Abstract
Metastasis to distant organs and not the primary tumor itself is usually the cause of death for cancer patients. Hence, studying the key molecules and molecular pathways involved in metastasis is essential. Metastasis is a complex process in which cancer cells detach from the original tumor, migrate and invade through surrounding tissues and metastasize to other sites of the body through circulation. Cell-extracellular matrix (ECM) adhesion proteins play a fundamental role in this process as cancer cells need to weaken their adhesions in order to dissociate from the ECM as well as the neighboring cells within the tumor and finally form new adhesions and invade surrounding tissues. Ras suppressor-1 (RSU-1) was originally identified as a suppressor of Ras-dependent oncogenic transformation and found to be localized to cell-ECM adhesions where it binds to PINCH-1, a focal adhesion involved in cell survival. Although RSU-1 was connected to cancer early on, little is known with regard to its expression in various cancer types or its role in metastasis. In this article, we review the recent literature regarding the expression of RSU-1 in various cancer types and its potential role in metastasis, discussing interesting findings and issues that still need to be addressed.
Keywords: extracellular matrix, cell-matrix adhesion, PINCH-1, breast cancer, hepatocellular carcinoma
Cancer metastasis to distant organs, and not the primary tumor, is the main cause of death for cancer patients. Metastasis is a complex biological process involving many different steps in which connections to the extracellular matrix (ECM) are fundamental. More specifically, the already disrupted cell-ECM and cell-cell adhesions in the primary tumor undergo further changes (1–3) in order for cancer cells to be able to detach from the original tumor mass, degrade adjacent ECM and invade through neighboring tissues to finally enter the circulation and form distant metastasis (Figure 1). Hence, integrins and ECM-related adhesion proteins play important role in metastasis and invasive behavior of cancer cells (1).
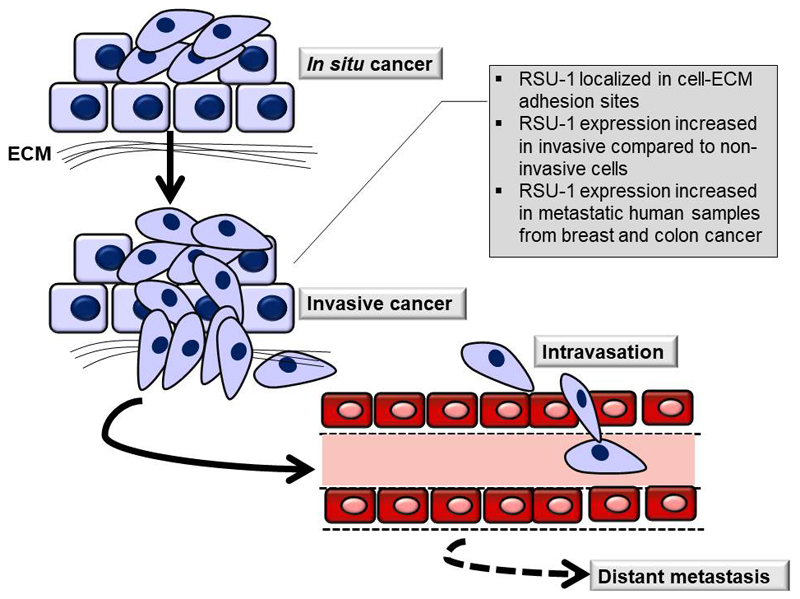
Figure 1. Schematic showing the initial stages of metastasis where RSU-1 is involved.
Cancer cells in in situ cancer proliferate within the tumor while in invasive cancer cell adhesion is disrupted and cells dissociate from the original tumor to migrate and invade through surrounding tissues until they reach the circulation. Once in circulation, cells are transferred to distant sites of the body where they colonize the sites and form metastasis. RSU-1 localizes to cell-ECM adhesions and its expression is increased in invasive compared to non-invasive cells.
I. Introduction to Ras suppressor-1
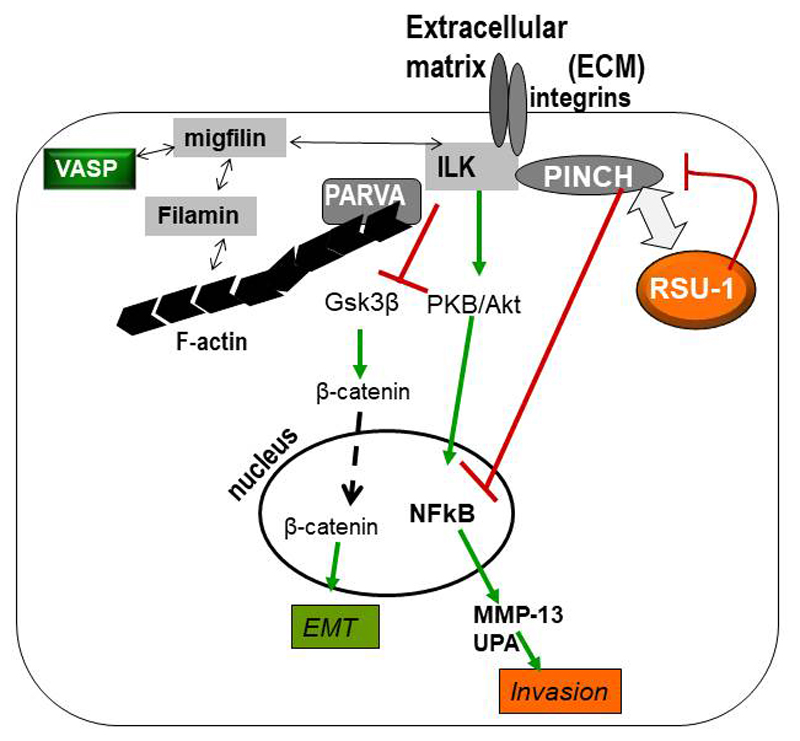
Ras Suppressor-1 (RSU-1) gene was originally identified to suppress Ras-dependent oncogenic transformation (4) and was found to be localized to human chromosome 10 (5). Importantly, RSU-1 protein was later shown to be localized to cell-ECM adhesions through its interaction with Particularly Interesting New Cysteine-Histidine-rich protein-1 (PINCH-1) (6), a cell-ECM adhesion adaptor protein vital for cell survival, apoptosis resistance and cell shape modulation (7, 8). PINCH-1 is known to form a stable ternary complex at cell-ECM adhesions along with adhesion proteins Integrin Linked Kinase (ILK) and alpha-parvin (PARVA), which are also known to play important roles in cell survival (9). Importantly, PARVA binds directly to actin connecting the whole IPP-RSU-1 complex to the actin cytoskeleton and thus to functions such as cell spreading, migration and invasion that require actin involvement and movement co-ordination (Figure 2).
Figure 2. Schematic representation of the protein-protein interactions at the cell-ECM adhesion sites involving RSU-1.
RSU-1 binds to PINCH-1 which is connected to the IPP complex (ILK-PINCH-1-PARVA). PARVA in turn binds directly to actin connecting the IPP-RSU-1 complex to actin cytoskeleton and thus to functions such as cell spreading, migration and invasion.
RSU-1 was immediately connected to Ras-dependent oncogenic transformation since its cloning in 1992 (4, 5), while there is consensus among researchers with regard to the fact that RSU-1 has anti-tumorigenic effects suppressing cancer cell growth (4, 10–12). However, little is known regarding its expression in cancer tissues, as well as its involvement in metastasis and the underlining molecular mechanism.
A. Gene expression of RSU-1 in cancer cells and tissues
Regarding the expression of RSU-1 in cancer cells and tissues, results are limited (Table 1). A study involving colon cancer samples demonstrated that RSU-1 mRNA expression was dramatically up-regulated in metastatic colon cancer samples compared to healthy controls as well as compared to primary colon cancer samples (13). Another study in breast cancer samples showed that RSU-1 expression level was elevated both at the mRNA and protein level in samples from breast cancer patients compared to adjacent normal tissue of the same patient. Moreover, it was shown that RSU-1 was more dramatically upregulated in metastatic samples compared to non-metastatic (14) being associated with metastasis of breast cancer cells to the lymph nodes (14). RSU-1 was also shown to be upregulated both at the protein and mRNA level in aggressive cancer cell lines. For instance, it was found upregulated in MDA-MB-231 breast cancer cells compared to less aggressive MCF-7 cells (14), as well as in the aggressive HepG2 hepatocellular carcinoma cells compared to the less invasive PLC/PRF/5 (Alexander) hepatoma cells (15).
Table 1.
Summary of literature findings on RSU-1 expression and cancer
| Cancer type | RSU-1 expression | Reference No |
|---|---|---|
| Breast cancer | Increased in 32 samples from breast cancer patients compared to respective adjacent normal tissue and lymph node-metastatic samples compared to non-metastatic | 14 |
| Increased in aggressive MDA-MB-231 breast cancer cells compared to less aggressive MCF-7 cells | 14 | |
| Colon cancer | Increased in metastatic colon cancer samples compared to healthy controls or primary colon cancer samples | 13 |
| Hepatocellular carcinoma | Increased in aggressive HepG2 hepatocellular carcinoma cells compared to the less invasive PLC/PRF/5 (Alexander) hepatoma cells | 15 |
B. Effect of RSU-1 on metastasis-related properties
Given the fact that RSU-1 is upregulated in more invasive cancer cells as well as in several metastatic tumors, it would be rational to hypothesize that it was involved in metastasis. A few years ago it was demonstrated that depletion of RSU-1 from aggressive MDA-MB-468 breast cancer cells enhances cell migration (16), while its depletion from normal mammary MCF-10A cells significantly reduces migration (17), suggesting a possibly different mechanism between cancer cell lines or a different function of RSU-1, depending on the aggressiveness of the cell line.
Regarding its role in cell invasion, little was known until recently, when another in vitro study in hepatocellular carcinoma cells, indeed showed that RSU-1 depletion from the highly invasive hepatocellular carcinoma HepG2 cells leads to increased cell proliferation and reduced cell adhesion and invasion (15). This indicates that RSU-1 is implicated in HCC cell invasive behavior. Furthermore, it was shown lately that RSU-1 siRNA-mediated silencing also downregulates two major proteases known to be involved in the degradation of matrix and the facilitation of invasion, namely, urokinase Plasminogen Activator (uPA), and metalloproteinase-13 (18). More importantly, in the same study, tumor spheroids were generated from three different breast cancer cell lines of different invasive potential and were then embedded in three dimensional (3D) collagen gels. Strikingly, spheroids formed from RSU-1-depleted cells exhibited dramatic reduction in their invasive capacity (18), confirming the involvement of RSU-1 in metastasis-related processes. Finally, Kaplan-Meier survival plot analysis using the in silico online tool Kaplan Meier plotter, corroborated that high RSU-1 expression is associated with poor prognosis for distant metastasis-free and remission-free survival in breast cancer patients (18).
C. Known connections to main molecular pathways
Nevertheless, the molecular mechanism underlying RSU-1 functions within cells remains vague. Most of the studies are focusing on its involvement in growth inhibition, as it has been shown that overexpression of RSU-1 in breast cancer MCF-7 cells induces upregulation of cell cycle regulator, p21CIP CDK inhibitor, which in turn suppresses cell proliferation and tumor growth (11). In addition to that, another study showed that RSU-1 apart from inhibiting breast cancer cell proliferation, functions as an apoptosis-promoter by upregulating the pro-apoptotic gene p53-upregulated-modulatore-of-apoptosis (PUMA) (14). Furthermore, RSU-1 overexpression in response to Epidermal growth factor (EGF) stimulation, led to increased Extracellular signal Regulated Kinase-2 (ERK) kinase activation (11, 19), decreased activation of Jun kinase (11, 19) and reduced Rho kinase (ROCK) activity (11). Regarding p38 Mitogen Activated Protein Kinase (MAPK) signaling, RSU-1 depletion was demonstrated to activate p38 signaling in one study, suggesting that RSU-1 is an inhibitor of p38 signaling (6), while RSU-1 depletion in another more recent study was shown to abolish p38 signaling, suggesting RSU-1 can act as p38 activator (17).
Other RSU-1 functions also seem more complex. For instance, in one study RSU-1 depletion resulted in reduced expression level of PINCH-1, a known binding partner of RSU-1 (17), and in elevated expression of PINCH-1 in another study (14).
II. Concluding Remarks
RSU-1 is emerging as a novel and promising target of metastasis that should be evaluated also as a potential metastasis biomarker. Current data point to the direction that RSU-1 is a “friend” of metastasis promoting it in cancer cells. However, inhibiting RSU-1 seems to abolish the cell invasion making potential inhibitory agents a “foe” of metastasis. Definitely more research is needed in order to decipher the exact molecular mechanism involved and many questions remain still unanswered. For instance, is RSU-1 upregulated in other cancer types apart from breast and colon cancer? Apart from PINCH-1, are there any other binding partners that interact with RSU-1 and what is the proposed molecular mechanism of its action? Could RSU-1 be a potent metastasis biomarker? If yes, for what type of cancer? Is its role cell/tumor type-dependent? Lastly, since all the studies performed so far on RSU-1 have been performed in vitro, would in vivo experiments blocking RSU-1 in animal cancer models confirm in vitro findings?
Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement no 336839-ReEngineeringCancer.
Abbreviations
- ECM
extracellular matrix
- ERK
extracellular signal Regulated Kinase
- PINCH-1
Particularly Interesting New Cysteine-Histidine-rich protein-1
- PUMA
p53-upregulated-modulatore-of-apoptosis
- ROCK
Rho kinase
- RSU-1
Ras Suppressor-1
- uPA
urokinase plasminogen activator
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126(Pt 2):393–401. doi: 10.1242/jcs.100115. [DOI] [PubMed] [Google Scholar]
- 3.Ungefroren H, Sebens S, Seidl D, Lehnert H, Hass R. Interaction of tumor cells with the microenvironment. Cell Commun Signal. 2011;9:18. doi: 10.1186/1478-811X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutler ML, Bassin RH, Zanoni L, Talbot N. Isolation of rsp-1, a novel cDNA capable of suppressing v-Ras transformation. Mol Cell Biol. 1992;12(9):3750–6. doi: 10.1128/mcb.12.9.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuda T, Cutler ML. Human RSU1 is highly homologous to mouse Rsu-1 and localizes to human chromosome 10. Genomics. 1993;18(2):461–2. doi: 10.1006/geno.1993.1503. [DOI] [PubMed] [Google Scholar]
- 6.Dougherty GW, Chopp T, Qi SM, Cutler ML. The Ras suppressor Rsu-1 binds to the LIM 5 domain of the adaptor protein PINCH1 and participates in adhesion-related functions. Exp Cell Res. 2005;306(1):168–79. doi: 10.1016/j.yexcr.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda T, Chen K, Shi X, Wu C. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J Biol Chem. 2003;278(51):51324–33. doi: 10.1074/jbc.M309122200. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Fukuda T, Li Y, Zha X, Qin J, Wu C. Molecular dissection of PINCH-1 reveals a mechanism of coupling and uncoupling of cell shape modulation and survival. J Biol Chem. 2005;280(30):27631–7. doi: 10.1074/jbc.M504189200. [DOI] [PubMed] [Google Scholar]
- 9.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7(1):20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 10.Tsuda T, Marinetti MR, Masuelli L, Cutler ML. The Ras suppressor RSU-1 localizes to 10p13 and its expression in the U251 glioblastoma cell line correlates with a decrease in growth rate and tumorigenic potential. Oncogene. 1995;11(2):397–403. [PubMed] [Google Scholar]
- 11.Vasaturo F, Dougherty GW, Cutler ML. Ectopic expression of Rsu-1 results in elevation of p21CIP and inhibits anchorage-independent growth of MCF7 breast cancer cells. Breast Cancer Res Treat. 2000;61(1):69–78. doi: 10.1023/a:1006462323260. [DOI] [PubMed] [Google Scholar]
- 12.Donthamsetty S, Bhave VS, Mars WM, Bowen WC, Orr A, Haynes MM, Wu C, Michalopoulos GK. Role of PINCH and its partner tumor suppressor Rsu-1 in regulating liver size and tumorigenesis. PLoS One. 2013;8(9):e74625. doi: 10.1371/journal.pone.0074625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbazan J, Alonso-Alconada L, Muinelo-Romay L, Vieito M, Abalo A, Alonso-Nocelo M, Candamio S, Gallardo E, Fernandez B, Abdulkader I, de Los Angeles Casares M, et al. Molecular characterization of circulating tumor cells in human metastatic colorectal cancer. PLoS One. 2012;7(7):e40476. doi: 10.1371/journal.pone.0040476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giotopoulou N, Valiakou V, Papanikolaou V, Dubos S, Athanassiou E, Tsezou A, Zacharia LC, Gkretsi V. Ras suppressor-1 promotes apoptosis in breast cancer cells by inhibiting PINCH-1 and activating p53-upregulated-modulator of apoptosis (PUMA); verification from metastatic breast cancer human samples. Clin Exp Metastasis. 2015;32(3):255–65. doi: 10.1007/s10585-015-9701-x. [DOI] [PubMed] [Google Scholar]
- 15.Gkretsi V, Bogdanos DP. Elimination of Ras Suppressor-1 from hepatocellular carcinoma cells hinders their in vitro metastatic properties. Anticancer Res. 2015;35(3):1509–12. [PubMed] [Google Scholar]
- 16.Dougherty GW, Jose C, Gimona M, Cutler ML. The Rsu-1-PINCH1-ILK complex is regulated by Ras activation in tumor cells. Eur J Cell Biol. 2008;87(8–9):721–34. doi: 10.1016/j.ejcb.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Nieves R, Desantis AI, Cutler ML. Rsu1 contributes to regulation of cell adhesion and spreading by PINCH1-dependent and - independent mechanisms. J Cell Commun Signal. 2013 doi: 10.1007/s12079-013-0207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gkretsi V, Stylianou A, Louca M, Stylianopoulos T. Identification of Ras suppressor-1 (RSU-1) as a potential breast cancer metastasis biomarker using a three-dimensional in vitro approach. Oncotarget. 2017;8(16):27364–79. doi: 10.18632/oncotarget.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masuelli L, Cutler ML. Increased expression of the Ras suppressor Rsu-1 enhances Erk-2 activation and inhibits Jun kinase activation. Mol Cell Biol. 1996;16(10):5466–76. doi: 10.1128/mcb.16.10.5466. [DOI] [PMC free article] [PubMed] [Google Scholar]