Key Clinical Message
Upper extremity lymphedema and cutaneous spread are atypical behavior of prostate disease and should be kept in the differential for selected patients. This presentation in these patients may be underdiagnosed and potentially an ominous sign. Our case adds to our continued learning of possible prostate malignancy behavior.
Keywords: Adenocarcinoma, cutaneous, lymphedema, metastatic, prostate, recurrence
Introduction
Prostate cancer is the most common leading cause of death from cancer in men following lung cancer 1. The only three well documented risk factors for prostate cancer are race, family history, and older age 1. Metastasis is common to the pelvis, lumbar, thoracic vertebrae, ribs, and long bones. Visceral metastasis can occur to lungs, liver, and adrenal glands. Skin involvement is rare, with some authors reporting rates fewer than 0.1% 2. Very rarely does it spread to the axilla, with brachial plexus involvement being even rarer 3. Lymphedema associated with prostate cancer has been reported, but involving only the lower extremities, commonly following surgical intervention 4, 5, 6.
We report a case of metastatic prostate adenocarcinoma with axillary metastases and subsequent unilateral upper extremity lymphedema.
Case
Our patient is an 86‐year‐old man who was initially diagnosed with prostate cancer 17 years prior, with initial prostate specific antigen (PSA) level of 22 ng/mL, Gleason score of 4 + 5 in all sampled cores; 60% involvement of the left apex cores, 2 of 2 cores; and 60% involvement of right apex, 2 of 3 cores. At the time, computed tomography (CT) imaging of the abdomen and pelvis revealed an enlarged prostate along with left common iliac and left obturator adenopathy. He had no cutaneous, bone, or supradiaphragmatic involvement of disease (Fig. 1). He was subsequently treated with androgen deprivation therapy (ADT), followed by external beam radiation therapy (EBRT) to the pelvis, a total of 8100 cGy. The disease went into chemical remission, and the patient was treated with surveillance as an outpatient.
Figure 1.

Axial and coronal contrast‐enhanced CT showing no evidence of thoracic lymphadenopathy.
Five years following the completion of EBRT to the prostate, the patient was found to have a biochemical relapse of disease with a rising PSA level above 2.0 ng/mL. Repeat CT imaging of the chest had revealed no adenopathy, but did reveal a sclerotic lesion in the right scapula, suspicious of bone metastasis; CT imaging of the abdomen and pelvis revealed a new left peri‐aortic lymph node. He was subsequently started on ADT for biochemical relapse of disease, presumed metastatic, and followed as an outpatient.
He complained of pain in the right shoulder 8 years following EBRT to the prostate, which was postulated to be secondary to probable osseous metastasis seen on prior CT imaging. Repeat imaging revealed extensive right scapula metastases with an extraosseous soft tissue mass extending into the glenohumeral joint capsule and scapula notch, with a probable nondisplaced fracture of the scapular spine. Orthopedic surgeons had no acute interventions to offer. He was provided with a sling for immobilization and treated with radiation therapy (RT) to the lesion, a total of 3000 cGy. He was also continued on ADT at the time. His symptoms seemed to improve.
Fifteen years following the completion of EBRT to the prostate, the patient presented to our institution Urgent Care Center with right upper extremity pain again, but this time with associated swelling. A deep vein thrombus (DVT) was part of our differential, subsequently being ruled out with a negative upper extremity duplex ultrasound study. He was managed with opioids for pain, achieving good pain control for outpatient management. His PSA level had remained stable at this point, suggesting no biochemical progression of prostate cancer. Surveillance was continued as outpatient, with clinic appointment <1 week following discharge.
In the clinic, a CYP17 (17 alpha‐hydroxylase/C17,20‐lyase) was added to his medication regimen, and his disease was managed as castrate resistant at this point. However, the right upper extremity swelling persisted and progressed with the development of functional motor deficits. He had no sensory or any other neurologic deficits, and suspicion for a cerebrovascular event was low. Physical exam revealed right upper extremity nonpitting edema with reduced right shoulder motion and reduced dexterity of ipsilateral hand.
He was subsequently scheduled for lymphedema rehabilitation with minimal improvement. Imaging (Figs 2, 3, 4) of the right shoulder was also obtained, which was suggestive of metastasis occupying nearly the entire right scapula; with suspicion for a comminuted pathologic fracture; infiltration of the right axilla, around the axillary neurovascular bundle; and severe narrowing of the right axillary vein, but no gross encasement of the brachial plexus. Right supraclavicular, axillary, subpectoral, and chest wall nodal metastases were noted. Muscular and soft tissue tumor implants were seen. Muscular/fascial edema noted to be reactive edema or lymphedema was also seen. He received further irradiation to the area, 1400 cGy dose. Again, there was no acute surgical intervention to be offered (Fig. 5).
Figure 2.

Top: Axial contrast‐enhanced CT of the chest showing bilateral axillary lymphadenopathy (arrows) Bottom Left: Coronal reformatted images from a contrast‐enhanced CT scan of the chest showing bilateral lymphadenopathy (solid arrows). Note the irregular dermal thickening (dashed arrow) that was biopsy‐proven to be prostate cancer metastasis with lymphangitic spread. Bottom Right: Coronal reformatted images from a contrast‐enhanced CT scan of the right upper extremity showing encasement of the neurovascular bundle (arrows) with compression of the right axillary vein (lower arrow). Again, note the irregular dermal thickening of the left shoulder that was biopsy‐proven prostate cancer metastasis with lymphangitic spread.
Figure 3.

Axial contrast‐enhanced CT of the right upper extremity showing infiltrative soft tissue metastases encasing the neurovascular bundle. The axillary vein (solid arrow) is compressed and thrombosed. Axillary artery (dashed arrow) is also encased. Note the permeative, lytic appearance of the bones indicative of prostate cancer metastases.
Figure 4.

Axial contrast‐enhanced CT of the right upper extremity showing subpectoral axillary lymphadenopathy (arrows).
Figure 5.

Panel A showing axial views comparing images from the time of diagnosis (Left) and the time when the patient had the symptoms (Right). Panel B shows coronal views of the same, respectively. These are seen in Figures 1 and 2 and show the difference in soft tissue swelling in the right axilla.
His condition was then complicated by cellulitis of the right upper extremity, for which he was admitted and treated with antibiotics. Upon discharge, the main issues reported by the patient were pain, swelling, and serous discharge. At this point, the CYP17 (17 alpha‐hydroxylase/C17, 20‐lyase) inhibitor was discontinued, and he was trialed on a pure androgen receptor inhibitor for possible better control of disease. Physical examination revealed the right shoulder to be persistently firm and deformed with the right upper arm and forearm nonpitting edema with clear serous drainage; the skin was warm with distal sensation preserved, but range of motion of the shoulder joint was severely limited, along with poor hand dexterity, suggesting prior functional deficits to be persistent. He had superficial telangiectasias in the subcutaneous area of the right infraclavicular region, along with several cutaneous or subcutaneous nodules that were bland but firm. These findings were a progression from the prior exam, which was mostly significant for right arm edema and poor motor function (Fig. 5).
At this point, we postulated there was possible osteonecrosis contributing to the patient's symptoms, an infectious etiology for skin involvement could not be ruled out due to the serous drainage, tumor involvement was possible however unusual, and the possibility of radiation induced sarcoma. The patient's PSA and acid phosphatase started to increase at this time (Fig. 6).
Figure 6.

Graphs representing the trend of the patient's PSA and acid phosphatase over 1.5 years.
Repeat imaging revealed bilateral axillary adenopathy greater on the right than left, with a right‐sided hilar node and subcarinal node. All data suggested prostate cancer disease progression. We decided to obtain fine‐needle aspiration (FNA) of the skin nodule (see Fig. 2), to rule out prostate disease as a cause of symptoms. Pathology revealed malignant prostatic adenocarcinoma (see Fig. 7).
Figure 7.
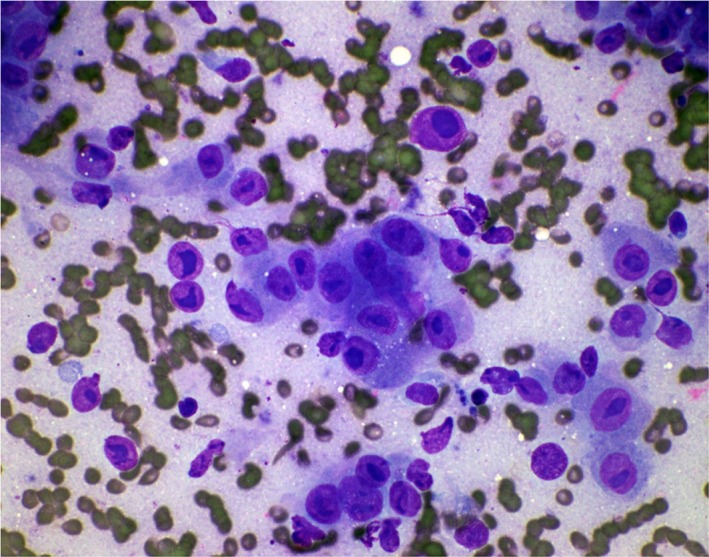
FNA of right axillary skin. Diff‐Quik Stain, x60. Description: Cellular specimen. Tridimensional clusters of large epithelial cells with prominent bizarre macronucleoli. Adenocarcinoma metastatic to skin, consistent with prostate primary.
At this point, the patient was treated as metastatic castrate‐resistant prostatic adenocarcinoma with bone involvement, cutaneous invasion, axillary lymph node involvement, and upper extremity lymphedema. Hormonal therapy options had been exhausted. Weekly chemotherapy with docetaxel was initiated. The patient did not respond to chemotherapy, and rapidly deteriorated, necessitating referral for hospice care. Sadly, the patient expired shortly after.
Discussion
Atypical presentation and metastasis of prostate cancer are well documented. The most common lymph nodes involved in prostate adenocarcinoma are pelvic and retroperitoneum nodes. In more advanced cases, peri‐aortic, intrathoracic, supraclavicular, and rarely axillary lymph nodes are involved 7. Some studies have reported the left supraclavicular fossa as the most common site of nonregional lymphatic spread, or nonosseous sites 8, 9. Theories behind spread to the left supraclavicular lymph node include spread via Batson's venous plexus. Another theory is that these nodes are located close to the entry of the thoracic duct into the subclavian vein predisposing to retrograde metastasis 9.
Of the cutaneous metastases, prostate cancer has been reported to represent approximately 1% by some authors 10. In general, once genitourinary cancers metastasize to the skin, the prognosis for the patient is poor 3. No significant association has been found between initial Gleason grade and the site of metastasis 11.
Thoracic wall involvement and axillary lymphadenopathy have been described in the literature, but no resultant upper extremity lymphedema to the best of our knowledge. Upper extremity lymphedema is usually an association made with breast cancer following surgical intervention. Some studies also report lymphedema following radiation therapy 12. However, views on lymphedema following radiation seem to be conflicting in the literature, with some authors reporting none following therapy 13. Of the reported axillary or supraclavicular lymphadenopathy from prostate cancer, the majority, if not all the cases, involve the left side 14, 15.
Our patient is atypical due to the involvement of the right supraclavicular nodes, the predominance of right axillary adenopathy, narrowing of the axillary venous drainage, and the resultant right upper extremity lymphedema. It is unclear in our case whether radiotherapy contributed to the development of lymphedema, but unlikely given the disease involvement with the neurovascular bundle as seen on imaging. Our patient also had clinical progression without biochemical progression initially, which is also unusual behavior in prostate cancer. This is evidenced in Fig. 1 where the PSA is seen to be stable during the time of development of the symptoms described. Failure to respond to chemotherapy implies a poor prognosis and aggressive disease in our patient. The case also highlights how we are still learning the metastatic behaviors of prostate cancer, as not too long ago we believed brain metastases to be rarer than what we know now 16, 17. Our case also suggests lymphedema in prostate cancer is not necessarily limited to lower extremities in patients who are status – post surgical intervention and radiotherapy, and may indeed be underdiagnosed. Our main aim was to increase awareness of this atypical metastases in this disease and expand the differential diagnosis in patients who may present as such in the correct clinical setting.
Authorship
CTM: Resident Physician under Primary Oncologist. BL: Radiology physician reviewing images. LK: Primary Oncologist for the patient. JMC: Pathologist who reviewed the FNA.
Conflict of Interest
The authors have no conflict of interest to disclose.
Clinical Case Reports 2018; 6(6): 1014–1019
References
- 1. Yasemin, B. , Mustafa S., and Kemal D.. 2011. A case of prostate adenocarcinoma in a 67‐year‐old man manifesting as generalized lymphadenopathy. Turk. J. Geriatr. 14:381–385. [Google Scholar]
- 2. Wu, J. , Huang D., Pang K., and Tyring S.. 2006. Color Photographs: cutaneous metastasis to the chest wall from prostate cancer. Int. J. Dermatol. 45:946–948. [DOI] [PubMed] [Google Scholar]
- 3. Ampil, F. L. 1986. Brachial plexus neuropathy from prostatic carcinoma metastasis. J. La. State Med. Soc. 138:38–40. [PubMed] [Google Scholar]
- 4. Lieskovsky, G. , Skinner D. G., and Weisenburger T.. 1980. Pelvic lymphadenectomy in the management of carcinoma of the prostate. J. Urol. 124:635–638. [DOI] [PubMed] [Google Scholar]
- 5. Kavoussi, L. R. , Sosa E., Chandhoke P., Chodak G., Clayman R. V., Hadley H. R., et al. 1993. Complications of laparoscopic pelvic lymph node dissection. J. Urol. 149:322–325. [DOI] [PubMed] [Google Scholar]
- 6. Rainwater, L. M. , and Zincke H.. 1988. Radical prostatectomy after radiation therapy for cancer of the prostate: feasibility and prognosis. J. Urol. 140:1455–1459. [DOI] [PubMed] [Google Scholar]
- 7. Collins, G. R. , Yamil L., and Fleurette A.. 2012. Prostatic adenocarcinoma metastatic to axillary lymph node diagnosed by fine‐needle aspiration biopsy. Diagn. Cytopathol. 40:751–753. [DOI] [PubMed] [Google Scholar]
- 8. Saeter, G. , Fosså S. D., Ous S., Blom G. P., and Kaalhus O.. 1984. Carcinoma of the prostate with soft tissue or non‐regional lymphatic metastases at the time of diagnosis: a review of 47 cases. Br. J. Urol. 56:385–390. [DOI] [PubMed] [Google Scholar]
- 9. Pinaquy, J. B. , Allard J. B., Cornelis F., Pasticier G., and De Clermont H.. 2015. Unusual lymph node metastases of prostate cancer detected by 18F‐Fluorocholine PET/CT. Clin. Nucl. Med. 40:e255–e257. [DOI] [PubMed] [Google Scholar]
- 10. Brown, G. , Kurtzman D., Lian F., and Sligh J.. 2014. Eruptive nodules of the head and neck: a case report of metastatic prostate cancer. Dermatol. Online J. 20:pii: doj_21544. [PubMed] [Google Scholar]
- 11. Folasire, A. , Ntekim A., Omikunie A., and Ali‐Gombe M.. 2015. Association of gleason risk groups with metastatic sites in prostate cancer. Afr. J. Biomed. Res. 18:189–196. [Google Scholar]
- 12. Cormier, J. N. , Askew R. L., Mungovan K. S., Xing Y., Ross M. I., and Armer J. M.. 2010. Lymphedema Beyond Breast Cancer A systematic review and meta‐ analysis of cancer‐related secondary lymphedema. Cancer 116:5138–5149. [DOI] [PubMed] [Google Scholar]
- 13. Greskovich, F. J. , Zagars G. K., Sherman N. E., and Johnson D. E.. 1991. Complications following external beam radiation therapy for prostate cancer: an analysis of patients treated with and without staging pelvic lymphadenectomy. J. Urol. 146:798–802. [DOI] [PubMed] [Google Scholar]
- 14. Cho, K. R. , and Epstein J. I.. 1987. Metastatic prostatic carcinoma to supradiaphragmatic lymph nodes. A clinicopathologic and immunohistochemical study. Am. J. Surg. Pathol. 11:457–463. [DOI] [PubMed] [Google Scholar]
- 15. Tell, D. T. , Khoury J. M., Taylor H. G., and Veasey S. P.. 1985. Atypical metastasis from prostate cancer clinical utility of the immunoperoxidase technique for prostate‐specific antigen. JAMA 253:3574–3575. [DOI] [PubMed] [Google Scholar]
- 16. Bubendorf, L. , Schöpfer A., Wagner U., Sauter G., Moch H., Willi N., et al. 2000. Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum. Pathol. 31:578–583. [DOI] [PubMed] [Google Scholar]
- 17. Catane, R. , Kaufman J., West C., Merrin C., Tsukada Y., and Murphy G. P.. 1976. Brain metastasis from prostatic carcinoma. Cancer 38:2583–2587. [DOI] [PubMed] [Google Scholar]


