Abstract
Most αβT-cells that form in the thymus are generated during mainstream conventional thymocyte development and involves the generation and selection of a diverse αβTCR repertoire that recognises self-peptide/MHC complexes. Additionally, the thymus also supports the production of T-cell subsets that express αβTCRs but display unique developmental and functional features distinct from conventional αβT-cells. These include multiple lineages of CD1d-restricted iNKT-cells that express an invariant αβTCR, branch off from mainstream thymocytes at the CD4+CD8+ stage, and are potent producers of polarising cytokines. Importantly, and despite their differences, iNKT-cells and conventional αβT-cells share common requirements for thymic epithelial microenvironments during their development. Moreover, emerging evidence suggests that constitutive cytokine production by iNKT-cells influences both conventional thymocyte development and the intrathymic formation of additional innate CD8+ αβT-cells with memory-like properties. Here, we review evidence for an intrathymic innate-lymphocyte network, in which iNKT-cells play key roles in multiple aspects of thymus function.
Introduction
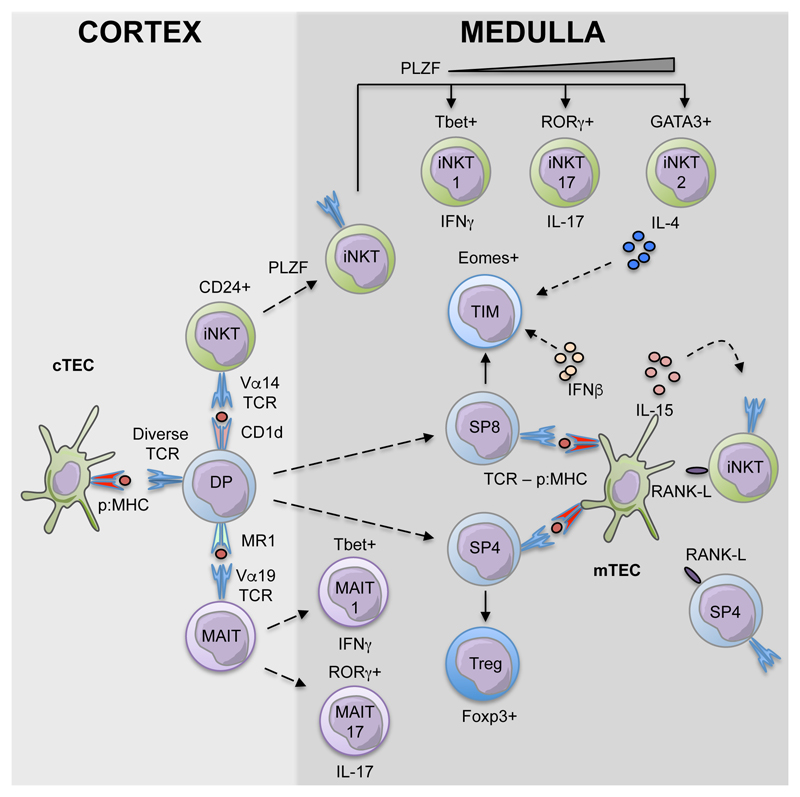
The thymus is a primary lymphoid organ that is specialised in its ability to support T-cell development. As the thymus contains no long-term haemopoietic stem cell populations, T-cell development depends upon the continuous importation of lymphoid progenitors from the bone marrow via the circulation (1, 2). While T-cell development represents a complex and multi-stage process, it can be simplified and measured by defined changes in cell surface phenotype that take place in developing thymocytes. Such a developmental program is perhaps most readily evident from analysis of conventional αβT-cell development. For example, early T-cell progenitors that lack expression of CD4 and CD8 undergo maturation into CD4+CD8+ intermediates, which is then followed by the generation of both MHC class I restricted CD8+ and MHC class II restricted CD4+ αβT-cells that represent essential cellular components in immune responses to invading pathogens (3, 4). Importantly, analysis of the stages in conventional αβT-cell development in relation to their positioning within intrathymic microenvironments has uncovered important information about the roles of defined thymic stromal cells in this process. Thus, development of cortex-resident CD4-CD8- and CD4+CD8+ thymocytes involves signals from cortical thymic epithelial cells (cTEC), while in the medulla interactions between CD4+ and CD8+ single positive thymocytes with medullary thymic epithelial cells (mTEC) are important (5, 6).
Collectively, these observations fit well with the idea that anatomical compartmentalisation within the thymus exists to support step-wise stages in conventional αβT-cell development, which is further supported by conventional αβT-cells being the dominant lineage produced during thymopoiesis. Interestingly however, the thymus also supports the development of other αβT-cell lineages that branch off from mainstream conventional thymocytes yet retain the requirement for particular thymic microenvironments for their development. For example, CD4+CD8+ thymocytes expressing the Vα14+ invariant αβTCR that recognise glycolipid/CD1d complexes represent progenitors of invariant NKT-cells (iNKT-cells) (7), with accumulating evidence indicating that these cells require and influence medullary thymic microenvironments (8–10). In this review, we summarise the role of the thymus medulla in αβT-cell development, focussing in particular on emerging evidence that indicates the importance of interplay between innate and adaptive αβT-cells within this site.
Intrathymic Selection Of Innate and Adaptive αβT-cells
Following low affinity αβTCR engagement in the cortex, positively selected CD4+CD8+ thymocytes undergo a program differentiation and guided migration, resulting in the generation of CD4+ and CD8+ thymocytes that reside in medullary thymic regions. The migration relies upon chemokine ligand responsiveness, typified by thymocyte upregulation of CCR7 and migration towards CCL21 produced by mTEC (11). Notably, entry of conventional αβT-cells to the medulla drives several key developmental processes, including mechanisms of central tolerance prior to T-cell export into peripheral tissues.
In addition to the clonal deletion of potentially autoreactive T-cell clones via the combined action of mTEC and dendritic cells (DC), the thymus medulla supports regulatory T-cell (T-Reg) development (12). Such intrathymic skewing of CD4+ αβT-cells towards the T-Reg lineage is associated with the upregulation of Foxp3 and acquisition of suppressive functions (13). The acquisition of effector function by T-Reg prior to thymic export stands in contrast to the process for conventional αβT cells. While conventional αβT cells undergo a process of progressive maturation during their medullary residency, associated with a gain in proliferative response to TCR triggering and capacity for cytokine secretion (14, 15), they are exported from the thymus in a naïve ‘vanilla’ state, only gaining specific effector function following peripheral T-cell priming. Whilst thymic T-Reg are arguably the most well defined subset of intrathymically generated diverse αβT-cells that acquire functional lineage specification prior to thymic exit, the thymic medulla also represents a critical developmental locale for the formation of additional ‘natural’ T-cell subsets including thymus-dependent RORγt+ CD4+ Th17 and Eomesodermin+ CD8+ memory-like T-cells (16–18), discussed below. The significance of pre-programming T-cell subsets prior to thymic exit likely corresponds with the ability of such subpopulations to rapidly exert effector functions following peripheral stimulation in an innate-like fashion. However, that the majority of TCR-diverse conventional αβT-cells exit the thymus in a base, naïve state presumably highlights the functional importance of possessing flexibility in effector function, which allows an effective T-cell response to be tailored towards defined pathogenic challenges. The beneficial nature of this process likely outweighs the negative impact of the time-lag associated with peripheral T-lineage effector programming, with this drawback at least in part being balanced by the rapid activity of both innate and innate-like systems of immunity, including αβT-cells that undergo naturally-acquired effector lineage-specification prior to thymus egress.
In addition to supporting the maturation of diverse, adaptive αβT-cells, the thymus supports the development of innate-like αβT-cells, including invariant natural killer T-cells (iNKT) and mucosal-associated invariant T cells (MAIT). Inline with conventional αβT-cells, iNKT and MAIT cells undergo positive selection within cortical compartments of the thymus. However, in contrast to positive selection of conventional αβT-cells by self-peptide/MHC complexes on cTEC, iNKT and MAIT cells undergo positive selection via their respective interactions with CD1d or MR1 MHC-I-like molecules expressed by CD4+CD8+ thymocytes (7, 19–21). In addition, the selection of CD4+CD8+ thymocytes towards the iNKT and MAIT cell lineages involves semi-invariant TCR usage, which in mice is characterised by Vα14-Jα18 and Vα19-Jα33 respectively (22, 23). Interestingly, semi-invariant innate-like MAIT and iNKT-cells can acquire effector function prior to thymic export. This process is characterised by the expression of defined transcription factors, such as RORγt and Tbet, and associated capacity to secrete effector cytokines including IL-17 and IFNγ (24, 25).
Reciprocal Dependency Of The Thymus Medulla With iNKT And Adaptive αβT-Cells
The bidirectional dependency of mTEC and single positive thymocytes is well studied, with early reports highlighting the loss of mTEC compartments in mice lacking mature thymocytes (26). More recently, the molecular basis for such developmental crosstalk between mTEC and conventional SP αβT-cells was shown to include signalling via tumour necrosis factor receptor superfamily (TNFRSF) members expressed by mTEC, including RANK, CD40 and LTβR, and the provision of TNFSF ligands by CD4+ thymocytes (27–31). In parallel with conventional thymocyte/mTEC crosstalk, quantitative loss of mature mTEC in adult CD1d-deficient mice (8) indicates that mTEC development is additionally supplemented by the provision of RANKL by iNKT-cells. While these findings suggest both conventional and innate-like αβT-cells act cooperatively to condition medullary microenvironments in the postnatal thymus, in the embryo the mTEC compartments are regulated by distinct innate lymphoid lineages, including RORγt+ lymphoid tissue inducer cells (LTi) and thymic Vγ5+ dendritic epidermal T-cells (DETC) (32, 33). Whilst such data highlight distinct cellular mechanisms for fetal and adult crosstalk involving mTEC, the molecular basis for mTEC maturation via TNFRSF signalling appears to be a process that is conserved in pre- and post-birth stages. At a functional level, the conditioning of medullary microenvironments at fetal stages by innate-like cells may be a contributing mechanism that helps to pre-establish mTEC compartments, at least to a certain degree, prior to the first cohorts of conventional single positive thymocytes transiting through the thymus.
Intact mTEC compartments are critical for the development of thymic Foxp3+ T-Reg (12), and similarly, thymic maturation of iNKT-cells is also dependent upon the presence of the thymus medulla (8). Whilst the requirement for mTEC in Foxp3+ T-Reg development is at least in part dependent on MHC presentation of self-peptides (34), the importance of iNKT TCR driven interactions with CD1d molecules in medullary microenvironments remains to be fully determined. Although the role of TCR triggering for mTEC-dependent iNKT maturation is uncertain, the requirement for iNKT mTEC-dependency can, at least in part, be substituted by supplementation of mTEC-deficient mice with IL15-IL15R complexes, suggesting a dominant role for mTEC provision of cytokines for quantitative iNKT development (8). However, whether IL15-IL15R supplementation in the context of mTEC-deficiency leads to the re-establishment of qualitatively normal iNKT sub-populations remains an open question. In an additional layer of complexity, recent studies have highlighted that type I interferons are critical for the development of thymic effector primed CD8+ Eomesodermin+ T-Innate memory cells (TIM) in adult mice (35). Given that mature mTEC provide a constitutive source of type I IFN (36, 37) and mTEC themselves are regulated by both conventional thymocytes and iNKT-cells, the development of effector primed diverse αβT-cells would also appear to form part of this complex medullary-dependent developmental network (Figure 1).
Figure 1. Innate and Adaptive αβT-cell Development In The Thymus.
αβT-cells that are produced in the thymus are heterogeneous, and consist of multiple sublineages that are phenotypically and functionally distinct. Conventional αβT-cells, MAIT and iNKT-cells all derive from cortex-resident CD4+CD8+ (DP) thymocytes, with both MAIT and iNKT-cells being generated via TCR ligand recognition on thymocytes rather than cortical thymic epithelial cells (cTEC). The medulla represents an important microenvironment for both αβT-cells and iNKT-cells, where the latter regulate RANK-mediated mTEC development and the development of Eomesodermin+ CD8+ innate memory T-cells (TIM).
Pathways During Intrathymic iNKT-Cell Development
Whilst initial reports suggested that T-cells expressing Vα14+ TCR transcripts characteristic of iNKT cells were present early in embryonic development in the embryonic body, yolk sac and fetal liver prior to their detection in the thymus (38), it is now clear that conventional αβT-cells and iNKT-cells primarily develop intrathymically from CD4+CD8+ progenitors (39). However, recent studies have also identified an alternative developmental pathway in the thymus, in which iNKT-cells stem directly from CD4-CD8- thymocytes, providing evidence for developmental heterogeneity during iNKT-cell maturation (40). The thymus dependency of iNKT-cells is clear from studies demonstrating their absence in the spleen and liver of nude mice, and thymectomized mice (41, 42). Previous discrepancies were perhaps due, at least in part, to technical limitations in the tools and mouse strains used to study iNKT-cells. More recently however, the introduction of experimental approaches using tetramer reagents, composed of glycolipids loaded onto CD1d molecules, has facilitated the accurate identification of these cells (43). Indeed, and consistent with their intrathymic origin, use of this approach has shown that CD1d tetramer+ iNKT-cells can be first identified in the thymus at postnatal day 5, and subsequently appear in peripheral tissues such as the liver and spleen by postnatal day 8 (41).
In relation to developmental progression of iNKT-cells in the thymus, early studies used the markers NK1.1, CD44 and CD24 to establish a linear model where distinct developmental stages are defined by differential expression of these markers (22). In this model, CD1d tetramer+ stage 0 iNKT-cells can display a potential combined phenotype characterised as CD24highCD44lowNK1.1-CD69+CCR7+ and Erg-2high (44, 45). A CD24-CD69-CD44lowNK1.1- phenotype was then used to define stage 1 iNKT-cells, which could be further divided into IL17RB- and IL17RB+ cells (46) while stage 2 and stage 3 cells were defined as CD24-CD69-CD44hiNK1.1- and CD24-CD69-CD44hiNK1.1+ respectively (22, 47). Importantly however, combined analysis of transcription factor/cytokine production capabilities in CD1d tetramer+ cells has recently shown that the thymus generates multiple iNKT-cell sublineages that cannot be explained by a linear model of development. Thus, within CD1d tetramer+ cells, T-bet+ cells that produce IFNγwere defined as iNKT1, RORγt+ cells producing IL17 were iNKT17, and GATA3+ cells producing IL4 and IL13 were iNKT2 (24, 48). Based on this definition, detailed molecular analysis of each of the effector iNKT cell populations has been performed (49, 50), and overlap linear model ‘stages’ was highlighted, such that NKT1 cells were most like stage 3, NKTp and NKT17 most like stage 2 and finally NKT2 most like both stage 1 and 2 (49). Importantly, and in line with the importance of mTEC during iNKT-cell development, direct visualisation of CD1d tetramer+ cells in thymic tissue sections demonstrated that most iNKT1, iNKT2 and iNKT17 cells locate to the thymus medulla (51). However, it is also interesting to note that some iNKT-cells are detectable in thymic cortical areas, and at present the functional significance of this is not known. In relation to medulla-resident iNKT-cells, it is also not clear whether this localisation, including the potential for interactions with differing medullary stromal cells, dictates their expression of cytokines and/or function.
While these studies provide a better understanding of intrathymic iNKT-cell heterogeneity in the thymus, the nature of iNKT-cell progenitors that give rise to such distinct iNKT-cell lineages, and the signals drive their continued development and their migration into the medulla remain poorly defined. As mentioned earlier, as iNKT-cells derive from CD4+CD8+ thymocytes, pre-selection iNKT-cells may be defined as CD1d tetramer+ CD24hi cells that reside within the thymic cortex (7, 51). Following CD1d recognition, these cells may upregulate their expression of CCR7, a chemokine receptor known to be important in medullary localisation of conventional thymocytes (44) together with PLZF, a member of the BTB/POZ-ZF family of transcription factors described as a master regulator of iNKT-cell development (52). Indeed, expression of PLZF coincides with downregulation of CD69 and CD24 (52), and is expressed at high levels in CD24lowCD44-NK1.1- cells, with iNKT-cell development in PLZF-deficient mice failing to progress beyond this stage (52, 53). Thus, PLZF and CCR7 expression may be a potential means to define iNKT-cell progenitors (iNKTp) that give rise to all (iNKT1, iNKT2, iNKT17) intrathymic mature iNKT-cell lineages.
Interestingly, Lee et al and Engel et al (49, 50) produced detailed studies using RNAseq of 4 subsets of iNKT-cells. Engel et al looked at a single cell level in the 4 linear subsets stage 0-3, while Lee et al used T-bet/IL-4 reporter mice to identify and analyse NKTp (PLZFhiIL17RB-IL4- cells), NKT1, NKT2 and NKT17 cells. Both studies highlighted the difference between iNKT-cell precursors/iNKT stage 0 and their more mature counterparts. Interestingly, evidence exists for the regulation of iNKT-cell precursors by cMyc (49, 50), as well as their inability to produce cytokines (49), unlike their more mature counterparts. While these studies have provided insight into the properties and developmental requirements of NKTp, further analysis of early stages in iNKT-cell development should aid in the identification and characterisation of this poorly defined population.
iNKT-Cells As Regulators Of Thymus Function
While iNKT-cells have been shown to play important roles in peripheral tissues, emerging evidence also suggests that these cells also display functional properties intrathymically, and influence both stromal microenvironments and other innate αβT-cell populations.
Influence On T-Innate Memory Cells (TIM)
T-cells are often divided into naïve or memory populations based on the phenotypic changes that occur as a result of TCR engagement. For example, naïve T-cells (TN), express the lymph node homing receptors CCR7 and CD62L, and following antigen encounter they express high levels of CXCR3 and CD44 to allow their entry into peripheral tissues as antigen-experienced memory T-cells (TM) (54, 55). Interestingly, while the majority of T-cells in non-immunised germ-free (GF) mice have a naïve phenotype, 10-20% of CD8+ T-cells possess hallmark features of memory cells (56), indicating their presence is not due to exposure to commensal or environmental microbes. Importantly, similar cells have also been described in the thymus, and have been termed Innate Memory T-Cells (TIM), while in peripheral tissues, these cells represent a combination of TIM and Virtual Memory T Cells (VIM). While TIM and VIM are phenotypically indistinguishable outside the thymus, making their respective roles a challenge to clarify, thymic CD8+ TIM are characterised phenotypically as CD8+CD62L+CD44hiCD122+CD24loCD69-CD25-Eomesodermin+Tbet- (57–59).
Intrathymic generation of TIM was first examined using mice deficient in Inducible T Cell Kinase (Itk). These mice exhibited an alteration in the composition of the SP8 population, manifested by increased frequencies of CD44hiCD25loCD8+ TIM (57, 58). In addition, mice deficient in several other genes, for example Kruppel-like factor 2 (KLF2) (60), cAMP responsive element binding protein (CBP) (61), CD155 and its ligand CD226 (62), have a disrupted population of CD8+ TIM in the thymus. The involvement of these genes in the generation of PLZF+ iNKT cells, or the production of IL4 by iNKT cells, has helped establish the requirement for NKT2 to generate CD8+ TIM. This was conclusively demonstrated by the absence of CD8+ TIM in iNKT cell deficient CD1d-/-, IL4Rα-/- (63) and IL4-/- mice (64). In addition, the close interplay between iNKT cells and CD8+ TIM is clearly visible in inbred strains of WT mice, for example BALB/c mice, which have a prominent population of PLZF+ NKT2, and a corresponding enlarged population of CD8+ TIM (60). PLZF+CD4+ thymocytes are also generated in the human fetal thymus, however these cells do not express a restricted αβTCR, nor do they bind CD1d/αGalCer tetramers, indicating they are not iNKT-cells (65). Within the same gestation period, Eomesodermin+ CD8+ T-cells are present in the human thymus (66), and interestingly, the frequency of both cells decline during fetal and early postnatal development. These results indicate a dependency on PLZF+CD4+ T-cells to drive the development of CD8+ TIM in both mouse and human.
Despite difficulties in the identification of TIM and TVM as two distinct populations of cells within peripheral tissues, a clear difference is evident from their reliance on cytokines for their generation. As previously discussed, CD8+ TIM depend on IL4 production for their development. However, CD8+ TVM are present albeit at a reduced frequency, in IL4 deficient BALB/c mice (67, 68). In addition, IL15-/- mice completely lack CD8+ TVM, and in particular IL15 transpresentation by CD8α+ DC has been shown to be required for their development in the periphery (69). Furthermore, the functional capabilities of CD8+ TIM and CD8+ TVM are similar in that both populations can produce IFNγ upon antigen binding (59, 69), however CD8+ TVM have been shown to respond to additional stimuli that CD8+ TIM have not. Studies using Nur77-GFP reporter mice, in which GFP expression levels indicate TCR signal strength (70), have shown that CD8+ TVM can produce IFNγ when they are stimulated by IL-12, IL-15 and IL-18, independently of TCR triggering (71). CD8+ TVM can also elicit cytotoxic responses by their release of perforin and granzyme, features which have not been attributed to TIM (71, 72).
Influence On Conventional Thymocyte Egress
Experiments involving engraftment of thymic lobes under the kidney capsule of congenic mice has been used as a system to show the rapid export of conventional αβT-cells, followed by repopulation with host derived T-cell precursors (73, 74). This is in contrast to iNKT-cells, where in such grafting systems, some donor cells can remain resident for 12 weeks following transplantation (73), suggesting their presence in the thymus is long-lived. CXCR3 has been identified as an example of a chemokine receptor that is required for this retention of iNKT-cells within the thymus. For example, direct injection of FITC into the thymus of WT and CXCR3-/- mice revealed an increase in FITC+ iNKT-cell recent thymic emigrants (RTE) in the latter. In the same experiments, numbers of FITC+ conventional αβT-cell RTE were unaltered, suggesting that CXCR3 may act to specifically retain iNKT-cells in the thymus (75). Although this study provides evidence of a distinct mechanism controlling the egress of iNKT and conventional T-cells, similarities do exist. One such similarity is the role of TNFRSF member LTβR, which has been proposed to control the egress of both iNKT-cells and conventional αβT-cells (9, 27, 76). Despite no alterations in the frequency of thymic iNKT-cells in LTβR-/- mice, reduced iNKT-cell numbers have been described in the liver and spleen (9, 76). To study this further, bone marrow chimeras were generated to restrict LTβR expression to stromal cells. These chimeras recapitulated the phenotype seen in LTβR-/- mice, providing evidence for a requirement of stromal cell expression of LTβR in the regulation of the peripheral iNKT-cell pool. Reduced peripheral expansion was ruled out as an explanation for this phenotype, as transfer of CFSE labelled congenic CD4+ T-cells showed the same extent of proliferation in WT and LTβR-/- mice. Instead, intrathymic injection of FITC into LTβR-/- mice revealed a reduction in FITC+ iNKT RTE compared to WT mice. Interestingly, this study also reported normal numbers of FITC+ conventional T-cell RTE in LTβR-/- mice, which contrasts to earlier work indicating the accumulation of mature conventional SP thymocytes in the LTβR-/- thymus occurs as a result of reduced thymic egress (27). Furthermore, both conventional αβT-cells and iNKT-cells express S1PR1, and share S1P-mediated dependency for thymic egress, as seen by a thymic emigration defect in both populations in p56LckCreS1P1fl/fl mice. Interestingly however, unlike conventional αβT-cells, the S1P axis is not required to control the distribution of iNKT-cells across peripheral sites (77).
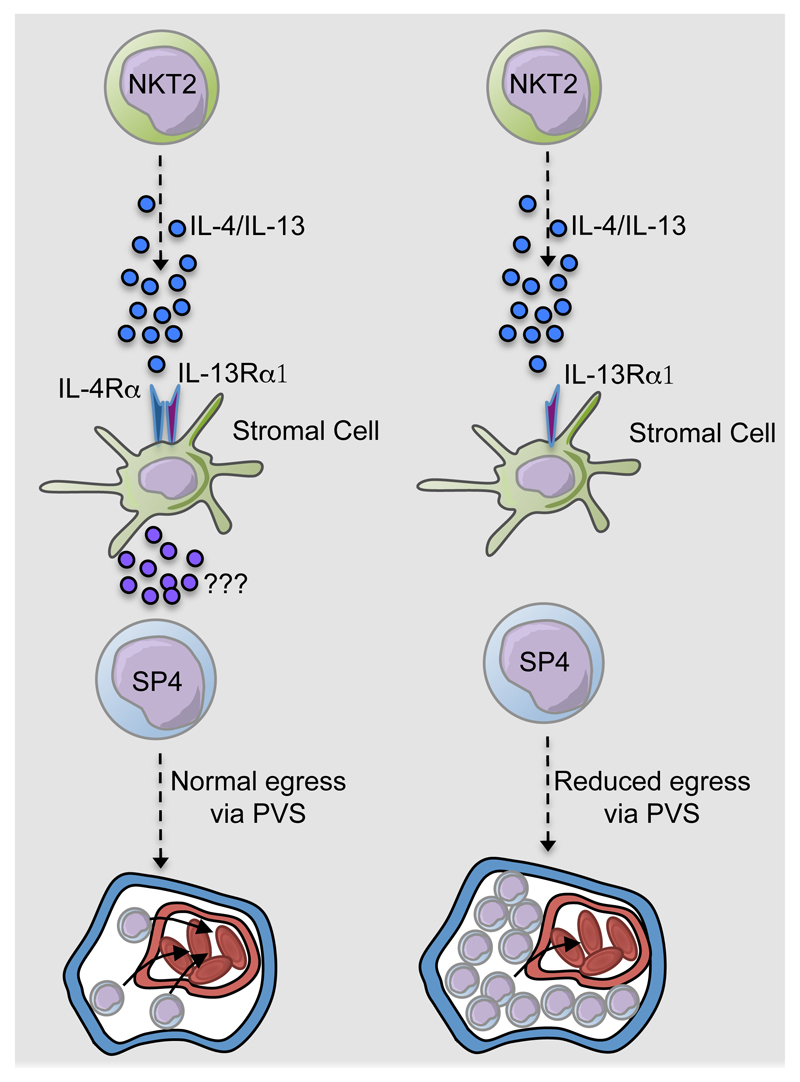
In relation to intrthymic functions of iNKT-cells, recent work has demonstrated their importance in the regulation of egress of conventional αβT-cells from the thymus (10). Thus, in CD1d-/- mice, accumulations of mature CD4+ thymocytes within the thymic perivascular space (PVS) were observed. Moreover, mTEC were shown to express the Type 2 IL4R, and respond to iNKT-cell derived cytokines IL4 and IL13. Indeed, analysis of thymus emigration in IL4Rα-/- mice provided evidence for a mechanism of thymic egress in which IL4/IL13 production by long term thymus resident iNKT-cells triggers Type 2 IL4R signalling in thymic microenvironments to control the effective emigration of mature conventional thymocytes (Figure 2). Interestingly, mature thymocytes from IL4Rα-/- mice had an intact ‘thymus exit phenotype’, including normal levels of KLF2, CD69 and S1PR1. The latter finding was of particular significance as it indicated the requirement for Type 2 ILR4 signalling is distinct from that of the S1P-S1PR1 axis. Consistent with this, treatment of with the S1PR1 agonist FTY720 promoted further intrathymic accumulation IL4Rα-/- mice. Thus, intrathymic microenvironments appear to regulate thymocyte egress from the adult thymus via at least two separate pathways. Firstly, intrathymic S1P levels are kept low to create a S1P gradient that directs mature thymocytes to blood vessel exit points in the medulla, while IL4/IL13 from iNKT-cells aids in the trafficking of thymocytes from the perivascular space into the circulation. As mentioned earlier, in addition to their specialised cytokine-producing properties, IL4/IL13-producing iNKT-cells can remain in the thymus for long periods (10, 73), and this intrathymic retention may provide some insight into their involvement in conventional αβT-cell migration from the thymus. For example, as current evidence suggests that single positive thymocytes are 4-5 days old prior to emigration, with an intrathymic conveyor belt ensuring oldest thymocytes exit first (78), the availability of tissue resident iNKT-cells may maintain this process by ensuring ordered access to the perivascular space around thymic exit points. While the lower Rag2GFP levels on IL4Rα-/- thymocytes are indicative of prolonged medulla dwell time, further experiments are required to determine whether the ordered programme of emigration is altered in iNKT-cell deficient CD1d-/- mice and IL4Rα-/- mice. Significantly, while this study demonstrated a role for IL4Rα signalling in CD4+ thymocyte egress, the defect in Eomesodermin+ CD8+ TIM development means that the requirement for stromal cell expressed IL4Rα in conventional CD4+ thymocyte emigration was not assessed. Furthermore, while the downstream regulators that are triggered by IL4/IL13 signalling in thymic stroma to regulate thymocyte emigration are not clear, it is interesting to note that Type 2 IL4R signalling in mTEC triggers expression of the chemokines CXCL10 and CCL21 (10). Given that CCL21 production in the thymus occurs within mTEClow (79), and the localisation of IL4 secreting NKT2 in the thymic medulla (51), it is possible that CCL21 influences the intrathymic positioning of iNKT-cells. Finally, as CXCL10 and CCL21 have been shown previously to influence long-term resident iNKT-cells and mature conventional thymocytes in mice (75, 80), this may also suggest that Type 2 IL4R signalling controls chemokine availability for innate and adaptive αβT-cells, which influences their respective thymus retention and emigration. In comparison to murine studies, the development and functional significance of iNKT-cells in the human thymus is less well defined. While initial studies suggested that numbers of thymic iNKT-cells decline rapidly during gestation (81), subsequent studies using more stringent gating to identify iNKT-cells, in conjunction with matched thymus and blood samples, showed the presence of iNKT-cells in the postnatal thymus, with their numbers stable in the thymus up to 9 years post birth (82, 83). Although human iNKT-cells undergo maturation in the periphery (82), the functionality of iNKT-cells in the human thymus, including their potential for regulation of conventional αβTCR+ thymocyte emigration, has yet to be addressed.
Figure 2. Cytokine Production By Intrathymic iNKT2 Cells Controls Conventional Thymocyte Egress Via The Type 2 IL4R.
In wildtype (WT) mice, IL4/IL13 production by type 2 iNKT-cells triggers Type 2 IL4R in signalling in mTEC for normal transit of fully mature conventional thymocytes from the medulla to the circulation via the perivascular space (PVS). While the downstream mediators of this process are not known, IL4Rα signalling in mTEC is known to trigger the expression of chemokines that include CXCL10 and CCL21. In IL4Rα-/- mice, lack of type 2 IL4R signalling in thymic stroma results in the intrathymic accumulation of mature thymocytes within the PVS, and a reduction in recent thymus emigrants (RTE).
Conclusions
The thymus is well known as an important site for the production of conventional αβT-cells. In this review, we have summarised how the thymus medulla, a key site for central tolerance during conventional αβT-cell development, represents an important feature in the intrathymic development of CD1d-restricted iNKT-cells. It is now clear that, as with conventional thymocytes, iNKT-cells influence the development and function of mTEC, demonstrating that thymic crosstalk in the adult thymus involves both innate and adaptive αβT-cells. In addition, a clearer definition of intrathymic iNKT-cell heterogeneity has helped to show their involvement in the regulation of thymic egress and the intrathymic production of Eomesodermin+ innate memory CD8+ T-cells. Further examination of how the links between iNKT-cells and the thymus medulla will be an important step in understanding the mechanisms that control the development and function of this site.
Acknowledgments
We thank all lab members for helpful discussions during the preparation of this manuscript.
This work was supported by a Wellcome Trust Seed Award to AJW, a BBSRC project grant (WEJ) and an MRC programme grant (GA).
Abbreviations
- cTEC
cortical thymic epithelial cell
- mTEC
medullary thymic epithelial cell
- iNKT
invariant natural killer T-cell
- MAIT
Mucosa-associated invariant T-cell
References
- 1.Krueger A. Thymus Colonization: Who, How, How Many? Arch Immunol Ther Exp (Warsz) 2017 doi: 10.1007/s00005-017-0503-5. [DOI] [PubMed] [Google Scholar]
- 2.Zlotoff DA, Bhandoola A. Hematopoietic progenitor migration to the adult thymus. Ann N Y Acad Sci. 2011;1217:122–138. doi: 10.1111/j.1749-6632.2010.05881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 4.Anderson G, Takahama Y. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol. 2012;33:256–263. doi: 10.1016/j.it.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 6.Takaba H, Takayanagi H. The Mechanisms of T Cell Selection in the Thymus. Trends Immunol. 2017;38:805–816. doi: 10.1016/j.it.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, Bendelac A, Littman DR. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 8.White AJ, Jenkinson WE, Cowan JE, Parnell SM, Bacon A, Jones ND, Jenkinson EJ, Anderson G. An essential role for medullary thymic epithelial cells during the intrathymic development of invariant NKT cells. J Immunol. 2014;192:2659–2666. doi: 10.4049/jimmunol.1303057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franki AS, Van Beneden K, Dewint P, Hammond KJ, Lambrecht S, Leclercq G, Kronenberg M, Deforce D, Elewaut D. A unique lymphotoxin {alpha}beta-dependent pathway regulates thymic emigration of V{alpha}14 invariant natural killer T cells. Proc Natl Acad Sci U S A. 2006;103:9160–9165. doi: 10.1073/pnas.0508892103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White AJ, Baik S, Parnell SM, Holland AM, Brombacher F, Jenkinson WE, Anderson G. A type 2 cytokine axis for thymus emigration. J Exp Med. 2017;214:2205–2216. doi: 10.1084/jem.20170271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozai M, Kubo Y, Katakai T, Kondo H, Kiyonari H, Schaeuble K, Luther SA, Ishimaru N, Ohigashi I, Takahama Y. Essential role of CCL21 in establishment of central self-tolerance in T cells. J Exp Med. 2017;214:1925–1935. doi: 10.1084/jem.20161864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. J Exp Med. 2013;210:675–681. doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogquist KA, Xing Y, Hsu FC, Shapiro VS. T Cell Adolescence: Maturation Events Beyond Positive Selection. J Immunol. 2015;195:1351–1357. doi: 10.4049/jimmunol.1501050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing Y, Wang X, Jameson SC, Hogquist KA. Late stages of T cell maturation in the thymus involve NF-kappaB and tonic type I interferon signaling. Nat Immunol. 2016;17:565–573. doi: 10.1038/ni.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rafei M, Hardy MP, Williams P, Vanegas JR, Forner KA, Dulude G, Labrecque N, Galipeau J, Perreault C. Development and function of innate polyclonal TCRalphabeta+ CD8+ thymocytes. J Immunol. 2011;187:3133–3144. doi: 10.4049/jimmunol.1101097. [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson WE, McCarthy NI, Dutton EE, Cowan JE, Parnell SM, White AJ, Anderson G. Natural Th17 cells are critically regulated by functional medullary thymic microenvironments. J Autoimmun. 2015;63:13–22. doi: 10.1016/j.jaut.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, Craft J. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seach N, Guerri L, Le Bourhis L, Mburu Y, Cui Y, Bessoles S, Soudais C, Lantz O. Double-positive thymocytes select mucosal-associated invariant T cells. J Immunol. 2013;191:6002–6009. doi: 10.4049/jimmunol.1301212. [DOI] [PubMed] [Google Scholar]
- 20.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 21.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 22.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 23.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koay HF, Gherardin NA, Enders A, Loh L, Mackay LK, Almeida CF, Russ BE, Nold-Petry CA, Nold MF, Bedoui S, Chen Z, et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol. 2016;17:1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- 26.van Ewijk W, Shores EW, Singer A. Crosstalk in the mouse thymus. Immunol Today. 1994;15:214–217. doi: 10.1016/0167-5699(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 27.Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White AJ, Withers DR, Parnell SM, Scott HS, Finke D, Lane PJ, Jenkinson EJ, Anderson G. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur J Immunol. 2008;38:942–947. doi: 10.1002/eji.200738052. [DOI] [PubMed] [Google Scholar]
- 29.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, et al. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, et al. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, et al. RANK signals from CD4(+)3(-) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204:1267–1272. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, McConnell FM, Desanti GE, Benezech C, Parnell SM, Cunningham AF, et al. Rank signaling links the development of invariant gammadelta T cell progenitors and Aire(+) medullary epithelium. Immunity. 2012;36:427–437. doi: 10.1016/j.immuni.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 2010;11:512–519. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 35.Martinet V, Tonon S, Torres D, Azouz A, Nguyen M, Kohler A, Flamand V, Mao CA, Klein WH, Leo O, Goriely S. Type I interferons regulate eomesodermin expression and the development of unconventional memory CD8(+) T cells. Nat Commun. 2015;6:7089. doi: 10.1038/ncomms8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lienenklaus S, Cornitescu M, Zietara N, Lyszkiewicz M, Gekara N, Jablonska J, Edenhofer F, Rajewsky K, Bruder D, Hafner M, Staeheli P, et al. Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J Immunol. 2009;183:3229–3236. doi: 10.4049/jimmunol.0804277. [DOI] [PubMed] [Google Scholar]
- 37.Otero DC, Baker DP, David M. IRF7-dependent IFN-beta production in response to RANKL promotes medullary thymic epithelial cell development. J Immunol. 2013;190:3289–3298. doi: 10.4049/jimmunol.1203086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makino Y, Kanno R, Koseki H, Taniguchi M. Development of Valpha4+ NK T cells in the early stages of embryogenesis. Proc Natl Acad Sci U S A. 1996;93:6516–6520. doi: 10.1073/pnas.93.13.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bezbradica JS, Hill T, Stanic AK, Van Kaer L, Joyce S. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc Natl Acad Sci U S A. 2005;102:5114–5119. doi: 10.1073/pnas.0408449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dashtsoodol N, Shigeura T, Aihara M, Ozawa R, Kojo S, Harada M, Endo TA, Watanabe T, Ohara O, Taniguchi M. Alternative pathway for the development of Valpha14(+) NKT cells directly from CD4(-)CD8(-) thymocytes that bypasses the CD4(+)CD8(+) stage. Nat Immunol. 2017;18:274–282. doi: 10.1038/ni.3668. [DOI] [PubMed] [Google Scholar]
- 41.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(-)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol. 2000;164:2412–2418. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- 43.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen-specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–1903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowan JE, McCarthy NI, Parnell SM, White AJ, Bacon A, Serge A, Irla M, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G. Differential requirement for CCR4 and CCR7 during the development of innate and adaptive alphabetaT cells in the adult thymus. J Immunol. 2014;193:1204–1212. doi: 10.4049/jimmunol.1400993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, Glimcher LH. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10:306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, Yoshida H, Kubo M, Kawamoto H, Koseki H, Taniguchi M. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012;10:e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 48.Michel ML, Mendes-da-Cruz D, Keller AC, Lochner M, Schneider E, Dy M, Eberl G, Leite-de-Moraes MC. Critical role of ROR-gammat in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci U S A. 2008;105:19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee YJ, Starrett GJ, Lee ST, Yang R, Henzler CM, Jameson SC, Hogquist KA. Lineage-Specific Effector Signatures of Invariant NKT Cells Are Shared amongst gammadelta T, Innate Lymphoid, and Th Cells. J Immunol. 2016;197:1460–1470. doi: 10.4049/jimmunol.1600643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Engel I, Seumois G, Chavez L, Samaniego-Castruita D, White B, Chawla A, Mock D, Vijayanand P, Kronenberg M. Innate-like functions of natural killer T cell subsets result from highly divergent gene programs. Nat Immunol. 2016;17:728–739. doi: 10.1038/ni.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee YJ, Wang H, Starrett GJ, Phuong V, Jameson SC, Hogquist KA. Tissue-Specific Distribution of iNKT Cells Impacts Their Cytokine Response. Immunity. 2015;43:566–578. doi: 10.1016/j.immuni.2015.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bjorkdahl O, Barber KA, Brett SJ, Daly MG, Plumpton C, Elshourbagy NA, Tite JP, Thomsen LL. Characterization of CC-chemokine receptor 7 expression on murine T cells in lymphoid tissues. Immunology. 2003;110:170–179. doi: 10.1046/j.1365-2567.2003.01727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu JK, Kagari T, Clingan JM, Matloubian M. Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proc Natl Acad Sci U S A. 2011;108:E118–127. doi: 10.1073/pnas.1101881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ. The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL. Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 59.Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci U S A. 2013;110:13498–13503. doi: 10.1073/pnas.1307572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fukuyama T, Kasper LH, Boussouar F, Jeevan T, van Deursen J, Brindle PK. Histone acetyltransferase CBP is vital to demarcate conventional and innate CD8+ T-cell development. Mol Cell Biol. 2009;29:3894–3904. doi: 10.1128/MCB.01598-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Georgiev H, Ravens I, Shibuya A, Forster R, Bernhardt G. CD155/CD226-interaction impacts on the generation of innate CD8(+) thymocytes by regulating iNKT-cell differentiation. Eur J Immunol. 2016;46:993–1003. doi: 10.1002/eji.201546073. [DOI] [PubMed] [Google Scholar]
- 63.Lai D, Zhu J, Wang T, Hu-Li J, Terabe M, Berzofsky JA, Clayberger C, Krensky AM. KLF13 sustains thymic memory-like CD8(+) T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. J Exp Med. 2011;208:1093–1103. doi: 10.1084/jem.20101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Renkema KR, Lee JY, Lee YJ, Hamilton SE, Hogquist KA, Jameson SC. IL-4 sensitivity shapes the peripheral CD8+ T cell pool and response to infection. J Exp Med. 2016;213:1319–1329. doi: 10.1084/jem.20151359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee YJ, Jeon YK, Kang BH, Chung DH, Park CG, Shin HY, Jung KC, Park SH. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. J Exp Med. 2010;207:237–246. doi: 10.1084/jem.20091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Min HS, Lee YJ, Jeon YK, Kim EJ, Kang BH, Jung KC, Chang CH, Park SH. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. J Immunol. 2011;186:5749–5757. doi: 10.4049/jimmunol.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J Immunol. 2012;188:2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tripathi P, Morris SC, Perkins C, Sholl A, Finkelman FD, Hildeman DA. IL-4 and IL-15 promotion of virtual memory CD8(+) T cells is determined by genetic background. Eur J Immunol. 2016;46:2333–2339. doi: 10.1002/eji.201646404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sosinowski T, White JT, Cross EW, Haluszczak C, Marrack P, Gapin L, Kedl RM. CD8alpha+ dendritic cell trans presentation of IL-15 to naive CD8+ T cells produces antigen-inexperienced T cells in the periphery with memory phenotype and function. J Immunol. 2013;190:1936–1947. doi: 10.4049/jimmunol.1203149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, Bevan MJ, Zehn D, Prlic M. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gordon SM, Carty SA, Kim JS, Zou T, Smith-Garvin J, Alonzo ES, Haimm E, Sant'Angelo DB, Koretzky GA, Reiner SL, Jordan MS. Requirements for eomesodermin and promyelocytic leukemia zinc finger in the development of innate-like CD8+ T cells. J Immunol. 2011;186:4573–4578. doi: 10.4049/jimmunol.1100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berzins SP, McNab FW, Jones CM, Smyth MJ, Godfrey DI. Long-term retention of mature NK1.1+ NKT cells in the thymus. J Immunol. 2006;176:4059–4065. doi: 10.4049/jimmunol.176.7.4059. [DOI] [PubMed] [Google Scholar]
- 74.Cowan JE, McCarthy NI, Anderson G. CCR7 Controls Thymus Recirculation, but Not Production and Emigration, of Foxp3(+) T Cells. Cell Rep. 2016;14:1041–1048. doi: 10.1016/j.celrep.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drennan MB, Franki AS, Dewint P, Van Beneden K, Seeuws S, van de Pavert SA, Reilly EC, Verbruggen G, Lane TE, Mebius RE, Deforce D, et al. Cutting edge: the chemokine receptor CXCR3 retains invariant NK T cells in the thymus. J Immunol. 2009;183:2213–2216. doi: 10.4049/jimmunol.0901213. [DOI] [PubMed] [Google Scholar]
- 76.Vallabhapurapu S, Powolny-Budnicka I, Riemann M, Schmid RM, Paxian S, Pfeffer K, Korner H, Weih F. Rel/NF-kappaB family member RelA regulates NK1.1- to NK1.1+ transition as well as IL-15-induced expansion of NKT cells. Eur J Immunol. 2008;38:3508–3519. doi: 10.1002/eji.200737830. [DOI] [PubMed] [Google Scholar]
- 77.Allende ML, Zhou D, Kalkofen DN, Benhamed S, Tuymetova G, Borowski C, Bendelac A, Proia RL. S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. FASEB J. 2008;22:307–315. doi: 10.1096/fj.07-9087com. [DOI] [PubMed] [Google Scholar]
- 78.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lkhagvasuren E, Sakata M, Ohigashi I, Takahama Y. Lymphotoxin beta receptor regulates the development of CCL21-expressing subset of postnatal medullary thymic epithelial cells. J Immunol. 2013;190:5110–5117. doi: 10.4049/jimmunol.1203203. [DOI] [PubMed] [Google Scholar]
- 80.Ueno T, Hara K, Willis MS, Malin MA, Hopken UE, Gray DH, Matsushima K, Lipp M, Springer TA, Boyd RL, Yoshie O, et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16:205–218. doi: 10.1016/s1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 81.Sandberg JK, Stoddart CA, Brilot F, Jordan KA, Nixon DF. Development of innate CD4+ alpha-chain variable gene segment 24 (Valpha24) natural killer T cells in the early human fetal thymus is regulated by IL-7. Proc Natl Acad Sci U S A. 2004;101:7058–7063. doi: 10.1073/pnas.0305986101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, Weinberg KI, Metelitsa LS. Distinct homeostatic requirements of CD4+ and CD4- subsets of Valpha24-invariant natural killer T cells in humans. Blood. 2004;104:4150–4156. doi: 10.1182/blood-2004-04-1629. [DOI] [PubMed] [Google Scholar]
- 83.Berzins SP, Cochrane AD, Pellicci DG, Smyth MJ, Godfrey DI. Limited correlation between human thymus and blood NKT cell content revealed by an ontogeny study of paired tissue samples. Eur J Immunol. 2005;35:1399–1407. doi: 10.1002/eji.200425958. [DOI] [PubMed] [Google Scholar]