Abstract
Innate immunity includes a cellular and a humoral arm. PTX3 is a fluid phase pattern recognition molecule (PRM) conserved in evolution which acts as a key component of humoral innate immunity in infections of fungal, bacterial and viral origin. PTX3 binds conserved microbial structures and self-components under conditions of inflammation and activates effector functions (complement, phagocytosis). Moreover, it has a complex regulatory role in inflammation, such as ischemia/reperfusion injury and cancer-related inflammation, as well as in extracellular matrix organization and remodeling, with profound implications in physiology and pathology. Finally, PTX3 acts as an extrinsic oncosuppressor gene by taming tumor promoting inflammation in murine and selected human tumors. Thus, evidence suggests that PTX3 is a key homeostatic component at the crossroad of innate immunity, inflammation, tissue repair and cancer.
Dissecting the complexity of PTX3 pathophysiology and human genetics paves the way to diagnostic and therapeutic exploitation.
Keywords: pentraxins, PTX3, inflammation, innate immunity, complement activation, cancer-related inflammation
1. Introduction
Innate immune responses are key to resistance against infectious agents and orchestrate response to tissue damage and repair (99). Moreover, innate immunity sensors respond to dysmetabolic conditions triggering inflammation. The innate immune system includes a cellular and a humoral arm. Polymorphonuclear leukocytes and macrophages, the main cells involved in innate immunity and inflammation, recognize microbes, tissue and cell damage, and products of dysmetabolism (e.g. uric acid crystals) via pattern recognition receptors (PRR) strategically located on the cell membrane, endosomal compartment and cytoplasm. PRRs belong to different classes including toll-like receptors, dectins, inflammasomes and nucleic acid sensors (79, 97, 103, 122).
The innate immune system includes a humoral arm, complementary to cellular recognition and effector function. Humoral, fluid phase pattern recognition molecules (PRM) are as diverse as cellular sensors. Humoral PRM include Complement components, mannose binding lectin (MBL), surfactant proteins, ficolins and pentraxins. The pentraxin C reactive protein (CRP) was in fact the first PRM to be recognized as an antibody-like molecule recognizing the C type polysaccharide of pneumococcus (1, 141). In general, humoral PRM behave as functional ancestors of antibodies, “ante-antibodies”. They recognize microbial moieties and tissue damage, activate Complement, promote phagocytosis (opsonic activity) and regulate inflammation.
The long pentraxin PTX3 is a prototypic PRM, highly conserved in evolution (51, 96), which has served as a tool to dissect recurrent themes in humoral innate immunity. Evidence suggests that this molecule has complex roles in pathophysiology which range from essential homeostatic functions (reproduction) to defense against infectious agents, tissue repair and regulation of carcinogenesis. Dissection of the molecular properties and complex role in pathophysiology of PTX3 has paved the way to ongoing translational efforts.
2. Gene and protein organization
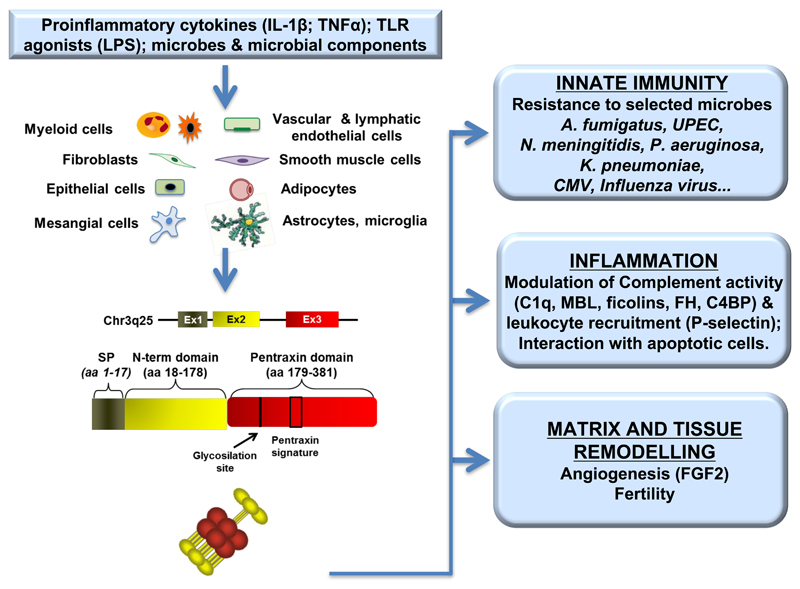
Human PTX3 gene is located on chromosome 3q25 and is organized into three exons (Fig. 1), the first two coding for a leader peptide and the long N-terminal domain (amino acids 18-178), while the third exon codes for the C-terminal pentraxin like domain (amino acids 179-381). The proximal promoter contains multiple binding sites for various transcription factors, including PU.1, AP-1, NF-κB, Sp1, and NFIL-6, all targets of proinflammatory cytokines [mainly tumor necrosis factor-α (TNF-α) and interleukin 1 (IL-1)] and Toll like receptors (TLR) agonists, essential for the induction of PTX3. PTX3 transcription can be also activated by PI3K/Akt and JNK pathways (60, 107), or downstream FUS/CHOP translocation (149).
Figure 1. Source and main functions of the long pentraxin PTX3.
Proinflammatory stimuli, TLR engagement and microbial recognition can induce PTX3 production by a wide variety of cell types, including cells of the myeloid lineage, vascular and lymphatic endothelial cells, smooth muscle cells, fibroblasts, epithelial cells, adipocytes, mesangial cells, astrocytes and cells of microglia. PTX3 gene is organized in three exons, the first coding the leader peptide, the second coding for the N-terminal domain and the third coding for the C-terminal, pentraxin like domain. The protein participates in matrix remodeling, plays a non-redundant role in the resistance to selected pathogens, has a regulatory role in inflammation and in fertility. The multifunctional properties of PTX3 are connected with the capacity to interact with different ligands.
Epigenetic mechanisms have been recently described that can be implicated in the regulation of human PTX3 gene expression. In particular hypermethylation of a PTX3 enhancer and of a region of PTX3 promoter is responsible of PTX3 gene silencing in human cancer (13, 143, 147), while lower methylation is associated to higher PTX3 plasma levels in coronary artery disease (58).
Single nucleotide polymorphisms (SNPs) in the human PTX3 have been investigated. SNPs described so far are mainly located in non-coding regions of PTX3 gene, with the exception of one exonic SNP causing an amino acid variation in position 48 (Asp48Ala). Specific PTX3 haplotypes have been associated to higher protein expression (7, 36), but the molecular mechanisms responsible of this association are still poorly understood.
The primary sequence of PTX3 protein is 381 amino acids long, including the 17 amino acids of the leader peptide (Fig 1). The C-terminal domain, homologous to the short pentraxins CRP and SAP, contains the 8 amino acid–long pentraxin signature (HxCxS/TWxS, where x is any amino acid), a consensus sequence highly conserved among all the members of the pentraxin superfamily. On the contrary, the long N-terminal domain has no sequence similarity to other known proteins. PTX3 primary sequence is highly conserved among animal species, indicating a strong evolutionary pressure to maintain its structure/function relationships (52, 69). In particular human and murine proteins share 92% of conserved amino acids. The murine PTX3 gene is located in the synthenic region of chromosome 3 (q24–28) and its expression profile resembles that of the human gene, strongly supporting the extension to humans of data obtained in ptx3-deficient mice.
A single N-glycosylation site has been identified in the C-terminal domain at Asn220, fully occupied by complex type oligosaccharides, in particular fucosylated and sialylated biantennary sugars and a minor fraction of tri- and tetraantennary glycans (66). In addition, the relative content of bi-, tri-, and tetra-antennary oligosaccharides and the level of sialylation in PTX3 isolates from different cellular sources vary to a great extent (66), thus suggesting that the glycosylation pattern of this long pentraxin might change depending on cell type and inducing stimuli.
As a member of the pentraxin superfamily, PTX3 is a homo-multimeric protein. Biophysical analysis revealed that PTX3 is an octameric molecule with a molecular mass of 340 kDa and composed of eight identical protomers interacting through intra- and inter-chain disulphide bonds (68). A low-resolution model of the full length PTX3 has been generated based on data from electron microscopy and Small Angle X-ray Scattering (SAXS). According to this model, the eight subunits of the protein are folded into an elongated structure with a large and a small domain interconnected by a stalk region (65). Prediction of N-terminal domain secondary structure suggested that this portion of the molecule is mainly folded in four α-helices involved in the formation of coiled-coil secondary structures (115). The asymmetric and octameric shape of PTX3 is unique amongst pentraxins inasmuch classical short pentraxins typically have a pentameric arrangement and only SAP from Limulus Polyphemus forms an octamer folding into a doubly stacked octameric ring (135).
As already mentioned, the C-terminal domain of PTX3 is homologous to the short pentraxins, with up to 57% similarity (20). Based on this similarity, a three-dimensional model of PTX3 C-terminal domain was generated, taking advantage of the crystallographic structures of CRP (PDBID:1b09) and SAP [PDBID:1sac; (56, 68, 69)]. This model shows a β-jelly roll topology for the C-terminal portion of PTX3, similar to that found in legume lectins (65). The structure is stabilized by three disulfide bonds involving six cysteine residues, two of which are highly conserved amongst pentraxins (68). Two cysteine residues are also described to form intra- and interchain disulfide linkages involving both the C-terminal and N-terminal portion of the molecule, thus supporting the quaternary structure of the PTX3 protein (see below) (68).
Despite the sequence similarities, PTX3 shows important differences in terms of producing cells and inducing stimuli compared to CRP and SAP. In fact, while CRP and SAP are made almost exclusively in the liver in response to IL-6, PTX3 is produced by different cell types in response to diverse stimuli (Fig. 1). The proinflammatory cytokines IL-1β and TNF-α, as well as TLR agonists induce PTX3 in cells of the myelomonocytic lineage, i.e. monocytes, macrophages, and myeloid dendritic cells [(DC; (39, 51)], while IL-6 is ineffective. Polymorphonuclear cells do not express PTX3 mRNA, however the protein is synthetized during the differentiation in the bone marrow and stored in lactoferrin-positive granules (72). Inflammatory signals and modified lipoproteins induce PTX3 in vascular endothelial cells and smooth muscle cells. In addition the protein is produced by fibroblasts, adipocytes, epithelial cells, neurons and glial cells (2, 60, 76, 88, 116).
The modular nature of PTX3 protomer provides a structural versatility that can support the interaction with a number of diverse ligands, mediating PTX3 biological activities (Fig. 1). For example, among the PTX3 ligands, fibroblast growth factor 2 (FGF2), inter-α-inhibitor (IαI), TNF-α–induced protein 6 (TNFAIP6 or TSG-6), myeloid differentiation protein 2 (MD-2), and conidia of Aspergillus fumigatus bind to the N-terminal domain of the protein (18, 52, 86, 100, 115, 133). On the other hand the pentraxin-like domain is mainly involved in the interaction with C1q, the first component of the classical complement pathway, and P-selectin (16, 41, 105), whereas both domains have been implicated in the interaction of PTX3 with complement factor H (FH), a major soluble inhibitor of the complement system (42).
A further fine tuning of PTX3 ligand binding properties is represented by the peculiar quaternary structure and the glycosidic moiety of PTX3. We have shown that the PTX3 octamer contains two FGF2 binding sites, with tetramers of the N-terminal domain acting as functional units in recognition and inhibition of this angiogenic factor (65). In addition, we have reported that dimers of the N-terminal domain are sufficient to mediate PTX3 binding to both IαI and TSG-6. This suggests that the octameric structure of PTX3 acts as nodal molecule in cross-linking hyaluronic acid in the extracellular matrix (6, 63). Interaction with both C1q and FH is modulated by the glycosylation status of PTX3 (42, 66). Most importantly, the N-linked glycosidic moiety of PTX3 is essential for the binding to P-selectin, an interaction mainly involved in regulation of leukocytes rolling and extravasation in animal models of acute lung injury and pleurisy (41). The sialylated glycans of PTX3 are also essential for the interaction of PTX3 with selected influenza A virus (IAV) strains (117). Thus changes in the glycosylation status of the protein might represent a strategy to fine tune the biological activities of PTX3 (67).
Therefore, the structural complexity and modular nature of the PTX3 protein probably explain the rather broad spectrum of ligands of this long pentraxin and the diversity of its biological roles as compared to the short pentraxins.
3. Humoral innate immunity
PRMs recognize microbes or tissue injury, activating the innate response. Cell-associated and soluble PRM have been described, the cellular receptors including TLR, NOD- and RIG-like receptors and scavenger receptors, while fluid-phase PRM includes members of the collectin, ficolin and pentraxin families (14, 50, 53, 62). CRP is the first PRM identified in the early 30’ as a molecule able to recognize the C-polysaccharide of Streptococcus pneumoniae through a direct interaction with posphorylcholine (PC), a major constituent of the C-type capsule. Besides S. pneumoniae, CRP binds various microbes, including bacteria, fungi, yeasts and parasites, through PC and glycan molecules, promoting complement activation, opsonisation, phagocytosis and resistance to infections (61). PTX3 shares the capacity to act as soluble PRM, recognizing microbial moieties and selected microorganisms, acting as an opsonin and regulating the inflammatory response.
3.1. Pattern recognition
The first description of PTX3 interaction with microbes is dated back to 2002, when it was reported that the higher susceptibility to infection with Aspergillus fumigatus observed in ptx3 deficient mice was associated to a direct binding of PTX3 to the fungus (52). Conidia from A. fumigatus were ingested more efficiently by alveolar macrophages following opsonisation with PTX3. PTX3 directly interacts with other fungal, bacterial and viral pathogens, including zymosan and Paracoccidoides brasiliensis (45), Pseudomonas aeruginosa (101), Klebsiella pneumoniae (73), Neisseria meningitidis (15), uropathogenic Escherichia coli (71), influenza virus (117), human and murine cytomegalovirus (17), murine hepatitis virus (59). Through the direct interaction with microbes, PTX3 acts as an opsonin, facilitating recognition and phagocytosis in an Fcγ receptor- and complement-dependent manner.
As expected for a PRM, PTX3 can recognize conserved microbial moieties which fulfill essential microbial functions. In particular PTX3 binds a member of the outer membrane proteins A (OmpA) family derived from K. pneumoniae [KpOmpA; (73)] or outer membrane vescicles (OMV) and meningococcal antigens from N. meningitidis (15).
In general, following recognition of selected microorganisms, PTX3 exerts a protective role that involves disposal of the pathogens by phagocytosis operated mainly by phagocytes, and activation of the complement cascades.
3.2. Effector mechanisms
3.2.1. Receptor(s)
Interaction of PTX3 with cells of the monocyte/macrophage lineage suggested the existence of a cellular receptor for this molecule (52). Attempts to identify a unique PTX3 receptors took advantage of the high homology with the short pentraxins CRP and SAP. Several functional studies suggested that SAP and CRP can directly interact with Fcγ receptors (FcγR) and these data were corroborated by structural analysis of SAP in complex with the extracellular domain of FcγRIIa (90). Surface plasmon resonance studies demonstrated that CRP and SAP directly interact with both activating FcγR (FcγRI, FcγR IIa and FcγRIII) and the inhibitory receptor FcγRIIb with affinity ranging from 10-5 to 10-7 M (90). In the same set of analysis it was shown that also PTX3 could recognize FcγR, particularly FcγRIII (KD 1.6 µM) and, with a lower affinity, FcγRIIa (KD 18.7 µM). Interestingly, the PTX3 opsonic activity is dependent on FcγRIIa and complement receptor 3 (CR3; CD11b/CD18) [see below; (52, 71, 100)], thus supporting a functional role for the interaction with these receptors.
FcγR are not the only receptors for SAP and CRP. Recently it has been shown that neutrophils, monocytes and macrophage still respond to SAP in the absence of FcγR. This occurs because of a specific interaction between SAP and dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin [(DC-SIGN); (34)]. SAP but not CRP binds DC-SIGN in a glycosylation-dependent manner, activating the receptor to regulate innate immune cells. Functional evidences, showing that PTX3 activates DC-SIGN and affects fibrocyte differentiation, suggested that also PTX3 could interact with DC-SIGN (34). However no data are available indicating a direct interaction of PTX3 with the receptor and we failed to validate the interaction of PTX3 with DC-SIGN.
3.2.2. Complement and other humoral PRMs
PTX3 shares with the classical short pentraxins CRP and SAP the capacity to modulate all three complement pathways (e.g the classical, the alternative and the lectin pathway) through a direct interaction with key molecules involved in activation and/or regulation of the complement cascades (16, 19, 35, 42, 46, 92–94, 105).
C1q, the first component of the classical complement pathway, was the first PTX3 ligand identified (16, 105). PTX3 bound C1q in a calcium-independent fashion, as opposed to CRP and SAP, that required calcium for their interaction with C1q (56), and interacts with the globular C1q domain (102, 125). Interaction with surface bound C1q resulted in activation of the classical complement cascade and deposition of C3 and C4. On the contrary, when interaction occurred in solution, PTX3 inhibited the complement cascade via competitive blocking of relevant interaction sites.
Modulation of the lectin complement pathway by PTX3 was due to specific interactions with ficolin-1, ficolin-2 and MBL. The interaction with ficolin-2 or MBL resulted in enhancement of complement deposition on the surface of Aspergillus fumigatus and Candida albicans, respectively (57, 92–94). After the formation of PTX3/MBL complexes, C1q was recruited and promoted C4 and C3 deposition on C. albicans. In addition PTX3/MBL complexes enhanced the phagocytosis of the pathogen. Similarly, immobilized PTX3 was able to trigger ficolin-1-dependent activation of the lectin complement pathway (57).
Finally PTX3 interacted with negative regulators of complement activation. Microtiter plate-based assay and surface plasmon resonance evidenced a direct interaction of PTX3 with FH. Two binding sites were identified, one in the short consensus repeat 7 (SCR7) and one in SCR19–20, at the most C-terminal region of FH (42). SCR19–20 interacted with the N-terminal domain of PTX3, while SCR7 bound the C-terminal domain, consistently with reports showing CRP interacting with the same FH region (109). Surface-bound PTX3 enhanced FH recruitment and iC3b deposition, modulating the activation of the alternative complement pathway and preventing an excessive inflammatory response to tissue injury, while increasing the deposition of opsonic molecules (42). Various diseases are associated to dysregulation of the alternative complement pathway due to mutation or polymorphisms of FH, in particular atypical hemolytic uremic syndrome (aHUS) and age-related macular degeneration (AMD). Interestingly, mutations in FH observed in patients with aHUS were associated with a reduced interaction between FH and PTX3 (82). Given the capability of PTX3 to recruit FH and to control excessive local complement activation, the defective interaction between the two molecules in aHUS patients amplified local complement-mediated inflammation essential in the pathogenesis of the disease. We also observed that a FH polymorphism in SCR7 (Tyr402His), associated with increased risk and incidence of AMD (153), did not influence the PTX3–FH interaction, in contrast to what observed when CRP was used. The altered interaction between variant FH and CRP was proposed as a pathogenetic mechanism in AMD causing decreased removal of drusen deposits and increased inflammation (83). Thus PTX3 could represent a possible substitute to ineffective CRP in individuals carrying the 402His variant.
Finally, PTX3 recruited C4 binding protein (C4BP) on apoptotic cells, thus reducing the deposition of the lytic C5b-9 terminal complex (19). PTX3 was capable of targeting functionally active C4BP to sites of tissue injury, thus limiting complement-mediated inflammation.
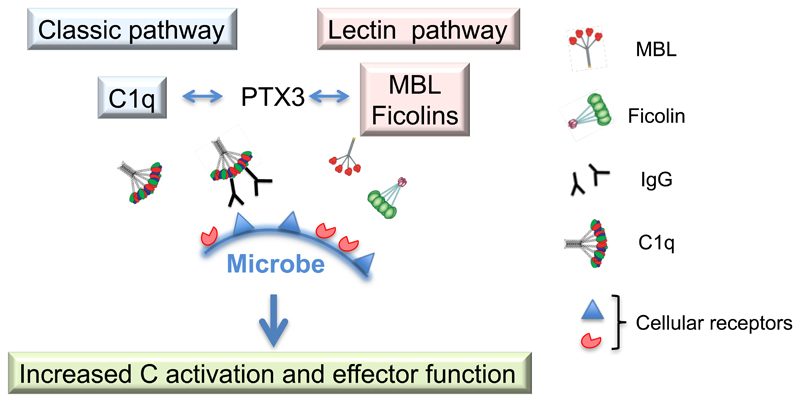
The analysis of the interaction of PTX3 with components of the humoral arm of innate immunity has broad implications in relation to the underlying logic of recognition and effector function. The interaction with other fluid phase PRM (ficolin 1 and 2; MBL; C1q) results in a broader repertoire of effective microbial recognition, synergism in pathogen disposal and plasticity in utilizing different Complement activation pathways (Fig. 2). Moreover, these results highlight the potential of PTX3 as a regulatory molecule of inflammatory reaction, as discussed below.
Figure 2. Synergistic interaction of PTX3 with other fluid phase PRM.
The interaction of PTX3 with other fluid phase PRM, namely C1q, MBL and ficolins, results in a broader repertoire of effective microbial recognition leading to increased Complement activation and effector functions.
4. Regulation of inflammation
The modulation of complement activity by PTX3, detailed above, is one of the main mechanisms through which this long pentraxin exerts its regulatory function on the inflammatory response. For example, PTX3 was show to amplify the inflammatory response induced in vivo by KpOmpA, once the microbial moiety was recognized by cellular receptors. The proinflammatory program induced by KpOmpA included production of PTX3, that in turns bound this microbial moiety and amplified inflammatory cells recruitment (73). This effect was abrogated by treatment with complement inhibitors, underlining the complement dependency of the amplification promoted by PTX3 (33).
Recently it was shown that PTX3 is induced by oxidative stress, a recognized cause of AMD, in the retinal pigmented epithelium (148). Upon induction, PTX3 regulated complement activation induced by oxidative stress through the interaction of FH. Oxidative stress in PTX3-deficient mice led to increased C3a levels, which in turn amplified NALP3 inflammasome activation and IL-1β production, and consequently macrophage recruitment in the choroid, demonstrating that PTX3 acts as essential brake for both the complement and the inflammasome pathways (148).
Besides complement activation, PTX3 regulates the inflammatory response acting on inflammatory cell recruitment. This effect was mediated by the capacity of PTX3 to interact with the adhesion molecule P-selectin, an interaction involving the N-linked glycosidic moiety of PTX3 (41). The interaction between PTX3 and P-selectin resulted in inhibition of leukocyte rolling on endothelium. Accordingly, PTX3 administration in vivo reduced leukocytes recruitment in models of pleurisy, acute lung injury and ischemia/reperfusion-induced kidney damage (41, 87).
Genetic models were essential to define the role of PTX3 in tissue damage both in sterile and infectious conditions. In different models of ischemia/reperfusion ptx3 deficiency was associated to higher tissue damage. In a model of post-ischemic renal injury, higher tissue damage was observed in ptx3-/- mice and administration of the recombinant protein reverted the phenotype, with enhanced recovery, suppression of glomerulosclerosis and inhibition of interstitial fibrosis (87, 151). In this model, renal injury was associated to a post-ischemic leukocyte recruitment completely abrogated by treatment with a neutralizing antibody against P-selectin (87). On the contrary, in a model of intestinal ischemia and reperfusion, the absence of PTX3 was associated with lower inflammation and lethality (138). The molecular mechanisms explaining these differences have not been addressed. A differential involvement of complement in kidney and intestinal ischemia might be responsible of the divergent effect of PTX3-deficiency in the two models.
In humans and in mice PTX3 was rapidly produced during acute myocardial ischemia. In a murine model of acute myocardial infarction, ptx3-/- animals developed more myocardial damage, with more neutrophil infiltration, decreased number of capillaries, increased number of apoptotic cardiomyocytes and higher C3 deposition in lesional tissue (130). Apolipoprotein E deficiency in ptx3-/- mice resulted in higher aortic lesions and a more pronounced inflammatory profile (108). Ablation of PTX3 in mice also aggravated coxsackievirus B3 (CVB3) -triggered inflammatory injury of the heart tissue and the associated cardiomyocyte apoptosis (111). PTX3 did not exert any direct antiviral effect, but rather facilitated the clearance of dead or dying cells.
It was previously reported that PTX3 bound apoptotic cells and enhanced complement-mediated clearance of the apoptotic debris (106, 126). This property plays a role also in a murine model of seizure-induced degeneration, where ptx3-/- mice displayed an increased number of dying neurons (apoptotic and/or necrotic) compared to wild type mice (116). Several studies have shown that efficient apoptosis inhibited the inflammatory response; therefore the capacity of PTX3 to affect engulfment of apoptotic cells could likely represent an additional mechanism of regulation of inflammation.
PTX3 gene inactivation strongly compromised the integrity of blood-brain barrier and the resolution of brain edema after ischemic injury. The defect in resolution was associated with impaired glial scar formation and alterations in scar-associated extracellular matrix production (121), highlighting the potential role of PTX3 in brain repair after cerebral ischemia.
Novel information on a critical role played by PTX3 has been provided by a recent report showing that ptx3 deletion aggravates allergic inflammation induced by ovoalbumin exposure. In ovoalbumin-induced experimental asthma, the absence of PTX3 resulted in an exaggerated allergen-induced inflammation (5), with enhanced recruitment of neutrophils and eosinophils, enhanced mucus production and higher secretion of IgE/IgG2a. A Th17-dominant CD4 T-cell response has been observed in ptx3-/- mice. As a matter of fact, ptx3-/- DC produced more IL-6 and IL-23, two cytokines with Th17 polarizing properties, providing a possible explanation for the Th17-dominan inflammation observed in ptx3-/- mice.
Contradictory results were obtained when PTX3 overexpressing mice were analyzed. In fact they showed greater resistance than wild-type mice to endotoxic shock induced by LPS and to polymicrobial sepsis caused by cecal ligation and puncture, but also an exacerbated inflammatory response in a model of intestinal ischemia and reperfusion (44, 138). In particular, in intestinal ischemia and reperfusion, PTX3 overexpressing mice displayed increased production of proinflammatory cytokines, higher degree of tissue damage and enhanced lethality compared to wild type animals. In a model of ventilator-induced lung injury, PTX3 overexpression resulted in increased inflammatory response (118). Finally PTX3 has been found able to induce endothelial dysfunction, inhibiting the vasorelaxation induced by acetylcholine and determining morphological changes in endothelial cells (25). Thus, in the context of ischemia and reperfusion injury, PTX3 could exert dual opposite roles, being protective or deleterious depending on specific tissues.
Collectively so far, available information points to the essential role of PTX3 in the regulation of the inflammatory response.
5. Tissue remodeling and repair
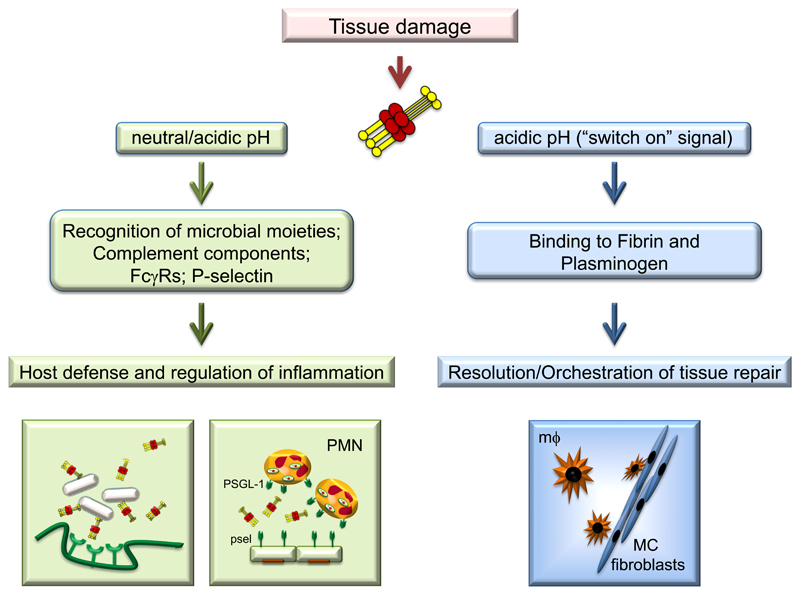
The role of PTX3 in tissue remodelling and repair has been recently investigated using different models of tissue damage (skin wound healing, chemically-induced sterile liver and lung injury, arterial thrombosis). In models of skin wounding, TLR and IL-1 receptor activation induced PTX3 expression in macrophages and mesenchymal cells, and the protein localized in the pericellular provisional fibrin matrix of these cells, where it promoted the directional migration and invasive phenotype of remodelling cells. Indeed, PTX3-deficient macrophages and mesenchymal cells showed defective pericellular fibrinolysis in vitro and failed to migrate within the fibrin matrix and remodel it in vivo. Excessive fibrin accumulation and subsequent increased collagen deposition were the major anomalies observed in skin, liver and lung injury models in PTX3-deficient mice, in line with the prominent role of fibrin as a provisional matrix protein guiding subsequent repair (21). These phenotypes were attributed to the interaction of PTX3 through the N-terminal domain with fibrinogen (FG)/fibrin and plasminogen (Plg) at acidic pH, which promoted fibrin degradation (Fig. 3). In agreement, selective inhibitors of fibrin deposition and platelet activation reverted the phenotype of PTX3-deficient mice in skin wound healing. Acidification of the injured tissue due to cell metabolic adaptation to tissue hypoperfusion and hypoxia, is necessary for the tripartite interaction among PTX3, fibrin and plasminogen, and this assures that the interaction occurs at sites of tissue damage and not in the circulation. These results have been supported by an in vivo study showing that PTX3 released from bone marrow-derived mesenchimal stem cells promoted mesenchimal stem cell pericellular fibrinolysis and migration within the fibrin-rich provisional matrix in a skin wound site favoring repair (23).
Figure 3. An acidic pH sets the PTX3 molecule in a tissue repair mode.
While at neutral pH PTX3 mainly participates to host defense and regulation of inflammation, at acidic pH PTX3 can interact with fibrin and plasminogen, promoting pericellular fibrinolysis by tissue remodelling cells and contributing to an appropriate tissue repair.
In a mouse model of arterial thrombosis, PTX3 derived from vascular endothelium localized within the thrombus and in the vessel wall, and dampened thrombogenesis. By targeting fibrinogen through its N-terminal domain, PTX3 inhibited platelet adhesion and aggregation (12). In addition, PTX3 was shown to play a protective role in a mouse model of brain ischemic injury, and to be involved in edema resolution and glial scar formation (121).
These findings provide a novel link between inflammation, immunity, haemostasis and tissue repair (48), and evidence that the recognition of microbial moieties and extracellular matrix molecules by the humoral arm of innate immunity are evolutionarily linked. Indeed, humoral PRMs interact with extracellular matrix components (e.g. C1q, collectins, CRP, SAP), or contain ancestral collagen and fibrinogen domains (e.g. ficolins, MBL, collectins) (14), and several extracellular matrix components recognize microbial moieties and have opsonic activity (e.g. fibronectin, mindin, osteopontin, vitronectin).
6. Role in female fertility
PTX3-deficiency is associated with a severe defect in female fertility (131). This unexpected phenotype has been attributed to defective assembly of the viscoelastic hyaluronan (HA)-rich matrix that is produced by cumulus oophorus and granulosa cells and deposited around the oocyte in the preovulatory follicle, and that is essential for successful in vivo fertilization. In this context, PTX3 is produced by mouse and human cumulus cells during cumulus expansion under the control of hormonal ovulatory stimuli (FSH or hCG), and oocyte-derived soluble factors, in particular by the TGFβ family members Growth Differentiation Factor-9 (GDF-9) and bone morphogenetic protein 15 (BMP15) (112, 131, 145), and localizes in the cumulus oophorus matrix. HA, a large glycosaminoglycan responsible for the viscoelastic properties of the cumulus matrix, represents the major component of this matrix, and the HA-binding proteins TSG-6 and IαI, together with PTX3, are required for the correct assembly of this matrix. In fact, deficiency of IαI, TSG-6 or PTX3 lead to impaired cumulus matrix formation and severe sub-fertility (128). In the process of matrix assembly, the heavy chains (HCs) of IαI become covalently linked to HA through reactions involving TSG-6. The long pentraxin PTX3, through its multimeric structure, establishes multiple contacts with the HCs and TSG-6, thus providing structural integrity to the cumulus matrix (68, 133). Recombinant PTX3 N-terminal domain, that forms tetramers and contains the HC-binding site, is sufficient to rescue the defective assembly of the cumulus matrix of PTX3-deficient cumuli (133). Interestingly, PTX3 genetic variants in humans have been associated with female fertility (98) and dizygotic twinning in Gambia (136).
Maternal circulating PTX3 levels slightly increase during pregnancy, likely reflecting the systemic inflammatory reaction associated with this condition (119). Compared to normal pregnancy, higher maternal PTX3 levels were observed in pregnancies complicated by preeclampsia (26, 127), possibly as a consequence of the endothelial dysfunction typical of this pregnancy disorder (132).
PTX3 plasma and vaginal levels were also increased during pregnancy complicated by spontaneous preterm delivery and in particular in the cases of placenta vasculopathy (3).
7. Infections
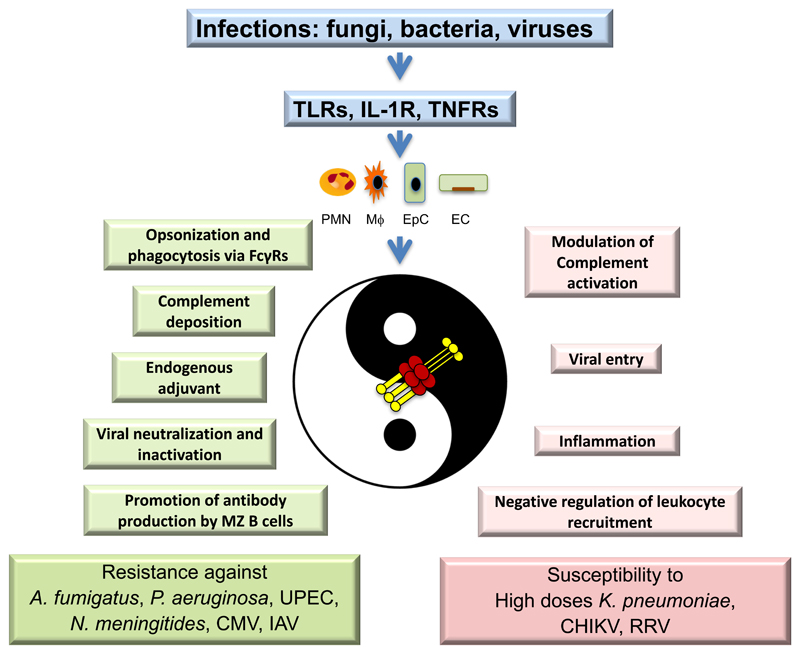
As mentioned above, PTX3 interacts with different fungi, bacteria and viruses. In most infectious conditions caused by recognized microorganisms, the deficiency of PTX3 in mice was associated with increased susceptibility to the infection (Figure 4). In particular, Ptx3-deficient mice were more susceptible to invasive pulmonary aspergillosis, showing higher mortality than wild-type mice (52) and developed an exacerbated T helper cell type 2 (Th2) response coupled with a low protective Th1 antifungal response (52). Treatment with recombinant PTX3 reversed this phenotype and induced a protective Th1 response, possibly by facilitating conidia internalization by antigen-presenting cells, thus participating in modulating the development of the immune responses to this fungus in immunocompetent mice, as well as in p47phox-/- mice, a model of chronic granulomatous disease (38, 52, 54).
Figure 4. The Yin-Yang of PTX3 in Innate Immunity.
Depending on the pathogen, functional effects and levels of production, PTX3 plays a Yin-Yang. In infectious diseases PTX3 can exert protective roles (in green) or may contribute to pathogenesis (in pink).
The analysis of cellular and molecular mechanisms underlying the protective role of PTX3 in this and other infections (e.g. A. fumigatus, P. aeruginosa, uropathogenic E. coli) showed that PTX3 has opsonic activity and facilitates phagocytosis by neutrophils of bound microorganisms through a FcγRII-, CD11b-, and complement-dependent mechanism (52, 72, 100). Indeed, PTX3 enhances the phagocytosis of conidia from A. fumigatus or P. aeruginosa by interacting with FcγRIIa, which have been proposed as pentraxin receptors (90), leading to the activation of CR3 (CD11b/CD18), thereby promoting the phagocytosis of C3-opsonised pathogens (100). PTX3 has therapeutic activity in a mouse model of chronic P. aeruginosa lung infection, a major cause of morbidity and mortality in cystic fibrosis patients, reducing the lung bacterial load and inflammation (lung proinflammatory cytokines and leukocytes) (101). PTX3 was found in high concentration in milk and in particular in colostrum. When administered via the oral route, mimicking the delivery of PTX3 through colostrum, PTX3 rapidly diffused in tissues and protected neonate mice from P. aeruginosa lung infection, thus contributing to innate immunity of neonates (70).
The role of PTX3 was studied in different infectious models by Gram-negative bacteria, namely uropathogenic E. coli, Neisseria meningitidis and K. pneumoniae. PTX3 is the first soluble PRM involved in resistance to urinary tract infections induced by uropathogenic E. coli (71). During urinary tract infection, inflammatory cells and uroepithelial cells produced PTX3 in a TLR4- and Myeloid differentiation primary response gene 88- (MyD88)-dependent manner and PTX3 facilitated phagocytosis of E. coli by neutrophils, thus controlling the bacterial load as well as tissue inflammation and damage (71). PTX3 also interacted with acapsular Neisseria meningitidis, through outer membrane vesicles and selected meningococcal antigens, and reduced the bacterial load in animal models of infection, by amplifying the responses to this bacterium (15). Finally, the infection by K. pneumoniae of PTX3-overexpressing mice caused increased inflammatory responses, leading to protection or faster lethality, depending on the bacterial load inoculated (137).
In cytomegalovirus (CMV) infection, PTX3 played a protective role by reducing viral entry and infectivity in DC in vitro and protecting mice from CMV primary infection and reactivation in vivo (17). PTX3 binds also the viral haemagglutinin glycoprotein of influenza virus strain H3N2 through the sialic acid residue present on its glycosidic moiety and inhibits hemagglutination and viral neuramidase activity (117). In contrast, PTX3 was inefficient in the defense against both seasonal and pandemic H1N1 influenza (78). The lack of effect of PTX3 was attributed to amino acid variations of viral hemagglutinin and neuraminidase and specificity for PTX3 sialic acid (77). In contrast with the described protective role of PTX3 in viral infections, PTX3 was shown to bind to arthritogenic alphaviruses (chikungunya virus and Ross River virus) and to facilitate viral entry and replication during the acute phase of infection, causing enhanced viral infectivity and prolonged disease (49).
SNPs of the PTX3 gene associated in particular haplotypes have been linked to susceptibility to different infections (see below), indicating that the functional role of PTX3 in innate resistance to infections is conserved in evolution and clinically relevant.
The results obtained in animal models of bacterial and viral infections suggest a Yin-Yang role for PTX3 in infections, since depending on the context and levels of production, PTX3 plays protective roles or may contribute to pathogenesis of infectious diseases (Fig. 4).
8. A link between innate and adaptive immunity
Innate and adaptive immunity are linked and it is therefore not surprising that PTX3 contributes to adaptive immune responses. PTX3-deficiency was responsible of the development of a defective protective Th1-skewed response and of an abnormal Th2-skewed response during A. fumigatus infection in vivo, which were attributed to defective DC activation by A. fumigatus conidia in the absence of PTX3 (see above and (52)). In addition, PTX3-deficient mice developed an impaired antibody response in vaccination protocols with N. meningitis Outer Membrane Vesicles, and co-administration of PTX3 increased the antibody response. These results suggest that PTX3, upon recognition of conserved microbial moieties of N. meningitidis, acts as an endogenous adjuvant of humoral responses to this bacterium (15).
Recently, PTX3 has been directly implicated in the regulation of antibody production (31). Together with B-1 cells, splenic marginal zone (MZ) B cells, an innate-like subset of antibody-producing lymphocytes located at the interface between the circulation and the adaptive immune system, homeostatically generate antibodies through an innate-like pathway: upon activation of B cell receptors, complement receptors, and TLRs, MZ B cells rapidly produce IgM and IgG with limited specificity for carbohydrate and lipid antigens through a T cell–independent pathway. PTX3 was shown to be released by a specific subset of neutrophils that surround the splenic MZ, to bind MZ B cells as well as and B-1 cells and immature B cells, activating FcγR-independent signals that triggered class-switching from IgM to IgG. PTX3 promoted MZ B cell differentiation into extrafollicular plasmablasts and plasmacells. As a consequence, PTX3 enhanced IgM and IgG responses to the blood-borne encapsulated bacterium Streptococcus pneumoniae, capsular polysaccharides or immunization with bacterial carbohydrates. These results indicate that PTX3 may bridge the humoral arms of the innate and adaptive immune systems by serving as an endogenous adjuvant for innate-like B cell subsets, including MZ B cells and B-1 cells (31).
9. Cancer
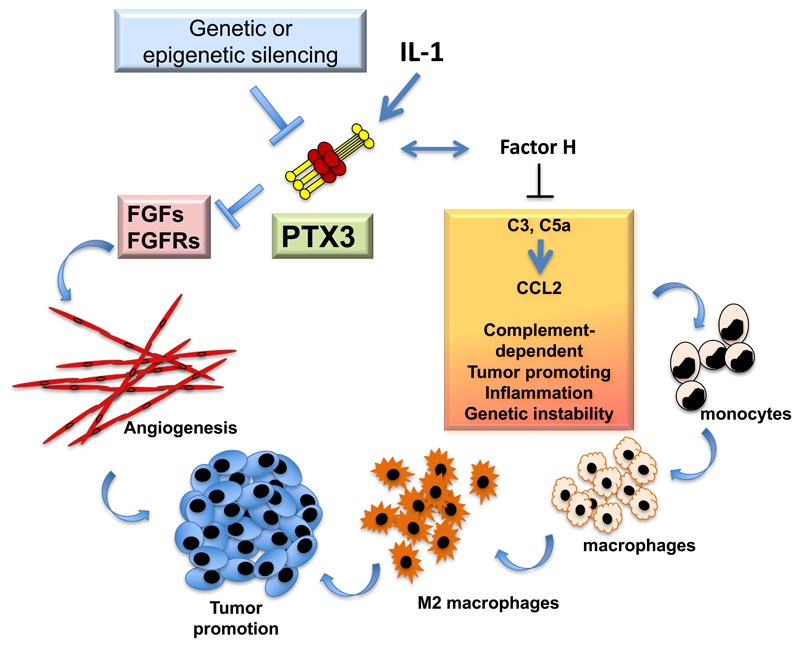
In the context of an effort to translate PTX3 to the clinic as a novel candidate antimicrobial agent in cancer patients at risk of opportunistic infections (36), we assessed its role in carcinogenesis and observed that PTX3-deficiency is associated with increased susceptibility to chemically-induced mesenchymal and epithelial carcinogenesis (13). In the models of 3-Methylcholanthrene (3-MCA)-induced carcinogenesis, and 7,12-dimethylbenz [α] anthracene/terephthalic acid (DMBA/TPA)-induced skin carcinogenesis, infiltrating myeloid cells and endothelial cells were a major source of PTX3 in response to locally produced IL-1. PTX3-deficient tumors showed enhanced macrophage infiltration, pro-inflammatory cytokine production, angiogenesis, complement C3 deposition and C5a levels, indicating increased cancer-related inflammation and complement activation. C3-genetic inactivation and CCL2-inhibition reverted the increased susceptibility to mesenchymal carcinogenesis and the M2-like phenotype and recruitment of tumor-associated macrophages in PTX3-deficient mice (Fig. 5). PTX3 was shown to regulate C3-deposition on sarcoma cells by interacting with and recruiting the negative regulator Factor H (13). Cancer-related inflammation has been proposed to contribute to cancer cell genetic instability (32). Interestingly, PTX3-deficiency was associated with increased DNA damage, as evidenced by increased mutations of Trp53, one of the genes targeted by 3-MCA, oxidative DNA damage and expression of DNA damage markers (13). Finally, in selected human mesenchymal and epithelial tumors, the PTX3 promoter and regulatory regions were highly methylated and this epigenetic modification caused transcriptional inactivation and silencing of PTX3 expression (13, 123, 147). In colorectal cancer PTX3 gene methylation and silencing is an early event, already detectable in adenomas and stage 1 neoplastic lesions, an observation consistent with a key role in pathogenesis (13). PTX3 interacts with several fibroblast growth factors (FGFs), such as FGF2, and FGF8b, and inhibits FGF-dependent angiogenic responses (115), including cancer-associated angiogenesis, and as a consequence, tumor growth (123, 124). In apparent contrast with these studies, it has been proposed that in specific models PTX3 has a pro-tumorigenic role, for instance by promoting tumor cell migration and invasion and macrophage chemotaxis (27, 28, 30, 81).
Figure 5. Role of PTX3 in cancer.
PTX3 regulates complement activation and tumor promoting inflammation, as well as FGF-dependent neo-angiogenesis and tumor growth. Indeed, genetic or epigenetic silencing of PTX3 results in increased tumor growth.
In different clinical studies, PTX3 emerged as a local or systemic marker of cancer-related inflammation. In particular, PTX3 was overexpressed in soft tissue sarcomas (55), lung cancer (64, 114), myeloproliferative neoplasms (8), pancreatic carcinoma (81), gliomas (89), and hepatocellular carcinoma (24), sometime correlating with cancer malignancy. In the case of lung cancer, different studies showed increased systemic and local PTX3 expression levels and a correlation with disease aggressiveness and progression (64, 114). Interestingly, in contrast with other epithelial cells that are poor producers of PTX3, lung epithelial cells express PTX3 in inflammatory conditions via JNK (60). This suggests that cancer-related inflammation may directly regulate PTX3 expression in lung cancer cells. Similarly, in myeloproliferative neoplasms, PTX3 expression correlated with mutant JAK2 (JAK2V617F) allele burden (8, 91), which is known to sustain leukocyte activation.
In conclusion, results in preclinical models and in selected human tumors (e.g. colorectal cancer) indicate that PTX3 acts as an extrinsic oncosuppressor gene, taming complement-driven macrophage-mediated tumor promotion. In other tumors, elevated PTX3 expression and levels appear to reflect cancer related inflammation or genetic events that drive carcinogenesis as is the case for JAK2 in myeloproliferative neoplasms. Further studies are needed to dissect the yin-yang of PTX3 in individual human tumors other than those mentioned above.
10. Human genetics and translation
The similarity with CRP, a widely used biomarker in several pathological conditions, prompted investigation of the usefulness of PTX3 as marker in diverse human pathologies with an infective or inflammatory origin. PTX3 in human plasma behaves as an acute phase protein, increasing more rapidly than CRP in inflammatory conditions. Evidence in acute myocardial infarction and meningococcal sepsis suggests that PTX3 increases already 6-8 h after insult whereas CRP increases with a lag of 24-30 h. The different behavior of the two cognate molecules correlate with the characteristics of their production. CRP is systemically produced by the liver in response to IL-6. On the contrary, the local production by different cell types and the release of the preformed protein by neutrophils in response to primary proinflammatory cytokines or microbial recognition, likely accounts for the rapidity of PTX3 increase. In healthy subjects, PTX3 plasma levels are around 2 ng/ml (152), and increase rapidly and dramatically up to hundreds of nanograms and even micrograms per ml during endotoxic shock, sepsis and other inflammatory and infectious conditions.
Increases of PTX3 plasma concentrations were described in pulmonary aspergillosis, meningococcal disease, tuberculosis, leptospirosis and dengue virus infection (4, 11, 36, 95, 139, 146). High levels of PTX3 were also associated with severe inflammatory response syndrome (SIRS) and sepsis (10, 22, 104, 144). In addition, in patients with acute myocardial infarction, PTX3 plasma levels increased rapidly, peaking within 8 hours from the onset of symptoms, and emerged as the only independent predictor of mortality among other established markers ([i.e. CRP, NT-proBNP, TroponinN, (85, 113)]. Other observations indicated the value of PTX3 in cardiovascular diseases, atherosclerosis (80, 134), and as predictor of all-cause mortality (47, 74, 75, 84). In general, data collected in different pathological conditions, evidenced an association between PTX3 plasma levels, disease severity and mortality. Besides cardiovascular diseases and sepsis, this is true for stroke (129), resuscitation from cardiac arrest (120) and kidney disease (142). In addition high PTX3 plasma levels predicted mortality in hemodialysis patients (140, 154) and hospitalized patients (9).
The role of PTX3 as marker of cancer-related inflammation has been mentioned above. In addition, in patients with hemato-oncological diseases undergoing hematopoietic stem cell transplantation, a strong increase in PTX3 plasma levels was observed at the onset of acute graft-versus-host disease (GvHD) (40). In this group of patients PTX3 plasma levels at GvHD onset predicted disease outcome, being significantly higher in those experiencing severe GvHD or those not responding to therapy. These results suggest that PTX3 could be used as a biomarker of GvHD severity and therapy responsiveness, a potentially important observation to tailor anti-GvHD therapy as soon as the pathology occurs.
In the pathological conditions detailed above, inflammation is the driving cause of PTX3 increases. However in the last years a number of observations analysed whether and how PTX3 genetic variants could have an impact on the susceptibility to diseases and/or on PTX3 blood levels. Focus was on three SNPs in the PTX3 gene: rs2305619, rs3816527 and rs1840680. As already mentioned, only one of the three SNPs analysed, (rs3816527) is located into exon 2 and causes an amino acid change (Asp48Ala) in a strategic position of the PTX3 N-terminal domain.
A protective haplotype was identified in a cohort of more than 400 patients infected with Mycobacterium tuberculosis (110) or in Pseudomonas aeruginosa infected individuals with cystic fibrosis (29). In human urinary tract infections, local and systemic levels of PTX3 correlated with clinical parameters of infection severity, and genetic variants were associated with susceptibility to acute pyelonephritis and cystitis (68). An association was found between genetic polymorphisms in the PTX3 locus and PTX3 blood levels in Ghanaian women (98), in patients with meningococcal sepsis (B. Bottazzi and T. Sprong, personal communication), and in lung-transplant recipients with primary graft dysfunction (43). An association was found between a donor haplotype in the PTX3 gene and incidence of invasive aspergillosis in patients undergoing hematopoietic stem cell transplantation. This haplotype was consistently associated with lower PTX3 levels in aspergillosis (36) and other clinical conditions (7). This observation was extended to solid organ transplanted patients and confirmed by two independent studies, where PTX3 polymorphisms behaved as independent risk factor for incidence of mold infections (37, 150). In agreement with these studies and with the role of PTX3 in promoting Ig production, sarcoidosis patients harboring a haplotype variant of the PTX3 gene associated with reduced PTX3 expression showed decreased capsular polysaccharides-reactive IgG in bronchoalveolar lavage fluids (31).
The impact of PTX3 genetic variation was also investigated in acute myocardial infarction patients. PTX3 SNPs did not significantly influence the risk of acute myocardial infarction in a European population, despite the association with high levels of circulating protein (7).
Epigenetic mechanisms, in particular promoter methylation, were recently described to affect PTX3 expression. In particular hypermethylation of PTX3 promoter was found in esophageal squamous cell carcinoma, in colorectal cancer and leiomyosarcomas (13, 147), and is associated to lower PTX3 expression. On the contrary, lower promoter methylation was observed in patients with coronary artery disease, resulting in higher PTX3 plasma concentrations.
Thus PTX3 gene polymorphisms are associated with protein levels and risk of developing selected infections, in particular fungal (36, 37, 150). Preclinical evidence suggests that PTX3 has therapeutic potential against infectious agents (e.g. A. fumigatus and P. aeruginosa), which represent a formidable clinical challenge, and that it can be additive or synergistic with conventional antimicrobial agents (38, 54). These results raise the issue of clinical translation of PTX3 as a diagnostic and/or therapeutic agent.
11. Concluding remarks
The humoral arm of innate immunity includes diverse molecules and diversity has obscured recurrent common themes. PTX3 is a key component of humoral innate immunity, characterized by high degree of conservation in evolution. Indeed, analysis of genetic associations in humans in terms of physiology (fertility) and pathology (susceptibility to infection) has yielded results mirroring results in gene modified mice. From an innate immunity perspective, PTX3 behaves as a functional ancestor of antibodies with shared key words and properties: microbial recognition, complement activation, opsonization, regulation of inflammation. We surmise that these key words are a recurrent unifying theme among fluid phase PRM and provide a framework for and a common denominator of diversity in humoral innate immunity.
However, innate antimicrobial resistance does recapitulate the physiological function of PTX3 and its evolutionary conservation. PTX3 plays a role in the extracellular matrix and the most dramatic reflection of this facet of this PRM is its physiological role in murine and human female fertility. It is reasonable to assume that its role in fertility has been key to its conservation in mammalian evolution. In the same vein, recent evidence suggests that PTX3 is essential for tissue repair by favoring timely dissolution of the provisional matrix represented by fibrin, and this function of PTX3 is switched on by the acid microenvironment typical of tissue damage and inflammation. This result indicates that cellular glycolytic metabolism by causing acidification sets PTX3 in a tissue repair and remodeling mode while retaining defense functions.
In an unexpected twist, PTX3 was found to act as an extrinsic oncosuppressor gene in the mouse and in selected human tumors (e.g. colorectal) by taming tumor promoting inflammation. The finding that an effector molecule of humoral immunity is a bona fide cancer gene provided a missing link in the connection between inflammation and cancer. In addition these and other results suggest that humoral innate immunity is a component of cancer-related inflammation. However, other emerging clinical data, such as the finding that PTX3 is a correlate of the JAK2 mutational load in chronic myelodisplastic neoplasms, point to potentially different function of this molecule in different tumors. Therefore the full spectrum of the yin yang role of PTX3 in carcinogenesis remains to be fully dissected in relation to its complexity in physiology.
The results summarized in this review indicate that a component of the humoral arm of innate immunity, PTX3, plays a complex role at the crossroad and interface between innate immunity, inflammation and extracellular matrix organization and remodeling, with profound implications in physiology and pathology. Preclinical evidence, genetic conservation and human genetic association provide a rationale for diagnostic and therapeutic translation.
Acknowledgements
The financial support of Fondazione Cariplo (Contract n. 2015-0564), Ministero della Salute (RF-2011-02348358 and RF-2013-02355470), Ministry of Education, University and Research (PRIN 2015YYKPNN), Cluster Alisei (MEDINTECH CTN01_00177_962865), the European Commission (FP7-HEALTH-2011-ADITEC-N°280873), the European Research Council (ERC – N° 669415 to AM), and the Italian Association for Cancer Research (AIRC, IG and 5x1000) is gratefully acknowledged. EM is recipient of fellowship from Fondazione Veronesi, AI is recipient of a Young Investigator Grant from Ministero della Salute (GR-2011-02349539)
Abbreviations
- 3-MCA
3-methylcholanthrene
- aHUS
atypical hemolytic uremic syndrome
- AMD
age-related macular degeneration
- BMP15
bone morphogenetic protein 15
- C4BP
C4 binding protein
- CCL2 or MCP-1
monocyte chemoattractant protein-1
- CMV
cytomegalovirus
- CVB3
coxsackievirus B3
- CR3
complement receptor 3
- CRP
C reactive protein
- CVB3
coxsackievirus B3
- DC
dendritic cells
- DC-SIGN
dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin
- DMBA/TPA
7,12-dimethylbenz [α] anthracene/terephthalic acid
- FcγR
Fcγ receptors
- FGF
fibroblast growth factor
- FG
fibrinogen
- FH
factor H
- FSH
follicle-stimulating hormone
- GDF-9
growth differentiation factor-9
- GvHD
graft-versus-host disease
- HA
hyaluronan
- hCG
human chorionic gonadotropin
- HCs
IαI heavy chains
- IAV
influenza A virus
- IL
interleukin
- IαI
inter-α-inhibitor
- JAK2
janus kinase 2
- MBL
mannose binding lectin
- MD-2
myeloid differentiation protein 2
- MyD88
myeloid differentiation primary response gene 88
- MZ
marginal zone
- NALP3
NACHT, LRR and PYD domains-containing protein 3
- NOD-like receptors
nucleotide-binding oligomerization domain-like receptors
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- OmpA
outer membrane proteins A
- OMV
outer membrane vescicles
- PC
posphorylcholine
- Plg
plasminogen
- PRM
pattern recognition molecule
- PRR
pattern recognition receptors
- RIG-I-like receptors
retinoic acid-inducible gene I-like receptors
- SAP
serum amyloid P component
- SCR
short consensus repeat
- SNPs
single nucleotide polymorphisms
- SAXS
small angle X-ray scattering
- SIRS
severe inflammatory response syndrome
- TGFβ
transforming growth factor β
- Th
T helper
- TLR
Toll like receptors
- TNF-α
tumor necrosis factor-α
- TNFAIP6 or TSG-6
TNF-α–induced protein 6
References
- 1.Abernethy TJ, Avery OT. The occurence during acute infections of a protein non normally present in the blood. I. Distribution of the reactive protein in patients' sera and the effect of calcium on the flocculation reaction with C. Polysaccharide of Pneumococcus. J Exp Med. 1941;73:173–182. doi: 10.1084/jem.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberti L, Gilardini L, Zulian A, Micheletto G, Peri G, Doni A, Mantovani A, Invitti C. Expression of long pentraxin PTX3 in human adipose tissue and its relation with cardiovascular risk factors. Atherosclerosis. 2009;202:455–460. doi: 10.1016/j.atherosclerosis.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Assi F, Fruscio R, Bonardi C, Ghidini A, Allavena P, Mantovani A, Locatelli A. Pentraxin 3 in plasma and vaginal fluid in women with preterm delivery. Bjog. 2007;114:143–147. doi: 10.1111/j.1471-0528.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.Azzurri A, Sow OY, Amedei A, Bah B, Diallo S, Peri G, Benagiano M, D'Elios MM, Mantovani A, Del Prete G. IFN-gamma-inducible protein 10 and pentraxin 3 plasma levels are tools for monitoring inflammation and disease activity in Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:1–8. doi: 10.1016/j.micinf.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Balhara J, Shan L, Zhang J, Muhuri A, Halayko AJ, Almiski MS, Doeing D, McConville J, Matzuk MM, Gounni AS. Pentraxin 3 deletion aggravates allergic inflammation through a TH17-dominant phenotype and enhanced CD4 T-cell survival. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baranova NS, Inforzato A, Briggs DC, Tilakaratna V, Enghild JJ, Thakar D, Milner CM, Day AJ, Richter RP. Incorporation of pentraxin 3 into hyaluronan matrices is tightly regulated and promotes matrix cross-linking. J Biol Chem. 2014;289:30481–30498. doi: 10.1074/jbc.M114.568154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbati E, Specchia C, Villella M, Rossi ML, Barlera S, Bottazzi B, Crociati L, d'Arienzo C, Fanelli R, Garlanda C, Gori F, et al. Influence of Pentraxin 3 (PTX3) Genetic Variants on Myocardial Infarction Risk and PTX3 Plasma Levels. PLoS One. 2012;7:e53030. doi: 10.1371/journal.pone.0053030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbui T, Carobbio A, Finazzi G, Vannucchi AM, Barosi G, Antonioli E, Guglielmelli P, Pancrazzi A, Salmoiraghi S, Zilio P, Ottomano C, et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica. 2011;96:315–318. doi: 10.3324/haematol.2010.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastrup-Birk S, Munthe-Fog L, Skjoedt M-O, Ma YJ, Nielsen H, Køber L. Pentraxin-3 level at admission is a strong predictor of short-term mortality in a community-based hospital setting. J Intern Med. 2015;277 doi: 10.1111/joim.12294. [DOI] [PubMed] [Google Scholar]
- 10.Bastrup-Birk S, Skjoedt MO, Munthe-Fog L, Strom JJ, Ma YJ, Garred P. Pentraxin-3 serum levels are associated with disease severity and mortality in patients with systemic inflammatory response syndrome. PLoS One. 2013;8:e73119. doi: 10.1371/journal.pone.0073119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biagi E, Col M, Migliavacca M, Dell'Oro M, Silvestri D, Montanelli A, Peri G, Mantovani A, Biondi A, Rossi MR. PTX3 as a potential novel tool for the diagnosis and monitoring of pulmonary fungal infections in immuno-compromised pediatric patients. J Pediatr Hematol Oncol. 2008;30:881–885. doi: 10.1097/MPH.0b013e318180bc1d. [DOI] [PubMed] [Google Scholar]
- 12.Bonacina F, Barbieri SS, Cutuli L, Amadio P, Doni A, Sironi M, Tartari S, Mantovani A, Bottazzi B, Garlanda C, Tremoli E, et al. Vascular pentraxin 3 controls arterial thrombosis by targeting collagen and fibrinogen induced platelets aggregation. Biochim Biophys Acta. 2016;1862:1182–1190. doi: 10.1016/j.bbadis.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonavita E, Gentile S, Rubino M, Maina V, Papait R, Kunderfranco P, Greco C, Feruglio F, Molgora M, Laface I, Tartari S, et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160:700–714. doi: 10.1016/j.cell.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 15.Bottazzi B, Santini L, Savino S, Giuliani MM, Duenas Diez AI, Mancuso G, Beninati C, Sironi M, Valentino S, Deban L, Garlanda C, et al. Recognition of Neisseria meningitidis by the long pentraxin PTX3 and its role as an endogenous adjuvant. PLoS One. 2015;10:e0120807. doi: 10.1371/journal.pone.0120807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottazzi B, Vouret-Craviari V, Bastone A, De Gioia L, Matteucci C, Peri G, Spreafico F, Pausa M, D'Ettorre C, Gianazza E, Tagliabue A, et al. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. 1997;272:32817–32823. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- 17.Bozza S, Bistoni F, Gaziano R, Pitzurra L, Zelante T, Bonifazi P, Perruccio K, Bellocchio S, Neri M, Iorio AM, Salvatori G, et al. Pentraxin 3 protects from MCMV infection and reactivation through TLR sensing pathways leading to IRF3 activation. Blood. 2006;108:3387–3396. doi: 10.1182/blood-2006-03-009266. [DOI] [PubMed] [Google Scholar]
- 18.Bozza S, Campo S, Arseni B, Inforzato A, Ragnar L, Bottazzi B, Mantovani A, Moretti S, Oikonomous V, De Santis R, Carvalho A, et al. PTX3 binds MD-2 and promotes TRIF-dependent immune protection in aspergillosis. J Immunol. 2014;193:2340–2348. doi: 10.4049/jimmunol.1400814. [DOI] [PubMed] [Google Scholar]
- 19.Braunschweig A, Jozsi M. Human pentraxin 3 binds to the complement regulator c4b-binding protein. PLoS One. 2011;6:e23991. doi: 10.1371/journal.pone.0023991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breviario F, d'Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M, Della Valle G, et al. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component. J Biol Chem. 1992;267:22190–22197. [PubMed] [Google Scholar]
- 21.Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 22.Caironi P, Masson S, Mauri T, Bottazzi B, Leone R, Magnoli M, Barlera S, Mamprin F, Fedele A, Mantovani A, Tognoni G, et al. Pentraxin-3 in patients with severe sepsis or shock: the ALBIOS trial. Eur J Clin Invest. 2016 doi: 10.1111/eci.12704. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappuzzello C, Doni A, Dander E, Pasqualini F, Nebuloni M, Bottazzi B, Mantovani A, Biondi A, Garlanda C, D'Amico G. Mesenchymal Stromal Cell-Derived PTX3 Promotes Wound Healing via Fibrin Remodeling. The Journal of investigative dermatology. 2016;136:293–300. doi: 10.1038/JID.2015.346. [DOI] [PubMed] [Google Scholar]
- 24.Carmo RF, Aroucha D, Vasconcelos LR, Pereira LM, Moura P, Cavalcanti MS. Genetic variation in PTX3 and plasma levels associated with hepatocellular carcinoma in patients with HCV. J Viral Hepat. 2016;23:116–122. doi: 10.1111/jvh.12472. [DOI] [PubMed] [Google Scholar]
- 25.Carrizzo A, Lenzi P, Procaccini C, Damato A, Biagioni F, Ambrosio M, Amodio G, Remondelli P, Del Giudice C, Izzo R, Malovini A, et al. Pentraxin 3 Induces Vascular Endothelial Dysfunction Through a P-selectin/Matrix Metalloproteinase-1 Pathway. Circulation. 2015;131:1495–1505. doi: 10.1161/CIRCULATIONAHA.114.014822. discussion 1505. [DOI] [PubMed] [Google Scholar]
- 26.Cetin I, Cozzi V, Pasqualini F, Nebuloni M, Garlanda C, Vago L, Pardi G, Mantovani A. Elevated maternal levels of the long pentraxin 3 (PTX3) in preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2006;194:1347–1353. doi: 10.1016/j.ajog.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Chang WC, Wu SL, Huang WC, Hsu JY, Chan SH, Wang JM, Tsai JP, Chen BK. PTX3 gene activation in EGF-induced head and neck cancer cell metastasis. Oncotarget. 2015;6:7741–7757. doi: 10.18632/oncotarget.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi JY, Hsiao YW, Li CF, Lo YC, Lin ZY, Hong JY, Liu YM, Han X, Wang SM, Chen BK, Tsai KK, et al. Targeting chemotherapy-induced PTX3 in tumor stroma to prevent the progression of drug-resistant cancers. Oncotarget. 2015;6:23987–24001. doi: 10.18632/oncotarget.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiarini M, Sabelli C, Melotti P, Garlanda C, Savoldi G, Mazza C, Padoan R, Plebani A, Mantovani A, Notarangelo LD, Assael BM, et al. PTX3 genetic variations affect the risk of Pseudomonas aeruginosa airway colonization in cystic fibrosis patients. Genes Immun. 2010;11:665–670. doi: 10.1038/gene.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi B, Lee EJ, Park YS, Kim SM, Kim EY, Song Y, Kang SW, Rhu MH, Chang EJ. Pentraxin-3 Silencing Suppresses Gastric Cancer-related Inflammation by Inhibiting Chemotactic Migration of Macrophages. Anticancer Res. 2015;35:2663–2668. [PubMed] [Google Scholar]
- 31.Chorny A, Casas-Recasens S, Sintes J, Shan M, Polentarutti N, Garcia-Escudero R, Walland AC, Yeiser JR, Cassis L, Carrillo J, Puga I, et al. The soluble pattern recognition receptor PTX3 links humoral innate and adaptive immune responses by helping marginal zone B cells. J Exp Med. 2016;213:2167–2185. doi: 10.1084/jem.20150282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 33.Cotena A, Maina V, Sironi M, Bottazzi B, Jeannin P, Vecchi A, Corvaia N, Daha MR, Mantovani A, Garlanda C. Complement Dependent Amplification of the Innate Response to a Cognate Microbial Ligand by the Long Pentraxin PTX3. J Immunol. 2007;179:6311–6317. doi: 10.4049/jimmunol.179.9.6311. [DOI] [PubMed] [Google Scholar]
- 34.Cox N, Pilling D, Gomer RH. DC-SIGN activation mediates the differential effects of SAP and CRP on the innate immune system and inhibits fibrosis in mice. Proc Natl Acad Sci U S A. 2015;112:8385–8390. doi: 10.1073/pnas.1500956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csincsi AI, Kopp A, Zoldi M, Banlaki Z, Uzonyi B, Hebecker M, Caesar JJ, Pickering MC, Daigo K, Hamakubo T, Lea SM, et al. Factor H-related protein 5 interacts with pentraxin 3 and the extracellular matrix and modulates complement activation. J Immunol. 2015;194:4963–4973. doi: 10.4049/jimmunol.1403121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunha C, Aversa F, Lacerda JF, Busca A, Kurzai O, Grube M, Loffler J, Maertens JA, Bell AS, Inforzato A, Barbati E, et al. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. New England Journal of Medicine. 2014;370:421–432. doi: 10.1056/NEJMoa1211161. [DOI] [PubMed] [Google Scholar]
- 37.Cunha C, Monteiro AA, Oliveira-Coelho A, Kuhne J, Rodrigues F, Sasaki SD, Schio SM, Camargo JJ, Mantovani A, Carvalho A, Pasqualotto AC. PTX3-Based Genetic Testing for Risk of Aspergillosis After Lung Transplant. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61:1893–1894. doi: 10.1093/cid/civ679. [DOI] [PubMed] [Google Scholar]
- 38.D'Angelo C, De Luca A, Zelante T, Bonifazi P, Moretti S, Giovannini G, Iannitti RG, Zagarella S, Bozza S, Campo S, Salvatori G, et al. Exogenous pentraxin 3 restores antifungal resistance and restrains inflammation in murine chronic granulomatous disease. J Immunol. 2009;183:4609–4618. doi: 10.4049/jimmunol.0900345. [DOI] [PubMed] [Google Scholar]
- 39.Daigo K, Inforzato A, Barajon I, Garlanda C, Bottazzi B, Meri S, Mantovani A. Pentraxins in the activation and regulation of innate immunity. Immunol Rev. 2016;274:202–217. doi: 10.1111/imr.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dander E, De Lorenzo P, Bottazzi B, Quarello P, Vinci P, Balduzzi A, Masciocchi F, Bonanomi S, Cappuzzello C, Prunotto G, Pavan F, et al. Pentraxin 3 plasma levels at graft-versus-host disease onset predict disease severity and response to therapy in children given haematopoietic stem cell transplantation. Oncotarget. 2016;7:82123–82138. doi: 10.18632/oncotarget.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deban L, Castro Russo R, Sironi M, Moalli F, Scanziani M, Zambelli V, Cuccovillo I, Bastone A, Gobbi M, Valentino S, Doni A, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 42.Deban L, Jarva H, Lehtinen MJ, Bottazzi B, Bastone A, Doni A, Jokiranta TS, Mantovani A, Meri S. Binding of the long pentraxin PTX3 to Factor H: Interacting domains and function in the regulation of complement activation. J Immunology. 2008;181:8433–8440. doi: 10.4049/jimmunol.181.12.8433. [DOI] [PubMed] [Google Scholar]
- 43.Diamond JM, Meyer NJ, Feng R, Rushefski M, Lederer DJ, Kawut SM, Lee JC, Cantu E, Shah RJ, Lama VN, Bhorade S, et al. Variation in PTX3 is associated with primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2012;186:546–552. doi: 10.1164/rccm.201204-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dias AA, Goodman AR, Dos Santos JL, Gomes RN, Altmeyer A, Bozza PT, Horta MF, Vilcek J, Reis LF. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol. 2001;69:928–936. [PubMed] [Google Scholar]
- 45.Diniz SN, Nomizo R, Cisalpino PS, Teixeira MM, Brown GD, Mantovani A, Gordon S, Reis LF, Dias AA. PTX3 function as an opsonin for the dectin-1-dependent internalization of zymosan by macrophages. J Leukoc Biol. 2004;75:649–656. doi: 10.1189/jlb.0803371. [DOI] [PubMed] [Google Scholar]
- 46.Doni A, Garlanda C, Bottazzi B, Meri S, Garred P, Mantovani A. Interactions of the humoral pattern recognition molecule PTX3 with the complement system. Immunobiology. 2012;217:1122–1128. doi: 10.1016/j.imbio.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Dubin R, Li Y, Ix JH, Shlipak MG, Whooley M, Peralta CA. Associations of pentraxin-3 with cardiovascular events, incident heart failure, and mortality among persons with coronary heart disease: data from the Heart and Soul Study. American heart journal. 2012;163:274–279. doi: 10.1016/j.ahj.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 49.Foo SS, Chen W, Taylor A, Sheng KC, Yu X, Teng TS, Reading PC, Blanchard H, Garlanda C, Mantovani A, Ng LF, et al. Role of pentraxin 3 in shaping arthritogenic alphaviral disease: from enhanced viral replication to immunomodulation. PLoS Pathog. 2015;11:e1004649. doi: 10.1371/journal.ppat.1004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 51.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 52.Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R, Maccagno A, Riva F, Bottazzi B, Peri G, Doni A, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–186. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 53.Garred P, Genster N, Pilely K, Bayarri-Olmos R, Rosbjerg A, Ma YJ, Skjoedt MO. A journey through the lectin pathway of complement—MBL and beyond. Immunol Rev. 2016;274:74–97. doi: 10.1111/imr.12468. [DOI] [PubMed] [Google Scholar]
- 54.Gaziano R, Bozza S, Bellocchio S, Perruccio K, Montagnoli C, Pitzurra L, Salvatori G, De Santis R, Carminati P, Mantovani A, Romani L. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob Agents Chemother. 2004;48:4414–4421. doi: 10.1128/AAC.48.11.4414-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Germano G, Frapolli R, Simone M, Tavecchio M, Erba E, Pesce S, Pasqualini F, Grosso F, Sanfilippo R, Casali PG, Gronchi A, et al. Antitumor and Anti-inflammatory Effects of Trabectedin on Human Myxoid Liposarcoma Cells. Cancer Res. 2010;70:2235–2244. doi: 10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]
- 56.Goodman AR, Cardozo T, Abagyan R, Altmeyer A, Wisniewski HG, Vilcek J. Long pentraxins: an emerging group of proteins with diverse functions. Cytokine Growth Factor Rev. 1996;7:191–202. doi: 10.1016/1359-6101(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 57.Gout E, Moriscot C, Doni A, Dumestre-Perard C, Lacroix M, Perard J, Schoehn G, Mantovani A, Arlaud GJ, Thielens NM. M-ficolin interacts with the long pentraxin PTX3: a novel case of cross-talk between soluble pattern-recognition molecules. Journal of immunology. 2011;186:5815–5822. doi: 10.4049/jimmunol.1100180. [DOI] [PubMed] [Google Scholar]
- 58.Guo TM, Huang LL, Liu K, Ke L, Luo ZJ, Li YQ, Chen XL, Cheng B. Pentraxin 3 (PTX3) promoter methylation associated with PTX3 plasma levels and neutrophil to lymphocyte ratio in coronary artery disease. Journal of geriatric cardiology : JGC. 2016;13:712–717. doi: 10.11909/j.issn.1671-5411.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han B, Ma X, Zhang J, Zhang Y, Bai X, Hwang DM, Keshavjee S, Levy GA, McGilvray I, Liu M. Protective effects of long pentraxin PTX3 on lung injury in a severe acute respiratory syndrome model in mice. Lab Invest. 2012;92:1285–1296. doi: 10.1038/labinvest.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han B, Mura M, Andrade CF, Okutani D, Lodyga M, dos Santos CC, Keshavjee S, Matthay M, Liu M. TNFalpha-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J Immunol. 2005;175:8303–8311. doi: 10.4049/jimmunol.175.12.8303. [DOI] [PubMed] [Google Scholar]
- 61.Hirschfield GM, Pepys MB. C-reactive protein and cardiovascular disease: new insights from an old molecule. Q J Med. 2003;96:793–807. doi: 10.1093/qjmed/hcg134. [DOI] [PubMed] [Google Scholar]
- 62.Holmskov U, Thiel S, Jensenius JC. Collectins and ficolins: humoral lectins of the innate immune defense. Annu Rev Immunol. 2003;21:547–578. doi: 10.1146/annurev.immunol.21.120601.140954. [DOI] [PubMed] [Google Scholar]
- 63.Ievoli E, Lindstedt R, Inforzato A, Camaioni A, Palone F, Day AJ, Mantovani A, Salvatori G, Salustri A. Implication of the oligomeric state of the N-terminal PTX3 domain in cumulus matrix assembly. Matrix Biol. 2011;30:330–337. doi: 10.1016/j.matbio.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Infante M, Allavena P, Garlanda C, Nebuloni M, Morenghi E, Rahal D, Roncalli M, Cavuto S, Pesce S, Monari M, Valaperta S, et al. Prognostic and diagnostic potential of local and circulating levels of pentraxin 3 in lung cancer patients. Int J Cancer. 2016;138:983–991. doi: 10.1002/ijc.29822. [DOI] [PubMed] [Google Scholar]
- 65.Inforzato A, Baldock C, Jowitt TA, Holmes DF, Lindstedt R, Marcellini M, Rivieccio V, Briggs DC, Kadler KE, Verdoliva A, Bottazzi B, et al. The angiogenic inhibitor long pentraxin PTX3 forms an asymmetric octamer with two binding sites for FGF2. Journal of Biological Chemistry. 2010;285:17681–17692. doi: 10.1074/jbc.M109.085639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inforzato A, Peri G, Doni A, Garlanda C, Mantovani A, Bastone A, Carpentieri A, Amoresano A, Pucci P, Roos A, Daha MR, et al. Structure and function of the long pentraxin PTX3 glycosidic moiety: fine-tuning of the interaction with C1q and complement activation. Biochemistry. 2006;45:11540–11551. doi: 10.1021/bi0607453. [DOI] [PubMed] [Google Scholar]
- 67.Inforzato A, Reading P, Barbati E, Bottazzi B, Garlanda C, Mantovani A. The “sweet” side of a long pentraxin: how glycosylation affects PTX3 functions in innate immunity and inflammation. Frontiers in immunology. 2013;3:407. doi: 10.3389/fimmu.2012.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inforzato A, Rivieccio V, Morreale AP, Bastone A, Salustri A, Scarchilli L, Verdoliva A, Vincenti S, Gallo G, Chiapparino C, Pacello L, et al. Structural characterization of PTX3 disulfide bond network and its multimeric status in cumulus matrix organization. J Biol Chem. 2008;283:10147–10161. doi: 10.1074/jbc.M708535200. [DOI] [PubMed] [Google Scholar]
- 69.Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, Peri G, Breviario F, Salmona M, De Gregorio L, Dragani TA, et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–1872. [PubMed] [Google Scholar]
- 70.Jaillon S, Mancuso G, Hamon Y, Beauvillain C, Cotici V, Midiri A, Bottazzi B, Nebuloni M, Garlanda C, Fremaux I, Gauchat JF, et al. Prototypic long pentraxin PTX3 is present in breast milk, spreads in tissues, and protects neonate mice from Pseudomonas aeruginosa lung infection. J Immunol. 2013;191:1873–1882. doi: 10.4049/jimmunol.1201642. [DOI] [PubMed] [Google Scholar]
- 71.Jaillon S, Moalli F, Ragnarsdottir B, Bonavita E, Puthia M, Riva F, Barbati E, Nebuloni M, Cvetko Krajinovic L, Markotic A, Valentino S, et al. The Humoral Pattern Recognition Molecule PTX3 Is a Key Component of Innate Immunity against Urinary Tract Infection. Immunity. 2014;40:621–632. doi: 10.1016/j.immuni.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 72.Jaillon S, Peri G, Delneste Y, Fremaux I, Doni A, Moalli F, Garlanda C, Romani L, Gascan H, Bellocchio S, Bozza S, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, Maina V, Magistrelli G, Haeuw JF, Hoeffel G, Thieblemont N, et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 74.Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2009;29:594–599. doi: 10.1161/ATVBAHA.108.178947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jenny NS, Blumenthal RS, Kronmal RA, Rotter JI, Siscovick DS, Psaty BM. Associations of pentraxin 3 with cardiovascular disease: the multi-ethnic study of atherosclerosis. J Thromb Haemost. 2014;12:999–1005. doi: 10.1111/jth.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeon H, Lee S, Lee WH, Suk K. Analysis of glial secretome: the long pentraxin PTX3 modulates phagocytic activity of microglia. J Neuroimmunol. 2010;229:63–72. doi: 10.1016/j.jneuroim.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Job ER, Bottazzi B, Short KR, Deng YM, Mantovani A, Brooks AG, Reading PC. A single amino acid substitution in the hemagglutinin of H3N2 subtype influenza A viruses is associated with resistance to the long pentraxin PTX3 and enhanced virulence in mice. J Immunol. 2014;192:271–281. doi: 10.4049/jimmunol.1301814. [DOI] [PubMed] [Google Scholar]
- 78.Job ER, Deng YM, Tate MD, Bottazzi B, Crouch EC, Dean MM, Mantovani A, Brooks AG, Reading PC. Pandemic H1N1 influenza A viruses are resistant to the antiviral activities of innate immune proteins of the collectin and pentraxin superfamilies. J Immunol. 2010;185:4284–4291. doi: 10.4049/jimmunol.1001613. [DOI] [PubMed] [Google Scholar]
- 79.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 80.Knoflach M, Kiechl S, Mantovani A, Cuccovillo I, Bottazzi B, Xu Q, Xiao Q, Gasperi A, Mayr A, Kehrer M, Willeit J, et al. Pentraxin-3 as a marker of advanced atherosclerosis results from the Bruneck, ARMY and ARFY Studies. PLoS One. 2012;7:e31474. doi: 10.1371/journal.pone.0031474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kondo S, Ueno H, Hosoi H, Hashimoto J, Morizane C, Koizumi F, Tamura K, Okusaka T. Clinical impact of pentraxin family expression on prognosis of pancreatic carcinoma. British Journal of Cancer. 2013;109:739–746. doi: 10.1038/bjc.2013.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kopp A, Strobel S, Tortajada A, Rodriguez de Cordoba S, Sanchez-Corral P, Prohaszka Z, Lopez-Trascasa M, Jozsi M. Atypical hemolytic uremic syndrome-associated variants and autoantibodies impair binding of factor h and factor h-related protein 1 to pentraxin 3. Journal of Immunology. 2012;189:1858–1867. doi: 10.4049/jimmunol.1200357. [DOI] [PubMed] [Google Scholar]
- 83.Laine M, Jarva H, Seitsonen S, Haapasalo K, Lehtinen MJ, Lindeman N, Anderson DH, Johnson PT, Jarvela I, Jokiranta TS, Hageman GS, et al. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J Immunol. 2007;178:3831–3836. doi: 10.4049/jimmunol.178.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Latini R, Gullestad L, Masson S, Nymo SH, Ueland T, Cuccovillo I, Vardal M, Bottazzi B, Mantovani A, Lucci D, Masuda N, et al. Pentraxin-3 in chronic heart failure: the CORONA and GISSI-HF trials. Eur J Heart Fail. 2012;14:992–999. doi: 10.1093/eurjhf/hfs092. [DOI] [PubMed] [Google Scholar]
- 85.Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, Vago L, Pasqualini F, Signorini S, Soldateschi D, Tarli L, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 86.Leali D, Inforzato A, Ronca R, Bianchi R, Belleri M, Coltrini D, Di Salle E, Sironi M, Norata GD, Bottazzi B, Garlanda C, et al. Long pentraxin 3/tumor necrosis factor-stimulated gene-6 interaction: a biological rheostat for fibroblast growth factor 2-mediated angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:696–703. doi: 10.1161/ATVBAHA.111.243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lech M, Rommele C, Grobmayr R, Eka Susanti H, Kulkarni OP, Wang S, Grone HJ, Uhl B, Reichel C, Krombach F, Garlanda C, et al. Endogenous and exogenous pentraxin-3 limits postischemic acute and chronic kidney injury. Kidney Int. 2013;83:647–661. doi: 10.1038/ki.2012.463. [DOI] [PubMed] [Google Scholar]
- 88.Lee GW, Lee TH, Vilcek J. TSG-14, a tumor necrosis factor- and IL-1-inducible protein, is a novel member of the pentaxin family of acute phase proteins. J Immunol. 1993;150:1804–1812. [PubMed] [Google Scholar]
- 89.Locatelli M, Ferrero S, Martinelli Boneschi F, Boiocchi L, Zavanone M, Maria Gaini S, Bello L, Valentino S, Barbati E, Nebuloni M, Mantovani A, et al. The long pentraxin PTX3 as a correlate of cancer-related inflammation and prognosis of malignancy in gliomas. Journal of neuroimmunology. 2013;260:99–106. doi: 10.1016/j.jneuroim.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 90.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcgammaR by innate pentraxins. Nature. 2008;456:989–992. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lussana F, Carobbio A, Salmoiraghi S, Guglielmelli P, Vannucchi AM, Bottazzi B, Leone R, Mantovani A, Barbui T, Rambaldi A. Driver mutations (JAK2V617F, MPLW515L/K or CALR), pentraxin-3 and C-reactive protein in essential thrombocythemia and polycythemia vera. J Hematol Oncol. 2017;10:54. doi: 10.1186/s13045-017-0425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma YJ, Doni A, Hummelshoj T, Honore C, Bastone A, Mantovani A, Thielens NM, Garred P. Synergy between ficolin-2 and pentraxin 3 boosts innate immune recognition and complement deposition. Journal of Biological Chemistry. 2009;284:28263–28275. doi: 10.1074/jbc.M109.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ma YJ, Doni A, Romani L, Jurgensen HJ, Behrendt N, Mantovani A, Garred P. Ficolin-1-PTX3 complex formation promotes clearance of altered self-cells and modulates IL-8 production. Journal of immunology. 2013;191:1324–1333. doi: 10.4049/jimmunol.1300382. [DOI] [PubMed] [Google Scholar]
- 94.Ma YJ, Doni A, Skjoedt MO, Honore C, Arendrup M, Mantovani A, Garred P. Heterocomplexes of mannose-binding lectin and the pentraxins PTX3 or serum amyloid P component trigger cross-activation of the complement system. J Biol Chem. 2011;286:3405–3417. doi: 10.1074/jbc.M110.190637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mairuhu AT, Peri G, Setiati TE, Hack CE, Koraka P, Soemantri A, Osterhaus AD, Brandjes DP, van der Meer JW, Mantovani A, van Gorp EC. Elevated plasma levels of the long pentraxin, pentraxin 3, in severe dengue virus infections. J Med Virol. 2005;76:547–552. doi: 10.1002/jmv.20397. [DOI] [PubMed] [Google Scholar]
- 96.Martinez de la Torre Y, Fabbri M, Jaillon S, Bastone A, Nebuloni M, Vecchi A, Mantovani A, Garlanda C. Evolution of the pentraxin family: the new entry PTX4. J Immunol. 2010;184:5055–5064. doi: 10.4049/jimmunol.0901672. [DOI] [PubMed] [Google Scholar]
- 97.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 98.May L, Kuningas M, van Bodegom D, Meij HJ, Frolich M, Slagboom PE, Mantovani A, Westendorp RG. Genetic variation in pentraxin (PTX) 3 gene associates with PTX3 production and fertility in women. Biol Reprod. 2010;82:299–304. doi: 10.1095/biolreprod.109.079111. [DOI] [PubMed] [Google Scholar]
- 99.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 100.Moalli F, Doni A, Deban L, Zelante T, Zagarella S, Bottazzi B, Romani L, Mantovani A, Garlanda C. Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood. 2010;116:5170–5180. doi: 10.1182/blood-2009-12-258376. [DOI] [PubMed] [Google Scholar]
- 101.Moalli F, Paroni M, Veliz Rodriguez T, Riva F, Polentarutti N, Bottazzi B, Valentino S, Mantero S, Nebuloni M, Mantovani A, Bragonzi A, et al. The therapeutic potential of the humoral pattern recognition molecule PTX3 in chronic lung infection caused by Pseudomonas aeruginosa. J Immunol. 2011;186:5425–5434. doi: 10.4049/jimmunol.1002035. [DOI] [PubMed] [Google Scholar]
- 102.Moreau C, Bally I, Chouquet A, Bottazzi B, Ghebrehiwet B, Gaboriaud C, Thielens N. Structural and Functional Characterization of a Single-Chain Form of the Recognition Domain of Complement Protein C1q. Frontiers in immunology. 2016;7:79. doi: 10.3389/fimmu.2016.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Motta V, Soares F, Sun T, Philpott DJ. NOD-like receptors: versatile cytosolic sentinels. Physiol Rev. 2015;95:149–178. doi: 10.1152/physrev.00009.2014. [DOI] [PubMed] [Google Scholar]
- 104.Muller B, Peri G, Doni A, Torri V, Landmann R, Bottazzi B, Mantovani A. Circulating levels of the long pentraxin PTX3 correlate with severity of infection in critically ill patients. Crit Care Med. 2001;29:1404–1407. doi: 10.1097/00003246-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 105.Nauta AJ, Bottazzi B, Mantovani A, Salvatori G, Kishore U, Schwaeble WJ, Gingras AR, Tzima S, Vivanco F, Egido J, Tijsma O, et al. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur J Immunol. 2003;33:465–473. doi: 10.1002/immu.200310022. [DOI] [PubMed] [Google Scholar]
- 106.Nauta AJ, Daha MR, van Kooten C, Roos A. Recognition and clearance of apoptotic cells: a role for complement and pentraxins. Trends Immunol. 2003;24:148–154. doi: 10.1016/s1471-4906(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 107.Norata GD, Marchesi P, Pirillo A, Uboldi P, Chiesa G, Maina V, Garlanda C, Mantovani A, Catapano AL. Long pentraxin 3, a key component of innate immunity, is modulated by high-density lipoproteins in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:925–931. doi: 10.1161/ATVBAHA.107.160606. [DOI] [PubMed] [Google Scholar]
- 108.Norata GD, Marchesi P, Pulakazhi Venu VK, Pasqualini F, Anselmo A, Moalli F, Pizzitola I, Garlanda C, Mantovani A, Catapano AL. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 109.Okemefuna AI, Nan R, Miller A, Gor J, Perkins SJ. Complement factor H binds at two independent sites to C-reactive protein in acute phase concentrations. The Journal of biological chemistry. 2010;285:1053–1065. doi: 10.1074/jbc.M109.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Olesen R, Wejse C, Velez DR, Bisseye C, Sodemann M, Aaby P, Rabna P, Worwui A, Chapman H, Diatta M, Adegbola RA, et al. DC-SIGN (CD209), Pentraxin 3 and Vitamin D Receptor gene variants associate with pulmonary tubercolosis risk in West-Africans. Genes and Immunity. 2007;8:456–467. doi: 10.1038/sj.gene.6364410. [DOI] [PubMed] [Google Scholar]
- 111.Paeschke A, Possehl A, Klingel K, Voss M, Voss K, Kespohl M, Sauter M, Overkleeft HS, Althof N, Garlanda C, Voigt A. The immunoproteasome controls the availability of the cardioprotective pattern recognition molecule Pentraxin3. Eur J Immunol. 2016;46:619–633. doi: 10.1002/eji.201545892. [DOI] [PubMed] [Google Scholar]
- 112.Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A. 2013;110:E776–785. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F. PTX3, a prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102 doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- 114.Planque C, Kulasingam V, Smith CR, Reckamp K, Goodglick L, Diamandis EP. Identification of five candidate lung cancer biomarkers by proteomics analysis of conditioned media of four lung cancer cell lines. Mol Cell Proteomics. 2009;8:2746–2758. doi: 10.1074/mcp.M900134-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Presta M, Camozzi M, Salvatori G, Rusnati M. Role of the soluble pattern recognition receptor PTX3 in vascular biology. J Cell Mol Med. 2007;11:723–738. doi: 10.1111/j.1582-4934.2007.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ravizza T, Moneta D, Bottazzi B, Peri G, Garlanda C, Hirsch E, Richards GJ, Mantovani A, Vezzani A. Dynamic induction of the long pentraxin PTX3 in the CNS after limbic seizures: evidence for a protective role in seizure-induced neurodegeneration. Neuroscience. 2001;105:43–53. doi: 10.1016/s0306-4522(01)00177-4. [DOI] [PubMed] [Google Scholar]
- 117.Reading PC, Bozza S, Gilbertson B, Tate M, Moretti S, Job ER, Crouch EC, Brooks AG, Brown LE, Bottazzi B, Romani L, et al. Antiviral Activity of the Long Chain Pentraxin PTX3 against Influenza Viruses. J Immunol. 2008;180:3391–3398. doi: 10.4049/jimmunol.180.5.3391. [DOI] [PubMed] [Google Scholar]
- 118.Real JM, Spilborghs GM, Morato-Marques M, de Moura RP, Negri EM, Camargo AA, Deheinzelin D, Dias AA. Pentraxin 3 accelerates lung injury in high tidal volume ventilation in mice. Molecular Immunology. 2012;51:82–90. doi: 10.1016/j.molimm.2012.02.113. [DOI] [PubMed] [Google Scholar]
- 119.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 120.Ristagno G, Varpula T, Masson S, Greco M, Bottazzi B, Milani V, Aleksova A, Sinagra G, Assandri R, Tiainen M, Vaahersalo J, et al. Elevations of inflammatory markers PTX3 and sST2 after resuscitation from cardiac arrest are associated with multiple organ dysfunction syndrome and early death. Clin Chem Lab Med. 2015;53:1847–1857. doi: 10.1515/cclm-2014-1271. [DOI] [PubMed] [Google Scholar]
- 121.Rodriguez-Grande B, Swana M, Nguyen L, Englezou P, Maysami S, Allan SM, Rothwell NJ, Garlanda C, Denes A, Pinteaux E. The acute-phase protein PTX3 is an essential mediator of glial scar formation and resolution of brain edema after ischemic injury. J Cereb Blood Flow Metab. 2014;34:480–488. doi: 10.1038/jcbfm.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roers A, Hiller B, Hornung V. Recognition of Endogenous Nucleic Acids by the Innate Immune System. Immunity. 2016;44:739–754. doi: 10.1016/j.immuni.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 123.Ronca R, Alessi P, Coltrini D, Di Salle E, Giacomini A, Leali D, Corsini M, Belleri M, Tobia C, Garlanda C, Bonomi E, et al. Long pentraxin-3 as an epithelial-stromal fibroblast growth factor-targeting inhibitor in prostate cancer. J Pathol. 2013;230:228–238. doi: 10.1002/path.4181. [DOI] [PubMed] [Google Scholar]
- 124.Ronca R, Giacomini A, Di Salle E, Coltrini D, Pagano K, Ragona L, Matarazzo S, Rezzola S, Maiolo D, Torrella R, Moroni E, et al. Long-Pentraxin 3 Derivative as a Small-Molecule FGF Trap for Cancer Therapy. Cancer Cell. 2015;28:225–239. doi: 10.1016/j.ccell.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 125.Roumenina LT, Ruseva MM, Zlatarova A, Ghai R, Kolev M, Olova N, Gadjeva M, Agrawal A, Bottazzi B, Mantovani A, Reid KB, et al. Interaction of C1q with IgG1, C-reactive protein and pentraxin 3: mutational studies using recombinant globular head modules of human C1q A, B, and C chains. Biochemistry. 2006;45:4093–4104. doi: 10.1021/bi052646f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rovere P, Peri G, Fazzini F, Bottazzi B, Doni A, Bondanza A, Zimmermann VS, Garlanda C, Fascio U, Sabbadini MG, Rugarli C, et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000;96:4300–4306. [PubMed] [Google Scholar]
- 127.Rovere-Querini P, Antonacci S, Dell'Antonio G, Angeli A, Almirante G, Cin ED, Valsecchi L, Lanzani C, Sabbadini MG, Doglioni C, Manfredi AA, et al. Plasma and tissue expression of the long pentraxin 3 during normal pregnancy and preeclampsia. Obstet Gynecol. 2006;108:148–155. doi: 10.1097/01.AOG.0000224607.46622.bc. [DOI] [PubMed] [Google Scholar]
- 128.Russell DL, Salustri A. Extracellular matrix of the cumulus-oocyte complex. Semin Reprod Med. 2006;24:217–227. doi: 10.1055/s-2006-948551. [DOI] [PubMed] [Google Scholar]
- 129.Ryu W-S, Kim CK, Kim BJ, Kim C, Lee S-H, Yoon B-W. Pentraxin 3: A novel and independent prognostic marker in ischemic stroke. Atherosclerosis. 2012;220:581–586. doi: 10.1016/j.atherosclerosis.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 130.Salio M, Chimenti S, De Angelis N, Molla F, Maina V, Nebuloni M, Pasqualini F, Latini R, Garlanda C, Mantovani A. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 131.Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P, Salvatori G, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 132.Sargent IL, Borzychowski AM, Redman CW. NK cells and human pregnancy--an inflammatory view. Trends Immunol. 2006;27:399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 133.Scarchilli L, Camaioni A, Bottazzi B, Negri V, Doni A, Deban L, Bastone A, Salvatori G, Mantovani A, Siracusa G, Salustri A. PTX3 interacts with inter-alpha-trypsin inhibitor: implications for hyaluronan organization and cumulus oophorus expansion. J Biol Chem. 2007;282:30161–30170. doi: 10.1074/jbc.M703738200. [DOI] [PubMed] [Google Scholar]
- 134.Shindo A, Tanemura H, Yata K, Hamada K, Shibata M, Umeda Y, Asakura F, Toma N, Sakaida H, Fujisawa T, Taki W, et al. Inflammatory biomarkers in atherosclerosis: pentraxin 3 can become a novel marker of plaque vulnerability. PloS one. 2014;9:e100045. doi: 10.1371/journal.pone.0100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shrive AK, Metcalfe AM, Cartwright JR, Greenhough TJ. C-reactive protein and SAP-like pentraxin are both present in Limulus polyphemus haemolymph: crystal structure of Limulus SAP. J Mol Biol. 1999;290:997–1008. doi: 10.1006/jmbi.1999.2956. [DOI] [PubMed] [Google Scholar]
- 136.Sirugo G, Edwards DR, Ryckman KK, Bisseye C, White MJ, Kebbeh B, Morris GA, Adegbola RA, Tacconelli A, Predazzi IM, Novelli G, et al. PTX3 genetic variation and dizygotic twinning in the Gambia: could pleiotropy with innate immunity explain common dizygotic twinning in Africa? Ann Hum Genet. 2012;76:454–463. doi: 10.1111/j.1469-1809.2012.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Soares AC, Souza DG, Pinho V, Vieira AT, Nicoli JR, Cunha FQ, Mantovani A, Reis LF, Dias AA, Teixeira MM. Dual function of the long pentraxin PTX3 in resistance against pulmonary infection with Klebsiella pneumoniae in transgenic mice. Microbes Infect. 2006;8:1321–1329. doi: 10.1016/j.micinf.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 138.Souza DG, Amaral FA, Fagundes CT, Coelho FM, Arantes RM, Sousa LP, Matzuk MM, Garlanda C, Mantovani A, Dias AA, Teixeira MM. The long pentraxin PTX3 is crucial for tissue inflammation after intestinal ischemia and reperfusion in mice. Am J Pathol. 2009;174:1309–1318. doi: 10.2353/ajpath.2009.080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sprong T, Peri G, Neeleman C, Mantovani A, Signorini S, van der Meer JW, van Deuren M. Ptx3 and C-Reactive Protein in Severe Meningococcal Disease. Shock. 2009;31:28–32. doi: 10.1097/SHK.0b013e31817fd543. [DOI] [PubMed] [Google Scholar]
- 140.Suliman ME, Qureshi AR, Carrero JJ, Barany P, Yilmaz MI, Snaedal-Jonsdottir S, Alvestrand A, Heimburger O, Lindholm B, Stenvinkel P. The long pentraxin PTX-3 in prevalent hemodialysis patients: associations with comorbidities and mortality. Qjm. 2008;101:397–405. doi: 10.1093/qjmed/hcn019. [DOI] [PubMed] [Google Scholar]
- 141.Tillet WS, Francis Tj. Serological reactions in pneumonia with a non protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–585. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tong M, C J, Qureshi AR, Anderstam B, Heimburger O, Barany P, Axelsson J, Alverstrand A, Stenvinkel P, Lindholm B, Suliman M. Plasma Pentraxin 3 in chronic kidney disease patients: association with renal function, protein-energy wasting, cardiovascular disease and mortality. Clin J Am Soc Nephrol. 2007;2:889–897. doi: 10.2215/CJN.00870207. [DOI] [PubMed] [Google Scholar]
- 143.Tsuji S, Midorikawa Y, Seki M, Takayama T, Sugiyama Y, Aburatani H. Network-based analysis for identification of candidate genes for colorectal cancer progression. Biochem Biophys Res Commun. 2016;476:534–540. doi: 10.1016/j.bbrc.2016.05.158. [DOI] [PubMed] [Google Scholar]
- 144.Uusitalo-Seppala R, Huttunen R, Aittoniemi J, Koskinen P, Leino A, Vahlberg T, Rintala EM. Pentraxin 3 (PTX3) is associated with severe sepsis and fatal disease in emergency room patients with suspected infection: a prospective cohort study. PLoS One. 2013;8:e53661. doi: 10.1371/journal.pone.0053661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–1167. doi: 10.1210/mend.16.6.0859. [DOI] [PubMed] [Google Scholar]
- 146.Wagenaar JF, Goris MG, Gasem MH, Isbandrio B, Moalli F, Mantovani A, Boer KR, Hartskeerl RA, Garlanda C, van Gorp EC. Long pentraxin PTX3 is associated with mortality and disease severity in severe Leptospirosis. J Infect. 2009;58:425–432. doi: 10.1016/j.jinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 147.Wang JX, He YL, Zhu ST, Yang S, Zhang ST. Aberrant methylation of the 3q25 tumor suppressor gene PTX3 in human esophageal squamous cell carcinoma. World J Gastroenterol. 2011;17:4225–4230. doi: 10.3748/wjg.v17.i37.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang L, Cano M, Datta S, Wei H, Ebrahimi KB, Gorashi Y, Garlanda C, Handa JT. PTX3 recruits complement factor H to protect against oxidative stress-induced complement and inflammasome overactivation. J Pathol. 2016 doi: 10.1002/path.4811. [DOI] [PubMed] [Google Scholar]
- 149.Willeke F, Assad A, Findeisen P, Schromm E, Grobholz R, von Gerstenbergk B, Mantovani A, Peri S, Friess HH, Post S, von Knebel Doeberitz M, et al. Overexpression of a member of the pentraxin family (PTX3) in human soft tissue liposarcoma. Eur J Cancer. 2006;42:2639–2646. doi: 10.1016/j.ejca.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 150.Wojtowicz A, Lecompte TD, Bibert S, Manuel O, Rueger S, Berger C, Boggian K, Cusini A, Garzoni C, Hirsch H, Khanna N, et al. PTX3 Polymorphisms and Invasive Mold Infections After Solid Organ Transplant. Clinical Infectious Diseases. 2015;61:619–622. doi: 10.1093/cid/civ386. [DOI] [PubMed] [Google Scholar]
- 151.Xiao Y, Yang N, Zhang Q, Wang Y, Yang S, Liu Z. Pentraxin 3 inhibits acute renal injury-induced interstitial fibrosis through suppression of IL-6/Stat3 pathway. Inflammation. 2014;37:1895–1901. doi: 10.1007/s10753-014-9921-2. [DOI] [PubMed] [Google Scholar]
- 152.Yamasaki K, Kurimura M, Kasai T, Sagara M, Kodama T, Inoue K. Determination of physiological plasma pentraxin 3 (PTX3) levels in healthy populations. Clin Chem Lab Med. 2009;47:471–477. doi: 10.1515/CCLM.2009.110. [DOI] [PubMed] [Google Scholar]
- 153.Zareparsi S, Branham KE, Li M, Shah S, Klein RJ, Ott J, Hoh J, Abecasis GR, Swaroop A. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005;77:149–153. doi: 10.1086/431426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhou Y, Zhang J, Zhu M, Lu R, Wang Y, Ni Z. Plasma Pentraxin 3 Is Closely Associated with Peripheral Arterial Disease in Hemodialysis Patients and Predicts Clinical Outcome: A 6-Year Follow-Up. Blood Purif. 2015;39:266–273. doi: 10.1159/000381254. [DOI] [PubMed] [Google Scholar]