Introductory paragraph
There is an urgent need to develop novel approaches for predicting and preventing the evolution of antibiotic resistance. Here we show that the ability to evolve de novo resistance to a clinically important β-lactam antibiotic, ceftazidime, varies drastically across the genus Pseudomonas. This variation arises because strains possessing the ampR global transcriptional regulator evolve resistance at a high rate. This does not arise because of mutations in ampR. Instead, this regulator potentiates evolution by allowing mutations in conserved peptidoglycan biosynthesis genes to induce high levels of β-lactamase expression. Crucially, blocking this evolutionary pathway by co-administering ceftazidime with the β-lactamase inhibitor avibactam can be used to eliminate pathogenic P. aeruginosa populations before they can evolve resistance. In summary, our study shows that identifying potentiator genes that act as evolutionary catalysts can be used to both predict and prevent the evolution of antibiotic resistance.
Antibiotic resistance in pathogenic bacteria poses a growing threat to human health, by increasing the mortality rate and economic burden associated with bacterial infections1. In light of this threat, there is an urgent need to develop new tools for predicting when resistance is likely to evolve in pathogen populations2. Research in this area has largely focused on understanding how differing antibiotic treatment strategies, such as mixtures and cycles, influence the evolutionary dynamics of resistance3–5. An alternative approach is to ask if there are specific genes that make bacteria more likely to evolve resistance to antibiotics6. Whole genome sequencing has highlighted the incredible genetic diversity of pathogenic bacteria7, but the impact of this diversity on the evolution of antibiotic resistance remains poorly understood. For example, recent work in Streptococcus pneumoniae has shown that genes that are important for resistance in one strain may be completely dispensable in another8. Although many genes are associated with clinical resistance, it is unclear to what extent other genes in the genome influence the evolution of resistance. For example, recent work has shown that some genes ‘potentiate’ the evolution of novel bacterial phenotypes by opening otherwise inaccessible routes to adaptation9,10. The existence of potentiator genes suggests that genomic background may play a key role in the evolution of antibiotic resistance.
In vitro selection experiments have emerged as an important tool for studying the evolution of antibiotic resistance1,3,5. However, these studies typically use selection lines derived from a single ancestral clone, making it difficult to understand the role that genetic background itself plays in the evolution of resistance. One approach to circumvent this difficulty is to use comparative experimental evolution, where a diverse collection of strains are challenged with adapting to a common selective pressure6. Using this approach, we recently demonstrated that genetic background influences the evolution of resistance to rifampicin by altering the spectrum and fitness effects of mutations in a highly conserved domain of RNA polymerase that confer resistance to rifampicin6,11. In this paper, we extend this approach to uncover resistance potentiator genes by challenging 8 strains that span the genus Pseudomonas with the β-lactam antibiotic ceftazidime.
Pseudomonas is a diverse genus of bacteria that includes P. aeruginosa, an important opportunistic pathogen of humans that is the primary cause of mortality in patients who suffer from cystic fibrosis. Crucially, it is possible to culture a wide range of Pseudomonas strains under a common set of lab conditions, making it possible to study evolutionary responses to antibiotics in these bacteria using tightly controlled and replicated experiments. We chose to study the evolution of resistance to ceftazidime for two reasons. First, ceftazidime is a clinically relevant antibiotic that is commonly used to treat Pseudomonas infections12 and ceftazidime resistance is common in clinical isolates of P. aeruginosa. Second, the mechanisms of ceftazidime action and resistance are well characterized. Ceftazidime inhibits cell wall biosynthesis by irreversibly binding to periplasmic penicillin-binding proteins, ultimately leading to cell death. In spite of this simple mechanism of action, Pseudomonas can use at least 4 routes to evolve resistance to ceftazidime: altering the structure of penicillin-binding proteins, upregulating the expression of efflux pumps, reducing permeability of the outer membrane and upregulating the expression of β-lactamase enzymes that break down the antibiotic13–15 (Figure S1). Mutations altering the structure of the β-lactamase enzyme itself do occur, but provide much lower increases in resistance16.
Here we use a serial passage experiment to challenge close to 1,000 populations of Pseudomonas with doses of ceftazidime that increased from sub-lethal to lethal concentrations over the course of 1 week. We then use extensive whole genome re-sequencing of evolved clones to identify genes and pathways that contribute to the rapid evolution of elevated ceftazidime resistance. Using selection experiments and competition assays with defined mutants, we directly test the evolutionary impact of key pathways to resistance identified from whole genome sequencing. Finally, we demonstrate that understanding the genetic drivers of resistance evolution can be used to design a simple drug mixture, consisting of ceftazidime coupled to a β-lactamase inhibitor, to prevent the evolution of resistance in vitro.
Results and discussion
Strain-specific variation in resistance evolution
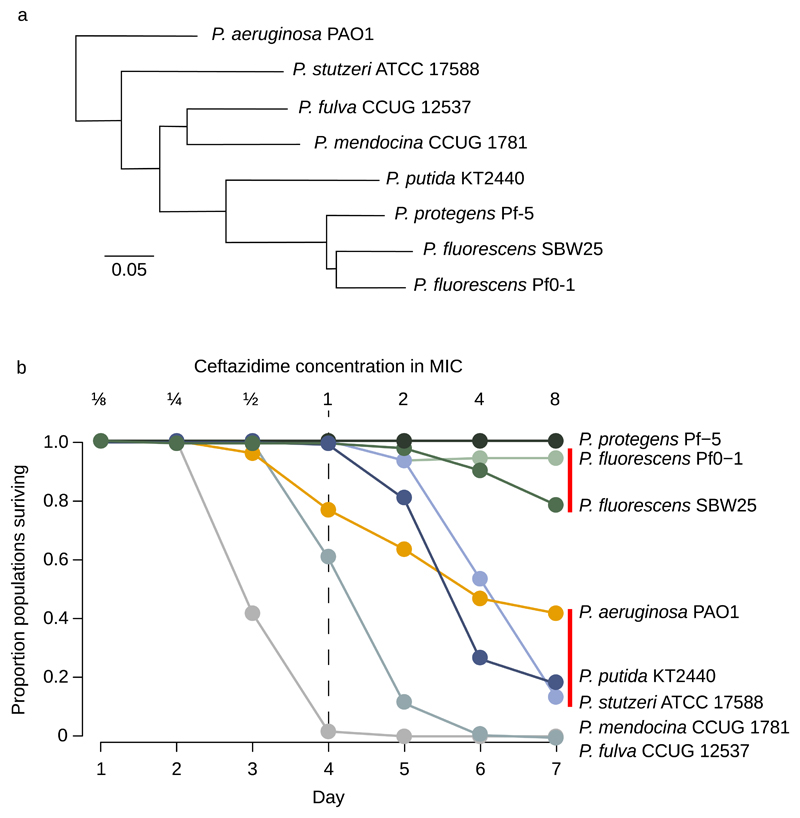
To test the role of genetic background in the evolution of antibiotic resistance, we challenged 120 populations of each of 8 strains that span the diversity of the genus Pseudomonas with ceftazidime (Figure 1a). This breadth of phylogenetic coverage allowed us to explore the impact of genome content on resistance evolution, and strains were chosen on the basis of variation in genome size, experimental tractability, and the availability of high-quality published reference genomes. Populations were serially passaged in standard lab culture medium supplemented with ceftazidime, the concentration of which was doubled daily from sub-lethal (1/8 minimal inhibitory concentration, or ‘MIC’) to super-lethal (8× MIC) levels over a 7 day selection experiment. The MIC of the parental strains varies (0.65-8 mg/L) and we controlled for this variation by standardizing antibiotic doses of selection lines to their appropriate parental strains. In this experimental design, populations can only avoid extinction if they evolve elevated antibiotic resistance, and we measured population survival at each day of the experiment. We define the rate of population extinction within strains as a measure of adaptive potential for resistance evolution, or ‘evolvability’. The rate of population extinction varied profoundly between strains (Figure 1b; Cox’s proportional hazard, likelihood ratio=1930, df=7, P<10-6). For example, all of the replicate populations went extinct in some strains, such as P. mendocina CCUG1781 and P. fulva CCUG12537, while at the other extreme, every population of P. protegens Pf-5 survived at up to 8× the MIC of the parental strain. Given that resistance evolved by selection on spontaneous mutations, one potential explanation for this result is that the ability to evolve ceftazidime resistance correlates to the mutation rate. However, evolvability does not correlate with mutation rate (r=0.33, F1,6=0.74, P=0.42, see supplementary table S1 for calculations) or mutation supply rate, which is the product of initial population size and mutation rate (r=0.22, F1,5=0.27, P=0.62). Additionally, there was no correlation between survival and the absolute difference between the temperature of the selection experiment (30° C) and published optimal growth temperatures for each strain (r=0.06, F1,6=0.027, P=0.88).
Figure 1. Responses of Pseudomonas to ceftazidime.
a Phylogeny of the strains used in this study, all nodes were supported with >99% confidence and the scale bar shows genetic distance (adapted from ref. 11 and 49 with permission under Creative Commons licence CC-BY-4.0). b The proportion of populations (n = 120 populations/strain) of each strain that survived exposure to increasing doses of ceftazidime. Doses were standardized relative to the MIC of the ancestral clone of each strain, and doses increased 2 fold daily up to 8× MIC. Evolvability differs between strains that are not connected by red lines (Post-hoc test on Cox’s proportional hazard, P<0.05).
Genomics of resistance evolution
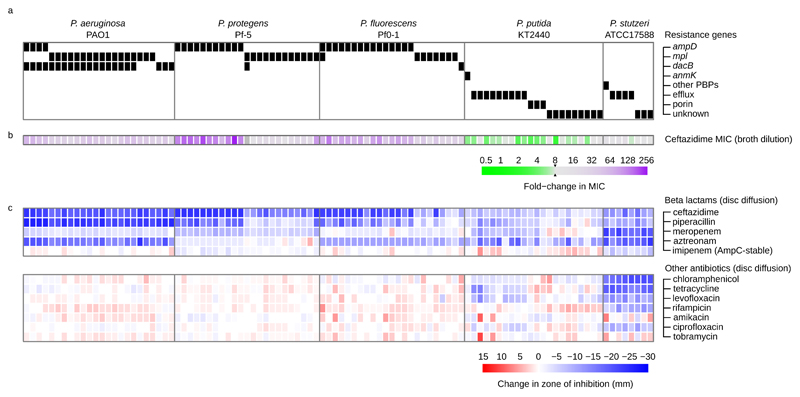
To determine the genetic basis of resistance evolution, we sequenced the genomes of 100 independently evolved clones from populations that survived selection for elevated resistance (n=14–24 clones/strain). We identified a total of 196 novel mutations in 69 unique genes (i.e. orthologs across strains are each counted once). Mutations included SNPs (n=80), short indels (n=71), insertion element insertions (n=15), larger insertions and deletions (n=7), and intergenic mutations (n=23). Several lines of evidence indicate that the mutations that we identified were predominantly beneficial. First, parallel evolution occurred both within and across strains. We identified a total of 25 genes mutated in two or more independent clones, and 76% of mutations occurred in these 25 genes. Second, all 80 SNPs observed in coding regions were non-synonymous, which is a clear hallmark of positive selection. A full list of the mutations we identified is given in supplementary data table S2. We categorized mutations according to known resistance mechanisms: (i) porin genes, (ii) penicillin binding proteins (PBPs), (iii) peptidoglycan biosynthesis genes and (iv) multidrug efflux pumps13–15. Almost all of the evolved clones (88/100) carry mutations in previously established ceftazidime resistance pathways. However, the distribution of mutations across these resistance pathways differs profoundly between strains, demonstrating strain-specific mechanisms of resistance evolution (Figure 2; χ2=139, df=12, P<10-6). P. protegens Pf-5 and P. fluorescens Pf0-1 adapt by mutations in genes involved in peptidoglycan biosynthesis and recycling (ampD and mpl), knockouts of which are known to increase the expression of the chromosomal ampC β-lactamase gene14,17. In addition to mutations in ampD and mpl, 21 of 24 clones of P. aeruginosa PAO1 carry mutations in a non-essential PBP (dacB/PBP4) that has also been shown to increase ampC expression when knocked out18. Consistent with this genetic data, clones from these strains have increased resistance to a broad spectrum of β-lactams, but retain sensitivity to imipenem, which is a poor substrate for the AmpC β-lactamase. In contrast, P. stutzeri ATCC17588 and P. putida KT2440 evolve resistance by mutations in efflux pump genes and, to a lesser extent, porins. Mutations in efflux pumps are associated with small increases in ceftazidime resistance and a multi-drug resistant phenotype, while porin mutations are predominantly associated with elevated β-lactam resistance (Figure 2). A substantial fraction (33.3%) of clones from these strains lack mutations in known resistance genes; however, these clones have resistance profiles that are similar to those of clones carrying mutations in known efflux pumps or porins.
Figure 2. Resistance in evolved clones.
Each column in this figure represents a single, randomly chosen clone from a population that survived until the end of the selection experiment (8× MIC). a Black boxes show the presence of mutations in known ceftazidime resistance genes, as determined by whole genome resequencing. Note that some clones carry mutations in multiple resistance genes, and that some clones lack mutations in known resistance genes (online supplementary data table S2). b Coloured boxes show the change in ceftazidime MIC of evolved clones (mean of n=3 replicates), and c changes in the zone of inhibition for a large panel of antibiotics, as determined by disc diffusion assay (mean of n=3 replicates).
The AmpR transcription factor increases evolvability
The key insight from whole genome sequencing, and phenotypic analysis of evolved clones, is that large increases in ceftazidime resistance are associated with mutations in the peptidoglycan biosynthesis pathway associated with increased β-lactamase production14. Importantly, the relevant peptidoglycan biosynthesis genes (ampD, mpl and dacB) are present in all of the strains, and the ampC β-lactamase gene is present in all of the strains except P. stutzeri ATCC17588 (which possesses another β-lactamase gene, blaZ).
These observations raise an interesting puzzle: if the key genes involved in adaptation are largely maintained, then why does evolvability vary across strains? An alternative approach to understanding why evolvability varies across strains is to take a functional approach to characterizing the effects of beneficial mutations. Inactivation of the peptidoglycan biosynthesis genes involved in adaptation in our experiment has been shown to increase ampC expression by causing an intracellular accumulation of peptidoglycan catabolites14,17. However, ampC induction via this mechanism requires the AmpR transcription factor; inactivation of ampR removes the ability to increase ampC expression in response to β-lactams17. Crucially, among our strains ampR is only present in the genomes of P. aeruginosa PAO1, P. protegens Pf-5, and the two P. fluorescens Pf0-1 and SBW25, and not found in the others. This simple association between the presence of the AmpR transcription factor and the probability of survival to the end of the experiment through adaptation suggests that regulation of ampC expression is key.
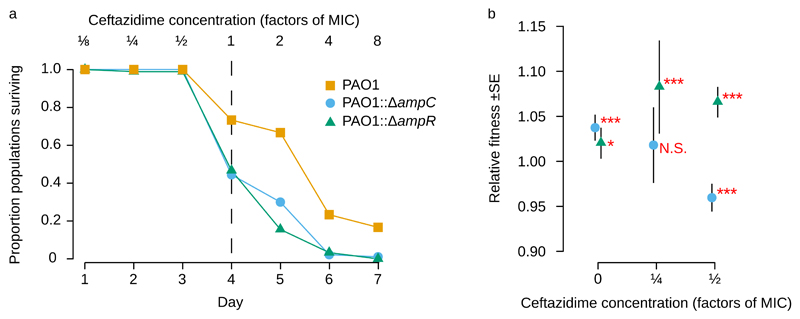
How does ampR increase evolvability? One simple possibility is that this regulator potentiates evolution by opening up new genetic paths to evolving elevated ceftazidime resistance9,10. Specifically, ampR could potentiate the evolution of ceftazidime resistance by allowing mutations in peptidoglycan biosynthesis genes, such as ampD, mpl, and dacB to increase levels of ampC expression. Consistent with this hypothesis, mutations in peptidoglycan biosynthesis genes and dacB are known to only increase resistance in the presence of ampR17,18. This hypothesis generates two simple predictions that can be tested using our method. First, if elevated expression of ampC is a key mechanism for evolving ceftazidime resistance, then deleting ampC should decrease evolvability. Second, if the AmpR regulator is required to drive the evolution of increased ampC expression, then deleting ampR should reduce evolvability by the same amount as deleting the ampC. To test these predictions, we challenged populations of ΔampR and ΔampC mutants of P. aeruginosa PAO1 with increasing doses of ceftazidime, as in our initial experiment (Figure 3a). Both of the mutants have dramatically reduced evolvability compared to their isogenic P. aeruginosa PAO1 control (Cox’s proportional hazard, likelihood ratio=23.82, df=2, P=6×10-6), providing conclusive evidence that both the β-lactamase (ampC) and its regulator (ampR) play key roles in driving the evolution of elevated ceftazidime resistance.
Figure 3. The AmpR transcription factor potentiates the evolution of ceftazidime resistance in P. aeruginosa PAO1.
a The survival of populations of an ampR deletion strain (PAO1::ΔampR; n = 90) relative to an isogenic PAO1 control (n = 30) under increasing doses of ceftazidime. The ampR deletion reduces evolvability to levels comparable to those observed in a mutant lacking the ampC β-lactamase (PAO1::ΔampC; n=90). b Relative fitness (mean +/- s.e; n = 9) of the PAO1::ΔampR mutant (grey triangles) and the PAO1::ΔampC mutant (blue circles) in direct competition with a PAO1 reference strain carrying a neutral YFP marker. Symbols denote statistical significance, as determined by a Bonferroni-corrected Wilcoxon rank sum test (N.S. = P>0.05,* = P<0.05, *** = P<0.001).
The low survival probability of P. aeruginosa PAO1 in comparison with the other strains that carry both ampR and ampC is also consistent with this hypothesis. Strains of P. fluorescens and P. protegens carry 2 homologs of ampD, which represses the expression of ampC, whereas P. aeruginosa PAO1 carries 3 homologs of this gene. The additional copy of ampD found in P. aeruginosa ensures that ampD mutations lead to weaker de-repression of ampC expression, and this is likely to translate into reduce evolvability in comparison to strains with only 2 ampD homologs; the ampD dosage effect has been demonstrated experimentally20. Consequently, most surviving P. aeruginosa strains possessed two loss of function mutations in the peptidoglycan biosynthesis pathway, in comparison with one only in the other ampR/ampC possessing strains (Figure 2).
Additionally, it is possible that adaptive plasticity in ampC expression mediated by ampR could increase evolvability22. Exposure to β-lactam antibiotics interferes with peptidoglycan biosynthesis by inhibiting PBPs, causing an AmpR-mediated increase in ampC expression17,19. This, in turn, may accelerate the genetic evolution of resistance by providing bacterial populations with the time to acquire ceftazidime resistance mutations. According to this explanation, ampR increases evolvability through ecological potentiation. The key assumption of this hypothesis is that the plasticity in ampC expression mediated by ampR must provide a benefit in the presence of ceftazidime. To test this hypothesis, we measured the effect of deleting ampR and ampC on fitness using short-term competition experiments (Figure 3b). Deleting ampC leads to a decrease in fitness the presence of ceftazidime, demonstrating that induced expression of this gene is beneficial. However, deleting ampR actually increases fitness in the presence of sub-MIC concentrations of ceftazidime, demonstrating that plasticity in gene expression cannot explain the link between ampR and increased evolvability. Indeed, as ampR expression is not particularly strongly induced by ceftazidime23, this suggests that ampR does not simply allow populations to ‘buy time’ to wait for an adaptive mutation. Although this result is counter-intuitive, it is important to emphasize that ampR is a global transcriptional regulator that affects the expression of 100s of genes24,25, including repressing another chromosomal β-lactamase, poxB25. In particular, ampR is involved in regulating quorum sensing factors, including lasR, several metabolic pathways, and the rpoS-mediated stress response pathway25. Although it is clear that inducing elevated levels of ampC expression in the presence of ceftazidime is beneficial, the fitness cost associated with the ampR regulator implies that the net fitness effect of all of the changes in gene expression caused by this regulator in the presence of ceftazidime is deleterious. The importance of ampR as a global regulator of expression perhaps explains why increased ampC expression did not arise through mutations in ampR itself, and why ampR mutations are not typically observed in clinical P. aeruginosa isolates26.
Inhibiting the evolution of ceftazidime resistance
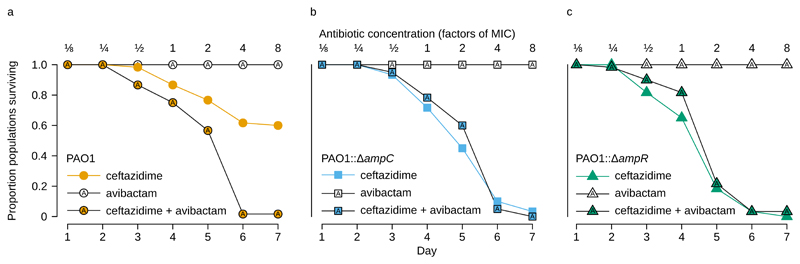
Given the important role that ampR mediated induction of ampC expression plays in the evolution of resistance, our results suggest that one possible strategy to prevent the evolution of cephalosporin resistance in P. aeruginosa infections would be to co-administer ceftazidime with AmpC β-lactamase inhibitors27. The rationale for this strategy is that a combination of a β-lactam and β-lactamase inhibitor will be active against both wild-type bacterial strains and mutants with elevated β-lactamase secretion. In other words, this strategy should effectively block a major evolutionary path to elevated resistance. To test this idea, we challenged P. aeruginosa PAO1 with ceftazidime in the presence of avibactam28, a recently developed AmpC inhibitor (Figure 4a). Unlike most β-lactamase inhibitors, avibactam does not possess any toxic effects on Pseudomonas28 and we did not detect any population extinction in the avibactam treated control populations. In support of our hypothesis, avibactam increased the rate of population extinction in the presence of ceftazidime compared to ceftazidime treated control populations (Cox’s proportional hazard, likelihood ratio test=78.968, df=1, P<10-6). We failed to detect any viable cells in 59 out of 60 populations of P. aeruginosa that were selected in 8× MIC ceftazidime supplemented with avibactam demonstrating that the effect of avibactam suppresses the evolution of elevated β-lactamase secretion just as effectively as knocking out ampC or ampR (Figure 3a). Importantly, this effect does not arise because avibactam increases the potency of ceftazidime. Surprisingly, we found that avibactam treatment actually increased the MIC of ceftazidime from 0.76 mg/L to 1.14 mg/L (Figure S2).
Figure 4. Blocking the evolution of ceftazidime resistance.
a Survival of populations of P. aeruginosa PAO1 that were challenged with increasing doses of ceftazidime in either the presence or absence of the AmpC-inhibitor avibactam (n = 60 populations/treatment). Avibactam was administered at a constant, non-inhibitory dose (4 mg/L). Avibactam increases the rate of population extinction in the presence of increasing doses of ceftazidime. b and c The survival of ampR or ampC deletion strains (PAO1::ΔampR and PAO1::ΔampC) under the same experimental conditions as for the isogenic wild-type POA1 (n = 60 populations/treatment for each strain). Avibactam had no effect on the survival of ampR or ampC deletion mutants.
As a final test of the role of ampR in evolvability, we challenged populations of ΔampR (Figure 4b) and ΔampC (Figure 4c) mutants of P. aeruginosa PAO1 with a combination of ceftazidime and avibactam, as in our experiment with wild-type P. aeruginosa PAO1. If our hypothesis is correct, then avibactam should have no effect on evolvability in these mutant strains, because they are effectively unable to increase ampC expression under our experimental conditions. Consistent with this idea, we found that avibactam does not have an effect on evolvability in either ΔampR or ΔampC mutants (Cox’s proportional hazard, likelihood ratio test=3.25, df=1, P= 0.071 and likelihood ratio test=0.02, df=1, P=0.876, respectively).
Conclusion
Whole genome sequencing is revolutionizing our understanding of the evolution and ecology of bacterial pathogens. One of the challenges that has arisen from this revolution is to understand the consequences of genetic diversity in pathogen populations. Here we show that comparative experimental evolution can be used to identify genes and pathways that influence the rate and mechanisms of adaptation to antibiotics. Our experiment addressed this problem at a fairly broad scale, by comparing the evolutionary responses of strains from different species. Our initial reasoning for working at this scale was that comparing divergent strains effectively maximizes the number of genes and SNPs that are included in the experiment, therefore maximizing the likelihood of detecting an impact of genetic background on evolvability. However, the sheer number of genetic differences between even the most closely related strains used in this study may have hindered our ability to detect more subtle genomic effects on evolvability. While it is clear that inducible ampC β-lactamase expression is an important driver of evolvability in this genus, it is clear that other genes must influence the ability to evolve ceftazidime resistance. For example, P. stutzeri ATCC17588 and P. putida KT2440, both of which lack ampR, have similar evolvability to P. aeruginosa PAO1. We are currently extending this research program by focusing on studying variation in evolvability between clones from the same species, and we hope that this approach will enable us to identify genetic drivers of evolvability in greater depth.
The differing modes of ampC expression among the pseudomonads affect their ability to evolve resistance to β-lactams by interacting with genes in the peptidoglycan biosynthesis pathway. In strains possessing ampR, the intracellular accumulation of peptidoglycan catabolites converts the AmpR transcription factor into an activator of ampC expression in response to peptidoglycan damage. Mechanistically, ampR increases evolvability by allowing mutations in peptidoglycan biosynthesis genes to induce high levels of β-lactamase expression, which effectively amplifies the ampC expression plasticity that occurs when cell wall synthesis is compromised by β-lactams20. From a more conceptual perspective, ampR can be thought of as a conduit that translates genetic variation in the peptidoglycan biosynthesis gene network into phenotypic variation in ampC expression. This suggests that response pathways that are involved in sensing environmental change may have a general role as evolutionary catalysts, linking plastic and mutational responses to environmental change. Intriguingly, these alternative expression modes are disseminated among the enterobacteria; however, insertion of the ampR gene into constitutive producers is not sufficient to restore inducible expression, suggesting a distinct regulatory mechanism in constitutive producers29. To avoid the evolution of high levels of ceftazidime resistance, and subsequent treatment failure, treatment with ceftazidime should be avoided in infections caused by strains with inducible ampC expression.
Understanding the evolutionary trajectory to high levels of ceftazidime resistance makes it possible to design a simple two-drug mixture consisting of ceftazidime and avibactam that can be used to effectively eliminate populations of the pathogen P. aeruginosa. We argue that this strategy is successful because avibactam effectively prevents mutations in peptidoglycan biosynthesis genes and dacB from increasing ceftazidime resistance, eliminating their fitness benefit. One possible solution to this evolutionary challenge would be to first evolve avibactam resistance, and then evolve ceftazidime resistance. However, avibactam does not have any detectable toxic effects on Pseudomonas at concentrations where it is able to effectively inhibit AmpC, rendering this evolutionary pathway to combined avibactam/ceftazidime resistance inaccessible. Although these results are encouraging, we emphasize that there are a number of confounding factors that may affect the efficacy of this combination of drugs in vivo. For example, the pharmacokinetic properties of the two drugs may make it difficult to effectively maintain the drug mixtures at the site of bacterial infections, and it is also possible that avibactam resistant alleles of ampC or other β-lactamases capable of hydrolyzing ceftazidime are already present in pathogen populations.
Predicting the evolution of antibiotic resistance is a challenging and important objective. Here we show that comparative experimental evolution can be used to identify genes and pathways that make some bacterial strains prone to evolving resistance, and to exploit this to design treatment strategies for preventing resistance evolution. High throughput sequencing is revolutionizing clinical microbiology30,31, and it may be possible to identify such potentiator genes in clinical pathogen populations and to use this information to optimize antimicrobial treatment strategies.
Materials and Methods
MIC Determination for parental strains
Three independent estimations of the MIC for each parental strain were determined in 96-well plates using the broth microdilution method. Briefly, 5-10 morphologically similar colonies of each strain were resuspended in sterile saline solution (NaCl 0.9%). The solution was adjusted to the adequate optical density so that it would contain approximately 1.5 × 108 cells/mL. This standardized inoculum and was diluted a further 200-fold in Mueller-Hinton 2 (MH2, Sigma-Aldrich, United Kingdom) broth containing ceftazidime (Sigma-Aldrich) at a concentration between 64 mg/L and 0.0625 mg/L. After 24h of incubation at 30 °C with shaking at 250 rpm, optical density at 595nm was determined for each well with a Synergy 2 plate reader (Biotek, Winooski, USA). We considered that bacterial growth had been inhibited if the optical density was less than 25% of that of antibiotic-free cultures. The lowest antibiotic concentration at which growth had been inhibited was considered the MIC. The measured MIC was used to calculate the ramping ceftazidime concentration regime in the selection experiment (see “Experimental evolution”).
Effect of avibactam on MIC
The effect of avibactam on MIC was evaluated by measuring growth inhibition by ceftazidime at the presence/absence of avibactam. The procedure was identical to MIC determination described above, except that one group of replicates was supplemented with 4 mg/L of avibactam (BioVision Inc. USA). No avibactam was added to a control group. The avibactam treatment and control groups were tested at concentrations ranging from 0.1 mg/L to 3.8 mg/L of ceftazidime with 4 replicates each.
Mutation rate estimation
Mutation rates were estimated by fluctuation assays, with the antibiotic rifampicin as the selection agent, using the method of Luria and Delbruck32. 480 replicate cultures were inoculated with approximately 50 cells from an overnight culture of each parental strain and incubated for 48 hours in 200 µl of KB media at 30 °C with constant shaking at 200 rpm. Approximately 107 cells from each culture were then plated onto KB-agar containing rifampicin at the appropriate MIC (minimum inhibitory concentration) for each strain (60 mg/L for P. aeruginosa PAO1, 30 mg/L for all other strains). For each strain the proportion of cultures yielding no mutants was scored, from which the mutation per culture was calculated using the negative natural logarithm. This value was then divided by the number of cells plated, which provides an estimate of the mutation rate per cell division.
Experimental evolution
To initiate the selection experiment, all parental strains were recovered from −80 °C stocks and cultured overnight in MH2 broth at 30 °C for 24 h with shaking at 250 rpm. Next, the cultures were diluted by 10-6 in MH2 broth and distributed on 96-well plates (200 μL per well). After 48 h of incubation at 30°C, we initiated the first transfer by diluting these cultures 1:100 in MH2 broth containing 1/8 MIC of ceftazidime, relative to the measured MIC of each strain. Bacterial populations were incubated for 24 h at 30°C with shaking at 250 rpm and diluted 1:100 for the next transfer. Every transfer ceftazidime concentration was doubled, reaching 8× MIC in the final transfer. Population survival was monitored during the course of the selection experiment by measuring optical density at 595 nm using a Synergy 2 microtiter plate reader (BioTek, Winooski, VT, USA). We additionally confirmed population survival after the last transfer by plating a 1 uL sample of each population on antibiotic-free MH2 agar plates that were scored for growth after overnight incubation at 30 °C. We performed the evolution experiment in two independent blocks. In each block we propagated 60 replicates populations of each strain that were challenged with increasing doses of ceftazidime and 12 replicate controls populations of each strain that were allowed to evolve in antibiotic-free MH2. At the end of the experiment, a maximum of 20 population per strain were streaked in MH2 agar plates and a clone was picked for each population was picked and amplified for further analyses. To avoid bias by conducting the experiment at different temperatures and incubators, a common growth environment (i.e. 30 °C, MH2) and growth medium (Mueller-Hinton 2) that supports the growth of all strains was chosen for all strains. Although the strains have different optimal growth temperatures (28 °C for P. protegens, P. fluorescens, and P. fulva; 30 °C for P. putida and P. mendocina, 35 °C for P. stutzeri and 37°C for P. aeruginosa), all were capable of vigorous growth in this environment, hence the number of generations per day (6-7) is instead dictated by the dilution factor (1/100).
Experimental evolution with ∆ampC and ∆ampR mutants
We obtained ∆ampC and ∆ampR mutants of P. aeruginosa PAO1 that were constructed following well-established procedures based on the cre-lox system for gene deletion and antibiotic resistance marker recycling33. We determined the MIC of these mutants using the microbroth dilution method, as above. To test evolvability of ∆ampC and ∆ampR mutants, we followed the same protocol as the main selection experiment, as outlined above. We challenged 90 replicate populations of each deletion mutant and 30 replicate populations of PAO1 wild-type with increasing doses of ceftazidime. In addition, we evolved 18 control populations per strain in antibiotic-free culture medium. This experiment was carried out in a single block.
Experimental evolution to test the effect of avibactam
The effect of avibactam on evolvability was tested for ΔampC and ΔampR mutants and for wild-type PAO1. 120 replicate populations of each strain were passaged following exactly the same procedure as in the two previous experiments. The ceftazidime concentration was doubled every transfer from 1/8 to 8× MICs. For each strain, half of the populations (60 replicates) were additionally challenged with avibactam (always 4 mg/L, BioVision Inc. USA). Population survival was monitored for 7 serial transfers by measuring optical density. We also included 20 control populations evolving at the presence of avibactam but without the antibiotic. There was no extinction observed in the control treatment.
Inhibition zone assays
Evolved clones were cultured in MH2 broth overnight (30 °C, 250 rpm). A sterile swab was dipped then into a 10-3 dilution of this overnight culture to and the swab was used to inoculate the surface of three MH2 agar plates. Then we placed four different antibiotic susceptibility testing discs (Oxoid) on each plate, testing a total of 12 antibiotics: ceftazidime, piperacillin, meropenem, imipenem, aztreonam, cloramphenicol, tetracycline, rifampicin, amikacin, tobramycin, ciprofloxacin and levofloxacin. After 24 h of incubation at 30 °C, the diameter of the different inhibition zones was measured with a ruler taking the average of three measurements in different axis. Assays were performed in 4 randomized blocks containing a similar number of evolved clones for each strain, and all ancestral strains were tested in each block as a control. Change in antibiotic sensitivity was estimated as the difference in diameter of the inhibition zone of each clone compared to its ancestor for each antibiotic.
DNA extraction and sequencing
DNA from the evolved clones surviving the duration of the experiment was extracted using the Wizard Genomic DNA Purification Kit (Promega, UK) as per the manufacturer’s instructions. To maximize phylogenomic coverage and reduce bias toward P. fluorescens strains, P. fluorescens SBW25 was excluded from sequencing due to being highly similar to P. fluorescens Pf0-1. We assessed the purity of DNA extractions by measuring absorbance at 230, 260, and 280 nm and by visualizing migration on a 0.7% agarose gel. The concentration of each genomic DNA in each sample was then accurately determined using QuantiFluordsDNA System (Promega, UK) and samples were diluted to 30 ng/μL in TE Buffer before sequencing.
Resequencing was done using Illumina HiSeq2000 with 100bp paired-end reads (Wellcome Trust Centre for Human Genetics, Oxford, UK). Sequencing analysis was performed using the pipeline first described in San Millan et al.34. Read filtering was done using the NIH-QCToolkit35. Read ends were trimmed if the Phred quality score was less than 20. We discarded reads <50bp after trimming, with >2% ambiguous bases, or with >20% bases of Phred score <20. BWA was used to map reads to the reference genome of each strain. Mapped reads were processed to increase the quality of the variant calling: 1) reads with multiple best hits were discarded; 2) duplicated reads were discarded using MarkDuplicates from the Picard package (http://picard.sourceforge.net); 3) reads around indels were locally realigned using RealignerTargetCreator and IndelRealigner from the GATK package to correct for misalignment; and 4) mate pairs were sorted using FixMateInformation in the Picard package. Variant calling was performed with GATK UnifiedGenotyper36 and Samtools mpileup37. VCFtools vcf-annotate38, and GATK toolkit VariantFiltration39, were used to filter the raw variants for strand bias, end distance bias, base quality bias, SNPs around gaps, low coverage and erroneously high coverage. Variants were combined using GATK's CombineVariants (keeping any unfiltered). High quality variants not filtered were annotated using SnpEff40. Three approaches were used to detect structural variants: BreakDancer41 (indels, inversions and translocations), Pindel42(indels, inversions, tandem duplications and breakpoints), and ControlFREEC (copy number variants43 with mappability tracks generated by gem-mappability (GEM library44).
Comparative genomics of resistance pathways
Using pairwise reciprocal BLAST between the reference sequences of the sequenced strains, we determined their similarity in genome content. This approach was taken because the strains differ in the extent to which their genomes are annotated. Using the KEGG database45, we compared the genes in the β-lactam resistance and peptidoglycan recycling pathways (irrespective of whether they had mutated during selection).
Competition experiment with ∆ampC and ∆ampR mutants
To measure relative fitness of the deletion mutants, we performed a competition experiment. ∆ampC, ∆ampR and their isogenic PAO1 wild-type were competed against a YFP-marked tester strain PAO1 strain that carries a constitutively expressed YFP integrated at the mini-Tn7 insertion site14. Competition experiments were carried out in MH2 broth containing ceftazidime at a concentration of 0, 0.25 or 0.5 mg/L. All competition experiments were replicated 9 times. First, the strains were recovered from -80 °C stock and cultured overnight in MH2 broth medium at 30 °C with shaking at 250 rpm. The overnight cultures were diluted 1:50 in MH2 broth and used to prepare 1:1 mixtures of PAO1-YFP with each of the 3 strains to be tested. Before starting competition, we first estimated the exact starting proportion of strains using flow cytometry (for details see below). Next, we combined 10 µL of these mixtures and 190 µL of MH2 with a corresponding ceftazidime concentration (0, 1/4 and 1/2 MICs). This resulted in an additional 1:20 dilution. The bacterial strains were let to compete in 96-well plates for 24 h at 30 °C. The next day, the cultures were diluted 1:50 in saline solution (0.9% NaCl) and analyzed on a flow cytometer in order to estimate the resulting proportion of the YFP-labeled versus unlabelled cells after competition (see below).
Flow cytometry was performed on Accuri C6 (BD Biosciences, UK). The cell densities were adjusted to give around 1000 events per second. During data acquisition, a lower cut off was set at 10,000 for FSC-H and at 8000 for SSC-H. The data were exported as FCS-files and processed in R using a custom pipeline based on flowCore and flowViz packages46–48. In the pipeline, the events were automatically gated on size by retaining the cells within 2 standard deviations around the median in the bivariate normal distribution of FSC-A and SSC-A. Then, k-mean clustering algorithm was applied on fluorescence intensity FL1-H to differentiate fluorescent versus non-fluorescent cells. For each antibiotic concentration, we ensured that YFP-expressing strain can be well separated from non-fluorescent strains by overlaying non-mixed controls (overlap is usually less than 2% of the cells). Figure S3 shows a representative plot of the gating strategy.
Relative fitness was calculated according to the formula
where p0 is an initial proportion of an unlabelled stain, and p1 is a final proportion of an unlabelled stain after competition. 1000 is a dilution factor, which reflects a difference in cell density at the beginning and at the end of the competition.
Supplementary Material
One Sentence Summary.
Here we identify potentiator genes and pathways that make bacteria prone to evolving antibiotic resistance, and we exploit this to design treatment strategies for preventing resistance evolution.
Acknowledgements
This work was supported by funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant (StG-2011-281591) and by a Wellcome Trust Senior Research Fellowship (WT106918AIA) held by RCM.
Footnotes
Data availability
Data generated or analysed during this study are included in this published article (and its supplementary information files), with the exception of sequence data, which are deposited in Eurpoean Nucleotide Archive (PRJEB20060).
Statement of author contributions: This study was designed by RCM. Experiments were carried out by VF, AP and TV. Bioinformatics were done by DRG. VF, AP, DRG and RCM analyzed data. AO contributed reagents and expertise. The manuscript was written by RCM, DRG and VF.
Competing interests: The authors declare the absence of any competing interests.
References
- 1.O'Neill J. Tackling drug-resistant infections globally: Final report and recommendations. 2016. [Google Scholar]
- 2.Martinez JL, Baquero F, Andersson DI. Predicting antibiotic resistance. Nat Rev Microbiol. 2007;5:958–965. doi: 10.1038/Nrmicro1796. [DOI] [PubMed] [Google Scholar]
- 3.Baym M, Stone LK, Kishony R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science. 2016;351:aad3292. doi: 10.1126/science.aad3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer AC, Kishony R. Understanding, predicting and manipulating the genotypic evolution of antibiotic resistance. Nat Rev Genet. 2013;14:243–248. doi: 10.1038/nrg3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacLean RC, Hall AR, Perron GG, Buckling A. The population genetics of antibiotic resistance: integrating molecular mechanisms and treatment contexts. Nat Rev Genet. 2010;11:405–414. doi: 10.1038/Nrg2778. [DOI] [PubMed] [Google Scholar]
- 6.Vogwill T, Kojadinovic M, Furió V, MacLean RC. Testing the role of genetic background in parallel evolution using the comparative experimental evolution of antibiotic resistance. Molecular Biology and Evolution. 2014 doi: 10.1093/molbev/msu262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loman NJ, Pallen MJ. Twenty years of bacterial genome sequencing. Nat Rev Microbiol. 2015;13:787–794. doi: 10.1038/nrmicro3565. [DOI] [PubMed] [Google Scholar]
- 8.Van Opijnen T, Dedrick S, Bento J. Strain dependent genetic networks for antibiotic-sensitivity in a bacterial pathogen with a large pan-genome. PLOS Pathog. 2016;12(9):Ee005. doi: 10.1371/Jjurnal.Ppat.1005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blount ZD, Barrick JE, Davidson CJ, Lenski RE. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature. 2012;489:513–518. doi: 10.1038/nature11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lind PA, Farr AD, Rainey PB. Experimental evolution reveals hidden diversity in evolutionary pathways. Elife. 2015;4 doi: 10.7554/eLife.07074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogwill T, Kojadinovic M, MacLean RC. Epistasis between antibiotic resistance mutations and genetic background shape the fitness effect of resistance across species of Pseudomonas. Proc Biol Sci. 2016;283(1830):20160151. doi: 10.1098/rspb.2016.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giamarellou H, Kanellakopoulou K. Current therapies for Pseudomonas aeruginosa. Crit Care Clin. 2008;24:261–278. doi: 10.1016/j.ccc.2007.12.004. viii. [DOI] [PubMed] [Google Scholar]
- 13.Castanheira M, Mills JC, Farrell DJ, Jones RN. Mutation-driven beta-lactam resistance mechanisms among contemporary ceftazidime-nonsusceptible Pseudomonas aeruginosa isolates from U.S. hospitals. Antimicrob Agents Chemother. 2014;58:6844–6850. doi: 10.1128/AAC.03681-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ropy A, et al. Role of Pseudomonas aeruginosa low-molecular-mass penicillin-binding proteins in AmpC expression, beta-lactam resistance, and peptidoglycan structure. Antimicrob Agents Chemother. 2015;59:3925–3934. doi: 10.1128/AAC.05150-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabot G, et al. Evolution of Pseudomonas aeruginosa Antimicrobial Resistance and Fitness under Low and High Mutation Rates. Antimicrob Agents Chemother. 2016;60:1767–1778. doi: 10.1128/AAC.02676-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berrazeg M, Jeannot K, Enguéné VY, Broutin I, Loeffert S, Fournier D, Plésiat P. Mutations in β-lactamase AmpC increase resistance of Pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrobial agents and chemotherapy. 2015;59:6248–6255. doi: 10.1128/AAC.00825-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mark BL, Vocadlo DJ, Oliver A. Providing beta-lactams a helping hand: targeting the AmpC beta-lactamase induction pathway. Future Microbiol. 2011;6:1415–1427. doi: 10.2217/fmb.11.128. [DOI] [PubMed] [Google Scholar]
- 18.Moya B, et al. Beta-lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. Plos Pathog. 2009;5:e1000353. doi: 10.1371/journal.ppat.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadlamani G, et al. The beta-lactamase gene regulator AmpR is a tetramer that recognizes and binds the D-Ala-D-Ala motif of its repressor UDP-N-acetylmuramic acid (MurNAc)-pentapeptide. J Biol Chem. 2015;290:2630–2643. doi: 10.1074/jbc.M114.618199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moya B, Juan C, Albertí S, Pérez JL, Oliver A. Benefit of Having Multiple ampD Genes for Acquiring β-Lactam Resistance without Losing Fitness and Virulence in Pseudomonas aeruginosa. Antimicrob Agents Ch. 2008;52:3694–3700. doi: 10.1128/aac.00172-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.San Millan A, Escudero JA, Gifford DR, Mazel D, MacLean RC. Multicopy` plasmids potentiate the evolution of antibiotic resistance in bacteria. Nature Ecology & Evolution. 2016;1:0010. doi: 10.1038/s41559-016-0010. [DOI] [PubMed] [Google Scholar]
- 22.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology. 2007;21:394–407. doi: 10.1111/j.1365-2435.2007.01283.x. [DOI] [Google Scholar]
- 23.Balasubramanian D, et al. Deep sequencing analyses expands the Pseudomonas aeruginosa AmpR regulon to include small RNA-mediated regulation of iron acquisition, heat shock and oxidative stress response. Nucleic Acids Res. 2014;42:979–998. doi: 10.1093/nar/gkt942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong KF, et al. Pseudomonas aeruginosa AmpR is a global transcriptional factor that regulates expression of AmpC and PoxB beta-lactamases, proteases, quorum sensing, and other virulence factors. Antimicrob Agents Chemother. 2005;49:4567–4575. doi: 10.1128/AAC.49.11.4567-4575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumari H, Balasubramanian D, Zincke D, Mathee K. Role of Pseudomonas aeruginosa AmpR on β-lactam and non-β-lactam transient cross-resistance upon pre-exposure to subinhibitory concentrations of antibiotics. Journal of medical microbiology. 2014;63(4):544–555. doi: 10.1099/jmm.0.070185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell JIA, Ciofu O, Høiby N. Pseudomonas aeruginosa isolates from patients with cystic fibrosis have different β-lactamase expression phenotypes but are homogeneous in the ampC-ampR genetic region. Antimicrob Agents Chemother. 1997;41:1380–1384. doi: 10.1128/aac.41.6.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacoby GA. AmpC β-Lactamases. Clinical Microbiology Reviews. 2009;22:161–182. doi: 10.1128/cmr.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahiri SD, et al. Avibactam and class C beta-lactamases: mechanism of inhibition, conservation of the binding pocket, and implications for resistance. Antimicrob Agents Chemother. 2014;58:5704–5713. doi: 10.1128/AAC.03057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goering RV, Sanders CC, Sanders WE, Jr, Guay R, Guerin S. Heterogeneity in ampR-ampC gene interaction in Enterobacter cloacae. Rev Infect Dis. 1988;10(4):786–792. doi: 10.1093/clinids/10.4.786. [DOI] [PubMed] [Google Scholar]
- 30.Laabei M, et al. Predicting the virulence of MRSA from its genome sequence. Genome Res. 2014;24:839–849. doi: 10.1101/gr.165415.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Earle SG, et al. Identifying lineage effects when controlling for population structure improves power in bacterial association studies. Nature Microbiology. 2016;1:16041. doi: 10.1038/nmicrobiol.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luria SE, Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moya B, et al. Beta-lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. Plos Pathog. 2009;5:e1000353. doi: 10.1371/journal.ppat.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.San Millan AS, et al. Positive selection and compensatory adaptation interact to stabilize non-transmissible plasmids. Nature Communications. 2014;5 doi: 10.1038/Ncomms6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. Plos One. 2012;7:e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DePristo MA, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danecek P, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DePristo MA, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen K, et al. BreakDancer: an algorithm for high-resolution mapping of genomic structural variation. Nat Methods. 2009;6:677–681. doi: 10.1038/nmeth.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25:2865–2871. doi: 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boeva V, et al. Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics. 2012;28:423–425. doi: 10.1093/bioinformatics/btr670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marco-Sola S, Sammeth M, Guigo R, Ribeca P. The GEM mapper: fast, accurate and versatile alignment by filtration. Nat Methods. 2012;9:1185–1188. doi: 10.1038/nmeth.2221. [DOI] [PubMed] [Google Scholar]
- 45.Kanehisa, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45:D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hahne F, et al. flowCore: a Bioconductor package for high throughput flow cytometry. BMC Bioinformatics. 2009;10:1–8. doi: 10.1186/1471-2105-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarkar D, Le Meur N, Gentleman R. Using flowViz to visualize flow cytometry data. Bioinformatics. 2008;24:878–879. doi: 10.1093/bioinformatics/btn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2017. URL http://www.R-project.org/ [Google Scholar]
- 49.Vogwill T, Kojadinovic M, MacLean RC. Data from: Epistasis between antibiotic resistance mutations and genetic background shape the fitness effect of resistance across species of Pseudomonas. Dryad Digital Repository. 2016 doi: 10.5061/dryad.qn370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.