, Fu N, Villela NA. and Yang P. (2015). Maternal diabetes triggers DNA damage and DNA damage response in neurulation stage embryos through oxidative stress. Biochem Biophys Res Commun 467:407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Reece EA. and Yang P. (2015). Oxidative stress is responsible for maternal diabetes-impaired transforming growth factor beta signaling in the developing mouse heart. Am J Obstet Gynecol 212:650.e1–650.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y, Wang F, Fu M, Wang C, Quon MJ. and Yang P. (2015). Cellular stress, excessive apoptosis, and the effect of metformin in a mouse model of Type 2 diabetic embryopathy. Diabetes 64:2526–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Weng H, Xu C, Reece EA. and Yang P. (2012). Oxidative stress-induced JNK1/2 activation triggers proapoptotic signaling and apoptosis that leads to diabetic embryopathy. Diabetes 61:2084–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ron D. and Walter P. (2007). Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529 [DOI] [PubMed] [Google Scholar]
- 19.Lundqvist J, El Andaloussi-Lilja J, Svensson C, Gustafsson Dorfh H. and Forsby A. (2013). Optimisation of culture conditions for differentiation of C17.2 neural stem cells to be used for in vitro toxicity tests. Toxicol In Vitro 27:1565–1569 [DOI] [PubMed] [Google Scholar]
- 20.Li X, Xu C. and Yang P. (2013). C-Jun NH2-terminal kinase 1/2 and endoplasmic reticulum stress as interdependent and reciprocal causation in diabetic embryopathy. Diabetes 62:599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu H, Yu J, Dong D, Zhou Q, Wang JY. and Yang P. (2015). The miR-322-TRAF3 circuit mediates the pro-apoptotic effect of high glucose on neural stem cells. Toxicol Sci 144:186–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu H, Yu J, Dong D, Zhou Q, Wang JY, Fang S. and Yang P. (2016). High glucose-repressed CITED2 expression Through miR-200b triggers the unfolded protein response and endoplasmic reticulum stress. Diabetes 65:149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine EL, Horal M, Chang TI, Fortin G. and Loeken MR. (1999). Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes 48:2454–2462 [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Xu C, Reece EA, Li X, Wu Y, Harman C, Yu J, Dong D, Wang C, et al. (2017). Protein kinase C-alpha suppresses autophagy and induces neural tube defects via miR-129-2 in diabetic pregnancy. Nat Commun 8:15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denu RA. and Hematti P. (2016). Effects of oxidative stress on mesenchymal stem cell biology. Oxid Med Cell Longev 2016:2989076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen CT, Shih YR, Kuo TK, Lee OK. and Wei YH. (2008). Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26:960–968 [DOI] [PubMed] [Google Scholar]
- 27.Chou CT, Lin HT, Hwang PA, Wang ST, Hsieh CH. and Hwang DF. (2015). Taurine resumed neuronal differentiation in arsenite-treated N2a cells through reducing oxidative stress, endoplasmic reticulum stress, and mitochondrial dysfunction. Amino Acids 47:735–744 [DOI] [PubMed] [Google Scholar]
- 28.Kawada K, Iekumo T, Saito R, Kaneko M, Mimori S, Nomura Y. and Okuma Y. (2014). Aberrant neuronal differentiation and inhibition of dendrite outgrowth resulting from endoplasmic reticulum stress. J Neurosci Res 92:1122–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu H, Tsang KS, Wang Y, Chan JC, Xu G. and Gao WQ. (2014). Unfolded protein response is required for the definitive endodermal specification of mouse embryonic stem cells via Smad2 and beta-catenin signaling. J Biol Chem 289:26290–26301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson ES, Waller LE. and Kroll KL. (2014). Geminin loss causes neural tube defects through disrupted progenitor specification and neuronal differentiation. Dev Biol 393:44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]