Abstract
Atherosclerosis is the underlying cause of cardio-cerebrovascular disease. However, the mechanisms of atherosclerosis are still unclear. The modification of DNA methylation has an important role in atherosclerosis development. As a member of the Ten-eleven translocation (TET) family, TET methylcytosine dioxygenase 2 (TET2) can modify DNA methylation by catalyzing 5-methylcytosine to 5-hydroxymethylcytosine and mediate DNA demethylation. Recent findings suggest that TET2 is related to the phenotype transformation of vascular smooth muscle cells, endothelial dysfunction, and inflammation of macrophage, the key factors of atherosclerosis. Therefore, TET2 may be a potential target for atherosclerosis treatment. This review will elaborate the recent findings that suggest the role of TET2 in atherosclerosis.
Keywords: : TET2, DNA demethylation, atherosclerosis, inflammation
Introduction
Atherosclerosis, a multifactorial disease that preferentially occurs in large and medium-size arteries, is the main pathological basis of cardio-cerebrovascular diseases. It is also a chronic inflammatory arterial vessel disease, which is initiated by the accumulation of lipoproteins in the intimal layer of the arterial vascular wall. It is closely related to endothelial cell (EC) dysfunction, vascular smooth muscle cell (VSMC) proliferation and phenotype transformation, and macrophage invasion (van Diepen et al., 2013; Yu et al., 2013a; Wang et al., 2015; Studentova et al., 2016), but its underlying regulatory mechanism is less clear.
In recent years, the role of epigenetics in atherosclerosis has become a topic of intense research interest, especially DNA methylation modification. Newman first proposed that DNA methylation modification is related to atherosclerosis (Newman, 1999). As a reverse process of DNA methylation, DNA demethylation is a passive or active process. In 2009, the human Ten-Eleven translocation methylcytosine dioxygenase 1 (TET1) was discovered to catalyze the conversion of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) (Tahiliani et al., 2009). Then, other mouse TET family members (Tet1, Tet2, and Tet3) were found (Ito et al., 2010). The roles of TET in diseases, especially in cancer, have been widely studied in recent years (Rasmussen and Helin, 2016). Furthermore, studies also found that TET2 was downregulated in atherosclerotic lesions and involved in pathophysiological progression of atherosclerosis (Li et al., 2015). In this review, we summarized the studies on the protective role of TET2 in atherosclerosis, transcription regulation of TET2 gene expression, and activity regulation of TET2 enzyme. Moreover, we aimed to provide a novel effective regulator and potential intervention target for atherosclerosis.
Protective Role of TET2 in Atherosclerosis
Atherosclerosis is a chronic inflammatory disease that exhibits monocyte and foam cell infiltration, vascular EC dysfunction, and proliferation and phenotype transformation of VSMCs. Studies have found that almost all the above processes can be regulated by TET2 (Table 1, Fig. 1).
Table 1.
Role of Ten-Eleven Translocation Methylcytosine Dioxygenase 2 in Atherosclerosis
| Treatment | Object | TET2 expression level | Pathway | Effect on AS | Reference |
|---|---|---|---|---|---|
| Ox-LDL | THP-1-derived macrophages | mRNA↓ | Autophagy (Beclin1↓, Lc3-II↓) | AS↑ | Li et al. (2015) |
| protein↓ | |||||
| Rapamycin | Smooth muscle cell | Protein↓ | Knockdown of TET2 can inhibit key contractile genes (MYOCD, SRF), upregulation of synthetic gene transcription (KLF4) | / | Liu et al. (2013) |
| 5hmC↓ | |||||
| Ox-LDL | HUVECs | 5hmC↓ | Autophagy (Beclin1↓, Lc3↓), Autophagic flux↓ | AS↑ | Peng et al. (2016) |
| Low shear stress | Endothelial cell | protein↓ | Autophagy (Beclin1↓ and Lc3-II/I↓) | AS↑ | Yang et al. (2016) |
| LPS | Tet2-deficient BMDC and macrophages and Tet2-silenced human dendritic cells | / | Tet2 recruited Hdac2 and repressed transcription of IL-6 through histone deacetylation. (independent of DNA methylation and hydroxymethylation) | AS↓ | Zhang et al. (2015) |
| Bone marrow transplantation | LDLR−/− chimeric mice with 10% of TET2-deficient HSPCs | / | Inflammation (NLRP3 inflammasome-mediated IL-1β production) | AS↑ | Fuster et al. (2017) |
AS, atherosclerosis; BMDC, bone marrow-derived dendritic cells; IL-6, interleukin-6; HSPCs, hematopoietic stem progenitor cells; HUVECs, human umbilical vascular endothelial cell lines; SRF, serum response factor; TET2, ten-eleven translocation methylcytosine dioxygenase 2.
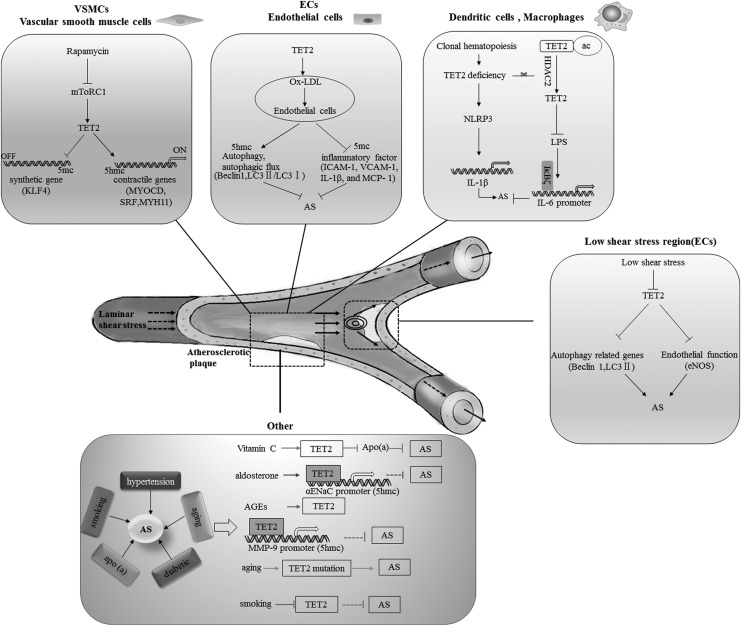
FIG. 1.
Schematic of TET2 signaling pathways involved in AS. TET2 as a potential target for regulation of AS processes, including the transformation of smooth muscle cells, autophagy of endothelial cells, endothelial dysfunction, and chronic inflammation. In addition, it is presumed that TET2 may indirectly regulate the development of AS by regulating the genes related to AS-related diseases. The dotted line represents the results to be verified, and the solid line represents the confirmed conclusion. AS, atherosclerosis; TET2, Ten-Eleven translocation methylcytosine dioxygenase 2.
TET2 regulates the transfer of smooth muscle cell phenotype
As a major component of atherosclerotic plaques, VSMCs play an important role in the initiation of atherosclerosis (Doran et al., 2008), especially the phenotype change (from contractile to synthetic phenotype) in the development and progression of atherosclerotic lesions. The synthetic phenotype has a stronger proliferation and migration ability than the contractile phenotype. Moreover, it can transfer VSMCs from the tunica media to the intima, leading to atherosclerosis (Bennett et al., 2016). Of course, the inhibition of VSMC phenotypic transformation is beneficial for atherosclerosis. TET2 and 5hmC are enriched in contractile VSMCs, but reduced in synthetic phenotype VSMCs. In addition, TET2 knockdown inhibits the expression of critical precontractual genes, such as myocardia (MYOCD) and serum response factor (SRF), and upregulates KLF4 simultaneously. TET2 and 5hmC are enriched in the CArG-rich regions of active SMC contractile promoters (MYOCD, SRF, and MYH11). In addition, the loss of TET2 prevents rapamycin-induced VSMC differentiation, whereas TET2 overexpression induces a contractile phenotype (Liu et al., 2013). These findings suggested that TET2 is a novel epigenetic regulator of VSMC phenotype transformation.
TET2 ameliorates vascular EC dysfunction
Endothelial damage and dysfunction is the starting point of vascular disease. The reduction of nitric oxide (NO) synthesis by endothelial nitric oxide synthase (eNOS) in vascular ECs is a marker of endothelial dysfunction (Hahn and Schwartz, 2008). As a crucial regulator of intracellular homeostasis, autophagy can serve as an antiatherosclerotic biological mechanism (Martinet and De Meyer, 2009). Autophagy inhibition can lead to EC dysfunction and atherosclerotic lesion formation. Studies have indicated that autophagy can be regulated by TET2 through demethylation modification in atherosclerosis. Autophagy was assessed primarily through detecting the expression level of Beclin1 and microtubule-associated protein1 light chain 3 (LC3). Beclin1 is a component of the class III PI3K complex that is required for autophagy initiation. LC3 is involved in the formation of autophagosome membranes, including two interconvertible forms, LC3-I and LC3-II. The proportion of LC3-II or LC3-II/LC3-I was positively correlated with the number of autophagosomes (Lamb et al., 2013). For example, low shear stress repressed EC autophagy through the downregulated expression of TET2 and decrease of autophagy-related gene (Beclin1, Lc3 II) expression (Yang et al., 2016). By contrast, autophagy level and autophagy-related gene (Beclin1, Lc3 II) expression were upregulated by TET2 overexpression, and also with an improvement of endothelial function, with increased eNOS expression and release of NO. In the ApoE−/− mice animal model, autophagy was upregulated by TET2 overexpression, whereas autophagy level decreased by TET2 silencing. Furthermore, in ox-LDL-treated vascular ECs, autophagy and autophagic flux were improved by TET2 overexpression and decreased by TET2 silencing (Peng et al., 2016).
TET2 inhibits inflammation
Interestingly, DNMT1 in macrophages has been reported to aggravate chronic inflammation release and atherosclerosis development by weakening the PPAR-γ signaling in mice (Yu et al., 2016). It indicated that inflammation may be regulated by methylation or demethylation. As an important demethylase, TET2 can regulate autophagy not only in ECs, but also in macrophages (Li et al., 2015). TET2 selectively mediates the repression of interleukin-6 (IL-6) transcription in dendritic cells and macrophages and several inflammatory mediators by histone deacetylation (Zhang et al., 2015). Clonal hematopoiesis of indeterminate potential, which is defined as the presence of expanded somatic blood cell clone in persons without other hematological abnormalities, is common in the elderly and is associated with an increased risk of blood cancer (Jaiswal et al., 2014). Clonal hematopoiesis contributes to the development of human atherosclerosis. Moreover, the LDLR−/− mice model, which was transplanted with bone morrow obtained from TET2+/− or TET2−/− mice, had larger atherosclerotic lesions in the aortic root and aorta than did mice that had received control bone marrow (Jaiswal et al., 2014, 2017). Interestingly, Fuster et al. (2017) also suggested a causal link between accelerated atherosclerosis and clonal hematopoiesis induced by somatic TET2 mutations. The results revealed that increased NLRP3 inflammasome-mediated IL-1β production is necessary for aggravated atherosclerosis in clonal hematopoiesis because of TET2 deficiency.
TET2 and other atherosclerotic pathogenic factors
Clonal hematopoiesis, associated with human aging, leads to the development of human atherosclerosis (Jaiswal et al., 2014, 2017), and somatic TET2 mutations in blood cells accelerate atherosclerosis development (Fuster et al., 2017). As an independent risk factor of atherosclerosis, the unique glycoprotein apolipoprotein(a) [apo(a)] composition of Lipoprotein(a) [Lp(a)] can be regulated by vitamin C, and enhances 5hmC generation in vivo and in cultured cells, most probably by acting as a cofactor for TET to hydroxylate 5 mC (Minor et al., 2013). Vitamin C can downregulate apo(a) expression through TET2-dependent DNA demethylation in HepG2 cells (Qu et al., 2017). A number of studies certified that TET2 is significantly increased in diabetic wound (Tan et al., 2016; Zhang et al., 2016), and advanced glycation end products can upregulate TET2 gene expression and activity in human keratinocytes. Additionally, TET2 proteins directly bind to the promoter region of MMP-9 and reduce the percentage of methylation in the MMP-9 promoter (Zhang et al., 2016), which results in the development of diabetic retinopathy (Kowluru et al., 2016). The epithelial sodium channel (ENaC) is a key player in the regulation of renal sodium homeostasis and blood pressure (Schild, 2010; Rossier, 2014). Aldosterone increases the reabsorption of sodium ions by increasing the transcription of αENaC in the collecting duct cells (Yu et al., 2013b). Previous studies have demonstrated that aldosterone stimulates the recruitment of TET2 to demethylate the αENaC promoter to induce αENaC transcription (Yu et al., 2013b). Martina et al. hypothesized that TET2 can also induce ENaC transcription, which affects cardiovascular outcomes and the regulation of blood pressure by hydrochlorothiazide (Chittani et al., 2015). In 2005, ∼673,000 people died of tobacco-related diseases in China, and the three major causes of deaths among 40–79-year-olds were cancer, cardiovascular diseases, and respiratory diseases (Chen, 2009). Wain et al. (2015) reported that the TET2 expression was higher in individuals who have not smoked than in heavy smokers (mean 25 pack-years). Unfortunately, the relationship between TET2 and smoking has not been well addressed in cardiovascular studies.
Factors Involved in the Regulation of TET2 Gene Expression and Enzyme Activity
With more evidences showing the importance of TET2 in atherosclerosis, it became very important to elucidate the factors involved in the expression and activity regulation of TET2 gene and enzyme. Some transcription factors can regulate TET2 gene expression and the activity of TET2 enzyme (Fig. 2).
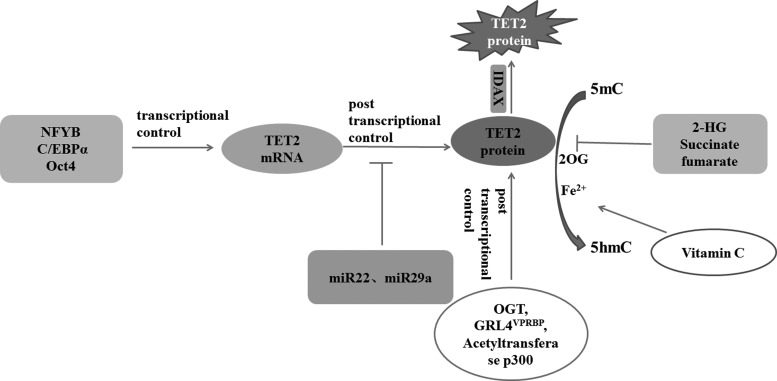
FIG. 2.
Factors involved in regulation of TET2 gene expression and enzyme activity. Transcription factors (NFYB, C/EBPα, Oct4) regulate TET2 mRNA levels in various developmental stages or cell types. microRNA inhibited TET protein expression in post-transcription level. TET protein can also be regulated by O-linked N-acetylglucosamine transferase or cullin-RING ubiquitin ligase 4-CRL4VprBP, acetyltransferase p300 in post-transcription level. IDAX results in TET2 protein degradation through caspase activation. Vitamin C acts as a cofactor for Fe2+ and 2-OG-dependent TET dioxygenase and can directly enhance the catalytic activity of TET protein. Pathological accumulation of three metabolites, namely, 2-hydroxyglutarete (2-HG), succinate, and fumarate, which are structurally similar to α-OG, leading to a competitive inhibition of α-OG-dependent TET activity. NFYB, nuclear transcriptional factor Y subunit beta.
Factors involved in TET2 gene expression regulation
DNA demethylation can be controlled by regulating the transcription of TET genes (Wu and Zhang, 2014). TET2 gene transcription is regulated by different transcription factors in various stages or cell types. TET2 is broadly expressed in adult tissues. For instance, hydrogen sulfide may facilitate the sulfhydration of the nuclear transcriptional factor Y subunit beta (NFYB) binding to TET2 promoters, resulting in increased transcription of the TET2 gene (Oh and Li, 2015). During the transformation of B cells into macrophages, CEBPα is directly associated with TET2 promoter and upregulated mRNA levels by binding to the TET2 enhancer (Kallin et al., 2012). In pluripotent stem cells, the key pluripotency factor Oct4 regulates TET2 transcription (Koh et al., 2011).
TET expression may also be post-transcriptionally regulated through miRNAs. miR-22 exerts its invasiveness and metastasis potential depending on the silencing of miR-200 through direct targeting of TET in breast cancer (Song et al., 2013c). In addition, miR-22 is upregulated in myelodysplastic syndrome (MDS), and its aberrant expression is correlated with poor survival. Conversely, TET2 is a key target of miR-22, and its ectopic expression suppresses miR-22-induced phenotypes. Hematopoietic stem cells exhibit increased self-renewal and are involved in MDS development because of the reduced levels of 5hmC by deregulating of TET2 proteins through miR-22 (Song et al., 2013b). Takayama et al. reported that the miR-29 family targets TET2, reduces TET2 or 5hmC expression levels in the animal model of hormone refractory PCa, and induces tumor growth (Takayama et al., 2015).
Factors involved in the regulation of TET2 enzymatic activity
TET proteins are the members of Fe2+/α-ketoglutarate (α-KG)-dependent dioxygenases, which require Fe2+ as a metal cofactor and α-KG as a cosubstrate. Withdrawing Fe2+ dramatically inhibited the conversion of 5mC to 5hmC (Ito et al., 2010). The activity of TET2 can also be regulated by metabolites. In various types of tumors, the pathological accumulation of three metabolites, namely, 2-hydroxyglutarate, succinate, and fumarate, which are structurally similar to α-KG, leads to a competitive inhibition of α-KG-dependent TET2 activity, resulting in a hypermethylation phenotype. Intraperitoneal injection of glucose in mice rapidly increased the levels of blood glucose and hepatic α-KG. Interestingly, genome-wide 5hmC levels were rapidly increased in various mouse tissues. However, the mRNA and protein level of TET2 was not changed in mouse livers after glucose injection. The results show that α-KG is important for activity regulation of TET2 enzyme (Yang et al., 2014).
O-linked N-acetylglucosamine transferase (OGT) modifies O-linked N-acetylglucosamine (O-GlcNAC) on various proteins, such as transcription factors, epigenetic regulators, and histones. Various studies have demonstrated that OGT as a protein interacts with TET2 proteins and catalyzes O-GlcNAcylation of TET2 (Chen et al., 2013; Deplus et al., 2013; Vella et al., 2013). O-GlcNAcylation occurs at serine or threonine residues of TET protein, and phosphorylation of TET protein can also happen in these amino acids. Interestingly, the phosphorylation extends of TET2 are higher than TET3. Phosphorylation of TET2 protein can be suppressed through O-GlcNAcylation by the glycosyltransferase OGT (Bauer et al., 2015). However, the relationship between TET2 phosphorylation and TET enzymatic activity has not been addressed in the literature.
The cullin-RING finger ligase-4 (CRL4) complex is crucial in regulating mammalian oocyte survival and reprogramming. Oocyte-specific deletion of the CRL4 linker protein DDB1 or its substrate adaptor VPRBP (also known as DCAF1) causes rapid oocyte loss, premature ovarian insufficiency, and silencing of fertility-maintaining genes (Yu et al., 2013). TET2 was highly expressed in oocytes and early embryos. The 5hmC generation was reduced in DDB1- or VPRBP-deleted oocytes and CRL4VPRBP might regulate TET2 activities. CRL4VPRBP enters the nucleus and activates TET2. Additionally, VPRBP-mediated monoubiquitylation plays a critical role in TET2 activation by promoting the ability of TET2 to bind DNA, but not the catalytic activity of TET2 enzyme. Then, deletion of the VPRBP gene causes substantial reduction of 5hmC, suggesting that the function of VPRBP is important for TET2 activity (Nakagawa et al., 2015).
The IDAX gene known as CXXC4 originates from the ancestral TET2 gene fission during vertebrate evolution and is transcribed in the opposite direction. The IDAX CXXC domain binds DNA sequences enriched in unmethylated CpG dinucleotides and interacts directly with the catalytic domain of TET2. IDAX binds DNA sequences containing unmethylated CpG dinucleotides in gene promoter and recruits TET2 to DNA, which results in caspase activation and TET2 protein degradation, without directly affecting the enzymatic activity of TET2 (Ko et al., 2013).
As an antioxidant, free radical scavenger, and a cofactor for Fe2+- and 2-αKG-dependent dioxygenases, vitamin C enhances 5hmC generation, most probably by acting as a cofactor for TET2 (Minor et al., 2013). It can directly enhance the catalytic activity of TET2 dioxygenases to oxidize 5 mC. Furthermore, the results showed that vitamin C can uniquely interact with the C-terminal catalytic domain of TET2 enzymes, which probably promotes their folding and/or recycling of the cofactor Fe2+. However, in the TET1/TET2 double-knockout cells, vitamin C did not alter 5 mC oxidation or the overall level of 5 mC (Yin et al., 2013). Blaschke demonstrated that the addition of vitamin C to mouse embryonic stem (ES) cells promotes TET activity, leading to the rapid and global increase in 5hmC. Interestingly, dot blot analysis revealed that TET1/TET2 double-knockout ESCs also show significantly reduced 5hmC signal, which was not increased by vitamin C treatment (Blaschke et al., 2013). These events indicated that the effects of vitamin C are TET dependent.
5hmC and 5fC are significantly enriched at the binding site of acetyltransferase p300 throughout the genome (Yu et al., 2012; Song et al., 2013a). TET2 is regulated by acetyltransferase p300, which enhances its enzymatic activity, protein stability, and partnering with DNMT1. The TET2/DNMT complex targeting chromatin is a key regulator during oxidative stress, which protects against abnormal DNA methylation (Zhang et al., 2017).
Conclusions and Perspectives
In this study, we showed that TET2 plays an important role in preventing atherosclerosis by repressing VSMC phenotype transformation, protecting ECs from damage and dysfunction, and inhibiting inflammation. The underlying mechanisms were mostly associated with the methylation/demethylation regulation of related gene expression, such as MYOCD, SRF, KLF4, eNOS, and autophagy-related genes. Also, several factors were associated with the expression and activity regulation of the TET2 gene and protein. TET2 gene expression could be regulated at the transcription and post-transcription level by NFYB, CEBPα, OCT4, and miRNAs. TET2 activity could be regulated by Fe2+, α-KG, OGT, VPRBP, vitamin C, IDAX, and acetyltransferase p300. The protective roles of TET2 in atherosclerosis are mentioned above. TET2 selectively mediates the repression of IL-6 transcription in dendritic cells and macrophages by histone deacetylation, and vitamin C can downregulate apo(a) expression through TET2 in HepG2 cells. TET2 enzyme activity-related factors, vitamin C, and acetyltransferase p300 may play an important role in atherosclerosis through regulating TET2 activity. In addition, miRNAs affect the pathogenesis of atherosclerosis. miRNAs may target TET2 and accelerate the process of atherosclerosis. Further studies should determine other regulatory factors related to TET2 and atherosclerosis.
Studies have confirmed that the early stage of atherosclerosis occurs during DNA methylation. Thus, DNA methylation is necessary for atherosclerosis development, or an epiphenomenon, and this condition has not been confirmed until now. Based on the effects of DNA methylation on the development of atherosclerosis, TET2, as an important demethylase, inevitably participates in most biological and pathological processes. However, the mechanisms related to atherosclerosis development have only been studied recently. We believe that in-depth studies of TET2 and its mechanisms related to atherosclerosis development will provide a new target for the effective prevention and treatment of atherosclerosis.
Acknowledgments
This study was supported by the Natural Science Foundation of China (No. 81070221) and the Innovative Research Team for Science and Technology in Higher Educational Institutions of Hunan Province and the Construct Program of the Key Discipline in Hunan Province.
Disclosure Statement
No competing financial interests exist.
References
- Bauer C., Gobel K., Nagaraj N., et al. (2015). Phosphorylation of TET proteins is regulated via O-GlcNAcylation by the O-linked N-acetylglucosamine transferase (OGT). J Biol Chem 290, 4801–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M.R., Sinha S., Owens G.K., (2016). Vascular smooth muscle cells in atherosclerosis. Circ Res 118, 692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke K., Ebata K.T., Karimi M.M., et al. (2013). Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 500, 222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Chen Y., Bian C., et al. (2013). TET2 promotes histone O-GlcNAcylation during gene transcription. Nature 493, 561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. (2009). Mortality attributable to smoking in China. N Engl J Med 360, 1911; author reply 1911. [DOI] [PubMed] [Google Scholar]
- Chittani M., Zaninello R., Lanzani C., et al. (2015). TET2 and CSMD1 genes affect SBP response to hydrochlorothiazide in never-treated essential hypertensives. J Hypertens 33, 1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplus R., Delatte B., Schwinn M.K., et al. (2013). TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J 32, 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran A.C., Meller N., and McNamara C.A. (2008). Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol 28, 812–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J.J., MacLauchlan S., Zuriaga M.A., et al. (2017). Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C., and Schwartz M. A. (2008). The role of cellular adaptation to mechanical forces in atherosclerosis. Arterioscler Thromb Vasc Biol 28, 2101–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., D'Alessio A.C., Taranova O.V., et al. (2010). Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Fontanillas P., Flannick J., et al. (2014). Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371, 2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S., Natarajan P., Silver A.J., et al. (2017). Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 377, 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallin E.M., Rodriguez-Ubreva J., Christensen J., et al. (2012). Tet2 facilitates the derepression of myeloid target genes during CEBPalpha-induced transdifferentiation of pre-B cells. Mol Cell 48, 266–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M., An J., Bandukwala H.S., et al. (2013). Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature 497, 122–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K. P., Yabuuchi A., Rao S., et al. (2011). Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell 8, 200–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru R.A., Shan Y., and Mishra M. (2016). Dynamic DNA methylation of matrix metalloproteinase-9 in the development of diabetic retinopathy. Lab Invest 96, 1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C.A., Yoshimori T., and Tooze S.A. (2013). The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol 14, 759–774 [DOI] [PubMed] [Google Scholar]
- Li G., Peng J., Liu Y., et al. (2015). Oxidized low-density lipoprotein inhibits THP-1-derived macrophage autophagy via TET2 down-regulation. Lipids 50, 177–183 [DOI] [PubMed] [Google Scholar]
- Liu R., Jin Y., Tang W.H., et al. (2013). Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 128, 2047–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet W., and De Meyer G.R. (2009). Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ Res 104, 304–317 [DOI] [PubMed] [Google Scholar]
- Minor E.A., Court B.L., Young J.I., et al. (2013). Ascorbate induces ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J Biol Chem 288, 13669–13674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Lv L., Nakagawa M., et al. (2015). CRL4 (VprBP) E3 ligase promotes monoubiquitylation and chromatin binding of TET dioxygenases. Mol Cell 57, 247–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman P.E. (1999). Can reduced folic acid and vitamin B12 levels cause deficient DNA methylation producing mutations which initiate atherosclerosis? Med Hypotheses 53, 421–424 [DOI] [PubMed] [Google Scholar]
- Oh S.A., and Li M.O. (2015). TETs link hydrogen sulfide to immune tolerance. Immunity 43, 211–213 [DOI] [PubMed] [Google Scholar]
- Peng J., Yang Q., Li A.F., et al. (2016). Tet methylcytosine dioxygenase 2 inhibits atherosclerosis via upregulation of autophagy in ApoE-/- mice. Oncotarget 7, 76423–76436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu K., Ma X.F., Li G.H., et al. (2017). Vitamin C down-regulate apo(a) expression via Tet2-dependent DNA demethylation in HepG2 cells. Int J Biol Macromol 98, 637–645 [DOI] [PubMed] [Google Scholar]
- Rasmussen K.D., and Helin K. (2016). Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev 30, 733–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier B.C. (2014). Epithelial sodium channel (ENaC) and the control of blood pressure. Curr Opin Pharmacol 15, 33–46 [DOI] [PubMed] [Google Scholar]
- Schild L. (2010). The epithelial sodium channel and the control of sodium balance. Biochim Biophys Acta 1802, 1159–1165 [DOI] [PubMed] [Google Scholar]
- Song C.X., Szulwach K.E., Dai Q., et al. (2013a). Genome-wide profiling of 5-formylcytosine reveals its roles in epigenetic priming. Cell 153, 678–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.J., Ito K., Ala U., et al. (2013b). The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell 13, 87–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.J., Poliseno L., Song M.S., et al. (2013c). MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 154, 311–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studentova H., Indrakova J., Petrova P., et al. (2016). Risk factors of atherosclerosis during systemic therapy targeting vascular endothelial growth factor. Oncol Lett 11, 939–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M., Koh K.P., Shen Y., et al. (2009). Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Misawa A., Suzuki T., et al. (2015). TET2 repression by androgen hormone regulates global hydroxymethylation status and prostate cancer progression. Nat Commun 6, 8219. [DOI] [PubMed] [Google Scholar]
- Tan Q., Wang W., Yang C., et al. (2016). Alpha-ketoglutarate is associated with delayed wound healing in diabetes. Clin Endocrinol (Oxf) 85, 54–61 [DOI] [PubMed] [Google Scholar]
- van Diepen J.A., Berbee J.F., Havekes L.M., et al. (2013). Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis. Atherosclerosis 228, 306–315 [DOI] [PubMed] [Google Scholar]
- Vella P., Scelfo A., Jammula S., et al. (2013). Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol Cell 49, 645–656 [DOI] [PubMed] [Google Scholar]
- Wain L.V., Shrine N., Miller S., et al. (2015). Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir Med 3, 769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Uryga A.K., Reinhold J., Figg N., et al. (2015). Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation 132, 1909–1919 [DOI] [PubMed] [Google Scholar]
- Wu H., and Zhang Y. (2014). Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 156, 45–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Lin H., Xu H., et al. (2014). TET-catalyzed 5-methylcytosine hydroxylation is dynamically regulated by metabolites. Cell Res 24, 1017–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Li X., Li R., et al. (2016). Low shear stress inhibited endothelial cell autophagy through TET2 downregulation. Ann Biomed Eng 44, 2218–2227 [DOI] [PubMed] [Google Scholar]
- Yin R., Mao S.Q., Zhao B., et al. (2013). Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc 135, 10396–10403 [DOI] [PubMed] [Google Scholar]
- Yu C., Zhang Y.L., Pan W.W., et al. (2013). CRL4 complex regulates mammalian oocyte survival and reprogramming by activation of TET proteins. Science 342, 1518–1521 [DOI] [PubMed] [Google Scholar]
- Yu J., Qiu Y., Yang J., et al. (2016). DNMT1-PPARgamma pathway in macrophages regulates chronic inflammation and atherosclerosis development in mice. Sci Rep 6, 30053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Hon G.C., Szulwach K.E., et al. (2012). Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell 149, 1368–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.H., Fu Y.C., Zhang D.W., et al. (2013a). Foam cells in atherosclerosis. Clin Chim Acta 424, 245–252 [DOI] [PubMed] [Google Scholar]
- Yu Z., Kong Q., and Kone B.C. (2013b). Aldosterone reprograms promoter methylation to regulate alphaENaC transcription in the collecting duct. Am J Physiol Renal Physiol 305, F1006–F1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yang C., Wang C., et al. (2016). AGE-induced keratinocyte MMP-9 expression is linked to TET2-mediated CpG demethylation. Wound Repair Regen 24, 489–500 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhao K., Shen Q., et al. (2015). Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.W., Wang Z., Xie W., et al. (2017). Acetylation enhances TET2 function in protecting against abnormal DNA methylation during oxidative stress. Mol Cell 65, 323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]