Abstract
Object
The aim of this study was to perform in vitro T2 mapping of serial dilutions of pharmaceutical surfactant.
Materials and Methods
MR imaging. MR scanning was performed on serial dilutions of surfactant on large bore clinical magnets at a field strength of 1.5T Philips and 3.0T (Achieva TX, Philips Healthcare, the Netherlands).
Results
The curves demonstrate a small increasing trend between surfactant concentration and R2 (shortened T2’s), with a 7.3% increase in R2 for each doubling of surfactant concentration (95% CI: 6.1-8.6%, p<0.001).
Conclusions
The increasing lung/ liver T2 signal ratio seen in fetal lungs with increasing gestational age is not due to increasing surfactant concentration.
Introduction
Fetal pulmonary hypoplasia is the most common complication of premature birth resulting in significant neonatal morbidity and mortality [1]. Given the severity of complications secondary to pulmonary immaturity the American College of Obstetrics and Gynecology recommends fetal pulmonary maturity testing prior to 39 weeks before elective delivery [2]. Currently, fetal lung function is assessed by amniocentesis, an invasive procedure with associated risks. A safe, non-invasive quantitative assay to assess fetal lung maturity is needed.
Premature delivery prior to 34 weeks requires evaluation of fetal pulmonary development to determine if glucocorticoids [3] should be administered prior to delivery to promote surfactant development. Surfactant, an amphiphilic compound, meaning a compound that is water soluble on one end and fat soluble on the other end reduces surface tension and prevents alveolar collapse [4]. Steroids given prior to birth decreases the number of neonates requiring intubation, positive airway pressure [5], and inhalational surfactant therapy [1] [6]. Surfactant solution is delivered directly into the lungs via an endotracheal tube primarily to treat respiratory distress syndrome [7].
The current gold standard for assessment of fetal lung maturity is measurement of surfactant levels within the amniotic fluid obtained by amniocentesis to evaluate the optimal timing of preterm delivery in high-risk pregnancies. However, results of amniocentesis may be erroneous, particularly if there is oligo- or polyhydramnios with corresponding concentration or dilution of amniotic fluid surfactant. Pulmonary hypoplasia is a dynamic process; any one measurement during the pregnancy may not be predictive of the degree of hypoplasia [8]. Since amniocentesis is invasive, obtaining serial measurements of amniotic fluid surfactant levels is not clinically feasible. A non-invasive quantitative measure to assess the degree of fetal lung maturity is crucially needed.
Recently, several publications [9, 10] indicated that T2 signal ratio from fetal lung to liver increases with gestational age. The T2 ratio may predict of the degree of fetal lung maturity [9, 11, 12]. The aim of this study was to perform in vitro T2 mapping of serial dilutions of pharmaceutical surfactant at 1.5T and 3.0T to determine whether increasing fetal surfactant concentration accounts for the clinically observed increase in lung to liver signal ratio.
Materials and Methods
Surfactant
Commercially available surfactant, Survanta (AbbVie Pharmaceuticals, Chicago IL) used to treat pulmonary hypoplasia in neonates was analyzed by Magnetic Resonance (MR) imaging. This agent is produced from bovine lung extract and consists of 25mg/ml of phospholipids including dipalmitoylphosphatidylcholine (the major component of human fetal surfactant), 11 mg/ml of desaturated phosphatidylcholine, 1 mg/ml of triglycerides, 1.5 mg/ml free fatty acids, and 1 mg/ml of bovine serum albumin protein. The standard dose for neonates is 100 mg of phospholipids/kg birth weight (4 ml/kg) delivered as mist through an endotracheal tube. Serial dilutions of surfactant were produced at 1:2, 1:4, 1:16, and 1:64 suspended in plasmalyte (Baxter Healthcare, Old Toongabbie, NSW Australia). These were placed into 5 mm outer diameter plastic NMR tubes (Figure 1).
Figure 1.


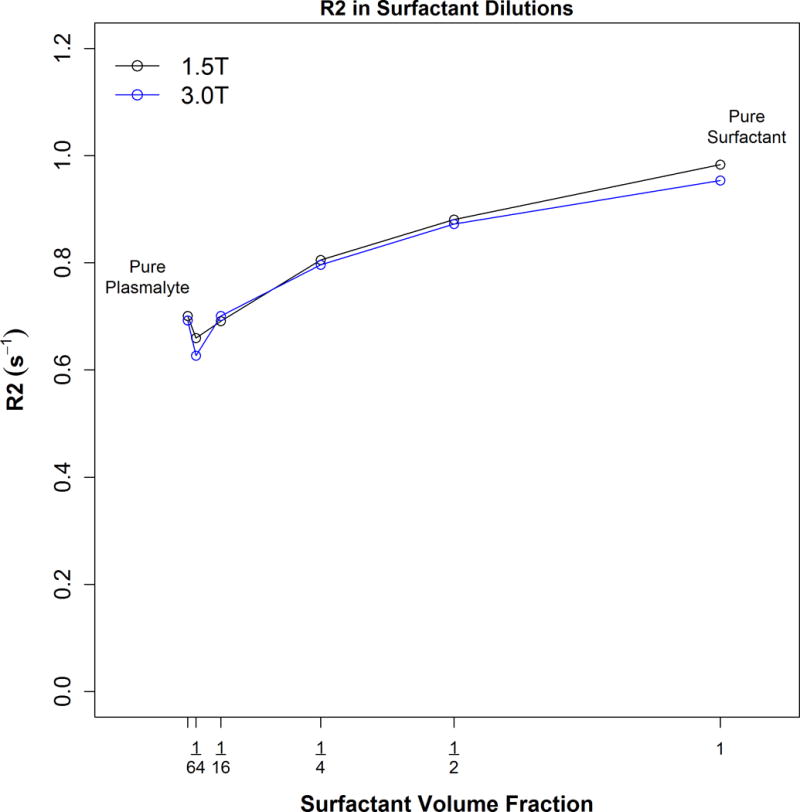
A graph of R2 (1/T2) vs. dilution showing that T2 becomes shorter as the surfactant concentration increases. Very little overall change in T2 occurs with changes in surfactant dilution, especially at physiologic concentrations best simulated at the dilution range of 1/4 to 1/16.
MR imaging
MR scanning was performed on large bore clinical magnets at a field strength of 1.5T Philips (Achieva TX, Philips Healthcare, the Netherlands) and 3.0T (Philips Achieva TX). Quantitative T2 mapping single slice turbo spin echo sequence was utilized. A total of 32 echoes spaced 20 ms apart was used with the following scan parameters: FOV 200 × 152, 3 mm slice thickness skip 1 mm for an in plane resolution of 1.0 × 1.0 mm, TR 2 sec (Fig 2). ROI signal intensity curves were fit to a monoexponential decay curve using offline software (Matlab, Natick MA).
Figure 2.
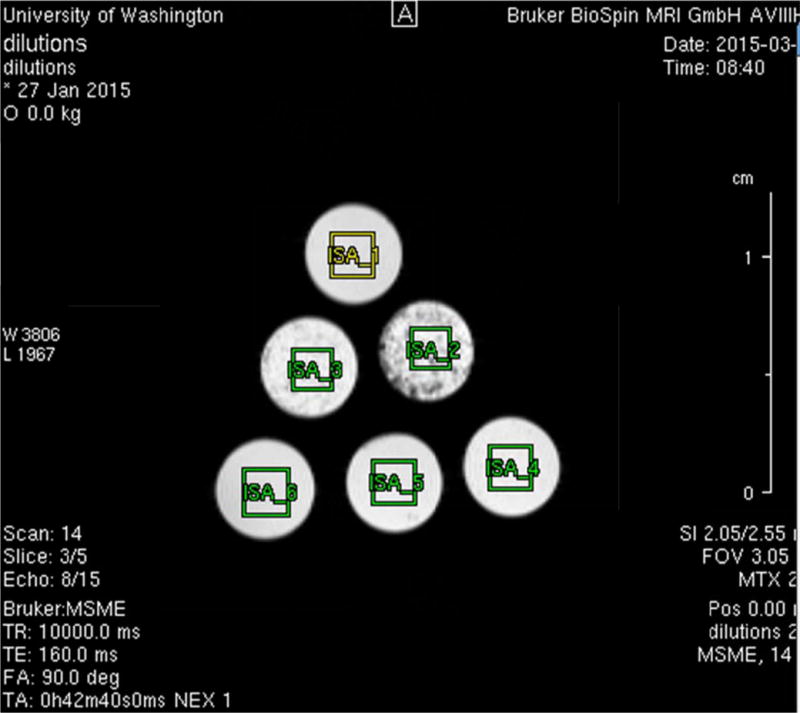
A T2 weighted MR image of the serial dilution tubes inside the MR scanner with rectangular regions of interest drawn over them for T2 mapping.
Statistical Analysis
First a standard linear model of R2 versus surfactant concentration) was fit to all of the data. This was then used to assess the relationship between surfactant concentration (independent variable) and R2 (dependent variable) at both field strengths (1.5T or 3.0T to determine the change in R2 as a function of surfactant concentration. Then a log log model in base 2 (meaning both the surfactant concentrations and R2 values were put into log format using base 2) so that the regression slope coefficient after exponentiation could be interpreted as the percent change in R2 per each doubling of the concentration. We then compared both analyses to each other to see if this changed the results (sensitivity analysis). All statistical calculations were conducted with the statistical computing language R (version 3.1.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
The T2 values for each dilution at 1.5 and 3.0T is shown in Table 1. Graphs of R2 (1/T2) versus surfactant dilution at 1.5T and 3.0T are shown in Fig 3 using the linear model and including all 6 data points. No significant difference was found between the intercepts (p=0.60) or slopes (p=0.97) for the two different field strength curves. A small increasing trend between R2 and surfactant concentration of 0.077 s−1 increase per 0.25 increase in surfactant concentration, p<0.001 is demonstrated using the linear model. We also found that R2 increased 7.3% for each doubling of the surfactant concentration (95% CI: 6.1-8.6%, p<0.001) as calculated by the log-log model. Therefore the conclusions were the same for either model.
Table 1.
R2 (s−1) estimates from each sample from pure plasmaltye to pure surfactant. Based on the generalized linear model, the standard deviation (SD) of each estimate is ± 0.033 s−1 and the SD of the mean is ± 0.024 s−1.
| Scanner | |||
|---|---|---|---|
| Sample | 1.5T | 3.0T | Mean |
| Pure plasmalyte | 0.701 | 0.692 | 0.697 |
| 1:64 dilution | 0.660 | 0.627 | 0.643 |
| 1:16 dilution | 0.692 | 0.701 | 0.696 |
| 1:4 dilution | 0.806 | 0.797 | 0.801 |
| 1:2 dilution | 0.881 | 0.873 | 0.877 |
| Pure surfactant | 0.983 | 0.954 | 0.968 |
Figure 3.
Discussion
Our data suggests that T2 weighted signal intensity should decrease with increasing amounts of surfactant [10]. This is contrary to what would be expected if increasing fetal surfactant concentration could be held accountable for the clinically observed increase in lung to liver signal ratio. As a general rule pure water has the longest T2 at all field strengths and our data demonstrates that pure plasmalyte has the highest T2 weighted signal whereas pure surfactant has the lowest. As more additives are placed into an aqueous solution - protein, fat, other chemicals – this causes more irreversible spin-spin dephasing, thus lengthening R2 (i.e. shortening T2). Thus our data is consistent with general MR principles.
The observed increase in lung/liver T2 signal ratio cannot be due to the increase in surfactant concentration in fetal lung development. Prior studies and our own data indicate that the liver signal remains constant throughout gestation [9]. Our data indicates that there is very minimal change in signal over a wide range of surfactant concentration. The change from the 1:4 to 1:16 dilution which most closely simulates the concentration present in mature fetal lungs of 2 mg/ml of lung tissue shows little change in T2 signal. Similarly, the T2 signal from the 1:64 dilution approaches that of pure plasmalyte which is a balanced crystalloid solution that most closely resembles amniotic fluid in composition and osmolarity. This data also suggests that the early gestation surfactant concentration change would not be detectable at clinically utilized magnetic field strengths.
The change in the signal ratio that we detected in our prior clinical study [10] must therefore be due to increasing concentrations of amniotic fluid within the lungs. As the fetal lung develops, the alveoli and bronchioles get thinner, alveolar epithelial cells produce increasing amounts of surfactant and proteins and there is less tissue per square millimeter as gestational age increases; hence, more amniotic fluid per volume of lung. The fluid within the fetal lung serves as a skeletal framework around which the alveoli and distal bronchioles form. This alveolar parenchymal thinning prepares the fetal lungs for gas exchange upon exposure to air.
The clinical utility of a noninvasive measure of fetal lung maturity would be of great value for several reasons. First, obstetricians still rely on amniocentesis results to decide the timing of preterm delivery in high-risk pregnancies when either maternal or fetal circumstances necessitate it. While generally much safer in the 3rd trimester compared to genetic amniocentesis earlier in the pregnancy, complications do occur including ruptured membranes, placental abruption, and fetal maternal hemorrhage (13). Since amniocentesis is an invasive procedure, obtaining serial measurements of amniotic fluid surfactant levels is not clinically feasible. In addition, no clinically feasible method has been developed to monitor the response of the fetal lung to the prenatal administration of steroids to improve pulmonary development. To date, no non-invasive quantitative measures have been established to assess the degree of fetal lung maturity. This particular manuscript is an attempt to understand the behavior of surfactant at MR imaging at physiologic concentrations and hopefully serve as a prelude to future investigations into the clinical utility of performing MR on fetuses to assess lung maturity.
The major limitation of this study is the assumption that the in vitro study of pharmaceutical surfactant simulates fetal lung surfactant physiology. To mitigate this limitation, we employed pharmaceutical surfactant as others have found that the properties of native pulmonary surfactant evaluated with NMR spectroscopy can be mimicked by synthetic lipid mixtures used for therapy. Surfactant forms micelles when suspended in solution which are structurally and functionally very similar to amniotic fluid filled fetal alveoli in utero. Farver, et al. concluded in their manuscript that the properties of native pulmonary surfactant can be mimicked by synthetic lipid mixtures used for therapy during NMR spectroscopy [13] which supports our use of pharmaceutical surfactant in this study. Fenton et al. compared the NMR spectra of mixtures of synthetic lecithin and saline to demonstrate that the peak at 3.2 ppm is due to lecithin in the amniotic fluid and that this peak is not seen in preterm amniotic fluid samples [14]. Attempts on our part to directly image fat within the fetal lungs by performing in vivo Dixon water suppression imaging, MR spectroscopy, diffusion imaging, and in and out of phase imaging have all been unsuccessful and it has proven difficult to collect meaningful MR data from living fetuses beyond the simple lung to liver T2 ratio signal which increases with gestational age. Therefore, we believe a suspension of surfactant in plasmalyte to be a satisfactory model to investigate the behavior of surfactant in solution than the in vitro methodology described herein.
Conclusion
The increasing lung/ liver T2 signal ratio seen with increasing gestational age is not due to increasing surfactant concentration, and is more likely due to increasing amniotic fluid within the fetal lungs. At physiologic surfactant concentrations, it is unlikely that MR imaging could detect subtle changes in concentration, particularly earlier in gestation. The behavior of surfactant in this serial dilution experiment is consistent with known behavior of solutions in general and while there is a limitation from performing in vitro studies to evaluate live fetuses, it seems reasonable to apply the conclusions from this study to the physiology of fetal lung development in vivo.
Acknowledgments
We thank Dr. Marie-Terese Little for scientific editing.
Footnotes
Author’s Contribution:
Dubinsky: Project development
Adams: Project development
Wilson: Data Collection, Data Management
Maki: Protocol development, Data Analysis
Moshiri: Project development
Hippe: Statistics, data analysis
Conflict of Interest:
The authors declare that they have no conflict of interest.
References
- 1.Polin RA, Carlo WA. Committee on Fetus, Newborn, American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–63. doi: 10.1542/peds.2013-3443. [DOI] [PubMed] [Google Scholar]
- 2.Luo G, Norwitz ER. Revisiting amniocentesis for fetal lung maturity after 36 weeks’ gestation. Rev Obstet Gynecol. 2008;1:61–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Martinerie L, Munier M, Le Menuet D, et al. The mineralocorticoid signaling pathway throughout development: expression, regulation and pathophysiological implications. Biochimie. 2013;95:148–57. doi: 10.1016/j.biochi.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Parra E, Perez-Gil J. Composition, structure and mechanical properties define performance of pulmonary surfactant membranes and films. Chem Phys Lipids. 2015;185:153–75. doi: 10.1016/j.chemphyslip.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Committee on F, Newborn. American Academy of P. Respiratory support in preterm infants at birth. Pediatrics. 2013;133:171–4. doi: 10.1542/peds.2013-3442. [DOI] [PubMed] [Google Scholar]
- 6.Tingay DG, Wallace MJ, Bhatia R, et al. Surfactant before the first inflation at birth improves spatial distribution of ventilation and reduces lung injury in preterm lambs. J Appl Physiol. 2014;116:251–8. doi: 10.1152/japplphysiol.01142.2013. [DOI] [PubMed] [Google Scholar]
- 7.Cuevas Guaman M, Sbrana E, Shope C, et al. Administration of antenatal glucocorticoids and postnatal surfactant ameliorates respiratory distress syndrome-associated neonatal lethality in Erk3(−/−) mouse pups. Pediatr Res. 2014;76:24–32. doi: 10.1038/pr.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman A, Phithakwatchara N, Shaaban A, et al. Fetal lung growth represented by longitudinal changes in MRI-derived fetal lung volume parameters predicts survival in isolated left-sided congenital diaphragmatic hernia. Prenat Diagn. 2015;35:160–6. doi: 10.1002/pd.4510. [DOI] [PubMed] [Google Scholar]
- 9.Mills M, Winter TC, Kennedy AM, et al. Determination of fetal lung maturity using magnetic resonance imaging signal intensity measurements. Ultrasound Q. 2014;30:61–7. doi: 10.1097/RUQ.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 10.Moshiri M, Mannelli L, Richardson ML, et al. Fetal lung maturity assessment with MRI fetal lung-to-liver signal-intensity ratio. AJR Am J Roentgenol. 2013;201:1386–90. doi: 10.2214/AJR.12.9679. [DOI] [PubMed] [Google Scholar]
- 11.Sebastia C, Gomez O, Salvador R, et al. Prognostic usefulness of derived T2-weighted fetal magnetic resonance imaging measurements in congenital diaphragmatic hernia. Radiologia. 2015;57:239–47. doi: 10.1016/j.rx.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Terui K, Omoto A, Osada H, et al. Prediction of postnatal outcomes in congenital diaphragmatic hernia using MRI signal intensity of the fetal lung. J Perinatol. 2011;31:269–73. doi: 10.1038/jp.2010.119. [DOI] [PubMed] [Google Scholar]
- 13.Farver S, Smith AN, Mills FD, et al. Delineation of the dynamic properties of individual lipid species in native and synthetic pulmonary surfactants. Biochim Biophys Acta. 2015;1848(1 Pt B):203–10. doi: 10.1016/j.bbamem.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Fenton BW, Lin CS, Seydel F, Macedonia C. Lecithin can be detected by volume-selected proton MR spectroscopy using a 1.5 T whole body scanner: a potentially non-invasive method for the prenatal assessment of fetal lung maturity. Prenat Diagn. 1998;18:1263–6. [PubMed] [Google Scholar]


