Abstract
Pregnancy and the postpartum period are times of profound behavioral change including alterations in cognitive function. This has been most often studied using hippocampal-dependent tasks assessing spatial learning and memory. However, less is known about the cognitive effects of motherhood for tasks that rely on areas other than the hippocampus. We have previously shown that postpartum females perform better on the extradimensional phase of an attentional set shifting task, a measure of cognitive flexibility which is dependent on the medial prefrontal cortex (mPFC). The present experiments aimed to extend this work by examining the importance of postpartum stage as well as offspring and parity in driving improved mPFC cognitive function during motherhood. We also examined whether the neuropeptide oxytocin, which plays a role in regulating numerous maternal functions, mediates enhanced cognitive flexibility during motherhood. Our results demonstrate that compared to virgin females, cognitive flexibility is enhanced in mothers regardless of postpartum stage and is not affected by parity since both first (primiparous) and second (biparous) time mothers showed the enhancement. Moreover, we found that improved cognitive flexibility in mothers requires the presence of offspring, as removal of the pups abolished the cognitive enhancement in postpartum females. Lastly, using an oxytocin receptor antagonist, we demonstrate that oxytocin signaling in the mPFC is necessary for the beneficial effects of motherhood on cognitive flexibility. Together, these data provide insights into the temporal, experiential and hormonal factors which regulate mPFC-dependent cognitive function during the postpartum period.
INTRODUCTION
Females of all mammalian species undergo dramatic behavioral changes during the transition to motherhood. Along with maternal behaviors directly related to the care of offspring, cognitive behaviors that may indirectly aid in the rearing of the offspring are also affected (Macbeth and Luine, 2010; Workman et al., 2012; Galea et al., 2014). This has been demonstrated in rodents most extensively using hippocampal-dependent tasks assessing spatial learning and memory. Overall, the evidence suggests that during the early (i.e. first week) postpartum period, when it may be advantageous for a new mother to stay close to the nest site, spatial cognition is impaired as compared to virgin (nulliparous) female rats without reproductive or maternal experience (Darnaudéry et al., 2007). In contrast, the middle (i.e. second week) to late (i.e. third week) postpartum periods are accompanied by improved spatial navigation, possibly to boost the mother’s abilities to forage so that she can efficiently procure the necessary resources and return to caring for and protecting her vulnerable young in the nest (Kinsley et al., 1999; Lambert et al., 2005; Cost et al., 2014). These cognitive benefits of motherhood are attributable to a combination of pregnancy and mothering and are long-lasting persisting after weaning well into aging (Kinsley et al., 1999; Gatewood et al., 2005; Lambert et al., 2005; Love et al., 2005; Lemaire et al., 2006; Pawluski et al., 2006a; Kinsley and Lambert, 2008; Macbeth et al., 2008a; Zimberknopf et al., 2011; Barha et al., 2015). Furthermore, improved spatial abilities have been shown to occur not only in first time (primiparous) mothers, but also in mothers with two (biparous) or more (multiparous) litters suggesting that parity in general promotes this aspect of cognitive function (Gatewood et al., 2005; Pawluski et al., 2006b; Macbeth et al., 2008b; Paris and Frye, 2008; Barha et al., 2015).
Executive functions, such as attention and cognitive flexibility, have been far less studied in mothers even though they are likely critical for maternal responsiveness and parenting quality not just in rodents but in humans as well (Barrett and Fleming, 2011; Lonstein et al., 2015). One way of assessing these functions in rodents is with attentional set shifting (Birrell and Brown, 2000; Fox et al., 2003). In this task, animals are required to learn a series of associations between stimuli of certain sensory modalities and food rewards and then switch these associations to other sensory modalities to effectively solve the task. Shifting attention from one sensory stimulus to another, referred to as an extradimensional shift (EDS), requires the medial prefrontal cortex (mPFC; Birrell and Brown, 2000; Fox et al., 2003). We have previously shown that compared to virgin female rats, primiparous females during the late postpartum period (20–24 days after parturition) perform better on the EDS phase of the attentional set shifting task suggesting that like hippocampal-dependent cognitive function, PFC-dependent cognitive function is also enhanced during motherhood (Leuner and Gould, 2010). However, it is unknown whether these changes occur earlier in the postpartum period and if they persist with additional parity. Furthermore, whether the effects of motherhood on cognitive flexibility are attributable to pregnancy alone or require mother-pup interactions has yet to be fully investigated.
The neuropeptide oxytocin (OT) is known to play a prominent role in regulating maternal care by acting on a distributed network of brain regions, including the mPFC (Bosch and Neumann, 2012; Sabihi et al., 2014). The mPFC abundantly expresses oxytocin receptors (OT-R; Insel and Shapiro, 1992; Liu et al., 2005; Smeltzer et al., 2006; Nakajima et al., 2014; Mitre et al., 2016) and receives long range axonal projections from OT producing neurons in the hypothalamus (Sofroniew, 1983; Knobloch et al., 2012). In addition, OT has been shown to mediate the motherhood-induced enhancement in spatial memory (Tomizawa et al., 2003) and research in non-parous animals has shown that OT can influence various aspects of learning and memory (Gur et al., 2014; Havranek et al., 2015), including cognitive flexibility which is impaired in oxytocin receptor (OT-R) knockout mice (Sala et al., 2011; Chini et al., 2014). However, no previous work has investigated whether OT contributes to improved cognitive flexibility during the postpartum period.
In the current study, we investigated the importance of postpartum stage as well as offspring, parity and OT in driving improved mPFC cognitive function during motherhood. To do so, attentional set shifting performance was assessed during two different weeks of the postpartum period, in postpartum females without offspring, as well as in both primiparous and biparous mothers. Lastly, cognitive flexibility was examined in mothers following blockade of OT-R in the mPFC.
MATERIALS AND METHODS
Animals
Age-matched virgin female (200-250g) and timed pregnant Sprague-Dawley rats purchased from Taconic (Germantown, NY) were used in Experiments 1 and 3. For experiment 2, parous females were generated in house. For primiparous females, one virgin female (200-250g) was paired with an adult male. Pregnancies were verified through daily vaginal swabs and microscopic identification of sperm. Upon positive determination of pregnancy, female rats were individually housed. For all experiments, the day of birth was designated as postpartum day 0 (PD0) and litters were culled to 10 pups (4–6 males, 4–6 females). To generate biparous females, groups of primiparous rats raised their litters to PD21 and were then remated. All parous females were age-matched such that primiparous rats during their first and only gestational period were the same age as biparous rats. Stages of estrous were monitored in virgin females through daily vaginal swabs and only those virgin females that had normal 4–5 d estrous cycles were included.
Except during breeding, all rats were housed individually in a temperature and humidity controlled room and maintained on a 12/12 light/dark cycle (lights on at 6 am). Rats initially had unrestricted access to food but were food restricted and maintained at 85% of their baseline weight for 9 d prior to testing to ensure sufficient motivation to perform the task. Water was available ad libitum throughout all experiments. All procedures were conducted in accordance with The Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and approved by The Ohio State University Institutional Animal Care and Use Committee.
Experiment 1: Effects of postpartum stage and offspring on attentional set shifting performance
Design
Four groups were tested: 1) Mothers with pups during the second week postpartum on PD10-11 (n = 10), 2) Mothers with pups during the third week postpartum on PD20-21 (n = 6), 3) PD10-11 mothers whose pups were permanently removed from the home cage at the time of culling (n = 9), and 4) Virgin females (n = 12).
Attentional set shifting
In attentional set shifting, rats are taken through a series of tasks in which they must dig in small terracotta flower pots to locate a hidden food reward. The testing apparatus (Figure 1, top) was a black, opaque Plexiglas box (50 × 40 × 30 cm). A removable, opaque divider separated one-third of the length of the arena from the rest, forming a start box. To begin each trial, a rat was placed in the start box, and given access to the rest of the arena by lifting this divider. A fixed divider separated the opposite third of the arena into two sections each of which contained one digging pot (diameter 11.4 cm; height 9.5 cm). All procedures were performed manually by a trained experimenter during the light phase beginning at 11 am. Digging was live scored and defined as a vigorous displacement of the medium to retrieve the reward buried within the pot. Simply investigating the rim of the pot or the surface of the digging medium with paws or snout without displacing material was not scored as a “dig”. During attentional set shifting, pups were kept warm in an incubator maintained at nest temperature.
Figure 1.
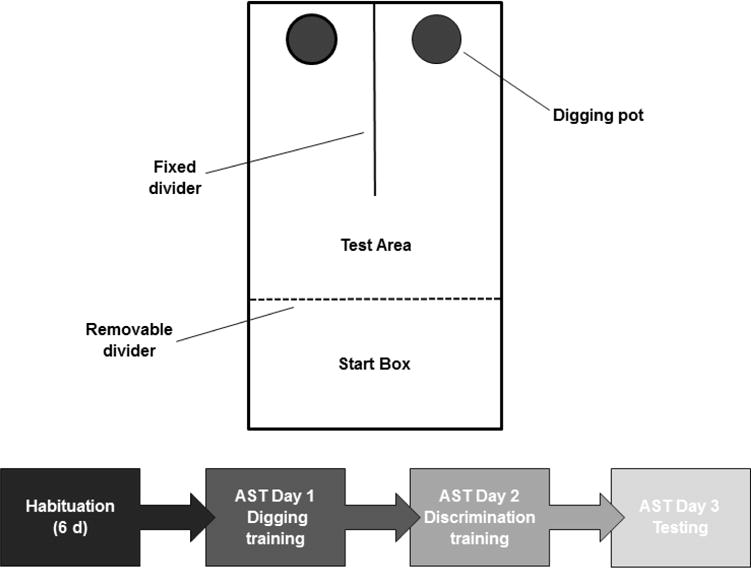
(Top) Schematic diagram of the attentional set shifting apparatus. A fixed Plexiglas panel divided the distal third of the arena into two sections, and a digging pot was placed in each section. A removable divider separated the proximal third of the arena from the rest, forming a start box. To begin a trial, a rat was placed in the start box, and the divider lifted. (Bottom) Timeline for attentional set shifting procedure.
Beginning on the day of food restriction, rats were habituated for 6 d by allowing them to freely explore the apparatus for 30 min per day. Following habituation, the attentional set shifting procedure took place over three consecutive days (Figure 1, bottom). On Day 1, rats were trained to dig reliably in untextured pots filled with cob bedding. Both pots were baited with the food reward (1/3 FrootLoop; Kellogs, Battle Creek, MI USA). Rats underwent training in four stages in which the reward was: 1) placed on top of the cob bedding; 2) placed under a thin layer of cob; 3) buried beneath ~2 cm of cob; 4) buried under ~4 cm of cob. Rats were initially given four exploratory trials (120 s each) to freely explore the chambers and dig in both pots. After the four exploratory trials, rats were given 120 s to retrieve one reward. If rats failed to do so, the trial was repeated. Progression to the next stage required that rats retrieve one reward within 120 s for six consecutive trials. On Day 2, rats underwent discrimination training in which they learned the location of the food reward in one of the pots based on a texture (sand paper vs duct tape covering the pots which were filled with cob digging media) or digging media (shredded paper vs shredded latex filling untextured pots). The order of the discriminations and the specific texture or digging medium (positive stimulus) that predicted the reward was determined randomly and represented equally across rats. The stimuli used in discrimination training were not used again. On Day 3, rats underwent testing on a series of five discriminations presented in a fixed order for all rats (Table 1). Testing began with a simple discrimination (SD) in which rats discriminated between two digging media in untextured containers, one of which was the positive stimulus. Next, in a compound discrimination (CD), an irrelevant texture dimension was introduced, but the positive stimulus was the same as in the SD. This was followed by an intradimensional attentional shift (IDS) involving two new stimuli from each stimulus dimension with digging medium remaining as the relevant dimension. The IDS was then reversed (REV), such that the formerly negative stimulus became the positive stimulus. Finally, in the extradimensional attentional shift (EDS), two new stimuli from each dimension were introduced, and the formerly task-irrelevant dimension (texture) became relevant. For both discrimination training and testing on days 2 and 3, a small quantity of powdered cereal was sprinkled into the digging medium of both pots at the start of the task to obviate the possibility that the rat may locate the bait by smell rather than by learning the discrimination. The left-right positioning of the baited container across trials was randomized. Rats were initially given four exploratory trials (120 s each) to explore both chambers and dig in both pots. Following the exploratory trials, the rat was given 120 s to dig in either pot. In the event of an incorrect choice (i.e. digging in the unbaited pot), the divider wall was immediately replaced so that the animal was not allowed access to the alternate pot. If an animal did not dig within 120 s, the partition was lowered, forcing the rat back into the waiting area. In either case, the trial was aborted and recorded as an error. Trials for each discrimination were continued until the animal reached a response criterion of 6 correct consecutive digs in the baited pot.
Table 1.
Representative example of stimulus pairs and the progression through the stages of the attentional set shifting protocol. Medium was the initial relevant stimulus dimension, shifting to texture in the EDS stage. For each stage, the positive stimulus is in bold, and was paired randomly across trials with the two stimuli from the irrelevant dimension.
| Discrimination Stage | Dimension | Example Combinations | ||
|---|---|---|---|---|
| Relevant | Irrelevant | (+) | (−) | |
| Simple (SD) | Medium | Beads | Gravel | |
| Compound (CD) | Medium | Texture | Beads/Leather | Gravel/Denim |
| Medium | Texture | Beads/Denim | Gravel/Leather | |
| Intradimensional Shift (ID) | Medium | Texture | Drierite/Fur | Wood shavings/Reverse Fur |
| Medium | Texture | Drierite/Reverse Fur | Wood shavings/Fur | |
| Reversal (REV) | Medium | Texture | Wood shavings/Fur | Drierite/Reverse Fur |
| Medium | Texture | Wood shavings/Reverse Fur | Drierite/Fur | |
| Extradimensional shift (EDS) | Texture | Medium | Velvet/Paper towel | Reverse Velvet/Hamster Bedding |
| Texture | Medium | Velvet/Hamster Bedding | Reverse Velvet/PaperTowel | |
Experiment 2: Effect of parity on attentional set shifting performance
Design
Three groups were tested on the attentional set shifting task as described above: 1) Primiparous females on PD10-11 (n = 6), 2) Biparous females on PD 10–11 (n = 4), and 3) Virgin females (n = 8).
Experiment 3: Effect of OT-R blockade on attentional set shifting performance in mothers
Design
Two groups were tested on the attentional set shifting task as described above: 1) Primiparous females on PD10-11 infused with saline in the mPFC (n = 8) and 2) Primiparous females on PD10-11 infused with an OT-R antagonist in the mPFC (n = 8).
Cannulation
On GD17, pregnant females were anesthetized with a 2–4% isoflurane gas/air mixture and aligned on a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). This timepoint for surgery is consistent with prior studies assessing behavioral changes during the postpartum period following drug administration via cannulation (Neumann et al., 2000; Lubin et al., 2003; Figueira et al., 2008). Body temperature was maintained throughout the surgery with a warming pad. Bilateral cannula guides (pedestal mounted 22-gauge stainless steel tubes with 1.5 mm separation and cut 3.5 mm below the pedestal; Plastics One, Roanoke, VA) were secured in a stereotaxic holder and lowered into the prelimbic mPFC (AP: + 3.2 mm, ML: ± 0.75 mm, DV: −3.2 mm; Paxinos and Watson, 1998). The cannula were secured by stainless steel screws and dental cement. A bilateral stainless steel obturator (0.35 mm diameter; Plastics One) extending 0.2 mm beyond the tip of the guide cannula was placed into the guide cannula after surgeries. The scalp was closed around the protruding portion of the cannula with surgical staples.
Infusions
On PD7, all rats were habituated to the handling and infusion procedures. During habituation, rats were removed from their home cage and handled for approximately 3 min while being lightly restrained in a terrycloth towel. The obturators were then removed and a 28-gauge bilateral injection cannula extending 0.2 mm beyond the tip of the guide cannula into the mPFC was inserted into the guide. The injection cannula were left in place for 3 min then removed and the obturator replaced. On Day 3 of AST (PD10-11), upon completion of the reversal task, obturators were removed and an injection cannula attached to two 1 μl Hamilton Syringes via PE-10 tubing was inserted into the guide (Donegan et al., 2014). Bilateral infusions were made using a Harvard Apparatus Pico Plus Elite infusion pump (Holliston, MA) which delivered 0.5μl of saline or 0.5μg/.5μL of the selective OT-R antagonist desGly-NH2-d(CH2)5[D-Tyr,Thr4]OVT (courtesy of Dr. Maurice Manning, University of Toledo; Toledo, OH) into each hemisphere at a rate of 0.33 μL/min. The injector was left in place for an additional 1 min before withdrawal to allow for diffusion. 10 min after injection, testing on the EDS phase was carried out. This dose and timing of infusions relative to testing was selected based on prior studies using OT-R antagonists (Lubin et al., 2003; Waldherr and Neumann, 2007; Sabihi et al., 2014).
Histology
Approximately 24 h after the cessation of testing, cannulated animals were over-dosed with an injection of Euthasol and transcardially perfused with 4% paraformaldehyde. Brains were removed, post-fixed for 24 h in 4% paraformaldehyde at 4°C and then transferred to 0.1M PBS and stored at the same temperature until 40 μm sections throughout the mPFC were obtained using a Vibratome (Leica). Sections were stained with 0.2% cresyl violet and cannula tract placement was verified. Examination under high magnification (40X) revealed limited to no damage at the tip of the cannula.
Statistics
Trials to criteria on the AST were analyzed using two-way repeated measures ANOVA with task phase (SD, CD, IDS, REV, EDS) as a within subject factor and group (Experiment 1: virgin, PD10-11 postpartum, PD20-21 postpartum, PD10-11 postpartum pups removed; Experiment 2: virgin, primiparous, biparous; Experiment 3: saline, OT-R antagonist) as a between subjects factor. Bonferonni post-hoc comparisons were applied to significant interactions. Effect size comparisons were performed for ANOVA (partial eta-squared, ηp2) and posthoc tests (Cohen’s d, d). An ηp2 greater than 0.14 and a d of greater than 0.8 are considered large effect sizes. For all statistical analyses, significance was determined at p values < 0.05.
RESULTS
Enhanced cognitive flexibility emerges during the second postpartum week and requires interactions with offspring
Postpartum females on PD10-11 with and without pups were compared to PD20-21 mothers with pups to assess the extent to which attentional set shifting performance in mothers is influenced by postpartum stage and requires offspring. Our results (Figure 2) show a main effect of group (F3,33 = 3.05, p < 0.05; ηp2 = 0.12) and task phase (F4,132 = 6.12, p < 0.001; ηp2 = 0.26) and a task phase x group interaction (F12,132 = 2.34, p < 0.01; ηp2 = 0.29). Post-hoc analysis revealed that PD10-11 mothers and PD21-22 mothers required fewer trials to reach criteria on the EDS phase of the AST as compared to virgin females (p’s < 0.05; d = 1.00, virgin and PD10-11; d = 1.19, virgin and PD21-22) and early postpartum females whose offspring were removed shortly after birth (p’s < 0.05; d = 1.56, PD10-11 and pups removed; d = 2.4, PD20-21 and pups removed) which did not differ from one another (p > 0.05). There were no significant differences among the groups on the SD, CD, IDS or REV task phases (p’s > 0.05).
Figure 2.
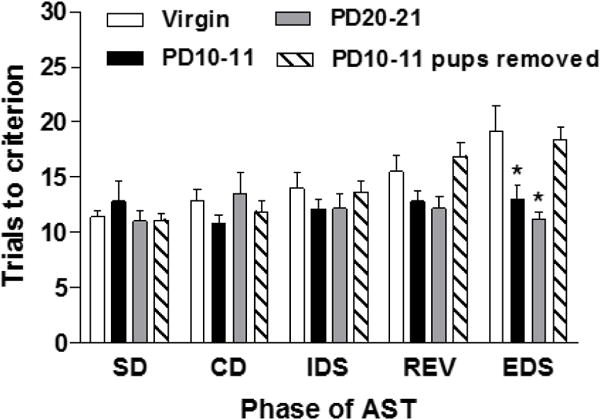
Mothers during the second (PD10-11) and third (PD20-21) postpartum weeks required fewer trials to reach criteria on the EDS phase of the AST as compared to virgin females and PD10-11 postpartum females whose offspring were removed shortly after birth. All groups performed similarly on the SD, CD, IDS and REV phases of the task. Bars represent mean + SEM, * p < 0.05.
Parity does not influence cognitive flexibility
To assess the effects of parity on mPFC-dependent cognitive function, primiparous and biparous mothers were tested on the attentional set shifting task. Our results (Figure 3) show a main effect of group (F2,15 = 8.63, p < 0.005; ηp2 = 0.22) and task phase (F4,60 = 7.33, p<0.001; ηp2 = 0.66) and a task phase x group interaction (F8,60 = 2.83, p < 0.01;ηp2 = 0.60). Post-hoc analysis revealed that primiparous and biparous mothers required fewer trials to reach criteria on the EDS phase of the AST as compared to virgin females (p’s < 0.05; d = 1.85, virgin and primiparous; d = 1.87, virgin and biparous) but did not differ from one another (p > 0.05). There were no significant differences among the groups on any other task phase (p’s > 0.05).
Figure 3.
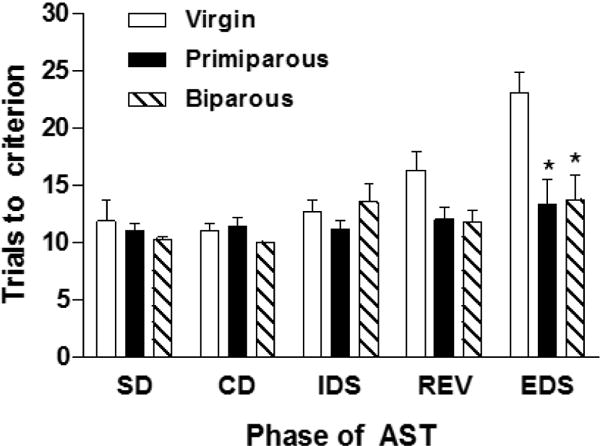
Both primiparous and biparous mothers during the second postpartum week (PD10-11) required fewer trials to reach criteria on the EDS phase than virgin females. Both groups of mothers performed similarly to virgins on all other phases of the task. Bars represent mean + SEM, * p < 0.05.
OT-R antagonism in the mPFC blocks enhanced cognitive flexibility in mothers
To determine whether OT signaling in the mPFC influences cognitive flexibility, an OT-R antagonist was infused bilaterally into the mPFC of PD10-11 mothers just prior to the EDS phase of the attentional set shifting task. Our results (Figure 4) show a main effect of drug (F1,14 = 6.092, p < 0.05; ηp2 = 0.14) and task phase (F4,56 = 11.11, p < 0.0001; ηp2 = 0.68) and a task phase × drug interaction (F4,56 = 6.894, p < 0.0005; ηp2 = 0.57). Post-hoc analysis revealed that saline-infused mothers required fewer trials to reach criteria on the EDS phase of the AST as compared to mothers that received an infusion of the OT-R antagonist (p < 0.0001; d = 2.65). There were no significant differences between the groups on any other task phase (p’s > 0.05). Locations of cannula placements within the mPFC are shown in Figure 4.
Figure 4.
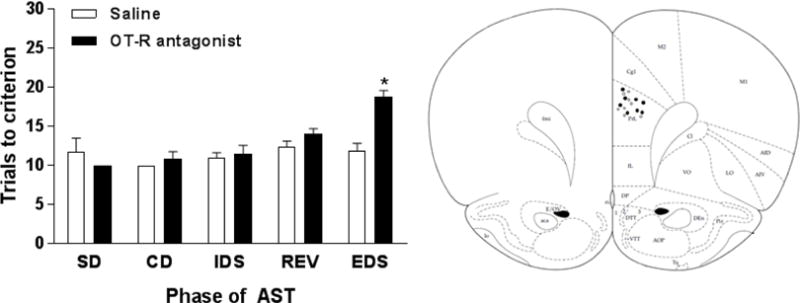
(Left) PD10-11 mothers that received a bilateral infusion of an OT-R antagonist into the mPFC immediately before the EDS phase of the attentional set shifting task required more trials to reach criterion than PD10-11 mothers that received a bilateral saline infusion. The number of trials to reach criterion for the other task phases did not differ between saline and OT-R antagonist mothers. (Right) Locations of cannula placements within the mPFC. White circles = saline; Black circles = OT-R antagonist. Bars represent mean + SEM, * p < 0.05.
DISCUSSION
In this study, we extend previous work which demonstrated that motherhood enhances extradimensional set shifting, a measure of cognitive flexibility that depends on the mPFC (Leuner and Gould, 2010). Specifically, we show that compared to virgin females, cognitive flexibility is enhanced in mothers regardless of postpartum stage and is not affected by parity. Moreover, we found that improved cognitive flexibility in mothers requires the presence of offspring and OT signaling in the mPFC. Together, these data provide insights into the temporal, experiential and hormonal factors which regulate mPFC-dependent cognitive function during the postpartum period.
Changes in cognitive function associated with motherhood have been primarily investigated using hippocampal-dependent spatial tasks. This work has shown that during the early (i.e. first week) postpartum period, spatial cognition is impaired compared to nulliparous controls (Darnaudéry et al., 2007) while the middle to late (i.e. second-third weeks) postpartum periods are accompanied by improved spatial cognition (Kinsley et al., 1999; Lambert et al., 2005; Cost et al., 2014). Here, using the attentional set shifting task, we found a selective enhancement in extradimensional set shifting, but not discrimination or reversal learning, which was evident in mothers during the third postpartum week as previously shown (Leuner and Gould, 2010) as well as earlier during the second week after parturition. It is important to note that SD, CD, IDS and REV do not require the PFC and thus, the absence any difference between virgin and maternal rats on these stages of the attentional set shifting task strongly suggests that motherhood has a specific effect on PFC function and does so without altering motivation to obtain food or other factors that could influence performance such as anxiety. Taken together, it appears that motherhood is generally beneficial across different domains of cognitive function at the mid-late stages of the postpartum period. It is important to note that because of technical limitations requiring adequate food restriction to ensure digging behavior, we did not test mothers at an earlier postpartum time point. Thus, with this task we cannot determine whether cognitive flexibility, like spatial cognition (Darnaudéry et al., 2007), would be similarly impaired during the first week postpartum.
Improved cognitive flexibility was evident in both primiparous and biparous mothers indicating beneficial effects of motherhood regardless of parity. Whether cognitive benefits on AST persist with additional maternal experience beyond two has yet to be examined. Nonetheless, these data are consistent with studies of spatial cognition which have found that parous rats outperform nulliparous rats with no reproductive experience (Gatewood et al., 2005; Pawluski et al., 2006b; Macbeth et al., 2008b; Paris and Frye, 2008; Barha et al., 2015). Some of these studies have further observed differences in cognitive performance between primiparity and multiparity, in some cases favoring primiparous dams (Pawluski et al., 2006b) and in other cases favoring multiparous dams (Gatewood et al., 2005). Here, attentional set shifting performance did not differ between the two maternally experienced groups which may suggest that certain cognitive skills may be differentially sensitive to varying amounts of parity.
The enhancement in EDS performance was dependent on the offspring as it was not evident in mothers whose pups were removed shortly after birth. Likewise, removal of pups prevents improved spatial learning and memory during motherhood (Pawluski et al., 2006a). These data indicate that pregnancy alone does not contribute to improved cognitive function but rather that interactions with offspring and maternal experience are important regardless of whether the task is hippocampal- or mPFC-dependent. It is possible that social and sensory stimulation provided by a litter of offspring is akin to an enriched environment, which is known to be beneficial to cognitive function (Kinsley et al., 1999). Another, not mutually exclusive, possibility is that the expression of maternal behavior itself may be an important factor driving the improvement in cognitive function. Future studies could address the role of sensory stimulation and the expression of maternal care by examining cognitive flexibility in sensitized virgin female rats that are exposed to pups. Such studies have been done using spatial tasks and these have shown enhancements in spatial memory after sensitization, pup-exposure, or pup-directed maternal behaviors alone, but these are transitory (Kinsley et al., 1999; Lambert et al., 2005; Pawluski et al., 2006a) unlike the improvements in hippocampal-dependent spatial learning that persist long after weaning in postpartum rats that experience pregnancy, parturition and mothering (Gatewood et al., 2005; Love et al., 2005; Lemaire et al., 2006; Kinsley and Lambert, 2008; Zimberknopf et al., 2011; Barha et al., 2015). In this regard, it is worth noting that maternal experience does not persistently alter PFC-dependent learning after weaning, at least as assessed using an operant set shifting task (Workman et al., 2013). Since both weaning and pup removal are also accompanied by a cessation of lactation, a role for lactation itself should also be considered. Nonetheless, the available data are consistent with the notion that factors associated with the postpartum period have an ‘activational,’ rather than ‘organizational’ effect on cognitive functioning that relies on the PFC (Workman et al., 2013; Cost et al., 2014).
A growing body of evidence implicates the mPFC and other cortical areas in various social and emotional behaviors linked to OT, including maternal care (Nakajima et al., 2014; Sabihi et al., 2014; Marlin et al., 2015). Our data show OT in the mPFC also contributes to improved mPFC-dependent cognitive function during motherhood. Indeed, central infusion of an OT-R antagonist into the mPFC of primiparous mothers was sufficient to attenuate the enhancement in postpartum cognitive flexibility during the EDS phase of the AST. Both maternal experience and OT have also been shown to enhance object recognition (Macbeth et al., 2008b; Havrenek et al., 2015) which could have contributed to the beneficial effect of motherhood-induced on EDS performance and its blockade by the OT-R antagonist although this is unlikely given that both digging pots on this phase have novel textural and digging stimuli. However, we cannot rule out the possibility that OT-R blockade may have affected cognitive flexibility by altering anxiety (Sabihi et al., 2014) or the motivation to obtain a food reward (Sabatier et al., 2013). Despite these caveats, these data expand upon prior work showing that central OT mediates the motherhood-induced enhancement in hippocampal-dependent spatial learning and memory (Tomizawa et al., 2003) and further point to OT as a mediator of cognitive flexibility (Sala et al., 2011; Chini et al., 2014), although more work is needed. For example, there are no studies to date that have examined how OT agonists in non-maternal animals affect cognitive flexibility on the attentional set shifting task or other tasks which rely on the prefrontal cortex. Furthermore, the mechanism by which OT regulates mPFC-dependent cognitive function is not known. Recent work has shown that within the cortex, OT-R, which are upregulated in the postpartum mPFC (Mitre et al., 2016), are expressed predominantly by cortical interneurons (Nakajima et al., 2014; Marlin et al., 2015) and at inhibitory synapses (Mitre et al., 2016). Given that GABA transmission in the PFC plays a key role in regulating cognitive function (Tse et al., 2015), OT-GABA interactions may be involved.
Altered cognitive function during motherhood is likely to have adaptive significance. For example, because effective and efficient foraging is highly dependent on spatial navigation, enhancements in these cognitive abilities would improve the chances of offspring survival (Pawluski et al., 2006a; Cost et al., 2014). The postpartum period is also a time when the survival and well-being of the offspring critically depends on the mother’s ability to attend to her infant’s needs which in turn requires that she be able to easily shift her attention depending on situational demands and adapt her behaviors accordingly (Barrett and Fleming, 2011; Olazábal et al., 2013). Thus, enhanced cognitive flexibility during motherhood may be another adaptive response that enables successful mothering. In support of this, it has been shown that mother rats who perform better on an attentional set shifting task are better mothers overall – they are less easily distracted, more attentive to their litter and lick their pups more (Lovic and Fleming, 2004). Similarly in humans, positive parenting behaviors during the postpartum period have been associated with better executive functions which genetic analyses have linked to OT-R polymorphisms (Tombeau Cost et al., 2016). On the other hand, reduced maternal sensitivity and difficulties during mother-infant interactions are associated with less cognitive flexibility (Atkinson et al., 2009; Gonzalez et al., 2012; Chico et al., 2014). Investigating whether dysfunctional OT signaling mediates these deficits in humans may provide a new target for therapeutic intervention to ultimately improve maternal functioning.
HIGHLIGHTS.
Cognitive flexibility is improved in mothers regardless of postpartum stage
Both primiparous and biparous mothers display enhanced cognitive flexibility
Enhanced cognitive flexibility in mothers is dependent on the presence of offspring
Blockade of OT-R in the mPFC prevents improved cognitive flexibility in mothers
Acknowledgments
This work was funded by NIH grants R00MH084148 and R21HD083791 to BL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkinson L, Leung E, Goldberg S, Benoit D, Poulton L, Myhal N, Blokland K, Kerr S. Attachment and selective attention: disorganization and emotional Stroop reaction time. Dev Psychopathol. 2009;21:99–126. doi: 10.1017/S0954579409000078. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Chow C, Galea LA. Multiparity-induced enhancement of hippocampal neurogenesis and spatial memory depends on ovarian hormone status in middle age. Neurobiol Aging. 2015;36:2391–2405. doi: 10.1016/j.neurobiolaging.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Chico E, Gonzalez A, Ali N, Steiner M, Fleming AS. Executive function and mothering: challenges faced by teenage mothers. Dev Psychobiol. 2014;56:1027–1035. doi: 10.1002/dev.21185. [DOI] [PubMed] [Google Scholar]
- Chini B, Leonzino M, Braida D, Sala M. Learning about oxytocin: pharmacologic and behavioral issues. Biol Psychiatry. 2014;76:360–366. doi: 10.1016/j.biopsych.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Cost KT, Lobell TD, Williams-Yee ZN, Henderson S, Dohanich G. The effects of pregnancy, lactation, and primiparity on onject-in-place memory of female rats. Horm Behav. 2014;65:32–39. doi: 10.1016/j.yhbeh.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Darnaudéry M, Perez-Martin M, Del Favero F, Gomez-Roldan C, Garcia-Segura LM, Maccari S. Early motherhood in rats is associated with a modification of hippocampal function. Psychoneuroendocrinology. 2007;32:803–812. doi: 10.1016/j.psyneuen.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Donegan JJ, Girotti M, Weingberg MS, Morilak DA. A novel role for brain interleukin-6: facilitation of cognitive flexibility in rat orbitofrontal cortex. J Neurosci. 2014;34:953–962. doi: 10.1523/JNEUROSCI.3968-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira RJ, Peabody MF, Lonstein JS. Oxytocin receptor activity in the ventrocaudal periaqueductal gray modulates anxiety-related behavior in postpartum rats. Behav Neurosci. 2008;122:618–628. doi: 10.1037/0735-7044.122.3.618. [DOI] [PubMed] [Google Scholar]
- Fox MT, Barense MD, Baxter MG. Perceptual attentional set-shifting is impaired in rats with neurotoxic lesions of posterior parietal cortex. J Neurosci. 2003;23:676–681. doi: 10.1523/JNEUROSCI.23-02-00676.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Leuner B, Slattery D. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26:641–648. doi: 10.1111/jne.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Jenkins JM, Steiner M, Fleming AS. Maternal early life experiences and parenting: the mediating role of cortisol and executive function. J Am Acad Child Adolesc Psychiatry. 2012;51:673–682. doi: 10.1016/j.jaac.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Gur R, Tendler A, Wagner S. Long-term social recognition memory is mediated by oxytocin-dependent synaptic plasticity in the medial amygdala. Biol Psychiatry. 2014;76:377–386. doi: 10.1016/j.biopsych.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Havranek T, Zatkova M, Lestanova Z, Bacova Z, Mravec B, Hodosy J, Strbak V, Bakos J. Intracerebroventricular oxytocin administration in rats enhances object recognition and increases expression of neurotrophins, microtubule-associated protein 2, and synapsin I. J Neurosci Res. 2015;93:893–901. doi: 10.1002/jnr.23559. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Lambert K. Reproduction-induced neuroplasticity: natural behavioral and neuronal alterations associated with the production and care of offspring. J Neuroendcocrinol. 2008;20:515–525. doi: 10.1111/j.1365-2826.2008.01667.x. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Berry AE, Griffins G, Amory-Meyers E, Madonia-Lomas L, Love G, Kinsley CH. Pup exposure differentially enhances foraging ability in primiparous and nulliparous rats. Physiol Behav. 2005;85:799–806. doi: 10.1016/j.physbeh.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Billard JM, Dutar P, George O, Piazza PV, Epelbaum J, Le Moal M, Mayo W. Motherhood-induced memory improvement persists across lifespan in rats but is abolished by a gestational stress. Eur J Neurosci. 2006;23:3368–3374. doi: 10.1111/j.1460-9568.2006.04870.x. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. J Neurosci. 2010;30:13499–13503. doi: 10.1523/JNEUROSCI.3388-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Pappas GD, Carter CS. Oxytocin receptors in brain cortical regions are reduced in haploinsufficient (+/−) reeler mice. Neurol Res. 2005;27:339–345. doi: 10.1179/016164105X35602. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Lévy F, Fleming AS. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Horm Behav. 2015;73:156–185. doi: 10.1016/j.yhbeh.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love G, Torrey N, McNamara M, Morgan M, Banks M, Hester NW, Glasper ER, Devries AC, Kinsley CH, Lambert KG. Maternal experience produces long-lasting behavioral modifications in the rat. Behav Neurosci. 2005;119:1084–1096. doi: 10.1037/0735-7044.119.4.1084. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task–reversal of effects with maternal-like licking stimulation. Behav Brain Res. 2004;148:209–219. doi: 10.1016/s0166-4328(03)00206-7. [DOI] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav Neurosci. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Gautreaux C, Luine VN. Pregnant rats show enhanced spatial memory, decreased anxiety, and altered levels of monoamingeric neurotransmitters. Brain Res. 2008a;1241:136–147. doi: 10.1016/j.brainres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Scharfman HE, Maclusky NJ, Gautreaux C, Luine VN. Effects of multiparity on recognition memory, monoaminergic neurotransmitters, and brain-derived neurotrophic factor (BDNF) Horm Behav. 2008b;54:7–17. doi: 10.1016/j.yhbeh.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Luine V. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2010;34:452–467. doi: 10.1016/j.neubiorev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behavior by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki CJ, Chao MV, Froemke RC. A Distributed Network for Social Cognition Enriched for Oxytocin Receptors. J Neurosci. 2016;36:2517–2535. doi: 10.1523/JNEUROSCI.2409-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Görlich A, Heintz N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell. 2014;159:295–305. doi: 10.1016/j.cell.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Torner L, Wigger A. Brain oxytocin: differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- Olazábal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, González-Mariscal G, Lévy F, Lucion AB, Morrell JI, Numan M, Uriarte N. Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci Biobehav Rev. 2013;37:1875–1892. doi: 10.1016/j.neubiorev.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Vanderbyl BL, Ragan K, Galea LAM. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res. 2006a;175:157–165. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Walker SK, Galea LA. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm Behav. 2006b;49:143–149. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Sabihi S, Dong SM, Durosko NE, Leuner B. Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front Behav Neurosci. 2014;8:258. doi: 10.3389/fnbeh.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Leng G, Menzies J. Oxytocin, feeding, and satiety. Front Endocrinol (Lausanne) 2013;4:35. doi: 10.3389/fendo.2013.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neuroscience Letters. 2006;394:146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Tombeau Cost K, Unternaehrer E, Plamondon A, Steiner M, Meaney M, Atkinson L, Kennedy JL, Fleming AS, MAVAN Research Team Thinking and doing: the effects of dopamine and oxytocin genes and executive function on mothering behaviours. Genes Brain Behav. 2016 doi: 10.1111/gbb.12337. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6:384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- Tse MT, Piantadosi PT, Floresco SB. Prefrontal cortical gamma-aminobutyric acid transmission and cognitive function: drawing links to schizophrenia from preclinical research. Biol Psychiatry. 2015;77:929–939. doi: 10.1016/j.biopsych.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Waldherr M, Neumann ID. Centrally released oxytocin mediates mating-induced anxiolysis in male rats. Proc Natl Acad Sci USA. 2007;104:16681–16684. doi: 10.1073/pnas.0705860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Barha CK, Galea LA. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci. 2012;126:54–72. doi: 10.1037/a0025538. [DOI] [PubMed] [Google Scholar]
- Workman JL, Crozier T, Lieblich SE, Galea LA. Reproductive experience does not persistently alter prefrontal cortical-dependent learning but does alter strategy use dependent on estrous phase. Horm Behav. 2013;64:439–447. doi: 10.1016/j.yhbeh.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Zimberknopf E, Xavier GF, Kinsley CH, Felicio LF. Prior parity positively regulates learning and memory in young and middle-aged rats. Comp Med. 2011;61:366–377. [PMC free article] [PubMed] [Google Scholar]


