Key Clinical Message
Penicillium non‐marneffei species rarely cause disease in humans and are encountered most commonly in the clinical laboratory as culture contaminants; however, recently they have emerged as opportunistic pathogens in immunocompromised hosts; therefore, it should not be routinely disregarded without a thorough investigation, especially if normally sterile sites are involved.
Keywords: Chemotherapy, immunosuppression, leukemia, lymphoma, Penicillium non‐marneffei, transplant
Introduction
Members of the genus Penicillium are ubiquitous in the environment, encountered in air, soil, and decaying material, and also they have been reported as the second most dominant fungal contaminant in the International Space Station. Penicillium spp. have also been described as components of human oral and gut microbiota 1. The conidia become easily aerosolized; this explains their role as environmental contaminants 2. Historically, non‐marneffei species rarely cause disease in humans and are encountered most commonly in the clinical laboratory as culture contaminants; however, recently they have emerged as opportunistic pathogens in immunocompromised hosts. Proof of clinical infection requires histological demonstration of tissue invasion.
Herein, we report a case in which a Penicillium non‐marneffei species pulmonary invasive fungal infection (IFI) was diagnosed synchronously to lymphoblastic lymphoma.
Case Report
A 16‐year‐old male was admitted with 4 weeks of systemic symptoms, bilateral pleural and pericardial effusions. A chest X‐ray and CT scan revealed pulmonary nodules and a mediastinal mass (Fig. 1A–C). The patient underwent bilateral thoracoscopy. A lung mass biopsy was performed, and lymphoblastic lymphoma was diagnosed with pathology and flow cytometry. Direct smears of the pulmonary nodules were negative, but on fungal culture (lung tissue was inoculated on Sabouraud dextrose agar at 30°C), a colony of rapidly growing mold was isolated, identified as Penicillium spp. (Fig. 1D and E). Multiple attempts to identify species were unsuccessful, including molecular techniques, analysis of sequences of recombinant DNA internal transcribed spacer (ITS 1 and ITS2), and MALDI‐TOF MS. The infection was confirmed with histological sections that stained with hematoxylin and eosin showing necrotic lung and pleural tissue with hemorrhagic areas, and Grocott methenamine silver showed numerous branching hyphae surrounded by suppurative granulomatous inflammation and vascular invasion (Fig. 1F and G). Antifungal susceptibility testing was determined according to CLSI criteria. In vitro susceptibility at 48 h reported minimal inhibitory concentrations for the following antifungals as follows: amphotericin B 1.0 μg/mL and voriconazole 2 μg/mL. The patient completed an 8‐week course of amphotericin B deoxycholate, followed by amphotericin B‐suppressive treatment during each of the remaining seven cycles of chemotherapy, without recurrence of infection. Nine months later, the patient underwent haploidentic hematopoietic stem cell transplantation. Suppressive treatment with amphotericin B was prescribed again, without recurrence of the infection. Up until the present date, 12 months after HSCT, the patient is in remission, without immunosuppression, and without relapse of the infection. We postulate this case represents infection by Penicillium non‐marneffei species as the sample was obtained from a surgical specimen in a sterile site, there was no other isolation, and the galactomannan was negative.
Figure 1.
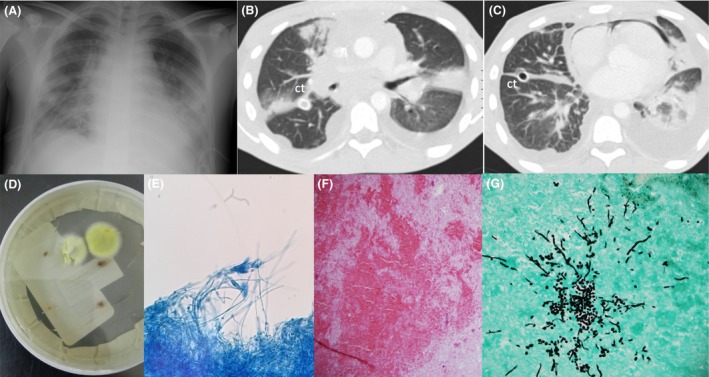
(A) Chest X‐ray showing large mediastinal mass, histologically shown to be malignant lymphoma of lymphoblastic type. (B) Computed tomography showing mediastinal mass surrounding vascular structures and the airway, pulmonary nodule in the right upper lobe, ct: chest tube; (C) pneumomediastinum and pneumopericardium secondary to previous surgery, intrapulmonary infiltrates in the left lower lobe and consolidation in the left upper lobe, and bilateral effusion due to invasive mycosis. (D) Yellow‐green smooth colony growing on the Sabouraud dextrose agar plate at 30°, at day 5. (E) Lactophenol cotton blue stain (x 40). (F) PAS showing necrotic lung and pleural tissue with hemorrhagic areas, accompanied by fibrin (x40). (G) Septate branching fungal mycelia in Grocott methenamine silver (x100).
Discussion
Talaromyces marneffei causes a typical clinical syndrome in endemic areas (Southeast and eastern regions of Asia) in patients with advanced HIV 3, specific immunodeficiency (autoantibody against interferon‐Gamma) 4, solid organ transplantation 5, 6, 7, 8, and, less commonly, in otherwise healthy individuals 9. This species was recently transferred to the genus Talaromyces together with other Penicillium species belonging to the subgenus Biverticillium as T. piceus and Talaromyces purpurogenus (previously P. piceum and P. purpurogenum) 10.
Penicillium‐like fungi are commonly recovered from clinical samples, in routine hospital air surveys and in clinical practice, and are often encountered as airborne contaminants of culture specimens 11, 12. Usually, Penicillium species isolated from samples of non‐AIDS patients are mostly discarded as environmental contaminants. However, in immunosuppressed patients, non‐marneffei species are being increasingly recognized as emerging opportunistic pathogens causing invasive fungal infections worldwide, with most reports involving P. citrinum, P. digitatum, and P. chrysogenum (Table 1) 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23.
Table 1.
Cases of Penicillium non‐marneffei species infections in hematological malignancy and transplant patients
| Ref | Underlying disease | Clinical presentation | Organism and identification | Type of Specimen positive | In Vitro Susceptibility (MIC) | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 13 |
AML 69/F |
Pulmonary IFI Pericarditis | P. citrinum | Sputum culture, lung tissue pathology postmortem | AMB, ITZ, FCZ, and 5‐FC >32 μg/mL | AMB and ITZ | Died |
| 14 |
ALL 19/F |
Pulmonary IFI | P. citrinum |
Lung tissue Histopathology postmortem |
AMB 12.5 μg/mL MCZ 0.78 μg/mL |
FCZ, MCZ 5‐FC |
Died |
| 15 |
AML 44/M |
Pulmonary IFI | T. purpurogenus | Culture of bronchial lavage x2 | NA | AMB | Cured of infection, death within 2 months from septic shock, no autopsy |
| 16 |
AML 16/M |
Pulmonary IFI | Penicillium sp. | Tissue cavitation | NA | AMB | Cured |
| 17 |
AML 44/M |
Pulmonary IFI | P. notatum | Culture forms bronchoalveolar lavage and lung tissue with fungal angioinvasion | NA | VCZ | Cured |
| 18 |
AML 12/F |
Pulmonary IFI and Hepatic abscess | P. oxalicum | Fine‐needle aspiration (FNA) hepatic lesion |
AMB <0.03 μg/mL VCZ 2 μg/mL ITZ 0.5 μg/mL ISA 8 μg/mL PCZ 0.125 μg/mL CSP 1 μg/mL |
6 weeks PCZ | Survived |
| 19 |
ALL 40/NA |
Disseminated disease | P. commune | Postmortem histology | NA | NA | Died |
| 20 |
ALL/BMT 21/F |
Pulmonary IFI Necrotic lung fungus ball | P. brevicompactum | Lung tissue and postmortem histopathology |
AMB 1.0 μg/mL ITZ 0.5 μg/mL 5‐FC 16 μg/mL |
AMB 5‐FC |
Died |
| 21 |
MM/BMT and Plasmocytoma 66/F |
Pulmonary IFI | T. purpurogenus | Sputum | Not performed | VCZ | Died |
| Present | Lymphoblastic lymphoma 16/M | Pulmonary IFI | Penicillium sp. | Lung tissue pulmonary biopsy | AMB 1.0 μg/mL, ITZ 0.25, VCZ 1 μg/mL | AMB | Cured |
| 22 |
Lung transplant 56/M |
Pulmonary IFI | P. chrysogenum | BAL |
AMB 16 μg/mL VCZ 0.25 μg/mL CSP 0.19 μg/mL PCZ 0.25 μg/mL |
PCZ and CSP | Died |
| 23 |
Kidney transplantation 37/M |
Fungemia | P. chrysogenum | Blood culture |
AMB <0.5 mg/L ITZ <0.5 mg/L 5‐FC <0.5 mg/L |
Postmortem diagnosis | Died |
ALL, acute lymphoblastic leukemia; BMT, bone marrow transplant; AML, acute Myeloid leukemia; MM, multiple myeloma; IFI, invasive fungal infection; NA, not available; BAL, bronchoalveolar lavage; AMB, amphotericin B deoxycholate; FCZ, fluconazole; VCZ, voriconazole; ITZ, itraconazole; PCZ, posaconazole; CSP, caspofungin; 5‐FC, 5‐flucytosine; MCZ, miconazole.
The clinical presentation of Penicilliosis caused by non‐marneffei species reported to date is different to that reported for T. marneffei species; although pulmonary involvement is common in both, non‐marneffei species, particularly in patients with hematological malignancies, usually cause disseminated infection in the setting of invasive pulmonary fungal infection, with high fatality rates.
In patients with hematological malignancies, they have emerged as potential opportunistic agents, mainly described in acute lymphoblastic and myeloid leukemia.
Pulmonary invasive fungal infection involving P. citrinum, P. purpurogenus, P. notatum, P. brevicompactum, P. oxalicum, and P. commune has been reported 13, 14, 15, 16, 17 ; including pericardial involvement 13, coinfection with P. jirovecii 17 , pulmonary and hepatosplenic involvement 18, and disseminated disease 19. In patients undergoing transplantation with hematopoietic progenitors, P. brevicompactum has been described causing invasive pulmonary infection 20, and, after autologous bone marrow transplantation for multiple myeloma, pulmonary infection due to T. purpurogenus has been described 21. In many cases, the diagnosis was made postmortem with an attributed mortality of 62% 13, 14, 19. In summary, according to the review, the most prevalent species causing infection in immunosuppressed patients is P. chrysogenum, which causes systemic and disseminated disease with invasive pulmonary infection. The susceptibility profile is not predictable and may vary according to isolates; some have high MICs to azoles and amphotericin B.
Confirming a diagnosis of Penicilliosis can be difficult by conventional phenotypic methods. Identification at the species level remains challenging, and the high number of species in these genera makes this task even more difficult 1. The use of molecular methods, however, provides a rapid and relatively simple method for the identification of Penicillium species. Matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry (MALDI‐TOF MS) has limitations for identification of dimorphic fungi but may identify species of Penicillium and differentiate T. marneffei from non‐marneffei species as P. brevicompactum and P. chrysogenum; however, the scores were below the cutoff required for species identification. This can be explained by the limited number of spectra for the two species so that expansion of MALDI‐TOF MS databases is needed 24.
Standard treatment for non‐marneffei species has not yet been established; antifungal susceptibility data for clinically available antifungal agents and treatment options for infections caused by Penicillium species are also poorly understood, aside from data published for T. marneffei. In a recent study of 118 clinical isolates, (mainly from the respiratory tract/human bronchoalveolar lavage) terbinafine (TRB) and the echinocandins showed the best in vitro activity against Penicillium species with MIC <0.03 μg/mL for TRB, 0.06 μg/mL for caspofungin and anidulafungin, and 0.125 for micafungin; amphotericin B showed intermediate activity with MIC of 2 μg/mL, and azoles revealed variable activity with MIC ranges of 0.5 μg/mL for posaconazole and 2 μg/mL for voriconazole and itraconazole 25.
In conclusion, Penicillium spp. isolates in immunosuppressed patients should not be routinely disregarded without a thorough investigation, especially if normally sterile sites are involved.
Conflict of Interest
The authors have no conflict of interest to declare.
Authorship
IR: contributed to the design, drafted, and revised the manuscript. AH: contributed to the design, drafted, and revised the manuscript. RC: contributed to the design, drafted, and revised the manuscript. All the authors: approved the submitted and final versions.
Clinical Case Reports 2018; 6(6): 1153–1157
References
- 1. Ghannoum, M. A. , Jurevic R. J., Mukherjee P. K., Cui F., Sikaroodi M., Naqvi A., et al. 2010. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathog. 6:e1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sautour, M. , Fournel I., Dalle F., Calinon C., L'Ollivier C., Goyer M., et al. 2012. Dynamics of fungal colonization in a new medical mycology laboratory. J. Mycol. Med. 22: 14–20. [DOI] [PubMed] [Google Scholar]
- 3. Anitnori, S. , Gianelli E., Bonaccorso C., Ridolfo L., Croce F., Sollima S., et al. 2006. Disseminated Penicillium marneffei infection in an HIV‐positive Italian patient and a review of cases reported outside endemic regions. J. Travel. Med. 13:181–188. [DOI] [PubMed] [Google Scholar]
- 4. Tang, B. S. , Chan J. F., Chen M., Tsang O. T., Mok M. Y., Lai R. W., et al. 2010. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin. Vaccine Immunol. 17:1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan, Y. H. , Wong K. M., Lee K. C., Kwok P. H., Chak W. L., Choi K. S., et al. 2004. Pneumonia and mesenteric lymphadenopathy caused by disseminated Penicillium marneffei infection in a cadaveric renal transplant recipient. Transpl. Infect Dis. 6:28–32. [DOI] [PubMed] [Google Scholar]
- 6. Stathakis, A. , Lim K. P., Boan P., Lavender M., Wrobel J., Musk M., et al. 2015. Penicillium marneffei infection in a lung transplant recipient. Transpl. Infect Dis. 17:429–434. [DOI] [PubMed] [Google Scholar]
- 7. Hart, J. , Dyer J. R., Clark B. M., McLellan D. G., Perera S., Ferrari P., et al. 2012. Travel‐related disseminated Penicillium marneffei infection in a renal transplant patient. Transpl. Infect Dis. 14:434–439. [DOI] [PubMed] [Google Scholar]
- 8. Chan, J. F. , Lau S. K., Yuen K. Y., and Woo P. C.. 2016. Talaromyces (Penicillium) marneffei infection in non‐HIV‐infected patients. Emerg. Microbes. Infect. 5:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saadiah, S. , Jeffrey A. H., and Mohamed A. L.. 1999. Penicillium marneffei infection in a non aids patient: first case report from Malaysia. Med. J. Malaysia 54:264–266. [PubMed] [Google Scholar]
- 10. Samson, R. A. , Yilmaz N., Houbraken J., Spierenburg H., Seifert K. A., Peterson S. W., et al. 2011. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus biverticillium . Stud. Mycol. 70:159–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. da Silva, A. , Porto J. C., Da Silva J. L., Morais K. F., Coelho F. A., de Sousa Lopes T., et al. 2016. Evaluation of disinfectants for elimination of fungal contamination of patient beds in a reference hospital in Piauí, Brazil. Environ. Monit. Assess. 188:644. [DOI] [PubMed] [Google Scholar]
- 12. Okten, S. , and Asan A.. 2012. Airborne fungi and bacteria in indoor and outdoor environment of the Pediatric Unit of Edirne Government Hospital. Environ. Monit. Assess. 184:1739–1751. [DOI] [PubMed] [Google Scholar]
- 13. Mok, T. , Koehler A. P., Yu M. Y., Ellis D. H., Johnson P. J., and Wickham N. W.. 1997. Fatal Penicillium citrinum pneumonia with pericarditis in a patient with acute leukemia. J. Clin. Microbiol. 35:2654–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mori, T. , Matsumura M., Kohara T., Watanabe Y., Ishiyama T., Wakabayashi Y., et al. 1987. A fatal case of pulmonary penicilliosis. Jpn. J. Med. Mycol. 28:341–348. [Google Scholar]
- 15. Breton, P. , Germaud P., Morin O., Audouin A. F., Milpied N., and Harousseau J. L.. 1998. Rare pulmonary mycoses in patients with hematologic diseases. Rev. Pneumol. Clin. 54:253–257. [PubMed] [Google Scholar]
- 16. Shamberger, R. C. , Weinstein H. J., Grier H. E., and Levey R. H.. 1985. The surgical management of fungal pulmonary infections in children with acute myelogenous leukemia. J. Pediatr. Surg. 20:840–844. [DOI] [PubMed] [Google Scholar]
- 17. Shokouhi, S. , Tehrani S., and Hemmatian M.. 2016. Mixed pulmonary infection with Penicillium notatum and Pneumocystis jiroveci in a patient with acute myeloid leukemia. Tanaffos 15:53–56. [PMC free article] [PubMed] [Google Scholar]
- 18. Chowdhary, A. , Kathuria S., Agarwal K., Sachdeva N., Singh P. K., Jain S., et al. 2014. Voriconazole‐resistant Penicillium oxalicum: an emerging pathogen in immunocompromised hosts. Open Forum Infect. Dis. 1:ofu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang, S. N. 1963. Acute disseminated penicilliosis. Report of a case and review of the pertinent literature. Am. J. Clin. Pathol. 39:167–174. [DOI] [PubMed] [Google Scholar]
- 20. de la Cámara, R. , Pinilla I., Muñoz E., Buendía B., Steegmann J. L., and Fernández‐Rañada J. M.. 1996. Penicillium brevicompactum as the cause of a necrotic lung ball in an allogeneic bone marrow transplant recipient. Bone Marrow Transplant. 18:1189–1193. [PubMed] [Google Scholar]
- 21. Atalay, A. , Koc A. N., Akyol G., Cakir N., Kaynar L., and Ulu‐Kilic A.. 2016. Pulmonary infection caused by Talaromyces purpurogenus in a patient with multiple myeloma. Infez. Med. 24:153–157. [PubMed] [Google Scholar]
- 22. Geltner, C. , Lass‐Flör C., Bonatti H., Müller L., and Stelzmüller I.. 2013. Invasive pulmonary mycosis due to Penicillium chrysogenum: a new invasive pathogen. Transplantation 95:e21–e23. [DOI] [PubMed] [Google Scholar]
- 23. Swoboda‐Kopec, E. , Wroblewska M. M., Rokosz A., and Luczak M.. 2003. Mixed bloodstream infection with Staphylococcus aureus and Penicillium chrysogenum in an immunocompromised patient: case report and review of the literature. Clin. Microbiol. Infect. 9:1116–1117. [DOI] [PubMed] [Google Scholar]
- 24. Lau, S. K. , Lam C. S., Ngan A. H., Chow W. N., Wu A. K., Tsang D. N., et al. 2016. Matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry for rapid identification of mold and yeast cultures of Penicillium marneffei . BMC Microbiol. 16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guevara‐Suarez, M. , Sutton D. A., Cano‐Lira J. F., García D., Martin‐Vicente A., Wiederhold N., et al. 2016. Penicillium‐like fungi from clinical samples in the USA and their antifungal susceptibility. J. Clin. Microbiol. 54:2155–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]


