Abstract
Borrelia burgdorferi is the causative bacterial agent of Lyme disease, the most prevalent tick-borne infection in North America. The ability of B. burgdorferi to cause disease is highly dependent on its capacity to evade the immune response during infection of the mammalian host. One of the ways in which B. burgdorferi is known to evade the immune response is antigenic variation of the variable major protein (VMP)-like sequence (Vls) E lipoprotein. Past research involving the B. burgdorferi antigenic variation system has implicated a gene-conversion mechanism for vlsE recombination, analyzed the long-term dynamic changes occurring within VlsE, and established the critical importance of antigenic variation for persistent infection of the mammalian host. However, a role for the VlsE protein other than providing an antigenic disguise is currently unknown, but it has been proposed that the protein may function in other forms of immune evasion. Although a substantial number of additional proteins reside on the bacterial surface, VlsE is the only known antigen that exhibits ongoing variation of its surface epitopes. This suggests that B. burgdorferi may use a VlsE-mediated system for immune avoidance of its surface antigens. Several recent experimental studies involving host reinfection, superinfection, and the importance of VlsE antigenic variation during the pathogen’s enzootic cycle have been used to address this question. Here, the cumulative results from these studies are reviewed, and the knowledge gaps that remain regarding the role of VlsE for immune avoidance are discussed.
Keywords: Lyme disease, Borrelia, immune evasion, antigenic variation, VlsE
I. INTRODUCTION
The multisystem disease known as Lyme disease is the most prevalent vector-borne infection affecting humans in both North America and Europe.1,2 Borrelia burgdorferi, the causative bacterial agent of the disease in the United States, is transmitted by hard-bodied ticks of the genus Ixodes. When feeding, infected ticks transmit B. burgdorferi to humans, which can result in a localized infection (erythema migrans) at the site of the tick bite. After transmission, disseminated and chronic stages of infection occur that are characterized by neurological, cardiological, and arthritic manifestations of disease. Infection with B. burgdorferi can last from months to years due to avoidance of the host immune response, and key to its successful evasion tactics is recombination within the vls locus located on the 28-kb linear plasmid (lp28-1).3–5
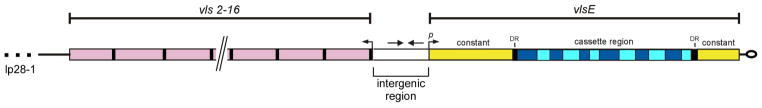
The vls locus consists of an expression site that encodes the 35-kDa variable major protein (VMP)-like sequence (Vls) E lipoprotein and a tandem array of 15 silent cassettes (vls2–16; each ~500 bp in length) that are oriented in the opposite direction from the vlsE gene (Fig. 1).3,5,6 A short intergenic region (~160 bp) separates the vlsE locus and the silent cassettes, and this intergenic space contains a near-perfect 51-bp inverted repeat sequence capable of forming a highly stable DNA stem loop.7 In addition, a portion of the promoter required for vlsE expression is located within this inverted repeat.7 The vlsE expression region is comprised of a central variable cassette (Fig. 1) that is flanked by constant regions. At the junction of the variable and constant regions are 17-bp direct repeats that are also found at either end of most of the silent cassettes. The vlsE cassette region exhibits a roughly 90% sequence identity with each of the silent cassettes,5 and the majority of sequence differences reside in six variable regions (Fig. 1) that are flanked by six highly conserved, or invariant, sequences.
FIG. 1.
The vls locus of B. burgdorferi B31. Arrangement of the vls expression site, vlsE, and the contiguous array of 15 silent cassettes comprising the vls locus on the right telomere end of lp28-1. The six variable regions of the central vlsE cassette and the six invariant regions. The bars flanking the vlsE cassette region and silent cassettes represent the 17-bp direct repeats. The silent cassettes (vls2–16) are not drawn to scale. Arrows positioned at the beginning of vlsE and silent cassettes indicate the respective orientations. Arrows located within the intergenic region denote the inverted DNA repeat. DR, 17-bp Direct repeat; p, vlsE promoter (reprinted with permission from Springer, Copyright 2012).8
It has been shown that recombination events can be detected as early as 4 d after infection of mice and continue to occur throughout infection.9 Previous work also demonstrated that antibodies specific for the variable regions of VlsE were produced during experimental infection of mice.10 Clones lacking lp28-1 have been shown to exhibit an intermediate infectivity phenotype, whereby these spirochetes are able to disseminate to tissue sites but unable to persist in the murine host.11,12 However, these same clones are capable of long-term survival in severe-combined immunodeficient (SCID) mice that lack an effective antibody response.13,14 lp28-1–Deficient isolates also grow normally in a dialysis membrane chamber implanted in the peritoneal cavity of rats, where exposure to either antibodies or immune cells is restricted.14 Finally, immunocompetent mice infected with an lp28-1− strain complemented with only the vlsE gene (lacking the vls silent cassette region) are able to clear infection, demonstrating that it is not the mere presence of VlsE that provides the capacity for persistent infection, but rather the ability to undergo vls gene conversion to produce VlsE variants.15
Infection experiments involving a vls deletion mutant demonstrated that this B. burgdorferi clone is completely cleared from immunocompetent C3H mice by d 21 after infection,16 matching the phenotype observed with clones that lacked lp28-1. Consistent with the findings that lp28-1 is not required for persistent infection in the absence of an adaptive immune response,13–15 the vls deletion mutant exhibits long-term survival in SCID mice. Targeted deletion was also used to obtain lp28-1 mutants containing mainly the vls locus and the genes necessary for autonomous replication of the plasmid. The only other potential genes retained on the mutant lp28-1 were eight very small open reading frames (ORFs; bbf19–22 and bbf27–30) predicted to encode for proteins consisting of only 82 amino acids or less. Infectivity experiments showed that these B. burgdorferi clones are fully infectious and persistent in immunocompetent mice, providing further evidence that the silent vls cassettes and vlsE are the primary lp28-1–resident genes involved in spirochete persistence.16 Moreover, these mutant clones carried out vlsE recombination in immunocompetent C3H mice, indicating that protein factors required for antigenic switching are likely not carried on lp28-1 but encoded elsewhere.
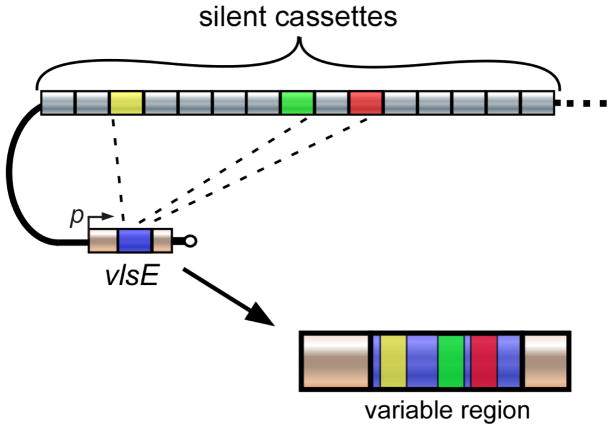
Although gene conversion has been implicated in vlsE antigenic variation, much is still unknown regarding the mechanistic details and proteins involved in recombination. The proposed model for vlsE antigenic switching involves a nonreciprocal gene-conversion mechanism, whereby segments within the vlsE central cassette region are replaced with sections of varied length and location from the silent cassettes (Fig. 2). Only the cassette region of vlsE displays sequence variation resulting from gene conversion; the sequence and organization of the silent cassettes remain unaltered.9 A detailed analysis of the vlsE sequence changes found that the vls antigenic variation system promotes both short and long recombination events within each cassette region. Along with gene-conversion events, template-independent changes that lead to additional sequence variation of vlsE also occur. The end result of these accumulated changes is a new vlsE sequence with a mosaic structure. Although disruption of a large number of genes involved in DNA recombination, repair, and replication ruled out the involvement of their respective encoded proteins in vlsE switching, it was discovered that the RuvAB complex of B. burgdorferi is required for vls antigenic variation, presumably by promoting branch migration of Holliday junctions during vlsE recombination.17,18
FIG. 2.
Overview of vlsE antigenic switching in B. burgdorferi. Variant-specific segments act as a source of DNA for nonreciprocal recombination events with the vlsE expression locus. Through this process, segments of the variable region are replaced by sections of varied length and location from the donor sequences. In the example shown, three sequential gene-conversion events (dashed lines) occur within each expression site through recombination with the colored donor sections to generate a new expression site sequence with a mosaic structure. p, vlsE promoter (reprinted with permission from John Wiley & Sons, Inc., Copyright 2009).4
An interesting study by Embers and coworkers reported that expression of the immunodominant outer-surface protein (Osp) C by an lp28-1–deficient B. burgdorferi clone was abnormally high in vivo, suggesting that down-regulation of this protein is impaired.19 It has been well documented that OspC is down-regulated in B. burgdorferi shortly after establishing infection in the animal host, and it has been suggested that this provides the spirochete with a mechanism to avoid clearance mediated by anti-OspC antibodies.20–22 The overall conclusion from the Embers et al. study was that failure of OspC repression by lp28-1–deficient spirochetes renders them susceptible to immune-mediated clearance, and this could potentially be responsible, in part, for the intermediate infectivity phenotype associated with these B. burgdorferi clones. Thus, the possibility was raised that one or more genes involved in ospC repression may be present on lp28-1.
As mentioned above, previous mutational analysis of lp28-1 did not address the potential role of the ORF regions bbf19–22 and bbf27–30 for B. burgdorferi persistence.16 However, it was recently reported that B. burgdorferi mutants lacking either region bbf19–22 or bbf27–30 were found to be capable of persistent infection of immunocompetent C3H mice for up to 91 d.23 Results from this study also showed that deletion of these gene regions had no effect on ospC expression during infection of mice. Together with previously published results involving additional lp28-1–deletion mutants,16 these findings increase the likelihood that the vls locus is the only lp28-1–resident genetic system responsible for persistence during infection of the mammalian host.
The overall strengths of past investigations include numerous independent analyses of VlsE sequence changes implicating a gene-conversion mechanism, the long-term dynamics of those changes, and the critical importance of VlsE antigenic variation for persistent infection by B. burgdorferi. However, a role for the VlsE protein other than providing an antigenic disguise is currently unknown. Additionally, no studies on the importance of VlsE antigenic variation for the pathogen’s enzootic cycle had previously been conducted until recently. This review focuses on recent investigations that have attempted to determine whether a VlsE-mediated immune avoidance system is at work in the Lyme disease pathogen and assess the importance of VlsE antigenic variation for the enzootic cycle of B. burgdorferi.
II. THE ROLE OF VlsE IN HOST REINFECTION AND SUPERINFECTION
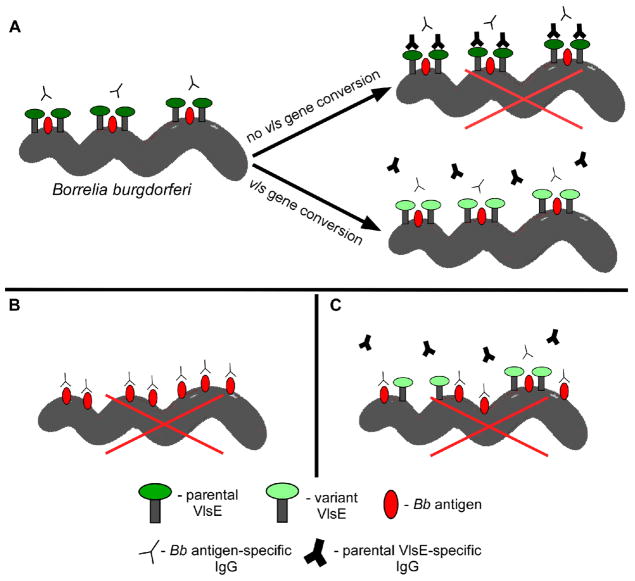
Despite the multitude of expressed surface proteins that are immunogenic, VlsE is the only known B. burgdorferi antigen that exhibits variation of its surface epitopes. A long-standing question regarding B. burgdorferi immune escape has been how such a feat is accomplished through sequence variation of this single lipoprotein, despite the presence of a substantial number of additional antigens residing on the bacterial surface. One possibility is that VlsE acts as a shield to obscure the epitopes of other surface antigens. In fact, crystallography data suggest that the binding of VlsE to other proteins on the membrane surface may block antibody binding to the lateral surface of VlsE.24 This in turn may protect other surface antigens that are tightly juxtaposed to VlsE from antibody recognition (Fig. 3A). A precedent for this type of interaction has been demonstrated in studies with the Borrelia protein P66, in which the protein is protected from proteolytic cleavage in Lyme Borrelia expressing high levels of OspA.25 It has also been shown that OspA potentially serves an antibody-shielding role in the tick vector during a blood meal uptake from an immune host.26
FIG. 3.
Model for VlsE-mediated protection of B. burgdorferi surface antigens. (A) Shortly after host infection, up-regulation of vlsE expression leads to surface localization of the encoded lipoprotein. Interaction of VlsE with other proteins results in a complex that works to shield epitopes of these surface antigens. Continued vls gene conversion leading to production of VlsE variants is necessary to avoid killing by antibodies raised against the parental and subsequent VlsE variants, allowing for sustained epitope masking. (B) Absence or (C) low expression of VlsE allows binding of antibodies to B. burgdorferi surface antigens that ultimately leads to spirochete death (red Xs). A legend indicating the identity of the various molecular depictions is provided at the bottom of the figure. IgG, immunoglobulin G (reprinted with permission from the American Society for Microbiology, Copyright 2016).27
A. Host Reinfection
With respect to VlsE-mediated shielding, experiments using either in vitro–grown or host-adapted wild-type B. burgdorferi were conducted to determine whether VlsE expression could provide Lyme disease spirochetes with the capacity to reinfect mice.28 The levels of VlsE expression were shown to be up-regulated by 32-fold during host infection relative to those measured in vitro.29 Unlike the highly susceptible in vitro–grown spirochetes, B. burgdorferi that have adapted within the animal host were demonstrated to be relatively invulnerable to the protective effects of immune sera.30,31 The reinfection study showed that cultured (low VlsE expressing) wild-type B. burgdorferi are unable to reinfect mice, whereas host-adapted (high VlsE expressing) wild-type spirochetes are fully competent for host reinfection (summarized in Table 1).28 To determine whether variable or static VlsE could provide a capacity for reinfection, researchers used a VlsE-deficient clone and a mutant clone expressing nonvariable or “static” VlsE.28 The results from these experiments involving wild-type and VlsE mutant clones found that only the wild type could reinfect mice initially infected and cleared of spirochetes devoid of VlsE. In other words, the immune response of these mice was sufficient to prevent reinfection by a VlsE-deficient clone but could not block reinfection by spirochetes capable of expressing variable VlsE (Fig. 3B). It was also shown that SCID mice treated with immune sera generated against a VlsE-deficient mutant were resistant to infection by this same clone but could be successfully challenged by host-adapted wild-type spirochetes expressing VlsE.28 The finding that passively transferred antibodies developed to non-VlsE surface antigens can provide immunity against the VlsE-deficient clone, but are not borreliacidal to wild-type spirochetes, may hint at a possible VlsE-mediated shielding mechanism. Moreover, the data from this study indicate that the adaptation state of infecting spirochetes, likely due to its respective effects on VlsE expression, can greatly influence B. burgdorferi evasion from the host antibody-mediated response (Fig. 3C).
TABLE 1.
Summary of findings regarding host reinfection and superinfection by B. burgdorferi wild-type and VlsE-mutant clones
| B. burgdorferi clone | VlsE status | Persistence? | Reinfection? | Superinfection? |
|---|---|---|---|---|
| In vitro–grown wild type | Parental (switchable) | Yes | No | Intrastrain, no Interstrain, yes |
| Host-adapted wild type | Variable | Yes | Yes | Intrastrain, no Interstrain, yes |
| VlsE deficient | Absent | No | No | Intrastrain, no Interstrain, yes |
| Static VlsE | Parental (nonswitchable) | No | No | Intrastrain, no Interstrain, yes |
It has also been proposed that VlsE may be a T-cell–independent antigen that could directly stimulate certain B-cell subsets.16,32 The resulting humoral response generated by VlsE may serve to override antibody production against other potential surface antigens. It has been observed that vls mutant B. burgdorferi clones complemented with a nonswitchable form of vlsE cleared more quickly in immunocompetent mice relative to non-VlsE–complemented mutants.16 It is conceivable that this outcome could be the result of direct stimulation of B cells by VlsE, and this modulating ability could result in more effective clearance of these spirochetes due to the absence of VlsE antigenic variation in those clones. In support of this, experiments from the reinfection study found that sera derived from nude mice infected with wild-type B. burgdorferi contained anti-VlsE T-cell independent antibodies at sufficient levels to prevent infection by in vitro–grown wild-type B. burgdorferi but were at inadequate quantities to prevent infection by the VlsE-deficient mutant clone.28 This finding suggests that the T-cell–independent antibody response is directed primarily to VlsE, with subdominant titers of these antibodies against non-VlsE surface antigens present, as demonstrated by their inability to prevent infection by the VlsE-deficient mutant clone.
B. Superinfection
Mixed infections with various B. burgdorferi genotypes have been reported in questing ticks,33–35 reservoir animals,36 and humans.37 In Peromyscus leucopus mice, infections by heterologous B. burgdorferi populations are fairly common and are potentially acquired by either coinfection or superinfection.36 Although host superinfection by heterologous strains has been experimentally established,36,38 the ability of homologous clones to superinfect a host has not been studied in the past. Additionally, a role for VlsE in host superinfection has not been previously investigated.
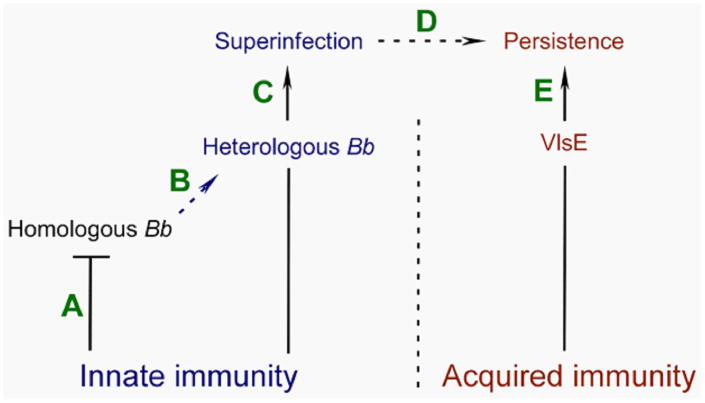
To address the above knowledge gaps regarding host superinfection, experiments were conducted using the homologous B31 wild-type and VlsE-deficient strains and the heterologous A297 wild-type strain.39 Results from experiments addressing the ability of homologous B31 wild-type and VlsE-mutant B. burgdorferi clones to superinfect various mouse models demonstrated an inability of intrastrain clones to superinfect immunocompetent mice (see Table 1). In contrast, heterologous B. burgdorferi strains exhibited the capacity to establish superinfection in immunocompetent mice, supporting findings from previous studies. Experiments also showed that homologous B. burgdorferi clones were unable to superinfect different types of antibody-deficient mice,39 suggesting that the host innate system is a factor in preventing B. burgdorferi superinfection. Importantly, data from additional immunodeficient mice indicate that murine complement is likely a major barrier to intrastrain superinfection. Finally, experiments involving VlsE-deficient mutant B. burgdorferi demonstrated that the presence of vlsE is not required for either intrastrain or interstrain superinfection.39 The data from this study also suggested that VlsE is likely a major specific target of the host antibody response during superinfection. Unlike the wild-type B31, the VlsE-deficient clone exhibited the capacity to establish spirochetemia in immunocompetent C3H mice persistently infected with the heterologous A297 wild-type clone. Thus, the ability of the VlsE-deficient clone to establish spirochetemia in an A297-infected host may indicate that the antibody response is directed primarily to VlsE, with antibodies against non-VlsE antigens potentially generated at subdominant levels. Despite the absence of spirochetes in blood, superinfecting wild-type B31 B. burgdorferi was detectable from other tissue sites, indicating an ability of heterologous wild-type B. burgdorferi to disseminate to and colonize occupied niches of the infected murine host.39 Given that spirochetes are presumably required to enter the bloodstream to travel to distal tissue sites, this result may suggest that superinfecting spirochetes are not completely prevented from transiently colonizing blood; instead, the titer of blood-residing spirochetes might simply be maintained at undetectable levels. Thus, during interstrain superinfection, host anti–B. burgdorferi antibodies seem to be responsible for primarily targeting B. burgdorferi that specifically express variable VlsE.
In nature, B. burgdorferi is propagated in a life cycle that involves an arthropod vector and mammalian reservoir host.40–42 The capacity to superinfect may provide B. burgdorferi with the advantage of being maintained in the enzootic cycle, especially in ecological situations when naive Peromyscus mice populations are of limited availability. The findings reported from the superinfection study suggested that murine complement is a barrier to superinfection by homologous, but not heterologous, B. burgdorferi. Host specificity by B. burgdorferi can be mediated by the alternative pathway of the complement system.44–46 Consequently, the repertoire of genes that encode high-affinity ligands for complement inhibitors can dictate the host range of B. burgdorferi.45,47 Thus, it is plausible that during superinfection, innate immunity in a reservoir host could act as a selective driving force for B. burgdorferi heterogeneity.
A proposed simplistic model based on the findings from this work illustrates this selection process (Fig. 4).39 In this model, the innate immune response of a persistently infected mouse impedes secondary infection by spirochetes that are homologous to primary-infecting B. burgdorferi. In contrast, heterogeneous B. burgdorferi have the capacity to overcome this innate barrier and thus eventually establish superinfection. Although both B31 wild-type and VlsE-deficient spirochetes have the capacity to superinfect A297-infected C3H mice, only the wild-type clone was able to persist. The ability of the wild-type B31 strain to establish persistence in the face of antibody response induced by heterologous B. burgdorferi may indicate that the VlsE-variant repertoire of B31 is not identical to that of A297 B. burgdorferi. Thus, once superinfection has been initially established, a distinct vls repertoire may be required for superinfecting B. burgdorferi to maintain persistence. Ultimately, only genetically diversified B. burgdorferi that have the capacity to overcome both innate and acquired immune responses will be the most likely candidates for continuation of the B. burgdorferi life cycle.
FIG. 4.
Innate immunity as a driving force for B. burgdorferi heterogeneity during superinfection. (A) Innate immunity of a persistently infected mouse host blocks (“T” [horizontal] end of the line) secondary-infecting spirochetes that are homologous to initial-infecting B. burgdorferi. (B) Immune pressure mediated by the host innate response acts as a driving force for selection of heterologous B. burgdorferi. (C) In turn, only heterologous B. burgdorferi have the capacity to overcome this innate barrier (arrow end of the line) and establish superinfection. (D) and (E) Initially, VlsE is uninvolved in this selection process. However, variable VlsE is required for evasion of the host-acquired immune response that leads to persistent B. burgdorferi superinfection. Established persistence of secondary-infecting B. burgdorferi in the reservoir host increases the likelihood of transmitting a selected B. burgdorferi heterogeneity to seasonally available questing ticks (reprinted with permission form the American Society for Microbiology, Copyright 2014).39
III. IMPORTANCE OF VlsE ANTIGENIC VARIATION FOR THE ENZOOTIC CYCLE OF B. BURGDORFERI
Survival of the Lyme pathogen in nature is completely dependent on its enzootic cycle. Thus, it is important to assess whether the variant-generating capacity of the vls system is a must for the Lyme pathogen to be efficiently and successfully perpetuated throughout its life cycle. Although previously untested at the time, the expected outcome would be that antigenic variation of VlsE is required for persistence in the natural reservoir host. Also unknown, but potentially more interesting, were the effects of vls mutation on the ability of B. burgdorferi to be acquired, persist, and be transmitted to naïve mice by Ixodes ticks.
The findings reported in a recent study (summarized in Fig. 5) show that devoid of the vls locus, both in vitro–grown and tick-transmitted B. burgdorferi lost the capacity to persist in Peromyscus mice,48 consistent with previous studies that showed an inability of VlsE-deficient B. burgdorferi to establish a persistent infection in laboratory strains of mice.16,28 Additionally, the data from this work demonstrated that antibodies generated in the natural reservoir were also borreliacidal to B. burgdorferi in the absence of the vls locus.48 Infection of SCID mice with the host-adapted VlsE-deficient clone was prevented by passive immunization with antibodies raised against this mutant clone in Peromyscus mice, whereas host-adapted wild-type B. burgdorferi was able to establish infection in the immunized animals. In contrast, an intact vls system was insufficient to allow tick-transmitted wild-type B. burgdorferi to resist these same antibodies, indicating that VlsE is unlikely to be functionally involved at the time of tick-mediated B. burgdorferi transmission. Indeed, vlsE recombination does not occur during infection of the tick vector,49 and very few spirochetes (<1%) express VlsE.50 Moreover, the level of VlsE expression is low in ixodid nymphs, compared to that found during murine infection, which further supports the insignificant role of the vls locus during the initial tick and murine host interaction.29,49–51 Overall, the combined findings provided further evidence of a VlsE-mediated immune avoidance system that functions to prevent B. burgdorferi surface antigens from being recognized by host antibodies once host infection has been established.
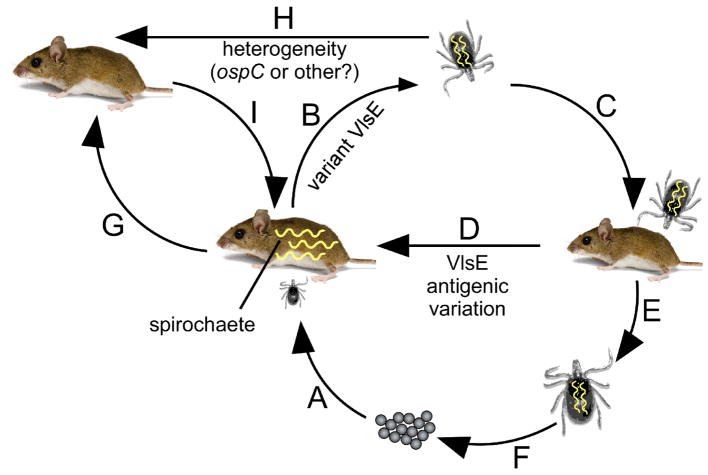
FIG. 5.
Summary of the importance of VlsE antigenic variation during the enzootic cycle of Borrelia burgdorferi. (A)–(I) Stages of the life cycle of B. burgdorferi involving the tick vector and reservoir host. (A) Acquisition of spirochetes occurs when hatched tick larvae feed on infected Peromyscus mice during the summer months. (B) The production of VlsE variants by spirochetes is necessary to escape anti-Borrelia antibodies present in the blood meal, allowing for efficient acquisition by larval ticks that are transstadially retained when the larvae molt into nymphs. (C) Infected nymphal ticks transmit spirochetes when feeding on young, uninfected mice during the spring. (D) Antigenic variation of VlsE by infecting B. burgdorferi in these young mice allows spirochetes to persist at least until the summer months to be acquired by tick larvae, thereby perpetuating the life cycle of the pathogen. (E) and (F) Infected nymphs molt into adult ticks, which are not considered to be important for maintaining B. burgdorferi in nature. Adults typically feed and mate on large mammals such as deer, resulting in the next generation of tick vectors. (G)–(I) Although not well studied, it is possible that immune clearance of spirochete infection occurs in certain numbers of mice. These mice may become reinfected by feeding nymphs that carry a strain of B. burgdorferi that is heterogeneous in some way to the original infecting strain. This capacity for reinfection might be highly advantageous for maintenance of the pathogen during ecological situations when immunologically naïve mammalian reservoir populations are of limited availability (reprinted with permission from PLoS, Copyright 2015).48
The B. burgdorferi enzootic cycle depends on efficient infection of not only the vertebrate host but also the arthropod vector to ensure continual maintenance of Lyme disease spirochetes in nature. Tick experiments involving VlsE-deficient and static VlsE clones demonstrated that Ixodes scapularis larvae were able to acquire both mutant clones.48 However, the acquisition rate for the static VlsE mutant was found to be significantly lower when larvae were allowed to feed on either C3H or Peromyscus mice, demonstrating that the presence of nonswitchable vlsE impaired the ability of these mutant spirochetes to infect larval ticks. In contrast, the VlsE-deficient mutant could be acquired by tick larvae from infected mice at levels comparable to those of the wild type. This latter finding correlates well with previously published data that demonstrated unimpaired tick acquisition of lp28-1–deficient B. burgdorferi clones.52,53 The higher tick acquisition rate of the VlsE-deficient clone compared to that of the static VlsE mutant from immunocompetent mice potentially indicates that a static VlsE variant constitutes a specific target of host antibodies present in the murine blood meal. This is supported by results showing that the static VlsE clone exhibits an acquisition rate comparable to that of wild-type B. burgdorferi when ticks were fed on SCID mice lacking an effective antibody response.48 The transstadial survival rates of both wild-type and VlsE-mutant B. burgdorferi clones in flat nymphs correlated well overall with the corresponding acquisition rates. This not only served to validate the acquisition rates but also suggested that the vls locus was not required for ticks to remain infected during the molting period.
The significant reduction in tick acquisition exhibited by the static VlsE mutant suggests that, in the long run, the presence of a fully functional vls system is an obligate requirement for Lyme disease spirochetes to be successfully propagated through continuous B. burgdorferi enzootic cycles. This study provided the first direct evidence for the significance of VlsE during the B. burgdorferi enzootic cycle and suggests that the variant-generating capacity of the vls system is crucial for the Lyme disease pathogen to be efficiently and successfully perpetuated throughout the B. burgdorferi life cycle.
IV. REMAINING KNOWLEDGE GAPS
Although the studies described above have provided support for the presence of VlsE-mediated immune avoidance in B. burgdorferi, experiments thus far have only offered indirect evidence for such a system. Future studies must focus on determining the actual identity of B. burgdorferi surface antigens that are potentially protected by the presence of VlsE. In turn, this would allow for more direct experimental approaches required to detect epitope shielding. Yet another remaining question is whether host molecules act in complex with VlsE to protect the epitopes of adjacent surface antigens from antibody recognition. As an example, the T-cell–independent immune response that is generated by VlsE may result in an IgM-bound VlsE complex that is large enough to effectively shield the epitopes of other surface antigens, thereby allowing escape from host IgG antibody recognition. A precedent for IgM masking of IgG epitopes was previously demonstrated for the malarial pathogen Plasmodium falciparum.54 These authors found that binding of IgM to the antigenically variable Plasmodium falciparum Erythrocyte Membrane Protein 1 (PfEMP1) protein Variant surface antigen 2-CSA (VAR2CSA), which is displayed on the surface of infected erythrocytes, allowed evasion from acquired immunity in the infected host. Interestingly, binding of IgM to this PfEMP1 protein did not compromise its function nor increase susceptibility to complement-mediated cell lysis.
As mentioned previously, the biological function of the VlsE protein is currently unknown, but an elegant study by Tilly et al. demonstrated that VlsE and OspC may share similar but distinct roles during host infection.55 Unfortunately, the exact function(s) of the OspC protein also remains undefined, so exactly what common role these two lipoproteins may share is still a mystery. Based on previous studies, it was speculated that they might serve to protect the pathogen from host defenses. Alternatively, they may aid in stabilizing the bacterial structure during host infection, similar to that recently demonstrated for OspA and B in lipid rafts on the spirochete membrane.56,57 Although OspC is not associated with these lipid rafts,57 high levels of either OspC or VlsE may still function in some fashion as an essential component for overall stability of the bacterial outer membrane.
Additional functions of VlsE independent of its antigenic variation properties have also been proposed in the past. Previous indications found that VlsE may be function as an adhesin protein that could serve a role in tissue tropism.58 It has been shown that in addition to a role in immune evasion, variable large protein (Vlp) / variable small protein (Vsp) variants can determine tissue tropism in the related relapsing fever agent Borrelia turicatae.59,60 Indeed, higher VlsE expression levels have been observed in spirochetes recovered from joint and skin tissues than from heart tissue.51 However, a previous study found no obvious differences in the amino acid sequence of VlsE variants recovered from different tissue sites, suggesting the absence of any VlsE role in tissue tropism.61 Despite this failure, a study by Baum et al. involving sera obtained from field-caught Peromyscus mice infected with either high- or low-prevalent B. burgdorferi strains demonstrated that the anti-VlsE antibody responses displayed limited overall reactivity.62 This is in sharp contrast to the more broadly cross-reactive responses observed in humans and laboratory strains of mice. Thus, it is possible that the highly segmental nature of VlsE antigenic variation that results in a vast number of variants during infection of laboratory mice has complicated efforts to associate specific protein sequences to a given tissue site.
In total, continued pursuit toward identifying the mechanism responsible for B. burgdorferi immune escape has important implications for the development of vaccines against the pathogen and other Borrelia species. If the protective effects of VlsE can be minimized using therapeutics, an effective vaccine can be developed and used in conjunction to prevent infection or reinfection by the Lyme disease spirochete. Additionally, such knowledge could lead to the development of novel strategies for targeting Lyme disease Borrelia in the tick vector and/or reservoir host. Overall, such future studies will significantly advance our knowledge of immune evasion by B. burgdorferi and in turn could have broad implications for other animal and human pathogens.
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Diseases (NIAID) grants R01 AI108704 and R03 AI087933.
ABBREVIATIONS
- vls
vmp-like sequence
References
- 1.Barbour AG. Borrelia: a diverse and ubiquitous genus of tick-borne pathogens. In: Scheld MW, Craig WA, Hughes JM, editors. Emerging infections 5. Washington, D.C: American Society for Microbiology; 2001. pp. 153–73. [Google Scholar]
- 2.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113(8):1093–101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norris SJ. Antigenic variation with a twist—the Borrelia story. Mol Microbiol. 2006;60(6):1319–22. doi: 10.1111/j.1365-2958.2006.05204.x. [DOI] [PubMed] [Google Scholar]
- 4.Palmer GH, Bankhead T, Lukehart SA. “Nothing is permanent but change”—antigenic variation in persistent bacterial pathogens. Cell Microbiol. 2009;11(12):1697–705. doi: 10.1111/j.1462-5822.2009.01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease Borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89(2):275–85. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JR, Norris SJ. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect Immun. 1998;66(8):3698–704. doi: 10.1128/iai.66.8.3698-3704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson CR, Frye JG, Quinn FD, Gherardini FC. Increased expression of Borrelia burgdorferi vlsE in response to human endothelial cell membranes. Mol Microbiol. 2001;41(1):229–39. doi: 10.1046/j.1365-2958.2001.02511.x. [DOI] [PubMed] [Google Scholar]
- 8.Bankhead T. Antigenic variation of VlsE in Borrelia burgdorferi. In: Embers ME, editor. The pathogenic spirochetes. New York: Springer; 2012. pp. 113–24. [Google Scholar]
- 9.Zhang JR, Norris SJ. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect Immun. 1998;66(8):3689–97. doi: 10.1128/iai.66.8.3689-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDowell JV, Sung SY, Hu LT, Marconi RT. Evidence that the variable regions of the central domain of VlsE are antigenic during infection with Lyme disease spirochetes. Infect Immun. 2002;70(8):4196–203. doi: 10.1128/IAI.70.8.4196-4203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labandeira-Rey M, Skare JT. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect Immun. 2001;69(1):446–55. doi: 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purser JE, Norris SJ. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2000;97(25):13865–70. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labandeira-Rey M, Seshu J, Skare JT. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect Immun. 2003;71(8):4608–13. doi: 10.1128/IAI.71.8.4608-4613.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purser JE, Lawrenz MB, Caimano MJ, Howell JK, Radolf JD, Norris SJ. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol Microbiol. 2003;48(3):753–64. doi: 10.1046/j.1365-2958.2003.03452.x. [DOI] [PubMed] [Google Scholar]
- 15.Lawrenz MB, Wooten RM, Norris SJ. Effects of vlsE complementation on the infectivity of Borrelia burgdorferi lacking the linear plasmid lp28-1. Infect Immun. 2004;72(11):6577–85. doi: 10.1128/IAI.72.11.6577-6585.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bankhead T, Chaconas G. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol Microbiol. 2007;65(6):1547–58. doi: 10.1111/j.1365-2958.2007.05895.x. [DOI] [PubMed] [Google Scholar]
- 17.Dresser AR, Hardy PO, Chaconas G. Investigation of the genes involved in antigenic switching at the vlsE locus in Borrelia burgdorferi: an essential role for the RuvAB branch migrase. PLoS Pathog. 2009;5(12):e1000680. doi: 10.1371/journal.ppat.1000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin T, Gao L, Edmondson DG, Jacobs MB, Philipp MT, Norris SJ. Central role of the Holliday junction helicase RuvAB in vlsE recombination and infectivity of Borrelia burgdorferi. PLoS Pathog. 2009;5(12):e1000679. doi: 10.1371/journal.ppat.1000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Embers ME, Alvarez X, Ooms T, Philipp MT. The failure of immune response evasion by linear plasmid 28-1-deficient Borrelia burgdorferi is attributable to persistent expression of an outer surface protein. Infect Immun. 2008;76(9):3984–91. doi: 10.1128/IAI.00387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung BP, McHugh GL, Leong JM, Steere AC. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun. 1994;62(8):3213–21. doi: 10.1128/iai.62.8.3213-3221.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang FT, Brown EL, Wang T, Iozzo RV, Fikrig E. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am J Pathol. 2004;165(3):977–85. doi: 10.1016/S0002-9440(10)63359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Q, Seemanapalli SV, McShan K, Liang FT. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect Immun. 2006;74(9):5177–84. doi: 10.1128/IAI.00713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magunda PR, Bankhead T. Investigating the potential role of non-vls genes on linear plasmid 28-1 in virulence and persistence by Borrelia burgdorferi. BMC Microbiol. 2016;16(1):180. doi: 10.1186/s12866-016-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eicken C, Sharma V, Klabunde T, Lawrenz MB, Hardham JM, Norris SJ, Sacchettini JC. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem. 2002;277(24):21691–6. doi: 10.1074/jbc.M201547200. [DOI] [PubMed] [Google Scholar]
- 25.Bunikis J, Barbour AG. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun. 1999;67(6):2874–83. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Battisti JM, Bono JL, Rosa PA, Schrumpf ME, Schwan TG, Policastro PF. Outer surface protein A protects Lyme disease spirochetes from acquired host immunity in the tick vector. Infect Immun. 2008;76(11):5228–37. doi: 10.1128/IAI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer GH, Bankhead T, Seifert HS. Antigenic variation in bacterial pathogens. Microbiol Spectr. 2016;4(1) doi: 10.1128/microbiolspec.VMBF-0005-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogovskyy AS, Bankhead T. Variable VlsE is critical for host reinfection by the Lyme disease spirochete. PLoS ONE. 2013;8(4):e61226. doi: 10.1371/journal.pone.0061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, Fikrig E. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun. 2004;72(10):5759–67. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barthold SW. Specificity of infection-induced immunity among Borrelia burgdorferi sensu lato species. Infect Immun. 1999;67(1):36–42. doi: 10.1128/iai.67.1.36-42.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Silva AM, Fikrig E, Hodzic E, Kantor FS, Telford SR, 3rd, Barthold SW. Immune evasion by tickborne and host-adapted Borrelia burgdorferi. J Infect Dis. 1998;177(2):395–400. doi: 10.1086/514200. [DOI] [PubMed] [Google Scholar]
- 32.Philipp MT, Bowers LC, Fawcett PT, Jacobs MB, Liang FT, Marques AR, Mitchell PD, Purcell JE, Ratterree MS, Straubinger RK. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J Infect Dis. 2001;184(7):870–8. doi: 10.1086/323392. [DOI] [PubMed] [Google Scholar]
- 33.Guttman DS, Wang PW, Wang IN, Bosler EM, Luft BJ, Dykhuizen DE. Multiple infections of Ixodes scapularis ticks by Borrelia burgdorferi as revealed by single-strand conformation polymorphism analysis. J Clin Microbiol. 1996;34(3):652–6. doi: 10.1128/jcm.34.3.652-656.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G, Liveris D, Brei B, Wu H, Falco RC, Fish D, Schwartz I. Real-time PCR for simultaneous detection and quantification of Borrelia burgdorferi in field-collected Ixodes scapularis ticks from the northeastern United States. Appl Environ Microbiol. 2003;69(8):4561–5. doi: 10.1128/AEM.69.8.4561-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151(1):15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmeister EK, Glass GE, Childs JE, Persing DH. Population dynamics of a naturally occurring heterogeneous mixture of Borrelia burgdorferi clones. Infect Immun. 1999;67(11):5709–16. doi: 10.1128/iai.67.11.5709-5716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liveris D, Varde S, Iyer R, Koenig S, Bittker S, Cooper D, McKenna D, Nowakowski J, Nadelman RB, Wormser GP, Schwartz I. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J Clin Microbiol. 1999;37(3):565–9. doi: 10.1128/jcm.37.3.565-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Derdakova M, Dudioak V, Brei B, Brownstein JS, Schwartz I, Fish D. Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl Environ Microbiol. 2004;70(11):6783–8. doi: 10.1128/AEM.70.11.6783-6788.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogovskyy AS, Bankhead T. Bacterial heterogeneity is a requirement for host superinfection by the Lyme disease spirochete. Infect Immun. 2014;82(11):4542–52. doi: 10.1128/IAI.01817-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rand PW, Lacombe EH, Smith RP, Jr, Rich SM, Kilpatrick CW, Dragoni CA, Caporale D. Competence of Peromyscus maniculatus (Rodentia: Cricetidae) as a reservoir host for Borrelia burgdorferi (Spirochaetares: Spirochaetaceae) in the wild. J Med Entomol. 1993;30(3):614–8. doi: 10.1093/jmedent/30.3.614. [DOI] [PubMed] [Google Scholar]
- 41.Peavey CA, Lane RS. Transmission of Borrelia burgdorferi by Ixodes pacificus nymphs and reservoir competence of deer mice (Peromyscus maniculatus) infected by tick-bite. J Parasitol. 1995;81(2):175–8. [PubMed] [Google Scholar]
- 42.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10(2):87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurtenbach K, Hanincova K, Tsao JI, Margos G, Fish D, Ogden NH. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol. 2006;4(9):660–9. doi: 10.1038/nrmicro1475. [DOI] [PubMed] [Google Scholar]
- 44.Kurtenbach K, De Michelis S, Etti S, Schafer SM, Sewell HS, Brade V, Kraiczy P. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 2002;10(2):74–9. doi: 10.1016/s0966-842x(01)02298-3. [DOI] [PubMed] [Google Scholar]
- 45.Kurtenbach K, Sewell HS, Ogden NH, Randolph SE, Nuttall PA. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun. 1998;66(3):1248–51. doi: 10.1128/iai.66.3.1248-1251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevenson B, El-Hage N, Hines MA, Miller JC, Babb K. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect Immun. 2002;70(2):491–7. doi: 10.1128/IAI.70.2.491-497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogovskyy AS, Casselli T, Tourand Y, Jones CR, Owen JP, Mason KL, Scoles GA, Bankhead T. Evaluation of the importance of VlsE antigenic variation for the enzootic cycle of Borrelia burgdorferi. PLoS ONE. 2015;10(4):e0124268. doi: 10.1371/journal.pone.0124268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Indest KJ, Howell JK, Jacobs MB, Scholl-Meeker D, Norris SJ, Philipp MT. Analysis of Borrelia burgdorferi vlsE gene expression and recombination in the tick vector. Infect Immun. 2001;69(11):7083–90. doi: 10.1128/IAI.69.11.7083-7090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohnishi J, Schneider B, Messer WB, Piesman J, de Silva AM. Genetic variation at the vlsE locus of Borrelia burgdorferi within ticks and mice over the course of a single transmission cycle. J Bacteriol. 2003;185(15):4432–41. doi: 10.1128/JB.185.15.4432-4441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crother TR, Champion CI, Wu XY, Blanco DR, Miller JN, Lovett MA. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect Immun. 2003;71(6):3419–28. doi: 10.1128/IAI.71.6.3419-3428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grimm D, Tilly K, Bueschel DM, Fisher MA, Policastro PF, Gherardini FC, Schwan TG, Rosa PA. Defining plasmids required by Borrelia burgdorferi for colonization of tick vector Ixodes scapularis (Acari: Ixodidae) J Med Entomol. 2005;42(4):676–84. doi: 10.1093/jmedent/42.4.676. [DOI] [PubMed] [Google Scholar]
- 52.Strother KO, Broadwater A, De Silva A. Plasmid requirements for infection of ticks by Borrelia burgdorferi. Vect Borne Zoonot Dis. 2005;5(3):237–45. doi: 10.1089/vbz.2005.5.237. [DOI] [PubMed] [Google Scholar]
- 53.Barfod L, Dalgaard MB, Pleman ST, Ofori MF, Pleass RJ, Hviid L. Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1. Proc Natl Acad Sci USA. 2011;108(30):12485–90. doi: 10.1073/pnas.1103708108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tilly K, Bestor A, Rosa PA. Lipoprotein succession in Borrelia burgdorferi: similar but distinct roles for OspC and VlsE at different stages of mammalian infection. Mol Microbiol. 2013;89(2):216–27. doi: 10.1111/mmi.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LaRocca TJ, Crowley JT, Cusack BJ, Pathak P, Benach J, London E, Garcia-Monco JC, Benach JL. Cholesterol lipids of Borrelia burgdorferi form lipid rafts and are required for the bactericidal activity of a complement-independent antibody. Cell Host Microbe. 2010;8(4):331–42. doi: 10.1016/j.chom.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toledo A, Crowley JT, Coleman JL, LaRocca TJ, Chiantia S, London E, Benach JL. Selective association of outer surface lipoproteins with the lipid rafts of Borrelia burgdorferi. MBio. 2014;5(2):e00899–14. doi: 10.1128/mBio.00899-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antonara S, Chafel RM, LaFrance M, Coburn J. Borrelia burgdorferi adhesins identified using in vivo phage display. Mol Microbiol. 2007;66(1):262–76. doi: 10.1111/j.1365-2958.2007.05924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cadavid D, Pachner AR, Estanislao L, Patalapati R, Barbour AG. Isogenic serotypes of Borrelia turicatae show different localization in the brain and skin of mice. Infect Immun. 2001;69(5):3389–97. doi: 10.1128/IAI.69.5.3389-3397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pennington PM, Allred CD, West CS, Alvarez R, Barbour AG. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65(1):285–92. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coutte L, Botkin DJ, Gao L, Norris SJ. Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection. PLoS Pathog. 2009;5(2):e1000293. doi: 10.1371/journal.ppat.1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baum E, Hue F, Barbour AG. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. MBio. 2012;3(6):e00434–12. doi: 10.1128/mBio.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]