Abstract
Background
Higher androgen and lower estrogen levels are associated with cardiovascular disease (CVD) risk factors in women. However, studies on sex hormones and incident CVD events in women have yielded conflicting results.
Objectives
We assessed the associations of sex hormone levels with incident CVD, coronary heart disease (CHD), and heart failure (HF) events among women without CVD at baseline.
Methods
We studied 2,834 post-menopausal women participating in MESA with testosterone, estradiol, dehydroepiandrosterone, and sex hormone binding globulin (SHBG) levels measured at baseline (2000–2002). We used Cox hazard models to evaluate associations of sex hormones with each outcome, adjusting for demographics, CVD risk factors, and hormone therapy use.
Results
The mean (SD) age was 64.9 (8.9) years. During 12.1 years of follow-up, 283 CVD, 171 CHD, and 103 HF incident events occurred. In multivariable-adjusted models, the Hazard Ratios (95% CI) associated with 1 SD greater log-transformed sex hormone level for the respective outcomes of CVD, CHD, and HF were as follows: Total testosterone: 1.14 (1.01–1.29), 1.20 (1.03–1.40), 1.09 (0.90–1.34); Estradiol: 0.94 (0.80–1.11), 0.77 (0.63–0.95), 0.78 (0.60–1.02); Testosterone/Estradiol ratio: 1.19 (1.02–1.40), 1.45 (1.19–1.78), 1.31 (1.01–1.70). Dehydroepiandrosterone and SHBG levels were not associated with these outcomes.
Conclusions
Among post-menopausal women, a higher testosterone/estradiol ratio was associated with an elevated risk for incident CVD, CHD, and HF events, higher levels of testosterone associated with increased CVD and CHD, while higher estradiol levels were associated with a lower CHD risk. Sex hormones levels after menopause are associated with women’s increased CVD risk later in life.
Condensed abstract
We studied 2,834 post-menopausal women in the Multi-Ethnic Study of Atherosclerosis. Higher total testosterone/estradiol ratio was independently associated with an elevated risk for incident CVD, CHD, and HF events. After >12 years of follow-up, the multivariable-adjusted Hazard Ratios (95% CI) associated with 1 SD greater log-transformed total testosterone/estradiol ratio for incident CVD, CHD and HF events were 1.19 (1.02–1.40), 1.45 (1.19–1.78), 1.31 (1.01–1.70), respectively. Sex hormones levels after menopause are associated with women’s increased CVD risk later in life.
Keywords: sex hormones, estradiol, testosterone, sex hormone binding globulin, cardiovascular disease, coronary heart disease, heart failure
Introduction
Atherosclerotic cardiovascular disease (CVD), which includes coronary heart disease (CHD) and ischemic stroke, affects men and women differently. In women, the risk of CVD is much lower than in men until 50 years of age, but it rises dramatically after menopause (1). It has been hypothesized that lower levels of endogenous estrogens and higher endogenous androgens that occur as a consequence of the menopausal transition might mediate the increased CVD risk later in life in post-menopausal women (1,2). This theory has been supported by observational studies that demonstrate associations between higher androgen and lower estrogen levels with CVD risk factors in post-menopausal women, including blood pressure, C-reactive protein (CRP), and insulin resistance (1,3–5).
Studies examining the associations between sex hormone levels and CVD events in postmenopausal women, however, have yielded conflicting results. Both high (6,7) and low androgen levels (8,9) have been associated with increased risk of CVD. With respect to estrogens, observational studies have demonstrated that estradiol levels in women were either not associated (7,10) or inversely associated with CVD events (6). As a result, the relationship of sex hormones with CVD events in post-menopausal women is still unclear.
Studies of the association between sex hormone levels and clinical CVD events in postmenopausal women, however, have been limited by suboptimal measurement of CVD events (10), use of hospital-based study populations (9,11), small sample size (11), or short duration of follow-up (7). Therefore, we used data from the Multi-Ethnic Study of Atherosclerosis (MESA) to evaluate the association of sex hormone levels with incident CVD, CHD, and heart failure (HF) events over 12 year follow-up in a community-based population of post-menopausal women free of CVD at baseline.
Methods
Study participants
MESA is a prospective cohort study of 6,814 men and women of 4 race/ethnicities (white, black, Hispanic and Chinese) who were aged 45–84 years and free of CVD at the baseline visit (2000–2002) (12). Participants were recruited from six centers across the United States (Los Angeles, California; St. Paul, Minnesota; New York City, New York; Chicago, Illinois; Forsyth County, North Carolina; Baltimore, Maryland). The present analysis included all post-menopausal women who attended the baseline exam (N=3,087). We then excluded 160 participants missing sex hormone measurements, 2 participants without information on CVD outcomes, and 91 participants missing other covariates. The final sample size was 2,834 postmenopausal women (Figure 1). The study was approved by the institutional review boards of all participating institutions, and written informed consent was obtained from all participants.
Figure 1. Flow chart of study participants.
The present analysis included all post-menopausal women who attended the baseline exam (N=3,087). After exclusions shown in Figure, the final sample size was 2,834 women.
Measurement of sex hormones
Sex hormone concentrations were measured from fasting serum samples that were drawn at the baseline exam between 7:30 AM and 10:30 AM and stored at −70°C until analysis. Biochemical analyses were performed at the University of Massachusetts Medical Center Sex Hormone Laboratory (Worcester, MA). Total testosterone and dehydroepiandrosterone (DHEA) were measured using radioimmunoassay kits. Sex hormone binding globulin (SHBG) was measured using a chemiluminescent enzyme immunometric assay (Immulite kits, Diagnostic Products Corporation, Los Angeles, CA). Estradiol was measured using an ultra-sensitive radioimmunoassay kit (Diagnostic System Laboratories, Webster, TX). Concentrations of bioavailable testosterone (the sum of SHBG-bound and albumin-bound testosterone) and free testosterone (reported as a percent of total testosterone) were calculated according to the method of Södergård (13). We divided total testosterone by estradiol to calculate total testosterone/estradiol ratio for each participant. Quality control serum was obtained from a ~10% blind pool. The coefficients of variation for total testosterone, SHBG, DHEA and estradiol were 12.3, 9.0, 11.2 and 10.5%, respectively (4).
CVD, CHD and HF assessment
Every 9–12 months, participants completed a telephone interview about interim hospital admissions, cardiovascular outpatient diagnoses and procedures, and deaths (12). Hospital records were obtained for 98% of reported hospitalized CVD events, and some medical record-based information was obtained for 95% of outpatient encounters. Two physicians independently reviewed and adjudicated events and assigned incidence dates. Outcome events were identified through December 31, 2013.
Incident CHD was defined as definite or probable myocardial infarction, resuscitated cardiac arrest, definite or probable angina (if followed by revascularization), and definite CHD death. Hard CHD events excluded definite or probable angina. Incident CVD events included incident CHD plus stroke, stroke death, other atherosclerotic death, and other CVD deaths. Hard CVD events included hard CHD, stroke, and stroke death. Ischemic stroke was determined by brain imaging with computed tomography or magnetic resonance imaging and included large vessel atherosclerosis, cardioembolism, small-vessel occlusion, acute ischemic stroke of other unknown cause.
Incident HF was defined as definite or probable HF and required symptoms such as shortness of breath or edema. In addition to symptoms, probable HF required a HF diagnosis by a physician and medical treatment for HF. Definite HF required 1 or more criteria, such as pulmonary edema or congestion by chest X-ray, ventricular dilation or poor left ventricular (LV) function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction. HF was classified as either HF with preserved ejection fraction (HFpEF, LVEF ≥45%) or with reduced EF (HFrEF, LVEF <45%) (14).
Measurement of other covariates
Demographic characteristics, lifestyle factors, and medication use including hormone therapy (HT) were collected through standardized questionnaires. Menopausal status was self-reported and corrected using information on age, self-reported hysterectomy, bilateral oophorectomy, and menopausal age. Only post-menopausal women were included in this analysis.
Level of education was classified as <high school, high school/technical school/associate degree, and college/graduate/professional school. Physical activity was estimated as the total amount of intentional moderate or vigorous exercise performed in a usual week, and measured in metabolic equivalent task–minutes. Smoking status was categorized into never, former, or current smoker.
Height, weight, waist circumference, hip circumference, and blood pressure were measured by trained staff. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist-hip ratio (WHR) was calculated as waist circumference/hip circumference. Seated resting blood pressure was measured three times using a Dinamap automated oscillometric sphygmomanometer, and the average of the 2nd and 3rd measurements was used.
Blood samples were obtained from participants at the baseline exam after a 12-hour fast. Details of measurements of clinical chemistry parameters are provided elsewhere (15). Diabetes was defined as a fasting serum glucose ≥126 mg/dl, a self-reported history of a physician-diagnosis of diabetes, or use of insulin or diabetes medications. Estimated glomerular filtration rate (eGFR) was calculated based on serum creatinine and cystatin C concentrations using the CKD-EPI equation (16). Inflammatory markers including CRP, interleukin-6 (IL-6), fibrinogen, and D-dimer were measured from stored samples, as previously described (17).
Statistical analysis
Normally distributed continuous variables were expressed as mean (standard deviation (SD)) and compared by CVD status using Student t test or ANOVA. Variables with skewed distributions were expressed as median (interquartile range (IQR)) and compared using the non-parametric Wilcoxon rank sum test. Categorical variables were expressed as percentage (%) and compared using χ2 test.
The associations between CVD risk factors and sex hormone levels were evaluated using multivariable-adjusted linear regression with loge-transformed sex hormone levels as dependent variables. The study endpoints were the development of incident CVD, CHD or HF. Participants were followed from the baseline visit until the development of a study endpoint, death, drop-out, or until December 31, 2013. Cox proportional hazards regression was used to estimate multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CI) for each study endpoint associated with a 1 SD greater loge-transformed sex hormone levels. We also evaluated the presence of non-linear dose-response relationships using restricted cubic splines with knots at the 5th, 35th, 65th and 95th percentiles of their sample distributions.
We used three models with increasing degrees of adjustment. Model 1 adjusted for demographic factors: age, race/ethnicity, study site, and HT use. Model 2 further adjusted for lifestyle variables: education, WHR, physical activity, and smoking. Model 3 further adjusted for intermediate CVD risk factors: systolic blood pressure, use of antihypertensive medications, total cholesterol, HDL-cholesterol, use of lipid lowering medication, diabetes, eGFR, CRP, IL-6, fibrinogen, and D-dimer. In the primary analyses, the associations between sex hormones and each of the CVD endpoints were modeled separately for each individual hormone. In secondary analyses, we included total testosterone, estradiol, DHEA, and SHBG in the fully-adjusted model to mutually account for one another.
We performed stratified analyses in pre-specified subgroups defined by age and race/ethnicity. We also performed sensitivity analyses excluding women who were using HT at baseline (because estradiol levels differ substantially among women taking vs. not taking HT), and using hard CVD and hard CHD events as endpoints instead of all CVD and CHD events. Additionally, we conducted analysis using non-CVD death as a competing risk. All reported p-values were two-sided, and the significance level was set at 0.05. Statistical analyses were performed using STATA version 14 (StataCorp LP, College Station, Texas).
Results
The baseline characteristics of the study population are presented in Table 1. The average age at baseline was 64.9 years (SD 8.9, IQR 58–72). The median (IQR) of sex hormone levels in nmol/L were: total testosterone 0.90 (0.59–1.32), estradiol 0.07 (0.05–0.16), DHEA 10.20 (6.97–14.50), and SHBG 59.50 (40.60–94.60), and for testosterone/estradiol ratio was 11.82 (4.88–22.21). The demographic-adjusted associations of CVD risk factors (independent variables) with sex hormone levels (dependent variables) at the baseline exam are presented in Online Table 1. Current smoking was associated with higher total testosterone, testosterone/estradiol ratio, and DHEA levels, and lower estradiol levels. Obesity and CRP were associated with higher total testosterone and estradiol, and with lower testosterone/estradiol ratio and SHBG levels.
Table 1.
Characteristics of post-menopausal women at the MESA baseline exam (2000–2002) followed for incident CVD through 2013.*,†,‡
| Incident CVD status | Overall (n = 2,834) |
No CVD (n = 2,551) |
CVD (n = 283) |
p-value |
|---|---|---|---|---|
| Age, years | 64.9 (8.9) | 64.4 (8.9) | 69.3 (8.6) | <0.001 |
| Total T, nmol/L‡ | 0.90 (0.59–1.32) | 0.90 (0.59–1.32) | 0.94 (0.59–1.39) | 0.06 |
| Bioavailable T, nmol/L‡ | 0.21 (0.10–0.35) | 0.21 (0.10–0.35) | 0.24 (0.14–0.38) | 0.04 |
| Estradiol, nmol/L‡ | 0.073 (0.048–0.16) | 0.073 (0.048–0.17) | 0.066 (0.044–0.11) | 0.05 |
| Total T/estradiol ratio | 11.82 (4.88–22.21) | 11.55 (4.79–22.06) | 15.42 (6.65–25.66) | 0.001 |
| DHEA, nmol/L‡ | 10.20 (6.97–14.50) | 10.31 (6.97–14.71) | 9.30 (6.25–13.05) | 0.003 |
| Free T, %‡ | 1.29 (0.89–1.70) | 1.28 (0.88–1.70) | 1.31 (0.90–1.70) | 0.45 |
| SHBG, nmol/L‡ | 59.50 (40.60–94.60) | 59.85 (40.70–95.50) | 58.10 (40.40–89.40) | 0.38 |
| Race/ethnicity | 0.16 | |||
| White | 1,087 (38.4) | 962 (37.7) | 125 (44.2) | |
| Chinese-American | 344 (12.1) | 317 (12.4) | 27 (9.5) | |
| Black | 781 (27.6) | 708 (27.8) | 73 (25.8) | |
| Hispanic | 622 (21.9) | 564 (22.1) | 58 (20.5) | |
| Education | <0.001 | |||
| <High school | 619 (21.9) | 541 (21.2) | 78 (27.6) | |
| High school, technical school, or associate degree | 1,428 (50.4) | 1,274 (50.0) | 154 (54.4) | |
| College, graduate or professional school | 785 (27.7) | 734 (28.8) | 51 (18.0) | |
| Smoking | 0.14 | |||
| Never | 1,673 (59.1) | 1,516 (59.5) | 157 (55.5) | |
| Former | 853 (30.1) | 767 (30.1) | 86 (30.4) | |
| Current | 306 (10.8) | 266 (10.4) | 40 (14.1) | |
| Total intentional exercise, | 0.004 | |||
| met-min/week‡ | 3540 (1755–6510) | 3630 (1803–6570) | 2925 (1305–6030) | |
| Waist to hip ratio | 0.91 (0.1) | 0.91 (0.1) | 0.94 (0.1) | <0.001 |
| BMI, kg/m2 | 28.6 (6.0) | 28.5 (6.0) | 29.6 (6.2) | 0.002 |
| Systolic BP, mm Hg | 129.5 (23.5) | 128.4 (23.2) | 139.7 (23.7) | <0.001 |
| Diastolic BP, mm Hg | 69.1 (10.3) | 68.9 (10.2) | 70.3 (11.1) | 0.03 |
| Total cholesterol, mg/dl§ | 201.5 (35.7) | 201.7 (35.6) | 199.5 (36.6) | 0.32 |
| HDL cholesterol, mg/dl§ | 56.7 (15.4) | 57.0 (15.5) | 54.0 (13.7) | 0.002 |
| LDL cholesterol, mg/dl§ | 118.4 (32.1) | 118.4 (32.2) | 118.5 (31.8) | 0.99 |
| Triglycerides, mg/dl | 131.7 (79.0) | 131.5 (80.2) | 133.3 (68.1) | 0.72 |
| eGFR, mL/min/1.73 m2 | 75.3 (15.7) | 75.8 (15.6) | 71.5 (16.8) | <0.001 |
| CRP, mg/l‡ | 2.6 (1.1–5.6) | 2.5 (1.1–5.5) | 3.2 (1.2–7.2) | 0.02 |
| D-Dimer, ug/ml‡ | 0.2 (0.1–0.4) | 0.2 (0.1–0.4) | 0.3 (0.2–0.6) | <0.001 |
| Fibrinogen, mg/dl‡ | 356 (311–4050) | 354 (309–403) | 371 (326–432) | <0.001 |
| IL6, pg/ml‡ | 1.3 (0.8–2.0) | 1.3 (0.8–1.9) | 1.6 (1.0–2.5) | <0.001 |
| Hormone therapy | 900 (32.5) | 829 (33.2) | 71 (25.7) | 0.01 |
| Hypertension medication | 1,179 (41.6) | 1,010 (39.6) | 169 (59.7) | <0.001 |
| Lipid lowering medication | 532 (18.8) | 463 (18.2) | 69 (24.5) | 0.01 |
| Diabetes | 349 (12.3) | 285 (11.2) | 64 (22.6) | <0.001 |
Abbreviations: MESA, Multi-Ethnic Study of Atherosclerosis; CVD, Cardiovascular Disease; T, Testosterone; DHEA, Dehydroepiandrosterone; SHBG, Sex Hormone Binding Globulin; BMI, body mass index; BP, blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; IL-6, interleukin-6
Data are mean (SD) or number (percentage) or
median (IQR)
To convert Total, HDL, and LDL cholesterol from mg/dl to mmol/L, divide by 38.67. To convert total testosterone, free testosterone, bioavailable testosterone DHEA from nmol/L to ng/dL, divide by 0.0347. To convert estradiol from nmol/L to ng/mL, divide by 3.67. To convert SHBG from nmol/L to mg/L, divide by 10.5263.
During 30,487 person-years of follow-up (median [IQR] follow-up of 12.1 [11.5, 12.7] years), the numbers of women who developed incident CVD, CHD and HF events were 283, 171 and 103, respectively (incidence rates of 9.3, 5.5 and 3.3 per 1,000 person-years, respectively). At baseline, women who developed incident CVD were more likely to be older, diabetic, hypertensive, have higher WHR, CRP, D-dimer, fibrinogen, IL-6, bioavailable testosterone levels, and testosterone/estradiol ratio, and have lower education, DHEA and HDL-cholesterol levels, eGFR, and physical activity (Table 1).
A higher total testosterone/estradiol ratio was independently associated with an increased risk of incident CVD, CHD, and HF (Table 2). The fully-adjusted HRs for CVD, CHD and HF associated with 1 SD greater log-transformed testosterone/estradiol ratio was 1.19 (95% CI 1.02–1.40), 1.45 (1.19–1.78), and 1.31 (1.01–1.70), respectively (Model 3). Total testosterone levels were also associated with an increased risk of incident CVD and CHD, but were not statistically significantly associated with HF. The corresponding HRs associated with 1 SD increase in log-transformed total testosterone were 1.14 (1.01–1.29), 1.20 (1.03–1.40) and 1.09 (0.90–1.34), respectively. Bioavailable testosterone was associated with CVD and CHD in demographic-adjusted models, but the associations were attenuated to null in fully-adjusted models. The corresponding HRs per 1 SD greater testosterone/estradiol ratio for the other endpoints were directionally consistent with the primary analyses as follows: hard CVD 1.29 (1.07–1.56), hard CHD 1.57 (1.22–2.02), and ischemic stroke 1.10 (0.81–1.48); Online Table 2 (Model 3).
Table 2.
Hazard Ratios (95% CI) for all CVD, CHD and HF associated with sex hormone levels in post-menopausal women: MESA 2000–2013*,†
| All CVD | |||
|---|---|---|---|
| (N events/N total= 283/2,834; IR = 9.3 per 1000 person-years) | |||
|
| |||
| Model 1‡ | Model 2§ | Model 3ǁ | |
| Total T (nmol/L) | 1.17 (1.04, 1.32) | 1.15 (1.02, 1.29) | 1.14 (1.01, 1.29) |
| Bioavailable T (nmol/L) | 1.17 (1.03, 1.33) | 1.10 (0.97, 1.25) | 1.07 (0.94, 1.22) |
| Free T (Percent) | 1.11 (0.96, 1.28) | 0.99 (0.85, 1.16) | 0.92 (0.78, 1.09) |
| Estradiol (nmol/L) | 1.03 (0.88, 1.21) | 0.99 (0.84, 1.16) | 0.94 (0.80, 1.11) |
| Total T/estradiol ratio | 1.13 (0.97, 1.31) | 1.15 (0.99, 1.34) | 1.19 (1.02, 1.40) |
| DHEA (nmol/L) | 0.96 (0.85, 1.09) | 0.93 (0.82, 1.04) | 0.91 (0.81, 1.03) |
| SHBG (nmol/L) | 0.89 (0.77, 1.03) | 0.99 (0.85, 1.15) | 1.07 (0.91, 1.25) |
|
| |||
| All CHD | |||
| (N events/N total= 171/2,834; IR = 5.5 per 1000 person-years) | |||
| Model 1‡ | Model 2§ | Model 3ǁ | |
| Total T (nmol/L) | 1.21 (1.04, 1.41) | 1.19 (1.02, 1.38) | 1.20 (1.03, 1.40) |
| Bioavailable T (nmol/L) | 1.18 (1.01, 1.39) | 1.10 (0.93, 1.30) | 1.08 (0.92, 1.28) |
| Free T (Percent) | 1.07 (0.89, 1.29) | 0.94 (0.77, 1.14) | 0.85 (0.69, 1.05) |
| Estradiol (nmol/L) | 0.90 (0.74, 1.10) | 0.84 (0.69, 1.03) | 0.77 (0.63, 0.95) |
| Total T/estradiol ratio | 1.28 (1.06, 1.55) | 1.33 (1.09, 1.61) | 1.45 (1.19, 1.78) |
| DHEA (nmol/L) | 0.95 (0.81, 1.11) | 0.91 (0.78, 1.06) | 0.92 (0.78, 1.07) |
| SHBG (nmol/L) | 0.93 (0.77, 1.11) | 1.06 (0.87, 1.28) | 1.17 (0.95, 1.44) |
|
| |||
| All HF | |||
| (N events/N total= 103/2,834; IR = 3.3 per 1000 person-years) | |||
| Model 1‡ | Model 2§ | Model 3ǁ | |
| Total T (nmol/L) | 1.12 (0.92, 1.36) | 1.09 (0.90, 1.33) | 1.09 (0.90, 1.34) |
| Bioavailable T (nmol/L) | 1.22 (0.99, 1.50) | 1.14 (0.92, 1.41) | 1.13 (0.90, 1.41) |
| Free T (Percent) | 1.10 (0.86, 1.40) | 0.98 (0.76, 1.27) | 0.91 (0.69, 1.20) |
| Estradiol (nmol/L) | 0.90 (0.70, 1.17) | 0.87 (0.67, 1.13) | 0.78 (0.60, 1.02) |
| Total T/estradiol ratio | 1.20 (0.93, 1.54) | 1.21 (0.93, 1.55) | 1.31 (1.01, 1.70) |
| DHEA (nmol/L) | 0.86 (0.71, 1.05) | 0.84 (0.69, 1.02) | 0.85 (0.70, 1.04) |
| SHBG (nmol/L) | 0.91 (0.71, 1.15) | 1.00 (0.78, 1.29) | 1.09 (0.83, 1.43) |
|
| |||
| HFpEF | |||
| (N events/N total= 55/2,821; IR = 1.8 per 1000 person-years) | |||
| Model 1‡ | Model 2§ | Model 3ǁ | |
| Total T (nmol/L) | 1.25 (0.96, 1.64) | 1.21 (0.92, 1.58) | 1.22 (0.91, 1.62) |
| Bioavailable T (nmol/L) | 1.21 (0.91, 1.61) | 1.08 (0.80, 1.46) | 1.10 (0.80, 1.51) |
| Free T (Percent) | 1.07 (0.76, 1.49) | 0.88 (0.62, 1.26) | 0.91 (0.62, 1.33) |
| Estradiol (nmol/L) | 1.14 (0.79, 1.66) | 1.10 (0.75, 1.62) | 0.98 (0.67, 1.43) |
| Total T/estradiol ratio | 1.13 (0.80, 1.60) | 1.13 (0.79, 1.61) | 1.22 (0.85, 1.75) |
| DHEA (nmol/L) | 1.18 (0.89, 1.58) | 1.15 (0.86, 1.52) | 1.21 (0.90, 1.63) |
| SHBG (nmol/L) | 0.94 (0.67, 1.30) | 1.12 (0.79, 1.60) | 1.10 (0.75, 1.60) |
|
| |||
| HFrEF | |||
| (N events/N total= 35/2,821; IR = 1.1 per 1000 person-years) | |||
| Model 1‡ | Model 2§ | Model 3ǁ | |
| Total T (nmol/L) | 1.10 (0.79, 1.55) | 1.09 (0.78, 1.53) | 1.11 (0.80, 1.56) |
| Bioavailable T (nmol/L) | 1.24 (0.86, 1.77) | 1.20 (0.83, 1.73) | 1.19 (0.82, 1.73) |
| Free T (Percent) | 1.11 (0.75, 1.67) | 1.07 (0.70, 1.64) | 1.01 (0.64, 1.59) |
| Estradiol (nmol/L) | 0.65 (0.44, 0.97) | 0.64 (0.43, 0.97) | 0.60 (0.39, 0.93) |
| Total T/estradiol ratio | 1.54 (1.02, 2.32) | 1.52 (1.00, 2.31) | 1.65 (1.07, 2.54) |
| DHEA (nmol/L) | 0.63 (0.48, 0.81) | 0.59 (0.45, 0.77) | 0.59 (0.44, 0.78) |
| SHBG (nmol/L) | 0.90 (0.61, 1.35) | 0.94 (0.62, 1.44) | 1.00 (0.64, 1.58) |
Abbreviations: CVD, cardiovascular disease; CHD, coronary heart disease; HF, heart failure; IR, Incidence Rates; T, Testosterone; DHEA, Dehydroepiandrosterone; SHBG, Sex Hormone Binding Globulin
All sex-hormones are log-transformed and standardized. Hazard Ratios are associated with 1 SD increase in log sex hormones. Bolded results are statistically significant.
Model 1: adjusts for age (year, continuous), race/ethnicity (white, black, Hispanic, and Chinese), study site (CA, MN, NY, IL, NC, and MD), and use of hormone therapy (yes, no).
Model 2: model 1 + education (<high school, high school, >high school), waist to hip ratio (continuous), physical activity, and smoking (never, former, current).
Model 3: model 2 + systolic blood pressure (mmHg, continuous), use of antihypertensive medications (yes, no), total cholesterol (mg/dl, continuous), HDL-cholesterol (mg/dl, continuous), use of lipid lowering medications (yes, no), diabetes (yes, no), eGFR (mL/min/1.73 m2), CRP (mg/l, log-transformed continuous), IL6 (pg/ml, log-transformed, continuous), fibrinogen (mg/dl, log-transformed continuous), D-dimer (ug/ml, log-transformed continuous).
Estradiol levels were not associated with overall CVD events, but were associated with a lower risk for CHD, with a non-significant trend for lower HF risk. The fully-adjusted HRs for CVD, CHD and HF associated with 1 SD greater log-transformed estradiol were 0.94 (0.80–1.11), 0.77 (0.63–0.95) and 0.78 (0.60, 1.02), respectively; Table 2 (Model 3). The corresponding HRs per 1 SD greater estradiol for other endpoints were as follows: hard CVD 0.88 (0.72–1.06), hard CHD 0.69 (0.53–0.88), and ischemic stroke 1.11 (0.80–1.55); Online Table 2 (Model 3).
DHEA and SHBG levels were not associated with CVD, CHD, all HF, hard CVD, hard CHD, or ischemic stroke in primary analyses.
Regarding the HF subtypes, testosterone/estradiol ratio was positively associated, and both estradiol and DHEA inversely associated, with HFrEF but not HFpEF (Table 2). The corresponding HRs for HFrEF per 1 SD greater sex hormone level were: testosterone/estradiol 1.65 (1.07–2.54), estradiol 0.60 (0.39, 0.93), and DHEA 0.59 (0.44–0.78).
In sensitivity analyses excluding women taking HT (Table 3), testosterone/estradiol ratio remained positively associated with CHD and HF, while estradiol was inversely associated with CHD, similar to the primary analyses. However, in this sensitivity analysis, free testosterone was now inversely associated with CHD [0.71 (0.54–0.93)] and SHBG levels were positively associated with CHD [1.39 (1.07–1.82)]. When all sex hormones were included in the same model, the associations remained similar (Online Table 3 for all women and Online Table 4 for women not on HT). Additional competing risk analyses yielded similar results to primary analysis (Online Table 5).
Table 3.
Hazard Ratios (95% CI) for all CVD, CHD and HF excluding women taking hormone therapy (n = 1,934)*,†,‡
| CVD | CHD | HF | |
|---|---|---|---|
| 212/1,934, IR = 10.3 | 127/1,934, IR = 6.1 | 81/1,934, IR = 3.9 | |
| Total T (nmol/L) | 1.13 (0.99, 1.31) | 1.19 (0.99, 1.43) | 1.17 (0.93, 1.48) |
| Bioavailable T (nmol/L) | 1.09 (0.93, 1.29) | 1.07 (0.87, 1.31) | 1.19 (0.91, 1.56) |
| Free T (Percent) | 0.86 (0.69, 1.08) | 0.71 (0.54, 0.93) | 0.77 (0.54, 1.09) |
| Estradiol (nmol/L) | 0.97 (0.78, 1.19) | 0.76 (0.58, 0.99) | 0.75 (0.54, 1.04) |
| Total T/estradiol ratio | 1.21 (0.99, 1.48) | 1.55 (1.20, 2.00) | 1.51 (1.10, 2.06) |
| DHEA (nmol/L) | 0.98 (0.84, 1.13) | 1.01 (0.83, 1.22) | 0.88 (0.69, 1.12) |
| SHBG (nmol/L) | 1.14 (0.93, 1.41) | 1.39 (1.07, 1.82) | 1.25 (0.89, 1.75) |
Abbreviations: CVD, cardiovascular disease; CHD, coronary heart disease; HF, heart failure; IR, incidence rates; T, testosterone; DHEA, dehydroepiandrosterone; SHBG, sex hormone binding globulin; HDL, high density lipoprotein; eGFR; estimated glomerular filtration rate; CRP, C-reactive protein; IL-6, interleukin-6.
All sex-hormones are log-transformed and standardized. Hazard Ratios are associated with 1 SD increase in log sex hormones.
Model adjusts for age (year, continuous), race/ethnicity (white, black, Hispanic, and Chinese), and study site (CA, MN, NY, IL, NC, and MD), education (<high school, high school, >high school), waist to hip ratio (continuous), physical activity, and smoking (never, former, current), systolic blood pressures (mmHg, continuous), use of antihypertensive medications (yes, no), total cholesterol (mg/dl, continuous), HDL-cholesterol (mg/dl, continuous), use of lipid lowering medications (yes, no), diabetes (yes, no), eGFR (mL/min/1.73 m2), CRP (mg/l, log-transformed continuous), IL-6 (pg/ml, log-transformed, continuous), fibrinogen (mg/dl, log-transformed continuous), D-dimer (ug/ml, log-transformed continuous).
In spline regression analyses, the risk of incident CVD and CHD increased progressively with total testosterone levels and testosterone/estradiol ratio (Figures 2A and 2C), and the risk of incident CHD and HF decreased progressively with estradiol levels (Figure 2B), adjusted for variables in Model 3. The P-values for the non-linear spline components of total testosterone for CVD, CHD and HF were 0.17, 0.08, and 0.22, respectively; for testosterone/estradiol ratio were 0.40, 0.13 and 0.006, respectively; for estradiol were 0.58, 0.49, and 0.06, respectively, indicating that the associations of sex hormones with these endpoints were approximately linear, except for testosterone/estradiol ratio and risk of HF which was U-shaped.
Figure 2. Hazard Ratios (HR) for incident CVD, CHD and HF by sex hormone levels*,†,‡.
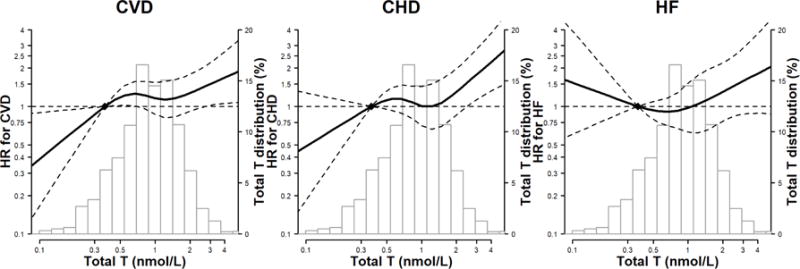
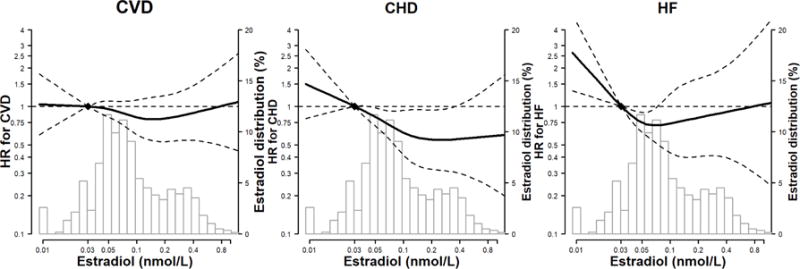
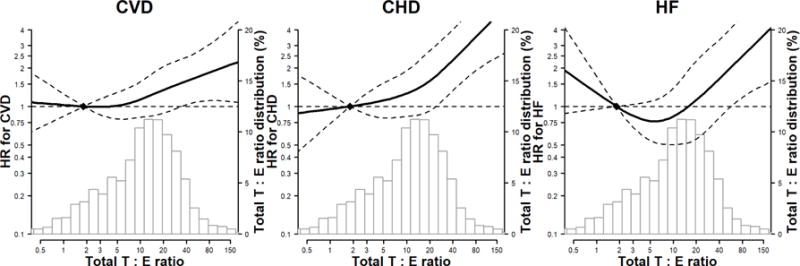
*Abbreviations: CVD, cardiovascular disease; CHD, coronary heart disease; HF, Heart Failure. †The curves represent the adjusted HR of CVD/CHD/HF by log sex hormone levels. The dose response association was estimated by using a linear and a cubic spline term for each sex hormone in the multivariable cox regression. Curves represent adjusted HR (solid line) and 95% CI (dashed lines) based on restricted cubic splines for sex hormones with knots at the 5th, 35th, 65th and 95th percentiles of their sample distributions. The reference values (diamond dots) were set at 10th percentile. ‡The model was adjusted for age, race/ethnicity, study center, education, waist to hip ratio, physical activity, smoking, systolic blood pressure, use of antihypertensive medications, total cholesterol, HDL-cholesterol, use of lipid lowering medications, diabetes, eGFR, use of hormone therapy, log(CRP), log(IL6), log(fibrinogen), and log(D-dimer).
Panel A: total testosterone (nmol/L)
Panel B: estradiol (nmol/L)
Panel C: testosterone-estradiol ratio
The associations between sex hormones and CVD outcomes were generally consistent across all age and race/ethnic subgroups, except that in younger participants (<65 years of age), the association of DHEA levels with CVD, CHD and HF endpoints was inverse while it was null in older participants (≥65 years); Figure 3 Panels A, B, C.
Figure 3. Associations between sex hormone levels with incident CVD, CHD and HF by age and race/ethnic subgroups.



Panel A: Hazard Ratio for CVD; Panel B: Hazard Ratio for CHD; Panel C: Hazard Ratio for HF. *Hazard Ratios are associated with 1 SD increase in log sex hormones. The model was adjusted for the covariates listed in footnote for Figure 2.
Discussion
Among racially/ethnically diverse post-menopausal women followed for >12 years, we found that higher testosterone/estradiol ratio were associated with an elevated risk for incident CVD, CHD, and HF events, higher total testosterone with increased risk of CVD and CHD, and higher estradiol levels were associated with a lower risk of CHD (Central Illustration). The associations persisted after adjustment for traditional cardiovascular risk factors, suggesting an independent role of sex hormones on cardiovascular events. The risks for CVD and CHD were approximately linear across the whole range of total testosterone, testosterone/estradiol ratio, and estradiol levels. However, there was a U-shaped association between testosterone/estradiol ratio and HF, with both extreme ends at higher risk for HF. Additionally, we found that testosterone/estradiol ratio was positively, and estradiol and DHEA were inversely, associated with risk of HFrEF.
Central Illustration. Testosterone/Estradiol Ratio and the Risk of Incident CVD, CHD, and HF in post-menopausal women: the Multi-Ethnic Study of Atherosclerosis*,†,‡.
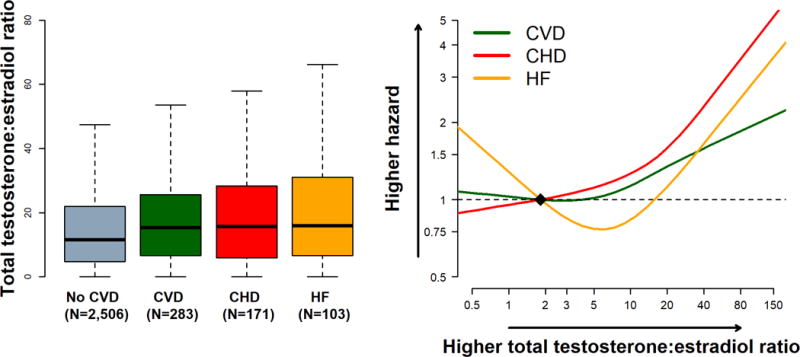
*Abbreviations: CVD, cardiovascular disease; CHD, coronary heart disease; HF, Heart Failure. †Left panel: Boxplot distribution of the testosterone/estradiol ratio by outcome status (unadjusted). The bottom and top of the box are the first and third quartiles, and the band inside the box is the second quartile (the median). The whiskers represent the 1st quartile −1.5*IQR and 3rd quartile +1.5*IQR. ‡Right panel: Adjusted Hazard Ratio of CVD/CHD/HF for testosterone/estradiol ratio using restricted cubic spline with knots at the 5th, 35th, 65th and 95th percentiles of the sample distribution. The reference value (diamond dot) was set at 10th percentile. The model was adjusted for age, race/ethnicity, study center, use of hormone therapy, education, waist to hip ratio, physical activity, smoking, systolic blood pressure, use of antihypertensive medications, total cholesterol, HDL-cholesterol, use of lipid lowering medications, diabetes, eGFR,, log(CRP), log(IL6), log(fibrinogen), and log(D-dimer).
Several biological mechanisms may underlie the association between endogenous sex hormones and CVD and its risk factors in women. Estrogens can promote vasodilation through increasing plasma concentrations of endothelium-derived relaxing factor nitric oxide (NO), and can inhibit the renin angiotensin system by reducing transcription of angiotensin converting enzyme (18). In addition to its favorable effects on lipids, estrogens can also reduce blood pressure through increasing endothelial vasodilator function and modulating autonomic function. Additionally, estrogen is thought to regulate specific inflammatory markers and cytokines (19). In contrast, testosterone can induce vasoconstriction and increased platelet aggregation through up-regulation of thromboxane (20). In population studies, androgens are associated with increased accumulation of visceral fat, lipid levels, and increased levels of cardiometabolic risk factors (2,3,21), including blood pressure, BMI, CRP, and insulin resistance in women (1,3–5). Furthermore, a history of early age of menopausal onset and shorter duration from menarche to menopause are associated with higher risk of cardiovascular outcomes, including CVD mortality, CHD and HF (22,23); this again suggests a role of sex hormones in disease pathogenesis.
In spite of plausible pathological pathways linking endogenous sex hormones and clinical CVD events, prospective studies have been contradictory. In a population-based study in Denmark (n=4,600 pre-menopausal and post-menopausal women), higher baseline total testosterone levels were associated with an increased risk of CHD and death over 30 years follow-up (6). In the Women’s Health Study (WHS), 200 post-menopausal women who developed CVD >3 years of follow-up had a higher baseline free androgen index compared to controls, but the association was null after adjustment for CVD risk factors (7), and a population-based cohort study in Germany (n=2,129 middle-aged women, 11-year follow-up) also concluded that baseline total testosterone was not associated with incident CVD or mortality (24). Furthermore, the Rancho Bernardo Study (8,10) (n=639 postmenopausal women, 12-year follow-up) and a hospital-based cohort study in Germany (9) (n=2,914 women aged between 18 and 75 years, 4.5-year follow-up) both found that lower baseline levels of total testosterone were associated with increased CVD events, which contrasts with our findings.
With respect to estrogens, a population-based study in Denmark found that lower estradiol levels were associated with increased risk of CHD and death (6), but the WHS (7), Rancho Bernardo Study (8,10) and a nested case-control study of elderly diabetic women (11) found no association between estradiol levels and CVD events.
Several methodological differences could explain the discrepancies in the associations observed in previous studies. First, previous studies have used different study designs, with a mixture of cohort (8,9,24), nested cohort (6), nested case-control designs (7), with follow-up times ranging from 4 to almost 30 years. Second, the study populations were very diverse, with mean age ranging from 49 to 74 years. Finally, these studies used different measurements of CVD events including self-reports (8,10,24), national patient registries (6), and medical records(7,9).
Our study supports an association of higher total testosterone levels and higher testosterone/estradiol ratio with increased CVD and CHD events in post-menopausal women, as well as an association of higher estradiol levels with reduced CHD and HF events, although the association between estradiol and HF did not reach statistical significance (HF events were fewer than CHD events, with therefore less power to detect associations). Among HF subtypes, we found that higher testosterone/estradiol ratio and lower estradiol levels were associated increased risk for HFrEF, but not HFpEF, the type of HF more commonly seen in older women. It is possible that the reduction in estradiol during menopause differentially affects vascular and cardiac remodeling processes that differentially lead to a HFrEF vs. HFpEF phenotype. Lower levels of estradiol are associated with risk for hypertension (25), a major risk factor for HFrEF. In our study estradiol was associated with CHD, and CHD may contribute more to HFrEF than to HFpEF. As mentioned, our results contradicted the two Germany studies and Rancho Bernardo Study that showed null or reversed association between total testosterone and CVD events (8,9,24). The difference may be due to the selection of study participants (younger women with mean age 49, and hospital based in the German study) or ascertainment of CVD outcome (self-report in Rancho Bernardo Study). Further studies are needed to confirm these findings and to better understand the associations and mechanisms between sex hormones and subtypes of HF.
In our study, SHBG and DHEA levels were not associated with CVD, CHD or overall HF. However, SHBG was associated with increased risk for CHD in women not taking HT. This result aligns with a previous MESA analysis showing that SHBG was positively associated with coronary artery calcification (15). Nevertheless, other studies have not found an association between SHBG and incident CVD (7,10,11,24). Additionally, in our study, DHEA was inversely associated with HFrEF risk, an association that needs to be confirmed in other studies. The role of DHEA on CVD is still undefined. It was hypothesized that lower DHEA levels might be related to increased risk of CVD (26), but this association was refuted by many studies, especially among women (27,28).
Although sex hormone levels may be linked to future CVD events, it is unclear what the best intervention is to modify sex hormone levels for risk reduction. While randomized clinical trials failed to conclusively show a beneficial effect of HT on CHD risk or CVD mortality in post-menopausal women in primary prevention (29,30), the trials didn’t consider compounds other than conjugated equine estrogen and medroxyprogesterone acetate, and the conclusions may not be generalized to all menopausal women in different ages (29,31,32). HT may not exert the same physiological effects as endogenous sex hormones, and these vary depending on the different formulations of estrogen and progestin (33). Also, the time of HT initiation may determine HT effects on CVD risk. It has been suggested that estrogen therapy may prevent subclinical atherosclerosis in peri-menopausal women when given around the time of the menopausal transition, but has no benefit on CVD risk in later life after menopause (29,34,35).
Our study has many strengths, such as the inclusion of a large, diverse, well-characterized multi-ethnic group of women prospectively followed for more than 10 years. We investigated the associations of sex hormones with a variety of CVD outcomes after careful adjustment for a number of potential confounders and use of HT. However, our results should be considered in the context of some limitations. First, in spite of the sample size and the long duration of followup, the number of events observed in our study was limited for some outcomes, particularly for HF subtypes and ischemic stroke, limiting our power to detect associations. Additionally, while large variation of associations across race/ethnicity were seen, the sample size may limit the detection of significant interaction between sex hormone levels and race/ethnicity. Second, we studied multiple sex hormones and outcomes, and some associations may have been observed by chance. However, we based our conclusions not only on significance tests, but also on biological plausibility, dose-response, and consistency of the associations. Third, although we adjusted for multiple potential confounders, we cannot exclude the possibility of residual confounding. Fourth, menopausal status was self-reported and subject to reporting bias, although we corrected self-reported menopausal status using an algorithm based on age, self-reported hysterectomy, bilateral oophorectomy, and menopausal age. Finally, sex hormone levels were only assessed once at baseline and were not repeatedly measured during follow-up. In particular, we did not have information on sex hormone levels prior to and during the menopausal transition. As a consequence, we could not examine the association of changes in sex hormone levels with CVD events.
In conclusion, our study suggests that, in post-menopausal women, higher testosterone/estradiol ratio are associated with the development of CVD, CHD, and HF events while estradiol is associated with a reduced risk of CHD and HFrEF. Sex hormones levels, especially higher total testosterone vs. estrogen after menopause, may contribute to women’s increased CVD risk later in life. However, in the absence of supportive interventional studies, the best strategy to modify sex hormone levels to affect CVD risk is still uncertain. Nonetheless, a more androgenic sex hormone profile may identify a woman at higher risk for CVD who may benefit from other risk reducing strategies.
Supplementary Material
Clinical Perspectives.
Competencies in Medical Knowledge
In post-menopausal women, higher total testosterone/estradiol ratio was associated with increased risk for incident CVD, CHD and HF. Total testosterone levels were independently associated with increased risk of incident CVD and CHD events while estradiol was associated with a reduced risk of CHD and HFrEF. Sex hormones levels after menopause may contribute to women’s increased CVD risk later in life.
Translational Outlook
Results from this observational study provide insight into the relationships among testosterone, estradiol, and the development of CVD in postmenopausal women. These results may help identify menopausal women at risk for CVD and possibly inform the design of future HT trials for CVD prevention in menopausal women.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. The authors thank American Heart Association Go Red for Women Strategic Focused Research Network for funding this study. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding: This work was partly supported by the American Heart Association Go Red for Women Strategic Focused Research Network contract AHA 16SFRN27870000. Drs. Zhao and Michos are also supported by the Blumenthal Scholars Fund for Preventive Cardiology Research. Dr. Shah is supported by NIH/NHLBI grants R01 HL107577 and R01 HL127028, and by AHA grant #16SFRN28780016. The MESA study was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by R01 HL074406 and R01 HL074338.
Disclosures outside of submitted work are as follows: Dr. Michos has received an honorarium from Siemens Diagnostics for being a blinded events adjudicator in a clinical trial. Dr. Ouyang has research grant support from Cordex Systems, Inc. Dr. Budoff has received research funds from GE Healthcare.
Abbreviations
- MESA
Multi-Ethnic Study of Atherosclerosis
- CVD
Cardiovascular Disease
- CHD
Coronary Heart Disease
- HF
Heart Failure
- HFpEF
Heart Failure with preserved Ejection Fraction
- HFrEF
Heart Failure with reduced Ejection Fraction
- LV
Left Ventricular
- T
Testosterone
- DHEA
Dehydroepiandrosterone
- SHBG
Sex Hormone Binding Globulin
- HT
Hormone Therapy
- BMI
Body Mass Index
- WHR
Waist Hip Ratio
- CRP
C-Reactive Protein
- IL-6
Interleukin-6
- eGFR
Estimated Glomerular Filtration Rate
- HR
Hazard Ratios
- CI
Confidence Intervals
- IR
Incidence Rates
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no conflicts of interest related to the topic of submitted work.
References
- 1.Crandall CJ, Barrett-Connor E. Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: a systematic review. Endocrinol Metab Clin North Am. 2013;42:227–53. doi: 10.1016/j.ecl.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Maturana MA, Breda V, Lhullier F, Spritzer PM. Relationship between endogenous testosterone and cardiovascular risk in early postmenopausal women. Metabolism. 2008;57:961–5. doi: 10.1016/j.metabol.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–9. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 4.Golden SH, Dobs AS, Vaidya D, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92:1289–95. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Szklo M, Folsom AR, Cook NR, Gapstur SM, Ouyang P. Endogenous sex hormones, blood pressure change, and risk of hypertension in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2012;224:228–34. doi: 10.1016/j.atherosclerosis.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benn M, Voss SS, Holmegard HN, Jensen GB, Tybjaerg-Hansen A, Nordestgaard BG. Extreme concentrations of endogenous sex hormones, ischemic heart disease, and death in women. Arterioscler Thromb Vasc Biol. 2015;35:471–7. doi: 10.1161/ATVBAHA.114.304821. [DOI] [PubMed] [Google Scholar]
- 7.Rexrode KM, Manson JE, Lee IM, et al. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–93. doi: 10.1161/01.CIR.0000091114.36254.F3. [DOI] [PubMed] [Google Scholar]
- 8.Laughlin GA, Goodell V, Barrett-Connor E. Extremes of endogenous testosterone are associated with increased risk of incident coronary events in older women. J Clin Endocrinol Metab. 2010;95:740–7. doi: 10.1210/jc.2009-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sievers C, Klotsche J, Pieper L, et al. Low testosterone levels predict all-cause mortality and cardiovascular events in women: a prospective cohort study in German primary care patients. Eur J Endocrinol. 2010;163:699–708. doi: 10.1530/EJE-10-0307. [DOI] [PubMed] [Google Scholar]
- 10.Barrett-Connor E, Goodman-Gruen D. Prospective study of endogenous sex hormones and fatal cardiovascular disease in postmenopausal women. BMJ. 1995;311:1193–6. doi: 10.1136/bmj.311.7014.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haffner SM, Moss SE, Klein BE, Klein R. Sex hormones and DHEA-SO4 in relation to ischemic heart disease mortality in diabetic subjects. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 1996;19:1045–50. doi: 10.2337/diacare.19.10.1045. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Sodergard R, Backstrom T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–10. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 14.Silverman MG, Patel B, Blankstein R, et al. Impact of Race, Ethnicity, and Multimodality Biomarkers on the Incidence of New-Onset Heart Failure With Preserved Ejection Fraction (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2016;117:1474–81. doi: 10.1016/j.amjcard.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang P, Vaidya D, Dobs A, et al. Sex hormone levels and subclinical atherosclerosis in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2009;204:255–61. doi: 10.1016/j.atherosclerosis.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whelton SP, Narla V, Blaha MJ, et al. Association between resting heart rate and inflammatory biomarkers (high-sensitivity C-reactive protein, interleukin-6, and fibrinogen) (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2014;113:644–9. doi: 10.1016/j.amjcard.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–41. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker L, Meldrum KK, Wang M, et al. The role of estrogen in cardiovascular disease. J Surg Res. 2003;115:325–44. doi: 10.1016/s0022-4804(03)00215-4. [DOI] [PubMed] [Google Scholar]
- 20.Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91:2742–7. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- 21.Wehr E, Pilz S, Boehm BO, Grammer TB, Marz W, Obermayer-Pietsch B. Low free testosterone levels are associated with all-cause and cardiovascular mortality in postmenopausal diabetic women. Diabetes Care. 2011;34:1771–7. doi: 10.2337/dc11-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall PS, Nah G, Howard BV, et al. Reproductive Factors and Incidence of Heart Failure Hospitalization in the Women’s Health Initiative. J Am Coll Cardiol. 2017;69:2517–2526. doi: 10.1016/j.jacc.2017.03.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muka T, Oliver-Williams C, Kunutsor S, et al. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016;1:767–776. doi: 10.1001/jamacardio.2016.2415. [DOI] [PubMed] [Google Scholar]
- 24.Schaffrath G, Kische H, Gross S, et al. Association of sex hormones with incident 10-year cardiovascular disease and mortality in women. Maturitas. 2015;82:424–30. doi: 10.1016/j.maturitas.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Dubey RK, Oparil S, Imthurn B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 26.Shufelt C, Bretsky P, Almeida CM, et al. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health–National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women’s Ischemia Syndrome Evaluation (WISE) J Clin Endocrinol Metab. 2010;95:4985–92. doi: 10.1210/jc.2010-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trivedi DP, Khaw KT. Dehydroepiandrosterone sulfate and mortality in elderly men and women. J Clin Endocrinol Metab. 2001;86:4171–7. doi: 10.1210/jcem.86.9.7838. [DOI] [PubMed] [Google Scholar]
- 28.Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA, Kenny AM. Effects of dehydroepiandrosterone (DHEA) on cardiovascular risk factors in older women with frailty characteristics. Age Ageing. 2010:451–8. doi: 10.1093/ageing/afq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 30.Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal Hormone Therapy and Long-term All-Cause and Cause-Specific Mortality: The Women’s Health Initiative Randomized Trials. JAMA. 2017;318:927–938. doi: 10.1001/jama.2017.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langer RD. The evidence base for HRT: what can we believe? Climacteric. 2017;20:91–96. doi: 10.1080/13697137.2017.1280251. [DOI] [PubMed] [Google Scholar]
- 32.Langer RD, Simon JA, Pines A, et al. Menopausal hormone therapy for primary prevention: why the USPSTF is wrong. Climacteric. 2017;20:402–413. doi: 10.1080/13697137.2017.1362156. [DOI] [PubMed] [Google Scholar]
- 33.Shufelt CL, Merz CN, Prentice RL, et al. Hormone therapy dose, formulation, route of delivery, and risk of cardiovascular events in women: findings from the Women’s Health Initiative Observational Study. Menopause. 2014;21:260–6. doi: 10.1097/GME.0b013e31829a64f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shufelt CL, Johnson BD, Berga SL, et al. Timing of hormone therapy, type of menopause, and coronary disease in women: data from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Menopause. 2011;18:943–50. doi: 10.1097/gme.0b013e3182113672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J Am Coll Cardiol. 2006;47:1741–53. doi: 10.1016/j.jacc.2005.10.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


