Abstract
Our objective was to compare several experimental preparations of a single injection of long-acting recombinant bovine FSH (rbFSH; types A and B) to a porcine pituitary-derived FSH (Folltropin) to superovulate Holstein dairy heifers. Nonlactating, nonpregnant virgin Holstein heifers (n = 56) aged 12 to 15 months were randomly assigned to one of four superstimulatory treatments. Beginning at a random stage of the estrous cycle, all follicles greater than 5 mm were aspirated. Thirty-six hours later, heifers received an intravaginal P4 device and superstimulatory treatments were initiated. Treatments were (1) 300 mg of pituitary-derived FSH (Folltropin) administered in eight decreasing doses over a period of 3.5 days; (2) a single injection of 50 μg of A-rbFSH; (3) a single injection of 100 μg of A-rbFSH; and (4) a single injection of 50 μg of B-rbFSH. All heifers received 25 mg PGF2α at 48 and 72 hours after the insertion of P4 device. At 84 hours after insertion, P4 devices were removed, and ovulation was induced 24 hours later with hCG (2500 IU). Heifers were inseminated at 12 and 24 hours after hCG treatment. The number of ovulatory follicles was greatest for heifers treated with Folltropin and B50-rbFSH, least for heifers treated with A50-rbFSH, and was intermediate for heifers treated with A100-rbFSH (25.7 ± 3.2, 18.9 ± 3.2, 5.9 ± 0.9, and 16.6 ± 3.1, respectively; P < 0.001). The number of corpora lutea was greatest for heifers treated with Folltropin, B50-rbFSH, and A100-rbFSH, and least for heifers treated with A50-rbFSH (19.1 ± 2.4, 16.1 ± 3.0, 15.9 ± 2.9, and 2.6 ± 0.9, respectively; P < 0.001). The number of good-quality embryos differed among treatments and was greatest for heifers treated with B50-rbFSH, Folltropin, and A100-rbFSH and least for heifers treated with A50-rbFSH (7.6 ± 2.4, 6.5 ± 1.7, 4.3 ± 1.5, and 0.8 ± 0.5, respectively; P < 0.001). In conclusion, a single injection of a preparation of long-acting rbFSH (either 100 μg of A-rbFSH or 50 μg of B-rbFSH but not 50 μg of A-rbFSH) produced similar superovulatory responses resulting in the production of good-quality embryos when compared with a pituitary-derived FSH preparation administered twice daily for 4 days. More studies using different types of cattle and different doses of rbFSH are needed to confirm the findings reported in this preliminary study.
Keywords: Superovulation, Recombinant FSH, Dairy heifer, Embryo
1. Introduction
Multiple ovulation and embryo transfer (MOET) is a reproductive biotechnology used worldwide and allows for increased production of offspring from genetically superior dams. Pituitary-derived porcine FSH has been used to superovulate cattle during the past few decades and is commercially available for use in cattle; however, because of its relative short half-life of approximately 5 hours in cows [1,2], pituitary-derived porcine FSH requires frequent administration (twice daily over a period of 4 days) to maintain adequate circulating levels of FSH to induce a successful superstimulatory response.
Since the early stages of MOET, researchers and practitioners have sought protocols requiring less handling of donor animals without compromising the yield of high-quality embryos. The development of hormonal synchronization protocols that allow for timed artificial insemination (AI) have had a considerable impact on the overall results of MOET programs and have facilitated donor management [3]. In addition, strategies allowing for successful superovulation with reduced frequency of treatments can decrease the handling of donors by up to 75%, thereby reducing costs per embryo produced [4]. Several approaches have been pursued to decrease the number of FSH treatments required to superovulate donors with interesting but varying results including once daily subcutaneous (sc) FSH injections [5]; once daily sc injections of FSH dissolved in saline and a gelatin gel [6]; a single sc FSH injection [7,8]; a single intramuscular (im) injection of FSH dissolved in polyvinylpyrrolidone [9]; a single [10] or slip-single [11] im injection of FSH dissolved in hyaluronan; or a single im injection of FSH dissolved in hydroxide gel [12]. All these studies used pituitary-derived FSH preparations, and superovulation studies using a single treatment of recombinant bovine FSH (rbFSH) have not been reported.
Preparations of pituitary-derived FSH might present batch-to-batch variations in the biologic activity and, or purity of the hormone, and the use of a recombinant FSH preparation might reduce the variation observed in the pituitary-derived FSH [4,13]. Several studies have used rbFSH to induce superstimulation of follicles in heifers and mature cows [14–16] with interesting results. Those studies did not use a long-acting rbFSH (LArbFSH) preparation, and multiple treatment with FSH over a period of 3 to 6 days was required to induce a superstimulatory response. In addition, repeated use of exogenous FSH from one species (e.g., swine) to induce superstimulation of donors in a different species (e.g., rabbits) might induce a humoral immune response, thereby reducing the superstimulatory response after repeated exposure of the donor, at least in goats [17], rabbits [18], and marsupials [19]. Equine chorionic gonadotropin (eCG) has been used to superstimulate cattle, and the use of repeated treatments with high doses of eCG induced a humoral immune response (reviewed by Baruselli et al. [20]). Exogenous recombinant FSH from the same species (e.g., ovine recombinant FSH used in ewes) may prevent this humoral immune response and transmission of diseases across species.
The objective of this experiment was to compare the efficacy of a single injection of two types (A and B) of LArbFSH using experimental doses to a standard pituitary-derived FSH preparation administered twice daily to superovulate Holstein heifers. Our hypothesis was that superstimulatory and superovulatory responses and the number of good-quality embryos produced would be similar between heifers treated with a single injection of LArbFSH compared with heifers treated twice daily with a standard pituitary-derived FSH preparation.
2. Materials and methods
2.1. Animals, feeding and housing
Nonlactating, nonpregnant virgin Holstein heifers aged 12 to 15 months from two commercial dairy herds (n = 56, farm A = 40 and farm B = 16) located in South-central Wisconsin were used. The experiment was conducted during July 2012. Heifers were housed in open lot corrals equipped with self-locking head gates at the feedline, and heifers had free access to feed and fresh water ad libitum throughout the experiment. Heifers were fed once daily a total mixed ration diet (similar at both locations) that consisted of grass hay and alfalfa silage as forage and concentrate based on corn and soybeans and supplemented with vitamins and minerals to meet or exceed the minimum requirements for Holstein heifers weighing 300 to 400 kg and gaining 0.6 to 0.8 kg/day of body weight [21]. All procedures were approved by the Animal Care Committee of the College of Agriculture and Life Sciences, University of Wisconsin–Madison.
2.2. Supplies and semen
Prostaglandin F2α (PGF2α; Lutalyse) was from Zoetis (New York, NY, USA). The hCG (chorionic gonadotropin, 3300 IU/dose; Chorulon) was from Merck Animal Health (Milsboro, DE, USA). The pituitary-derived FSH (NIH-FSH-P; Folltropin-V) from a single batch was from Bioniche Animal Health Canada Inc. (Belleville, Ontario, Canada). The experimental LArbFSH types A and B were provided by CEVA Animal Health. Intravaginal progesterone (P4) devices (Eazi-Breed CIDR; containing 1.38 g of progesterone) were from Zoetis (New York, NY, USA). Lidocaine (Lidocaine Hydrochloride Injectable 2%; 3 mL/dose) was from Phoenix Pharmaceutical Inc. (St. Joseph, MO, USA). Embryo filters (MiniFlush Embryo System) and y-tubing (Y-Junction Tubing) were from Minitube of America Inc. (Verona, WI, USA). Catheters (Silicon ET catheter CH16, 2-way Foley, 5 mL balloon) and embryo collection medium (BioLife Advantage Embryo Collection Medium, 2 L) were from Agtech, Inc. (Manhattan, KS, USA). Holding medium (Vigro Holding Plus) was from Bioniche Animal Health Canada Inc. (Pullman, WA, USA). All inseminations were performed using conventional frozen semen (20 × 106 sperm per straw) from three Holstein sires with high genetic merit from two AI companies. All AI sires had proven outstanding field fertility and were equally balanced among treatments.
2.3. Treatments, artificial insemination, and embryo collection and evaluation
Beginning at a random stage of the estrous cycle, all follicles of 5 mm in diameter or greater were ablated using an ultrasound-guided transvaginal needle (Aloka 900 V equipped with a 5 MHz convex-array transducer; Coromotrics Medical Systems Inc., Wallingford, CT) as described by Bergfelt et al. [22]. Heifers were randomly assigned to one of four treatments (Fig. 1; n = 14 heifers per treatment). Approximately 36 hours after follicle ablation, an intravaginal P4 device was inserted and superstimulatory treatments were initiated. Heifers in treatment 1 (Folltropin) received a total FSH dose equivalent to 300 mg of NIH-FSH-P1 (Folltropin-V) in eight decreasing doses (3.0, 3.0, 2.0, 2.0, 1.5, 1.5, 1.0, and 1.0 mL) administered im at 12-hour intervals over a 4 day period; heifers in treatment 2 (A50-LArbFSH) received 50 μg of LArbFSH (type A) in a single im injection; heifers in treatment 3 (A100-LArbFSH) received 100 μg of LArbFSH (type A) in a single im injection; and heifers in treatment 4 (B50-LArbFSH) received 50 μg of LArbFSH (type B) in a single im injection.
Fig. 1.
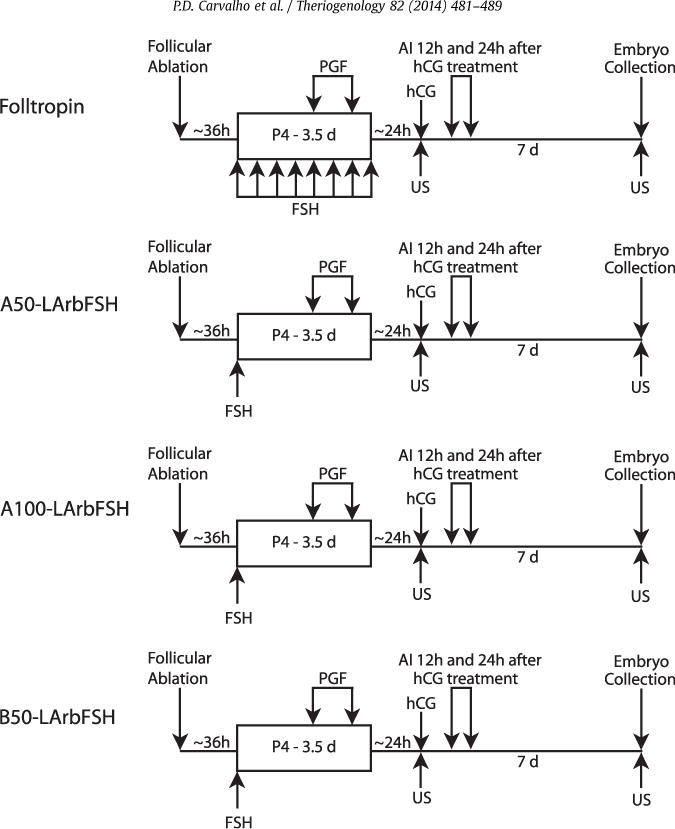
Schematic representation of experimental procedures (not to scale). Starting at a random stage of the estrous cycle, all follicles of 5 mm in diameter or greater were ablated and heifers were randomly assigned to one of four treatments (n = 14 heifers per treatment). Approximately 36 hours after follicle ablation, an intravaginal P4 device was inserted, and superstimulatory treatments were initiated. Heifers in treatment 1 (Folltropin) received a total FSH dose equivalent to 300 mg of NIH-FSH-P1 (Folltropin-V) in eight decreasing doses (3.0, 3.0, 2.0, 2.0, 1.5, 1.5, 1.0, and 1.0 mL) administered im at 12-hour intervals over a 4 day period, heifers in treatment 2 (A50-LArbFSH) received 50 μg of LArbFSH (type A) in a single im injection, heifers in treatment 3 (A100-LArbFSH) received 100 μg of LArbFSH (type A) in a single im injection, and heifers in treatment 4 (B50-LArbFSH) received 50 μg of LArbFSH (type B) in a single im injection. All heifers received two PGF2α injections at 48 and 72 hours after P4 device insertion (concomitant with the fifth and seventh FSH injection on treatment 1), and P4 devices were withdrawn 84 hours after insertion (concomitant with the last FSH injection in treatment 1). Twenty-four hours after P4 device removal, ovulation was induced with 2500 IU of hCG. Heifers were inseminated twice at 12 and 24 hours after hCG treatment by one technician. Seven days after hCG treatment, embryos were nonsurgically flushed.
All heifers received two PGF2α injections at 48 and 72 hours after P4 device insertion (concomitant with the fifth and seventh FSH injection on treatment 1), and P4 devices were withdrawn 84 hours after insertion (concomitant with the last FSH injection in treatment 1). Twenty-four hours after P4 device removal, ovulation was induced using 2500 IU of hCG administered im. Heifers were inseminated twice at 12 and 24 hours after hCG treatment by the same technician. Seven days after hCG treatment, ova and embryos were recovered from heifers nonsurgically using a uterine flushing technique, and the entire uterus was flushed with 1.5 L of embryo recovery medium. The flushing catheter was passed through the cervix, and the balloon was inflated with air just cranial to the cervix within the uterine body to allow the simultaneous flushing of the uterus and both uterine horns. Structures were collected into an embryo filter, and recovered structures were immediately searched for under a stereomicroscope and graded for quality according to the International Embryo Society standards [23]. All embryo searching and grading procedures were performed by the same treatment-blind technician. Embryos graded as 1, 2, and 3 were defined as viable embryos, whereas embryos graded 1 and 2 were classified as freezable embryos. The sequence of procedures and injections used to induce the superstimulation of follicles in the four treatments is summarized in Figure 1.
2.4. Ovarian ultrasonography
Ovarian ultrasonography was performed by a treatment-blinded technician using a portable ultrasound machine (Ibex Pro; E.I. Medical Imaging, Loveland, CO, USA) fitted with a 7.5 MHz linear-array transducer immediately before hCG treatment to assess number of ovulatory follicles (≥10 mm) and 7 days later immediately before embryo collection to assess the number of CL.
2.5. Definitions and statistical analysis
Heifers with three or more CL assessed by ultrasound at the time of embryo collection were considered to have responded to the superovulatory treatment. Heifers with no CL on the day of embryo collection were not flushed. These heifers were included in the analysis of number of ovulatory follicles, number of CL, and ovulation rate but were excluded from the analysis for ova/embryo characteristics. Percentage of fertilized structures was calculated by dividing the total number of cleaved structures by the total number of structures, percentage freezable embryos was calculated by dividing total number of grade 1 and 2 embryos by the total number of structures, percentage transferable embryos was calculated by dividing the total number of grade 1, 2, and 3 embryos by the total number of structures, and percentage degenerated embryos was calculated by dividing the total number of degenerated embryos by the total number of structures. The percentage transferable of the fertilized embryos was calculated by dividing the number of transferable embryos by the total number of fertilized structures. The percentage of fertilized embryos that were degenerated was calculated by dividing the number of degenerated embryos by the total number of fertilized structures. Ovulation rate was calculated by dividing the number of CL by the number of ovulatory follicles. All values are expressed as means ± SEM unless otherwise stated.
All statistical analyses were performed using SAS computational software, version 9.3 for Microsoft Windows [24]. The mixed procedure was used to compare embryo characteristics using heifer as the experimental unit and heifer within herd as a random effect. The initial model contained the variables for fixed effects of treatment, age, farm, and treatment by farm interaction. Model selection was performed using a stepwise backward elimination procedure that removed variables with P > 0.15 from the model. The final model included only treatment as a fixed effect. Data were analyzed for normality and were transformed when necessary. Percentage data were transformed using the arcsine square root transformation, whereas continuous data were ranked. Number of CL was analyzed in relation to the number of ovulatory follicles, and percentage transferable embryos and number of transferable embryos were analyzed in relation to the number of CL independent of treatments using the output statement of the REG procedure (Fig. 2). For the analysis of the number of CL in relation to the number of ovulatory follicles, all heifers except one that did not have any ovulatory follicles were included. For the analysis of the percentage transferable embryos and number of transferable embryos in relation to the number of ovulatory follicles, only heifers that were flushed were included in the analysis. A P value ≤0.05 was considered to be significant, whereas P values between 0.05 and 0.1 were considered a statistical tendency.
Fig. 2.
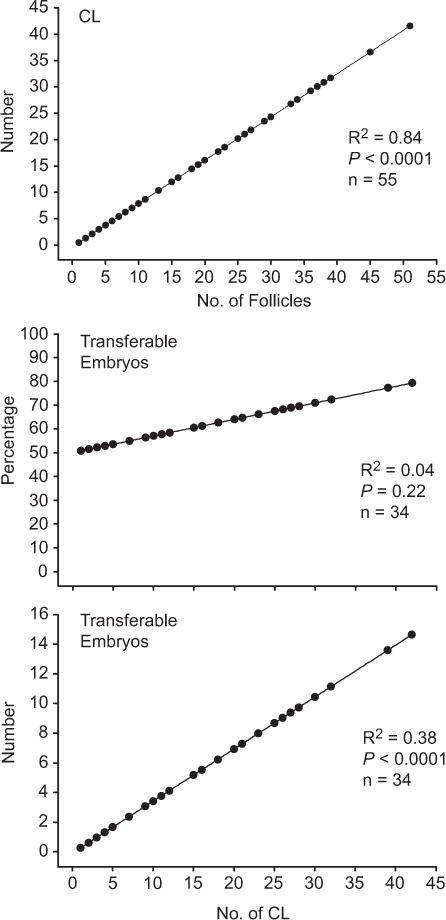
Linear regression lines for the number of CL on the number of ovulatory follicles (upper panel), for the percentage of transferable embryos on the number of CL (middle panel), and for the number of transferable embryos on the number of CL (lower panel), for heifers in which the uterine flushing was performed.
3. Results
3.1. Superstimulatory and superovulatory outcomes
The number of ovulatory follicles at the time of hCG treatment, number of CL at the time of embryo collection, proportion of heifers considered to have responded to the superstimulatory treatments, and ovulation rate are summarized in Table 1. The number of ovulatory follicles was greater for heifers treated with Folltropin than for heifers treated with A100-LArbFSH (P = 0.03) or A50-LArbFSH (P < 0.001). Interestingly, the number of ovulatory follicles did not differ between heifers treated with B50-LArbFSH and Folltropin (P = 0.12), or between heifers treated with B50-LArbFSH and A100-LArbFSH (P = 0.54); however, the number of ovulatory follicles was greater (P < 0.001) for heifers treated with B50-LArbFSH compared with heifers treated with A50-LArbFSH. In addition, the number of ovulatory follicles was greater (P < 0.001) for heifers treated with A100-LArbFSH than for heifers treated with A50-LArbFSH. The number of CL was similar (P > 0.10) among heifers treated with Folltropin, B50-LArbFSH, or A100-LArbFSH, but was less (P < 0.01) for heifers treated with A50-LArbFSH.
Table 1.
Effect of treatment on number of follicles and CL, percentage of superovulated heifers, and ovulation rate for Holstein heifers induced to superovulate using a long-acting recombinant bovine FSH preparation (LArbFSH) or a pituitary-derived FSH preparation (Folltropin).
| Item | Folltropin | A50-LArbFSH | A100-LArbFSH | B50-LArbFSH | P value |
|---|---|---|---|---|---|
| n | 14 | 14 | 14 | 14 | |
| Follicle numbera (min-max) | 25.7 ± 3.2a (7-51) | 5.9 ± 0.9c (0-11) | 16.6 ± 3.1b (2-39) | 18.9 ± 3.2ab (4-37) | <0.01 |
| CL numberb (min-max) | 19.1 ± 2.4a (7-32) | 2.6 ± 0.9b (0-11) | 15.9 ± 2.9a (2-42) | 16.1 ± 3.0a (2-39) | <0.01 |
| % Superovulatedc (n) | 100 ± 0.0a (14) | 28.6 ± 12.5b (4) | 85.7 ± 9.7a (12) | 92.9 ± 7.1a (13) | <0.01 |
| Ovulation rated | 76.0 ± 4.8a | 38.7 ± 10.6b | 88.0 ± 4.2a | 80.8 ± 4.9a | <0.01 |
Within a row, means with different superscripts (a, b) differ (P < 0.05).
Heifers treated with Folltropin received 300 mg of FSH (Folltropin) in eight decreasing doses administered im at 12-hour intervals over a 4-day period, heifers treated with A50-LArbFSH received 50 μg of LArbFSH (type A) in a single im injection, heifers treated with A100-LArbFSH received 100 μg of LArbFSH (type A) in a single im injection, and heifers treated with B50-LArbFSH received 50 μg of LArbFSH (type B) in a single im injection.
Measured immediately before hCG treatment.
Measured immediately before embryo collection.
Heifers with three or more CL at the time of embryo collection.
Measured immediately before embryo collection, one heifer treated with A50-LArbFSH was excluded because no follicles greater than 9 mm or CL were detected.
Figure 3 shows a distribution of the number of ovulatory follicles measured immediately before hCG treatment, and a distribution of number of CL measured immediately before embryo collection (upper and lower panel, respectively). At the time of embryo collection, four heifers (28.6%) treated with A50-LArbFSH did not have a CL, whereas all heifers in the other three treatments had at least two CL. In addition, the proportion of heifers with three or more CL on the day of embryo collection was similar (P > 0.10) among heifers treated with Folltropin, B50-LArbFSH, and A100-LArbFSH and was less (P < 0.01) for heifers treated with A50-LArbFSH. Ovulation rate was similar (P > 0.10) among heifers treated with Folltropin, B50-LArbFSH, and A100-LArbFSH; however, it was unexpectedly less (P < 0.001) for heifers treated with A50-LArbFSH.
Fig. 3.
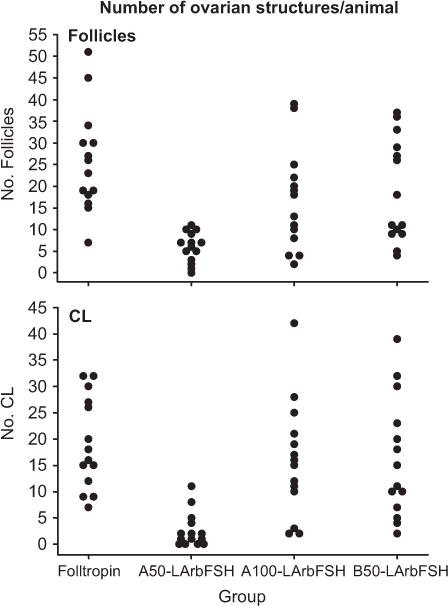
Scatter plot distributions of the number of ovulatory follicles measured immediately before induction of ovulation and the number of CL measured 7 days later and immediately before embryo collection (upper and lower panel, respectively).
Independent of treatment, as the number of ovulatory follicles increased, the number of ovulations as measured by the number of CL immediately before embryo collection also increased (r2 = 0.84, P < 0.001; Fig. 2, upper panel). The number of transferable embryos recovered per donor increased as the number of CL increased for heifers with at least one CL at the time of embryo collection (r2 = 0.38, P < 0.0001; Fig. 2, lower panel). Overall, the number of transferable embryos increased as the superstimulatory response increased. By contrast, the percentage of transferable embryos did not increase as the superstimulatory response increased (r2 = 0.04, P 0.22; Fig. 2, middle panel). Thus, more transferable embryos were recovered only because more total structures were recovered.
3.2. Ova and embryo recovery and characteristics
Results of ova and embryo recovery rate and characteristics are summarized in Table 2. The number of structures recovered per CL was greatest for heifers treated with B50-LArbFSH and was least for heifers treated with A50-LArbFSH (P = 0.04), whereas for heifers treated with Folltropin and A100-LArbFSH, recovery rate was intermediate and did not differ from the other treatments. Total structures recovered did not differ among treatments; however, more heifers were flushed and more of the flushed heifers had structures recovered when heifers were treated with B50-LArbFSH, Folltropin, and A100-LArbFSH compared with heifers treated with A50-LArbFSH. The number of fertilized ova was greater for heifers treated with B50-LArbFSH and less for heifers treated with A50-LArbFSH (P = 0.004), whereas heifers treated with Folltropin and A100-LArbFSH were intermediate and did not differ from the other treatments. Fertilization rate was greater for heifers treated with Folltropin and B50-LArbFSH than for heifers treated with A100-LArbFSH (P = 0.03), and was intermediate for heifers treated with A50-LArbFSH. The number of transferable embryos was greater for heifers treated with B50-LArbFSH and Folltropin than for heifers treated with A50-LArbFSH (P < 0.003) and was intermediate but did not differ from the other treatments for heifers treated with A100-LArbFSH. Percentage of transferable embryos from the total structures recovered was greater for heifers treated with Folltropin than for heifers treated with A50-LArbFSH (P = 0.05) and was intermediate but did not differ from the other treatments for heifers treated with B50-LArbFSH and A100-LArbFSH. Percent transferable of fertilized embryos, total degenerated, percentage degenerated of total, and percentage degenerated from the fertilized embryos did not differ among treatments.
Table 2.
Effect of treatment on recovery rate and embryo characteristics for Holstein heifers induced to superovulate using a long-acting recombinant bovine FSH preparation (LArbFSH) or a pituitary-derived FSH preparation (Folltropin).
| Item | Folltropin | A50-LArbFSH | A100-LArbFSH | B50-LArbFSH | P value |
|---|---|---|---|---|---|
| % Flushed (n) | 92.9 ± 7.1 (13) | 64.3 ± 11.3 (9) | 78.6 ± 11.4 (11) | 92.9 ± 7.1 (13) | 0.22 |
| % With structuresa (n) | 84.6 ± 10.4 (11) | 44.4 ± 17.6 (4) | 72.7 ± 14.1 (8) | 84.6 ± 10.4 (11) | 0.19 |
| Total ova and embryos recoveredb | 8.5 ± 2.2 | 4.3 ± 2.4 | 8.4 ± 2.3 | 10.1 ± 2.4 | 0.59 |
| % Ova and embryo per CLa | 36.4 ± 8.3ab | 28.3 ± 13.1b | 32.2 ± 8.8ab | 54.8 ± 10.0a | 0.22 |
| Fertilized ova | 8.0 ± 2.1ab | 2.0 ± 0.7b | 6.4 ± 2.1ab | 9.5 ± 2.3a | 0.28 |
| % Fertilized ova | 94.5 ± 2.2a | 71.6 ± 16.6ab | 63.8 ± 14.3b | 94.5 ± 2.0a | 0.06 |
| Transferable embryos | 6.5 ± 1.7a | 0.8 ± 0.5b | 4.3 ± 1.5ab | 7.6 ± 2.4a | 0.14 |
| % Transferable embryos | 78.0 ± 7.3a | 37.5 ± 23.9b | 44.7 ± 12.2ab | 66.1 ± 10.6ab | 0.12 |
| % Transferable of fertilized | 81.9 ± 6.8 | 50.0 ± 28.9 | 68.4 ± 8.7 | 69.5 ± 10.8 | 0.46 |
| Degenerated embryos | 1.5 ± 0.6 | 1.3 ± 0.9 | 2.1 ± 0.8 | 1.8 ± 0.5 | 0.87 |
| % Degenerated | 17.3 ± 6.4 | 34.1 ± 23.6 | 19.2 ± 6.5 | 28.4 ± 10.1 | 0.68 |
| % Degenerated of fertilized | 18.8 ± 6.7 | 50.0 ± 28.9 | 31.6 ± 8.7 | 30.5 ± 10.8 | 0.49 |
Within a row, means with different superscripts (a, b) differ (P < 0.05).
Heifers treated with Folltropin received 300 mg of FSH (Folltropin) in eight decreasing doses administered im at 12-hour intervals over a 4 day period, heifers treated with A50-LArbFSH received 50 μg of LArbFSH (type A) in a single im injection, heifers treated with A100-LArbFSH received 100 mg of LArbFSH (type A) in a single im injection, and heifers treated with B50-LArbFSH received 50 μg of LArbFSH (type B) in a single im injection.
Includes only flushed heifers.
Includes only flushed heifers with at least one structure recovered.
4. Discussion
This preliminary study evaluated the efficacy of a single injection of LArbFSH to superstimulate growth of ovarian follicles in Holstein heifers. Our hypothesis was that superstimulatory and superovulatory responses and the number of good-quality embryos recovered would be similar for heifers treated with a single injection of LArbFSH compared with heifers treated with a standard pituitary-derived FSH. Our results partially supported this hypotheses based on the following observations: (1) heifers treated with A100-LArbFSH and B50-LArbFSH had similar superstimulatory and superovulatory responses compared with heifers treated with Folltropin; (2) the proportion of heifers considered superovulated was similar among heifers treated with Folltropin, B50-LArbFSH, and A100-LArbFSH; and (3) heifers treated with A100-LArbFSH and B50-LArbFSH produced a similar number of transferable embryos compared with heifers treated with Folltropin. To our knowledge, this is the first experiment to compare a single injection of LArbFSH to a commercially available pituitary-derived FSH to superovulate dairy heifers.
Walsh et al. [5] compared twice daily im FSH injections to once daily sc FSH injections to superovulate beef heifers. Heifers treated with twice daily FSH injections had a greater superovulatory response, more recovered structures, and more embryos graded 1 and 2 compared with heifers treated with once daily FSH injections. Using a similar approach in beef heifers, Looney et al. [6] compared once daily sc injections of FSH dissolved in saline and a gelatin gel with twice daily im injections of FSH dissolved in saline. Heifers treated with once daily sc FSH injections dissolved in saline and a gelatin gel had more CL at the time of embryo collection compared with heifers treated with twice daily sc FSH injections in saline; however, the mean number of structures recovered per heifer and the mean number of fertilized ova per heifer did not differ between treatments [6].
Other studies have tried to further decrease the number of FSH treatments to a single administration with promising results. Bo et al. [7] using beef cows and Hockley et al. [8] using beef and dairy cows reported that a single sc injection of 400 mg of NIH-FSH-P1 administered behind the shoulder in beef cows resulted in similar superovulatory responses and number of embryos compared with a similar dose of FSH administered twice daily over a period of 4 days. Superovulatory responses and embryo production, however, was less for Holstein cows treated with a single sc injection of FSH compared with Holstein cows treated with twice daily FSH injections [8].
Reported differences between dairy and beef cattle might be related to differences in volume of adipose tissue and hormone absorption. In addition, others have tried with success to superovulate dairy cows with a single im injection of FSH dissolved in polyvinylpyrrolidone [9], to superovulate beef cattle with a single im [10] or slip-single im [11] injection of FSH dissolved in hyaluronan, or a single im injection of FSH dissolved in hydroxide gel [12]. In studies in which the FSH was dissolved in a slow-release formula [9–12], superovulatory responses and embryo production from animals treated with a single injection of FSH were similar to those treated with twice daily FSH injections over a period of 4 days. Similarly, in the present study in which a single im injection of an LArbFSH preparation was used, heifers treated with either B50-LArbFSH or A100-LArbFSH preparations had similar superovulatory responses and embryo production compared with heifers treated with pituitary-derived FSH administered twice daily over a period of 4 days.
The bioactivity of the B-LArbFSH molecule used in the present study was compared with Folltropin in an in vitro study [25]. Smaller doses of rbFSH (10 ng/mL) had similar in vitro bioactivity compared with larger doses of Folltropin (100 μg/mL). This greater activity at smaller doses of B-LArbFSH might explain why cows receiving this rbFSH preparation had similar ovulatory responses and embryo production compared with cows receiving a larger dose of Folltropin. Furthermore, the amino acid sequence of the rbFSH used in the present study is at least 85% homologous to native bovine FSH [25].
The degree of glycosylation of the FSH molecule plays a critical role in both the metabolic clearance rate by the liver and kidney and the in vitro and in vivo bioactivity of the molecule (for a review see [13,26–28]). Heifers treated with B50-LArbFSH had greater superovulatory responses and produced more embryos compared with heifers treated with A50-LArbFSH, but a similar response to heifers treated with A100-LArbFSH (Table 2). We speculate that type B LArbFSH might have a higher level of glycosylation compared with type A LArbFSH. Heifers treated with A100-LArbFSH received two times the FSH dose compared with heifers treated with A50-LArbFSH. As expected, heifers treated with A100-LArbFSH had a greater superstimulatory response and produced more embryos, and these results agree with the results of others [14–16] that compared animals treated with different doses of FSH; however, multiple rbFSH injections were administered over a period of 3 to 6 days in those studies, whereas a single injection was used in the present experiment.
Crowe et al. [14] used a model in which heifers were immunized against GnRH and reported that circulating FSH concentrations and the number of medium (5–9 mm) and large (>10 mm) follicles (8.4 vs. 2.0 and 9.2 vs. 4.3, respectively) increased as the dose of rbFSH increased. Wilson et al. [16] tested several treatment regimens using rbFSH and reported that embryo production increased as the dose of rbFSH administered increased. Interestingly, Bellow et al. [15] reported increased embryo production but similar superstimulatory responses in beef heifers treated with 24 mg rbFSH compared with heifers treated with 18 mg rbFSH. In the present experiment, the mean number of ovulatory follicles and CL for the three treatments (Folltropin, B50-LArbFSH, and A100-LArbFSH) considered able to induce a superstimulatory response ranged from 16.6 to 25.7 and 15.9 to 19.1, respectively (Table 1). Other studies using Holstein heifers treated with pituitary-derived FSH reported an average number of ovulatory follicles from 12.9 to 23.6, and an average number of CL at the time of embryo collection from 10.2 to 15.7 [29,30]. Taken together, results from the present experiment and those from others support the hypotheses that a pure preparation of rbFSH can induce growth of multiple follicles that achieve ovulatory capacity followed by ovulation, fertilization, and production of good-quality embryos.
Several studies reporting inconsistent results have evaluated the effect of various levels of LH during the superstimulation protocol ([31–33] among others). In the present experiment and several other experiments [14–16], a pure FSH preparation without any LH was used. In particular, the study by Crowe et al. [14] in which the level of endogenous LH was further reduced, growth of follicles greater than 10 mm was observed leading us to the question whether LH is necessary during superstimulatory treatments. These results may only apply to heifers receiving a superstimulatory dose of exogenous FSH and should be carefully interpreted because LH plays a critical role during follicle selection and is essential for follicular growth beyond the period of deviation when follicles reach about 8.5 mm in diameter [34,35]. In a recent study from our laboratory (Bender et al., unpublished data, 2012), lactating dairy cows fed ad libitum had poor embryo production when LH was increased during the final stages of follicular growth, whereas cows that were feed restricted had improved embryo production if a slightly greater amount of LH was administered at the end of the superstimulatory treatment.
In the present experiment, the number of transferable embryos recovered per heifer flushed averaged 3.7 for heifers treated with LArbFSH (6.5, 3.1, and 0.3 for B50-LArbFSH, A100-LArbFSH, and A50-LArbFSH, respectively); however, heifers treated with A50-LArbFSH negatively affected the overall results of the study. Other studies that used pituitary-derived FSH to superstimulate heifers reported mean numbers of transferable embryos from 2.3 to 4 [29,30,36]. Fertilization rate was lower in heifers treated with A100-LArbFSH. Souza et al. [29] suggested that increasing the dose of FSH can negatively affect oocyte quality and, or the reproductive tract, thereby reducing fertilization rate. Nonetheless, fertilization rate was approximately 85% in the present study, which is considered acceptable for superovulated animals. The fertilization rate observed in the present study is notable considering the excessively high ambient temperatures and humidity that occurred in Wisconsin during July when the experiment was performed (temperature humidity index = 75; data not shown).
Interestingly, the percentage of transferable embryos (Fig. 2,), percentage of degenerated embryos, and fertilization rate were not affected by the number of ovulations. In agreement with results from the present experiment, two other studies using lactating dairy cows ([37] and Bender et al., unpublished data, 2012) reported no effect of the number of ovulations on the percentage of transferable embryos. Hawk [38], however, reported that fertilization rate decreased as the number of ovulations increased, whereas two studies reported no effect of the number of ovulations on fertilization rate.
The protocol used in the present experiment has been developed for superovulation of cattle using twice daily FSH injections, and no attempt was made to adapt the timing of injections to the LArbFSH. In addition, we used experimental doses of the LArbFSH, and the doses were empirically selected. Several options are now available for researchers and practitioners that allow for reduced handling and fewer injections required for facilitating management of donors for a successful superovulatory response. Holstein heifers were used in the present experiment, and differences between types of animals (heifers vs. mature cows; dairy vs. beef cows; and Bos indicus vs. Bos taurus) should be taken into consideration when interpreting these results.
4.1. Conclusions
A single injection of LArbFSH induced superovulation and produced a similar number of good-quality embryos compared with a standard pituitary-derived FSH preparation. These results will be of interest for both commercial and private embryo transfer programs. More studies are needed to confirm the findings presented herein, to test the response to LArbFSH in other types of animals such as lactating dairy cows, and to determine the optimal dose of LArbFSH that maximizes embryo production in both cows and heifers.
Acknowledgments
The authors thank the Cottonwood Dairy and Wagner Dairy for use of their heifers to conduct this study and Peter Crump for statistical consultation. We also thank CEVA Animal Health for donating the LArbFSH. This study was supported by CEVA Animal Health, Hatch project WIS01171to P.M.F., and a fellowship from the Universidad Francisco de Paula Santander of Colombia to G.M. Baez (COLCIENCIAS-497).
Footnotes
Competing interests
The authors declare that they have no conflicts of interest that would prejudice the impartiality of this scientific work.
References
- 1.Demoustier MM, Beckers JF, Van Der Zwalmen P, Closset J, Gillard JL, Ectors F. Determination of porcine plasma follitropin levels during superovulation treatment in cows. Theriogenology. 1988;30:379–86. doi: 10.1016/0093-691x(88)90185-9. [DOI] [PubMed] [Google Scholar]
- 2.Laster DB. Disappearance and uptake of [125I]FSH in the rat, rabbit, ewe and cow. J Reprod Fertil. 1972;30:407–15. doi: 10.1530/jrf.0.0300407. [DOI] [PubMed] [Google Scholar]
- 3.Bó GA, Guerrero DC, Adams GP. Alternative approaches to setting up donor cows for superstimulation. Theriogenology. 2008;69:81–7. doi: 10.1016/j.theriogenology.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Looney CR, Pryor JH. Novel bovine embryo transfer technologies in the United States. Anim Reprod. 2012;9:404–13. [Google Scholar]
- 5.Walsh JH, Mantovani R, Duby RT, Overstrom EW, Dobrinsky JR, Enright WJ, et al. The effects of once or twice daily injections of pFSH on superovulatory response in heifers. Theriogenology. 1993;40:313–21. doi: 10.1016/0093-691x(93)90269-b. [DOI] [PubMed] [Google Scholar]
- 6.Looney CR, Boutte BW, Archbald LF, Godke RA. Comparison of once daily and twice daily FSH injections for superovulating beef cattle. Theriogenology. 1981;15:13–22. doi: 10.1016/s0093-691x(81)80014-3. [DOI] [PubMed] [Google Scholar]
- 7.Bo GA, Hockley DK, Nasser LF, Mapletoft RJ. Superovulatory response to a single subcutaneous injection of Folltropin-V in beef cattle. Theriogenology. 1994;42:963–75. doi: 10.1016/0093-691x(94)90119-4. [DOI] [PubMed] [Google Scholar]
- 8.Hockley DK, Bo GA, Palasz AT, Del Campo MR, Mapletoft RJ. Superovulation with a single subcutaneous injection of Folltropin in the cow: effect of dose and site of injection. Theriogenology. 1992;37:224. [Google Scholar]
- 9.Yamamoto M, Ooe M, Kawaguchi M, Suzuki T. Superovulation in the cow with a single intramuscular injection of FSH dissolved in polyvinylpyrrolidone. Theriogenology. 1994;41:747–55. doi: 10.1016/0093-691x(94)90184-k. [DOI] [PubMed] [Google Scholar]
- 10.Tríbulo A, Rogan D, Tribulo H, Tribulo R, Alasino RV, Beltramo D, et al. Superstimulation of ovarian follicular development in beef cattle with a single intramuscular injection of Folltropin-V. Anim Reprod Sci. 2011;129:7–13. doi: 10.1016/j.anireprosci.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Tríbulo A, Rogan D, Tríbulo H, Tríbulo R, Mapletoft RJ, Bó GA. Superovulation of beef cattle with a split-single intramuscular administration of Folltropin-V in two concentrations of hyaluronan. Theriogenology. 2012;77:1679–85. doi: 10.1016/j.theriogenology.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Kimura K, Hirako M, Iwata H, Aoki M, Kawaguchi M, Seki M. Successful superovulation of cattle by a single administration of FSH in aluminum hydroxide gel. Theriogenology. 2007;68:633–9. doi: 10.1016/j.theriogenology.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Hesser MW, Morris JC, Gibbons JR. Advances in recombinant gonadotropin production for use in bovine superovulation. Reprod Domest Anim. 2011;46:933–42. doi: 10.1111/j.1439-0531.2011.01767.x. [DOI] [PubMed] [Google Scholar]
- 14.Crowe MA, Enright WJ, Boland MP, Roche JF. Follicular growth and serum follicle-stimulating hormone (FSH) responses to recombinant bovine FSH in GnRH-immunized anoestrous heifers. Anim Sci (Penicuik, Scotland) 2001;73:115–22. [Google Scholar]
- 15.Bellow RA, Staigmiller RB, Wilson JM, Phelps DA, Darling A. Use of bovine FSH for superovulation and embryo production in beef heifers. Theriogenology. 1991;35:1069–82. [Google Scholar]
- 16.Wilson JM, Jones AL, Moore K, Looney CR, Bondioli KR. Superovulation of cattle with a recombinant-DNA bovine follicle stimulating hormone. Anim Reprod Sci. 1993;33:71–82. [Google Scholar]
- 17.Remy B, Baril G, Vallet JC, Dufour R, Chouvet C, Saumande J, et al. Are antibodies responsible for a decreased superovulatory response in goats which have been treated repeatedly with porcine follicle-stimulating hormone ? Theriogenology. 1991;36:389–99. doi: 10.1016/0093-691x(91)90467-r. [DOI] [PubMed] [Google Scholar]
- 18.Viudes De Castro MP, Cortell C, Mocé E, Marco-Jiménez F, Joly T, Vicente JS. Effect of recombinant gonadotropins on embryo quality in superovulated rabbit does and immune response after repeated treatments. Theriogenology. 2009;72:655–62. doi: 10.1016/j.theriogenology.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Magarey G, Rodger J, Buist J, Mate K. Effects of repeated superovulation and surgical oocyte collection on ovarian response and natural breeding ability of the tammar wallaby (Macropus eugenii) Reproduction. 2003;125:701–7. doi: 10.1530/rep.0.1250701. [DOI] [PubMed] [Google Scholar]
- 20.Baruselli PS, Ferreira RM, Sales JNS, Gimenes LU, Sá Filho MF, Martins CM, et al. Timed embryo transfer programs for management of donor and recipient cattle. Theriogenology. 2011;76:1583–93. doi: 10.1016/j.theriogenology.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 21.NRC. Nutrient requirements of dairy cattle Seventh rev ed. Washington, D.C: National Academy Press; 2001. [Google Scholar]
- 22.Bergfelt DR, Lightfoot KC, Adams GP. Ovarian synchronization following ultrasound-guided transvaginal follicle ablation in heifer. Theriogenology. 1994;42:895–907. doi: 10.1016/0093-691x(94)90113-w. [DOI] [PubMed] [Google Scholar]
- 23.Stringfellow DA, Givens MD. Manual of the International Embryo TransferSociety: a procedural guide and general information for the use of embryo transfer technology emphasizing sanitary precautions. Fourth. Champaign, IL: International Embryo Transfer Society; 2010. [Google Scholar]
- 24.Institute SAS. SAS user’s guide: statistics. Cary NC: SAS Ins Inc.; 2011. [Google Scholar]
- 25.Colgin MA, Donnelly RG, Stroud B. Methods for inducing superovulation in ungulates. Google Patents. 2013 [Google Scholar]
- 26.Rose MP, Gaines Das RE, Balen AH. Definition and measurement of follicle stimulating hormone. Endocr Rev. 2000;21:5–22. doi: 10.1210/edrv.21.1.0388. [DOI] [PubMed] [Google Scholar]
- 27.Ulloa-Aguirre A, Timossi C, Damián-Matsumura P, Dias J. Role of glycosylation in function of follicle-stimulating hormone. Endocrine. 1999;11:205–15. doi: 10.1385/ENDO:11:3:205. [DOI] [PubMed] [Google Scholar]
- 28.Perlman S, van den Hazel B, Christiansen J, Gram-Nielsen S, Jeppesen CB, Andersen KV, et al. Glycosylation of an N-terminal extension prolongs the half-life and increases the in vivo activity of follicle stimulating hormone. J Clin Endocrinol Metabol. 2003;88:3227–35. doi: 10.1210/jc.2002-021201. [DOI] [PubMed] [Google Scholar]
- 29.Souza AH, Sartori R, Guenther JN, Caraviello D, Monson R, Wiltbank MC. Effect of semen source and dose of FSH on superovulatory response and embryo production in Holstein heifers. Anim Reprod. 2007;4:70–6. [Google Scholar]
- 30.Sartori R, Suarez-Fernandez CA, Monson RL, Guenther JN, Rosa GJM, Wiltbank MC. Improvement in recovery of embryos/ova using a shallow uterine horn flushing technique in superovulated Holstein heifers. Theriogenology. 2003;60:1319–30. doi: 10.1016/s0093-691x(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 31.Donaldson LE, Ward DN. Effects of luteinizing-hormone on embryo production in superovulated cows. Vet Rec. 1986;119:625–6. [PubMed] [Google Scholar]
- 32.Chupin D, Combarnous Y, Procureur R. Antagonistic effect of LH on FSH-induced superovulation in cattle. Theriogenology. 1984;21:229. [Google Scholar]
- 33.Herrler A, Elsaesser F, Parvizi N, Niemann H. Superovulation of dairy-cows with purified FSH supplemented with defined amounts of LH. Theriogenology. 1991;35:633–43. doi: 10.1016/0093-691x(91)90459-q. [DOI] [PubMed] [Google Scholar]
- 34.Ginther OJ, Bergfelt DR, Beg MA, Kot K. Follicle selection in cattle: role of luteinizing hormone. Biol Reprod. 2001;64:197–205. doi: 10.1095/biolreprod64.1.197. [DOI] [PubMed] [Google Scholar]
- 35.Haughian JM, Ginther OJ, Diaz FJ, Wiltbank MC. GnRH, estradiol, and inhibin regulation of FSH and LH surges: implications for follicle emergence and selection in heifers. Biol Reprod. 2013;88:1–10. doi: 10.1095/biolreprod.112.107342. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez A, Lussier JG, Carruthers TD, Murphy BD, Mapletoft RJ. Superovulation of beef heifers with folltropinda new FSH preparation containing reduced LH activity. Theriogenology. 1990;33:519–29. doi: 10.1016/0093-691x(90)90509-r. [DOI] [PubMed] [Google Scholar]
- 37.Carvalho PD, Souza AH, Sartori R, Hackbart KS, Dresch AR, Vieira LM, et al. Effects of deep-horn AI on fertilization and embryo production in superovulated cows and heifers. Theriogenology. 2013;80:1074–81. doi: 10.1016/j.theriogenology.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawk HW. Gamete transport in the superovulated cow. Theriogenology. 1988;29:125–42. [Google Scholar]


