Abstract
Background and Purpose
We tested whether blood-brain barrier dysfunction in subcortical white matter is associated with white matter abnormalities, or risk of clinical dementia, in older people (n=126; mean age 86.4, SD: 7.7 years) in the MRC Cognitive Function and Ageing Study.
Methods
Using digital pathology we quantified blood-brain barrier dysfunction (defined by immunohistochemical labelling for the plasma marker fibrinogen). This was assessed within subcortical white matter tissue samples harvested from post mortem T2 MRI-detected white matter hyperintensities, from normal-appearing white matter (distant from co-existent MRI-defined hyperintensities) and from equivalent areas in MRI-normal brains. Histopathological lesions were defined using a marker for phagocytic microglia (CD68, clone PGM1).
Results
Extent of fibrinogen labelling was not significantly associated with white matter abnormalities defined either by MRI (OR 0.90; 95% CI 0.79, 1.03; p=0.130) or by histopathology (OR 0.93; 95% CI 0.77, 1.12; p=0.452). Among participants with normal MRI (no detectable white matter hyperintensities) increased fibrinogen was significantly related to decreased risk of clinical dementia (OR 0.74; 95% CI 0.58, 0.94; p=0.013). Among participants with histological lesions, increased fibrinogen was related to increased risk of dementia (OR 2.26; 95% CI 1.25, 4.08; p=0.007).
Conclusions
Our data suggest that some degree of BBB dysfunction is common in older people and that this may be related to clinical dementia risk, additional to standard MRI biomarkers.
Keywords: vascular cognitive impairment, dementia, white matter lesion, leukoaraiosis, neuropathology
Introduction
Diffuse white matter hyperintensities (WMH) are frequently seen on T2-weighted and Fluid-attenuated inversion recovery (FLAIR) MRI scans of older people. WMH are associated with increased risk of lacunar stroke, vascular cognitive impairment (VCI) and Alzheimer’s disease (AD)1.
The blood-brain barrier (BBB) is a property of cerebral blood vessels, resulting from endothelial expression of tight junction proteins and molecular transporters. BBB dysfunction has been proposed as a cause of WMH, VCI and dementia2, 3. MRI data suggest that leakage of intra-vascular contrast agent, a marker of BBB dysfunction, is associated with WMH and VCI4–8. Association of white matter pathology with BBB dysfunction, inferred from detection of extra-vascular plasma proteins (such as fibrinogen, albumin or IgG) is supported by some histological studies9, 10 though not others11, 12.
We tested the hypotheses that BBB dysfunction (measured using fibrinogen labelling) is associated i) with risk of white matter abnormalities (defined either by MRI or by histology) and ii) with risk of in-life dementia diagnosis. Throughout, we will maintain the distinction between radiologically-defined WMH and histological white matter lesions (WML) defined on the basis of phagocytic microglia.
We used systematic MRI-guided tissue sampling in subcortical white matter from participants in a large population of donated human brains, the MRC-CFAS Neuropathology study13–15.
Materials and Methods
Human tissue
The MRC-CFAS Neuropathology study is a prospective longitudinal population-based study of cognitive impairment and frailty in the elderly13–15. Here we sampled frontal and parietal subcortical white matter tissue blocks from donated brains. The 126 brains sampled represent all the donated brains from 3 (out of the 6) MRC-CFAS centres, Newcastle, Nottingham and Cambridge, for which: 1) formalin exposure was <10 years (median formalin time 5 y, range 3-7 y) and 2) luminal fibrinogen immunoreactivity was evident.
All cases reported (n=126) were over the age of 65 years (Table 1). Post mortem tissue was obtained from subjects who signed a Declaration of Intent to donate their brain after death. This study had approval from the UK National Research Ethics Service.
Table 1.
Demographic characteristics and neuropathological findings, according to clinical dementia status.
| No dementia | Dementia | Not Determined | |
|---|---|---|---|
| n | 47 | 69 | 10 |
| Number female (%) | 21 (44) | 47 (68) | 3 (30) |
| Age, median (inter-quartile range) | 82 (76-90) | 89 (85-93) | 84 (77-87) |
| AD pathology present | 8 (17) | 39 (56) | 2 (20) |
| APOEε4 present | 10 | 23 | 0 |
| Braak stage I/II | 23 (49) | 15 (22) | 4 (40) |
| Braak stage III/IV | 12 (26) | 16 (23) | 2 (20) |
| Braak stage V/VI | 1 (2) | 21 (30) | 0 (0) |
| Moderate-severe parenchymal CAA severity | 2 (4) | 10 (14) | 0 (0) |
| Moderate-severe meningeal CAA severity | 5 (11) | 16 (23) | 0 (0) |
Unless otherwise stated, data shown are number of participants (%). AD: Alzheimer’s disease, CAA: cerebral amyloid angiopathy. APOE genotype, data available for n=108 participants. Braak stage and CAA severity are defined in Supplementary Methods, Neuropathological Assessment.
Diagnosis of dementia
Dementia status at death was determined from cognitive assessments during the last years of life (based on the full GMS AGECAT diagnostic algorithm, see Supplementary Methods) combined with detailed retrospective interviews with a knowledgeable informant, and death certificates13, 14, 16. Clinical dementia status was recorded in 116 participants. Among these 69 (55%) had a clinical diagnosis of dementia during life (Table 1).
Post mortem MRI definition of WMH
MRI scans of fixed brain hemispheres were used to guide histological sampling of white matter tissue blocks, as detailed in previous reports15, 17. Briefly, fixed hemispheres were cut into 10 mm thick coronal slices (“thick slices”, Supplementary Figure I) for MRI. Scans were rated by consensus by three blinded observers using a modified Scheltens rating scale, as described previously15, 17. See Supplementary Methods. WMH were classified as “deep subcortical” or “periventricular”. In thick slices containing subcortical WMH, tissue blocks were cut from the thick slice in order to sample the MRI-defined WMH (these blocks inevitably also contained surrounding tissue). See Supplementary Figure I. In addition, tissue blocks were cut from the same brain, from MRI WMH-free locations (“normal appearing white matter”, NAWM) after WMH blocks had been sampled. NAWM blocks were anatomically remote from WMH and from periventricular areas. Equivalent white matter areas were sampled from “MRI normal” control brains, in which no WMH had been detected. All blocks sampled were approximately 20 mm x 20 mm x 5 mm in size. All blocks were taken from deep subcortical white matter of frontal or parietal cortex, avoiding the corpus callosum and internal capsule. Periventricular WMH and periventricular white matter were avoided.
Histopathology and immunohistochemical labelling
Paraffin wax embedded sections (6 µm thickness) were processed for immunohistochemistry as in our previous studies 11. Primary antibodies were to human fibrinogen (rabbit polyclonal A-0080)11, human IgG (rabbit polyclonal A-0423)11, human CD68 (mouse monoclonal, clone PGM1)15 and smooth muscle actin (mouse monoclonal, 1A4) all from Dako-Cytomation, Ely, UK; CD34 (mouse monoclonal, QBEnd10) and human IgG (mouse monoclonal, Clone RWP49)11 were from Novocastra-Leica Microsystems, Newcastle-upon-Tyne, UK.
Definition of histopathological lesions
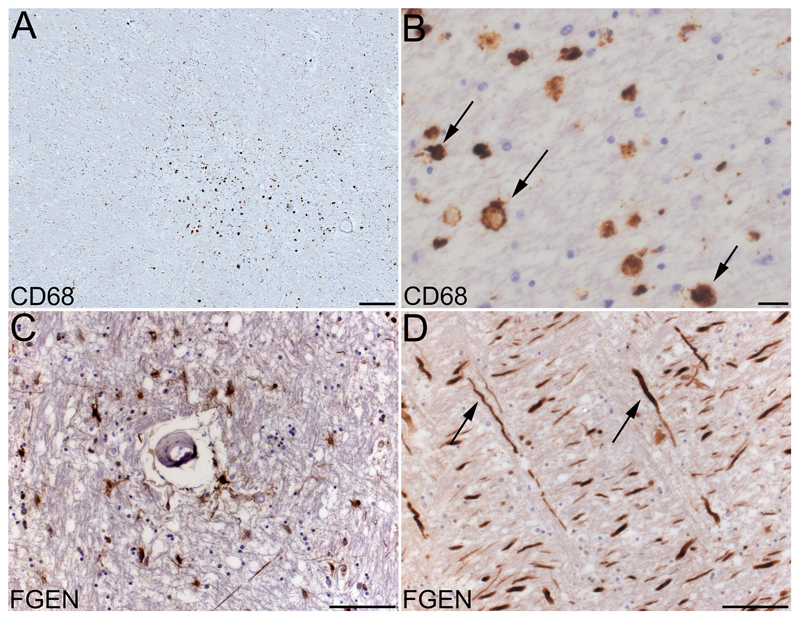
CD68-labelled sections from all tissue blocks were viewed by a registered senior neuropathologist (LRB) blind to MRI and clinical data. The CD68-PGM1 antibody is selective for phagocytic microglia and macrophages. CD68 positive cells with amoeboid microglial morphology in clusters of greatest diameter at least 1.1 mm, were defined as “histopathological lesion” (Figure 1A, B, Supplementary Figure II). This definition showed good inter-rater agreement when assessment of all sections was repeated by two independent, blinded cytopathologists (KE, JJ; Cohen’s κ = 0.97).
Figure 1.
Histopathology of white matter lesions and BBB dysfunction in subcortical white matter. A, CD68-PGM1 immunolabelled section shows a histopathological white matter lesion, defined by a cluster of amoeboid CD68 positive cells. B, CD68 positive amoeboid cells, assumed to be phagocytic microglia (arrows show examples) at higher magnification. C, fibrinogen positive cells and axons, around a small blood vessel. D, nerve axons strongly positive for fibrinogen (arrows show examples). Scale bars 200 µm (A), 20 µm (B) or 100 µm (C, D).
Histological image analysis
All sections were digitally scanned under a 20 x objective lens (Leica Slidescanner, UCL-Advanced Diagnostics, http://www.uclad.com/digital_pathology/). Scanned sections in SCN file format were viewed using Leica Slidepath Gateway free software (www.leicabiosystems.com/). Digital image analysis with NIH ImageJ free software (http://imagej.nih.gov/ij) was used to estimate the extent (percent area fraction) of labelling with fibrinogen. See Supplementary Methods, Supplementary Figures II and III.
Statistical analysis
All histochemical labelling and image analyses were performed blind to clinical and MRI data. Sample size of 120 was estimated to detect a 20% difference in fibrinogen labelled area fraction (α<0.05, β>0.8) based on previous studies using similar material11, 17.
Statistical analyses were performed using STATA, version 12. Analyses were performed by participant or by tissue block, as some participants had more than one brain region studied. As some participants contributed more than one tissue block, and each tissue block cannot be considered independent within the same brain, we used weighted analyses. All histological sections used to measure fibrinogen area fraction were used in the analyses and received a weight inversely proportional to the number of sections. For the comparison of categorical data, Fisher’s exact test was used as Cochran’s restrictions were present.
To test whether BBB dysfunction was a risk factor for MRI-defined WMH, we conducted weighted ordinal logistic regression analyses with WMH status as dependent variable (MRI-normal, NAWM, WMH) and fibrinogen area fraction as independent variable. These analyses were repeated with histopathologically-defined WML as dependent variable (none or present) and fibrinogen area fraction as independent variable. In addition we conducted weighted linear regression analyses with MRI classification (MRI normal, NAWM, WMH) as independent variable and fibrinogen area fraction as dependent variable. The same analyses were repeated with histologically-defined WML (none, present) as independent variable and also with combined MRI/histological methods (Supplementary Table I). In these analyses, control group (MRI normal or WML-free) was set as the reference.
To test whether BBB dysfunction was a risk factor for dementia we conducted weighted logistic regression analysis, with clinical dementia status (dementia, without dementia) as the dependent variable, and fibrinogen area fraction as independent variable.
All analyses were controlled for sex, age at death and formalin time (time during which post mortem tissue was stored in formalin). All tests were two-tailed, p<0.05 considered significant.
Results
We report a sample of older people (n=126; mean age 86.4 (SD: 7.7) years, Table 1) including 98 with MRI-detected WMH and 28 WMH-free (MRI-normal) individuals. Neuropathological data are reported for 151 tissue blocks. These included 28 blocks from MRI-normal donors, 82 blocks from MRI-defined WMH and 41 from normal appearing white matter (NAWM) areas anatomically distinct from any WMH. In 25 cases the WMH and NAWM areas came from the same donor. Histological white matter lesions detected on blind inspection of CD68-labeled sections by a registered neuropathologist (LRB) were reported in 17 out of 82 WMH-containing tissue blocks (20.7%). Histological WML were also reported in NAWM-derived tissue blocks (6/41 blocks, 14.6%) and in blocks from MRI normal brains (2/28 blocks, 7.1%).
Extravascular fibrinogen was frequently observed within parenchymal cells and axons, and also as a diffuse extracellular pattern, showing accentuation around vessels (Figure 1C, D; further examples in Supplementary Figures II, III and IV). Parenchymal cell labelling with fibrinogen was recorded as a histological feature in many tissue blocks (81/151, 54% of blocks examined). The fibrinogen labelling pattern was confirmed in neighbouring sections treated with another plasma marker, human IgG (polyclonal and monoclonal antibodies, Supplementary Figure IV) and was absent in neighbouring sections treated with an irrelevant primary antibody (rabbit anti-sheep IgG, not shown). Fibinogen labelled cells and axons were seen around small arteries (example in Figure 2) and around small veins (example in Supplementary Figure IV). We did not attempt to quantify the prevalence of fibrinogen labelling around a particular vessel type.
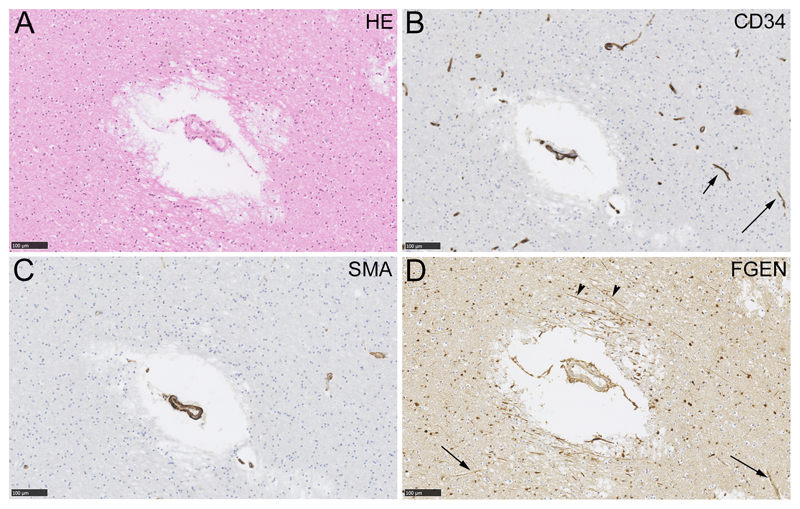
Figure 2.
Fibrinogen immunolabelling around a small artery in deep subcortical white matter. Neighbouring sections were labelled with haematoxylin and eosin (HE, panel A), an endothelial marker (CD34, panel B), a myocyte marker (smooth muscle action, SMA, panel C) or fibrinogen (FGEN, panel D). Capillaries are evident in the CD34-labelled section (arrows, B) and not in the SMA-labelled section. In panel D, fibrinogen labelling is evident around the small artery in cells and in axons (arrowheads). In this example, enhanced perivascular fibrinogen labelling is not seen around capillaries (arrows). Scale bars 100 µm.
The mean extent of fibrinogen labelling was 1.03 % (linearised SE 0.04 %) across tissue blocks. There was no significant difference between tissue groups defined by MRI or histopathology in terms of fibrinogen area fraction (F(3, 648)=0.21; p=0.892). We tested whether BBB dysfunction was a risk factor for white matter abnormalities. Fibrinogen labelling was not significantly related to white matter abnormalities, either as MRI-defined WMH (OR 0.90; 95% CI 0.79, 1.03; p = 0.130) or as CD68-defined histological lesions (OR 0.93; 95% CI 0.77, 1.12; p = 0.452). According to linear regression analyses, the presence of a white matter abnormality identified either by MRI, histology or combined methods was not significantly related to fibrinogen area fraction (Supplementary Table I).
We tested whether BBB dysfunction was associated with risk of clinical dementia. Across the cohort as a whole fibrinogen-labelled area fraction was not significantly associated with dementia status (OR 0.93; 95% CI (OR) 0.80, 1.09; p = 0.378). We repeated the analyses among MRI-defined subgroups and histology-defined subgroups (Table 2). Considering only participants who had no MRI-detected WMH, increased fibrinogen was significantly related to decreased risk of clinical dementia (OR 0.74; 95% CI 0.58, 0.94; p = 0.013, Table 2). Among participants where no CD68-defined WML was detected, fibrinogen was negatively associated with decreased dementia risk (OR 0.83; CI(OR) 0.70, 0.98, p=0.032, Table 2). Among participants where at least one CD68-defined WML was detected (irrespective of MRI-defined tissue categories) a greater extent of fibrinogen labelling was related to increased risk of dementia (OR 2.26; 95% CI 1.25, 4.08; p = 0.007, Table 2).
Table 2.
Relationship between extent of fibrinogen immunolabelling and risk (Odds Ratio, OR) of clinical dementia.
| OR | 95% CI (OR) | p-value | |
|---|---|---|---|
| MRI-defined WMH | |||
| MRI normal | 0.74 | (0.58; 0.94) | 0.013 |
| MRI NAWM | 0.96 | (0.56; 1.65) | 0.887 |
| MRI WMH | 1.27 | (0.92; 1.75) | 0.139 |
| Histopathology-defined white matter lesions | |||
| Histology non-lesion | 0.83 | (0.70; 0.98) | 0.032 |
| Histological lesion | 2.26 | (1.25; 4.08) | 0.007 |
| Combined MRI and histopathology | |||
| MRI normal, histology non-lesion | 0.72 | (0.56; 0.93) | 0.010 |
| MRI NAWM, histology non-lesion | 0.77 | (0.42; 1.40) | 0.391 |
| MRI WMH, histology non-lesion | 1.00 | (0.72; 1.38) | 0.988 |
| MRI WMH, histological lesion | 1.82 | (0.81; 4.09) | 0.146 |
To test BBB dysfunction as a risk factor for dementia we conducted weighted logistic regression analysis, with clinical dementia status (dementia, without dementia) as dependent variable and fibrinogen area fraction as independent variable. P<0.05 considered significant. Abbreviations: WMH: white matter hyperintensity. NAWM: normal appearing white matter.
Discussion
Fibrinogen immunolabelling of parenchymal cells and tissue was a frequent finding in this population-based study of older persons. We have interpreted this as indicative of BBB dysfunction, though clearly the degree of labelling is also dependent on tissue clearance pathways and cellular uptake and metabolism. In common with prior neuropathology studies10, 11, 18, 19 we routinely observed cellular and axonal labelling, suggesting an active uptake process. The concept that some degree of BBB permeability is common in older people is supported by extravasation of contrast agent in MRI studies4–8, 20 and biochemical detection of plasma markers in cerebrospinal fluid5, 20, 21. The concept is further supported by observations that brain amyloid can be depleted by systemic immunotherapy22, implying BBB penetration by circulating antibodies. Elevated BBB permeability may occur transiently, possibly in a regional mosaic, becoming more common with increasing age23, 24. Recent studies in experimental mice suggest that the healthy BBB exhibits some (small) degree of permeability to most solutes, across a wide range of molecular size (MW 86 – 150,000)25, 26. Efflux may be via defective tight junctions, or alternatively may be via a trans-cellular pathway27.
In our data, BBB dysfunction in brains harbouring histologically-defined white matter lesions was associated with greater risk of clinical dementia. This finding may be explained by toxic effects of plasma-derived fluid within areas of tissue damage (where transmembrane ionic gradients and transport pathways are likely to be disrupted). Our data agree with previous neuropathology cohort studies where markers of plasma extravasation were positively associated with dementia diagnosis10, 11, 18 or specifically with AD3, 19, 28, 29. Experiments in animals demonstrate cytotoxic effects of plasma-derived molecules such as fibrinogen on axons, myelin and other white matter components30, 31. Taken together, these findings suggest that BBB dysfunction may exacerbate the cognitive impact of active white matter lesions1.
On post hoc subgroup analysis, we found a significant negative association of BBB dysfunction with dementia risk among participants without detectable WMH (“MRI normal” brains, Table 2). This finding was at borderline significance (p = 0.013) and may represent a false-positive, due to multiple hypothesis testing. If correct, a negative association implies a possible “pre-conditioning” action of plasma extravasation. Brain tissue expresses a complex array of molecular mechanisms for degradation and clearance of parenchymal debris24. We speculate that in older persons without WMH, these clearance pathways may be augmented by modest plasma-derived fluid efflux.
Contrary to our hypothesis, the extent of fibrinogen labelling was not a risk factor for white matter abnormalities. Fibrinogen labelling within blocks cut from WMH, or within blocks containing CD68-defined histological lesions, did not significantly differ from other tissue types. Thus, our data do not support the hypothesis that BBB dysfunction is associated with WMH, or with white matter lesions. This contrasts with previous neuropathology studies in small cohorts9, 10. Unlike the present study, these were not targeted to subcortical white matter. In accord with the present findings, previous well-powered neuropathology studies found no relation of histological BBB markers with WMH11, 12. These findings resemble those in white matter of multiple sclerosis patients, where clusters of activated microglia were not significantly associated with heightened BBB dysfunction32.
Our data conflict with some previous MRI studies, where enhanced contrast agent extravasation was seen in patients with cerebrovascular disease4–7 8. Size-selective changes in BBB permeability may reconcile neuropathology data from our study and others11, 12 with these MRI findings. Size-selective BBB opening is observed in experimental mice subjected to chronic systemic treatment with low dose angiotensin II26, or where the tight junction protein claudin-5 was genetically deleted33. In the brains of these mice, efflux of water and small solutes (including MRI contrast agents, MW<1000) was evident, while large plasma protein molecules were retained11, 26, 33. Extravasation of small solutes may be associated with WMH while larger solutes (including proteins such as fibrinogen) are not.
The present study has several limitations. First, MRI scans were carried out post mortem and with low field strength (1.0 Tesla). Nevertheless, recent quantitative comparisons show reasonable correlation of post mortem T2-weighted images with ante mortem MRI34. Second, we report on just one, late-stage time-point for each participant. By the nature of a neuropathology study, we lack information on the longitudinal development of tissue lesions.
Third, the post hoc analyses of fibrinogen data raise the possibility of false-positive associations and should be viewed with caution. In addition, we did not include potential confounding covariates (such as APOE genotype).
Conclusions
Our data confirm that some degree of BBB dysfunction is common in older people. For the histological marker of BBB dysfunction that we used, we detected post hoc significant associations with dementia risk. No direct association with white matter changes was detected. If confirmed, these data raise the possibility that BBB dysfunction may be an independent marker for clinical dementia risk, additional to standard MRI biomarkers.
Supplementary Material
Acknowledgements
We gratefully thank our colleagues in St George’s Healthcare NHS Trust Cellular Pathology Service, St George’s Imaging Resource Facility and The Sheffield Institute for Translational Neuroscience for sharing their expertise with us. We are grateful to the MRC CFAS respondents and their families for their generous gift to medical research, which has made this study possible. We thank the interviewers, liaison officers, general practitioners and their staff, nursing and residential home staff, and the primary care trusts for their cooperation and support.
Funding
The Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) is supported by grants from the Medical Research Council (MRC, grant numbers G9901400, G0601022) and the United Kingdom (UK) Department of Health. The MRC CFAS Neuropathology brain tissue banks are supported by: the UK National Institute for Health Research (NIHR) Biomedical Research Centre for Ageing and Age-related Disease Award to the Newcastle-upon-Tyne Hospitals Foundation Trust; the NIHR Cambridge Biomedical Research Centre; The Cambridgeshire and Peterborough NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC); Nottingham University Hospitals NHS Trust; University of Sheffield and the Sheffield Teaching Hospitals NHS Foundation Trust.
AHH gratefully acknowledges financial support from Alzheimer’s Society (UK) (PG146/151), Alzheimer's Drug Discovery Foundation (Project Ref 20140901), Alzheimer’s Research UK (PPG2014A-8), St George’s Hospital Charity and The Neuroscience Research Foundation. HSM is supported by an NIHR Senior Investigator award, and by the Cambridge University Hospital Trusts NIHR Comprehensive Biomedical Research Centre.
Footnotes
Disclosures: None
Reference List
- (1).Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11:157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- (2).Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- (3).Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer's disease. J Cereb Blood Flow Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Munoz MS, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- (5).Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, et al. Blood-brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42:2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81:192–197. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- (7).Huisa BN, Caprihan A, Thompson J, Prestopnik J, Qualls CR, Rosenberg GA. Long-Term Blood-Brain Barrier Permeability Changes in Binswanger Disease. Stroke. 2015;46:2413–2418. doi: 10.1161/STROKEAHA.115.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Zhang CE, Wong SM, van de Haar HJ, Staals J, Jansen JF, Jeukens CR, et al. Blood-brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88:426–432. doi: 10.1212/WNL.0000000000003556. [DOI] [PubMed] [Google Scholar]
- (9).Simpson JE, Wharton SB, Cooper J, Gelsthorpe C, Baxter L, Forster G, et al. Alterations of the blood-brain barrier in cerebral white matter lesions in the ageing brain. Neurosci Lett. 2010;486:246–251. doi: 10.1016/j.neulet.2010.09.063. [DOI] [PubMed] [Google Scholar]
- (10).Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, et al. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer's disease patients. Stroke. 1996;27:2069–2074. doi: 10.1161/01.str.27.11.2069. [DOI] [PubMed] [Google Scholar]
- (11).Bridges LR, Andoh J, Lawrence AJ, Khoong CH, Poon WW, Esiri MM, et al. Blood-brain barrier dysfunction and cerebral small vessel disease (arteriolosclerosis) in brains of older people. J Neuropathol Exp Neurol. 2014;73:1026–1033. doi: 10.1097/NEN.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- (13).Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- (14).Richardson K, Stephan BC, Ince PG, Brayne C, Matthews FE, Esiri MM. The neuropathology of vascular disease in the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Curr Alzheimer Res. 2012;9:687–696. doi: 10.2174/156720512801322654. [DOI] [PubMed] [Google Scholar]
- (15).Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- (16).Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Fernando MS, O'Brien JT, Perry RH, English P, Forster G, McMeekin W, et al. Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol Appl Neurobiol. 2004;30:385–395. doi: 10.1111/j.1365-2990.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- (18).Utter S, Tamboli IY, Walter J, Upadhaya AR, Birkenmeier G, Pietrzik CU, et al. Cerebral small vessel disease-induced apolipoprotein E leakage is associated with Alzheimer disease and the accumulation of amyloid beta-protein in perivascular astrocytes. J Neuropathol Exp Neurol. 2008;67:842–856. doi: 10.1097/NEN.0b013e3181836a71. [DOI] [PubMed] [Google Scholar]
- (19).Hultman K, Strickland S, Norris EH. The APOE varepsilon4/varepsilon4 genotype potentiates vascular fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer's disease patients. J Cereb Blood Flow Metab. 2013;33:1251–1258. doi: 10.1038/jcbfm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Halliday MR, Pomara N, Sagare AP, Mack WJ, Frangione B, Zlokovic BV. Relationship between cyclophilin a levels and matrix metalloproteinase 9 activity in cerebrospinal fluid of cognitively normal apolipoprotein e4 carriers and blood-brain barrier breakdown. JAMA Neurol. 2013;70:1198–1200. doi: 10.1001/jamaneurol.2013.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- (23).Morita T, Mizutani Y, Sawada M, Shimada A. Immunohistochemical and ultrastructural findings related to the blood--brain barrier in the blood vessels of the cerebral white matter in aged dogs. J Comp Pathol. 2005;133:14–22. doi: 10.1016/j.jcpa.2005.01.001. [DOI] [PubMed] [Google Scholar]
- (24).Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Bien-Ly N, Boswell CA, Jeet S, Beach TG, Hoyte K, Luk W, et al. Lack of Widespread BBB Disruption in Alzheimer's Disease Models: Focus on Therapeutic Antibodies. Neuron. 2015;88:289–297. doi: 10.1016/j.neuron.2015.09.036. [DOI] [PubMed] [Google Scholar]
- (26).Faraco G, Sugiyama Y, Lane D, Garcia-Bonilla L, Chang H, Santisteban MM, et al. Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J Clin Invest. 2016;126:4674–4689. doi: 10.1172/JCI86950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- (29).Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Davalos D, Ryu JK, Merlini M, Baeten KM, Le MN, Petersen MA, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).van Horssen J, Singh S, van der Pol S, Kipp M, Lim JL, Peferoen L, et al. Clusters of activated microglia in normal-appearing white matter show signs of innate immune activation. J Neuroinflammation. 2012;9:156. doi: 10.1186/1742-2094-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Dawe RJ, Bennett DA, Schneider JA, Leurgans SE, Kotrotsou A, Boyle PA, et al. Ex vivo T2 relaxation: associations with age-related neuropathology and cognition. Neurobiol Aging. 2014;35:1549–1561. doi: 10.1016/j.neurobiolaging.2014.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.