Abstract
Alopecia is a dermatologic condition that affects the pilosebaceous unit in both men and women. In addition to a thorough medical history and physical examination, a host of diagnostic tools may be warranted to differentiate nonscarring and scarring alopecias. Female pattern hair loss represents the most common form of hair loss experienced by up to 40% of women by a certain age. Although alopecia is a benign disorder, even the most negligible amount of hair loss can be devastating to a patient’s self-esteem, self-image, and overall quality of life. We present this comprehensive review of quality of life studies in women with alopecia to describe the multitude of feelings and emotions associated with the disorder and remind dermatologists of the psychological impact it can have on women.
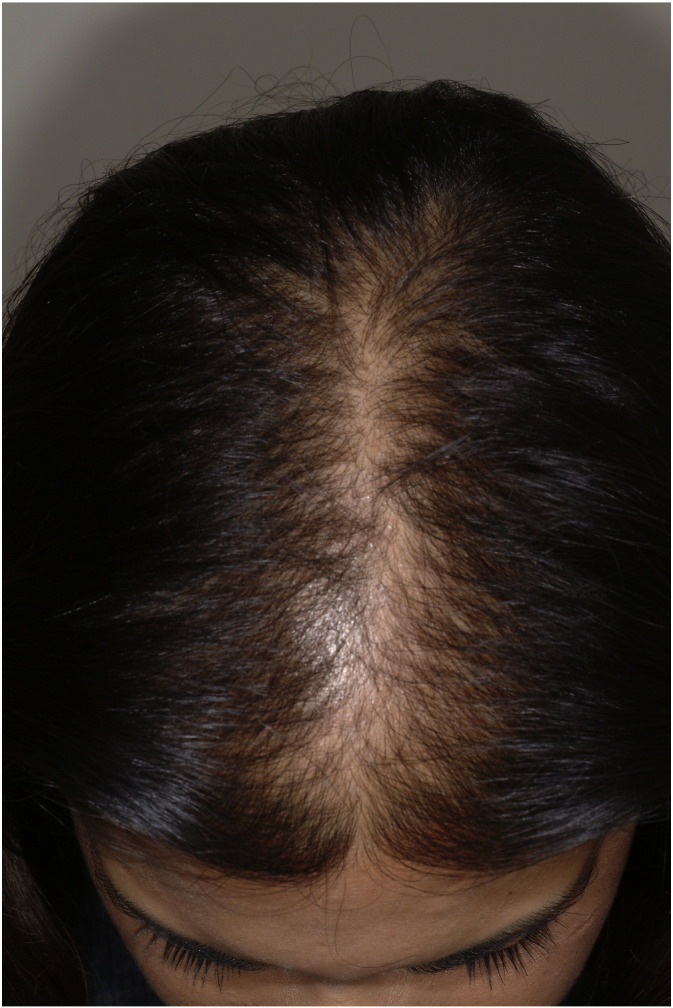
Alopecia is a dermatologic condition that affects the pilosebaceous unit in both men and women. There are several causes of alopecia; thus, a thorough medical history review, physical examination, laboratory evaluation, and scalp biopsy may be needed to establish a specific diagnosis. The most common cause of hair loss in women is female pattern hair loss (FPHL; also known as androgenetic alopecia; Fig. 1). The term FPHL is preferred because the relationship with androgens and inheritance is not clear in all women who have the phenotype of patterned, nonscarring, central scalp hair loss, especially given the early and late onset subtypes (Olsen, 2001). Alopecia occurs in approximately 12% of young female patients by 30 years of age and 30 to 40% of women by 60 to 69 years of age (Herskovitz and Tosti, 2013). The clinical picture may progress to greater severity in patients who present with alopecia at an earlier age compared with those whose hair loss begins later in life. The overall frequency and severity also increases with age (Olsen et al., 2003). Although alopecia is a benign disorder, even the most negligible amount of hair loss can be devastating to a patient’s self-esteem, self-image, and overall quality of life.
Fig. 1.
Female pattern hair loss. A middle-aged woman with a noticeably widened part central along the vertex scalp and extending anteriorly to the frontal scalp, which is accentuated by the triangular shape and resembles a Christmas tree pattern.
Alopecia can be divided into two classifications: nonscarring and scarring (cicatricial). Common causes of nonscarring alopecia include FPHL, telogen effluvium, anagen effluvium, and alopecia areata (AA). Scarring alopecias permanently destroy the pilosebaceous unit and are divided into primary and secondary causes. Primary scarring alopecias include lichen planopilaris (LPP), frontal fibrosing alopecia (FFA), central centrifugal cicatricial alopecia, chronic cutaneous lupus erythematosus (DLE), dissecting cellulitis, folliculitis decalvans, tufted folliculitis, and pseudopelade of Brocq. Secondary scarring alopecias occur when the hair follicles become an innocent bystander as a result of an infiltrative process such as infection (dermatophytes), trauma (burns or radiation), inflammatory processes (sarcoidosis), or malignancy (Olsen et al., 2003).
FPHL represents the most common form of hair loss experienced by women (Cash, 1999, Cash et al., 1993). FPHL is a nonscarring (noncicatricial) form of hair loss that is characterized by either diffuse central thinning with the frontal hair line preserved, accentuation along the frontal scalp in a Christmas tree-like pattern given the shape of the alopecic patch (Olsen, 1999), or recession of the hairline along the bilateral temporal regions (similar to Hamilton-type classification; Cash et al., 1993, Hamilton, 1951, Herskovitz and Tosti, 2013, Katoulis et al., 2015, Zhuang et al., 2013).
Medical treatments including a topical minoxidil 2% solution or foam and topical minoxidil 5% solution or foam (Blume-Peytavi et al., 2007) are available over the counter and approved by the U.S. Food and Drug Administration (FDA) for the treatment of FPHL. Oral spironolactone (antiandrogen; Famenini et al., 2015) and oral finasteride (5-alpha reductase inhibitor) are other effective treatments for patients with FPHL but these therapies are not specifically approved for this indication (Kelly et al., 2016). Cosmetic treatments include low-level laser therapy (Afifi et al., 2017, Avci et al., 2014) with either the FDA-cleared LaserComb (Schweiger et al., 2010), the iGROW helmet device (21 lasers diodes and 30 LEDs, 655 nm red laser; Esmat et al., 2017), Capillus hair regrowth laser cap or Theradome, platelet-rich plasma (PRP) injections (supported by preliminary data but still experimental; Laird et al., 2017, Puig et al., 2016), and hair restoration (Callender et al., 2014, Kelly et al., 2016, Rogers, 2015).
Even though alopecia is not life threatening and considered cosmetic in many cases, the effects on patients’ quality of life (QoL) are real. Throughout time, hair has evolved to not only represent beauty, youth, and health, it has also provided individuals with a sense of self-identity and self-esteem. To some individuals, hair may have a connection to a particular time period, ethnic group, social status, and even a sense of power. For women, hair portrays femininity and self-confidence. Williamson et al. (2001) demonstrated via the Dermatology Life Quality Index (DLQI) that the impact on QoL in patients with alopecia was equal to those with psoriasis. Although hair loss is considered a benign process, the loss has been shown to have a serious impact on individuals’ self-esteem, psychological social experiences, and overall QoL (Cash, 1999, Katoulis et al., 2015). A variety of measures to assess the effects of hair loss on patients’ lives are given in Table 1, which serves as a reference for all methods discussed in this article.
Table 1.
Review of types of alopecia and measuring tools used to assess the effect hair loss has on patients’ self-esteem
| Type of alopecia | Assessment scale | Outcome | Reference |
|---|---|---|---|
| FPHL |
|
|
Zhuang et al., 2013 |
| FPHL or telogen effluvium |
|
Fossati et al., 1993 | |
| FPHL |
|
|
Maffei et al., 1994 |
| FPHL |
|
|
Van Der Donk et al., 1994 |
| FPHL |
|
|
van der Donk et al., 1991 |
| FPHL |
|
|
Cash et al., 1993 |
| FPHL |
|
|
Sancak et al., 2016 |
| AA |
|
|
Al-Mutairi and Eldin 2011 |
| AA |
|
|
Jankovic et al., 2016 |
| AA |
|
|
Colon et al., 1991 |
| AA |
|
|
Ghajarzadeh et al., 2012 |
| AA |
|
|
Shi et al., 2013 |
| AA |
|
|
Liu et al., 2016 |
| AA |
|
|
Rencz et al., 2016 |
| Scarring alopecia |
|
|
Blume-Peytavi et al., 2007 |
| Scarring alopecia |
|
|
Dlova et al., 2016 |
| Scarring alopecia |
|
|
Chiang et al., 2015 |
AA, alopecia areata; DLQI, Dermatology Life Quality Index; FPHL, female pattern hair loss; QoL, quality of life; SF-36, Short Form 36; UCLA, University of California Los Angeles.
Over the years, more studies have investigated and quantified the impact of alopecia on QoL. One study assessed the QoL in 125 women with FPHL and the effectiveness of topical minoxidil to improve the patients’ QoL (Zhuang et al., 2013). At the time of the initial visit, all 125 women completed the Visual Analogue Scale (VAS) to assess their perception of hair loss severity and response to treatment (range, 0-100; high score supports complete satisfaction) as well as the Dermatology Life Quality Index (DLQI) questionnaire (a 10-question, validated, dermatology-focused questionnaire) to assess the impact of the skin disease on their QoL (range, 0-30; low score equals low impact on QoL).
The initial VAS score to evaluate patients’ perception of hair loss was 57.78 ± 18.06. The initial DLQI score was 9.62 ± 5.92 (Zhuang et al., 2013). This was a clinically significant correlation in severity of hair loss regardless of the age and disease duration of patients. The group of patients with the greatest severity also had the highest DLQI score, which supports that FPHL has maximum impact on QoL, and the lowest VAS score, which is a reminder for dermatologists to consider both the objective rating and subjective satisfaction when assessing clinical severity.
After the 12-month follow-up visit, 31 women from the same study were assessed after they received topical minoxidil treatment. The VAS indices prior to and after topical minoxidil treatment were 50.81 ± 14.61 and 72.52 ± 12.79, respectively, which shows an increase in satisfaction with the therapy. The DLQI scores prior to and after treatment were 8.94 ± 5.65 and 4.45 ± 3.36, respectively, which were statistically significant. This study proved that treatment with topical minoxidil may improve the QoL in women with FPHL with regard to how they feel during their daily activities and leisure time (Zhuang et al., 2013).
Another study of 285 men and women with either FPHL (20.7%) or telogen effluvium (79.3%) found that 75% of women displayed signs of a personality disorder, relative to 10.3% of the population base in Italy (on the basis of the diagnostic criteria of the Diagnostic Statistical Manual Mental Disorders, Revised Third Edition [DSM-III-R]). Both the Personality Disorders Questionnaire-Revised (PDQ-R; a validated self-rated 152-item true/false questionnaire) and the 6-point Symptom Checklist-90 (SCL-90, measures psychopathologic response to alopecia with scores that range from 0 for not at all to 5 for very much) were used to assess for personality disorders (Fossati et al., 1993). In a follow-up study, 76.3% of 116 patients with FPHL self-reported a personality disorder that overwhelmingly exceeded the population estimate (on the basis of both the PDQ-R and SCL-90 scales; Maffei et al., 1994).
In an initial study, van der Donk et al. (1991) compared 58 women with FPHL to women who sought treatment for other nonapparent dermatologic conditions and male patients with FPHL (using standardized psychological techniques via several self-reporting 5-6 point scales that assessed hair problems, dermatological complaints, self-esteem, and self-rated depression). The researchers found that women with FPHL experienced poor social adequacy and increased psychosocial problems, which they attributed to their hair loss, than patients in the comparable groups (van der Donk et al., 1991).
The same authors conducted a follow-up study that was composed of 58 women with FPHL who opted to participate in the psychological standardized interview portion of the study (Van Der Donk et al., 1994). The interview consisted of two parts. The first portion included open-ended questions on topics related to alopecia and the second portion consisted of close-ended questions about the participant’s behavior and feelings in certain situations related to their hair loss. In 88% of the study participants, alopecia had a negative effect on their daily life, 50% experienced social problems, and 75% reported that their hair problems caused a negative self-esteem (Van Der Donk et al., 1994).
Cash et al. (1993) conducted a study of 60 men and 96 women with FPHL to assess the psychological impact of diagnosis within each group using a series of standardized inventories including the 13-item Social Desirability Scale that assesses the influence of defensive response sets, the 69-item Multidimensional Body-Self Relations Questionnaire that assesses body image attitudes, the 16-item Texas Social Behavior Inventory that assesses social self-esteem, the 24-item Levenson Locus of Control Scale, the 13-item Self-Consciousness Scale, two 10-item indices to assess life satisfaction, and the 15-item Impact of Event Scale that measures the past week’s stressfulness of the condition for which the patient sought treatment. A total of 52% of women ranked their emotional stress due to FPHL as very-to-extremely upsetting. They also reported increased social anxiety, poorer self-esteem, a negative body image, and a sense of powerlessness because of their diagnosis (Cash, 1999).
Another case-controlled prospective study of 115 women with FPHL and 97 age-matched control patients found that even female sexual function was considerably compromised as a result of FPHL on the basis of a Turkish version of the Female Sexual Function Index that looks at six specific domains, including desire, arousal, lubrication, orgasm, satisfaction, and pain scores. A score of 2 was the lowest possible and 36 was the highest possible. A higher score indicates better function (Sancak et al., 2016).
For men, pattern hair loss may be the result of a normal phenomenon, but women believe just the opposite. Many authors have discussed that patients’ reactions to their diagnosis have more to do with self-perception than the objective clinical course because of the aforementioned feelings and the effects FPHL has on women’s self-image. At times, women use extraordinary measures to cope with and mask their diagnosis as well as feelings of being less attractive, including concealing thin areas with remaining scalp hair, covering thin areas with a hat, or using cosmetic products to camouflage the areas of hair loss (Cash, 1999). Patients may also avoid situations that can aggravate their distress, such as windy weather or brightly lit environments, to minimize bringing attention to themselves (Cash, 1999, Fossati et al., 1993). Compensatory measures such as men growing a beard, exercising to change one’s physique, and buying expensive clothes are also steps taken with the hope of improving one’s self-esteem (Famenini et al., 2015).
Several treatment options are available for patients with FPHL, including topical minoxidil (foam or solution; Blume-Peytavi et al., 2007), oral spironolactone (Famenini et al., 2015), oral finasteride (Kelly et al., 2016), low-level laser therapy (Afifi et al., 2017, Avci et al., 2014), PRP (Laird et al., 2017, Puig et al., 2016), microneedling (Kelly et al., 2016), and hair transplantation (Callender et al., 2014). Although a host of new emerging therapies is on the horizon, it is important to note that a substantial length of time is warranted to achieve a satisfactory outcome (Kelly et al., 2016).
The most common, localized, nonscarring alopecia is AA (Al-Mutairi and Eldin, 2011). One study observed 2962 new patients with AA including 1926 male and 1036 female patients. Women exhibited a 13.6% incidence of severe disease with marked disturbances in their social life, which forced them to miss school or social meetings, change their hairstyles, or alter their type of clothing. In another hospital-based, cross-sectional study, the QoL of 60 patients with AA (16 men and 44 women) was assessed via three self-administered questionnaires (DLQI, Short Form-36 [SF-36] Health Survey, and Skindex-29).
The SF-36 is a 36-item QoL questionnaire that looks at physical functioning, physical role activities, pain in different areas of the body, health perceptions, vitality, emotional role activities, mental health, and social functioning. A standardized scale measures all scores from 0 to 100, and a higher score correlates with a better QoL. The Skindex-29 is a specific 29-question tool used by dermatologists to assess how a skin disorder affects a patient’s burden of symptoms, emotional state, and social functioning. Scores range from 0 to 100 on the Skindex-29 scale, and a higher score correlates with a poorer QoL. Although the QoL was not as impaired when patients with psoriasis were compared with those with atopic dermatitis, the presence of alopecia universalis showed a poorer QoL in the results from the disease-specific questionnaires used in this study (Jankovic et al., 2016).
Other psychological problems including depression and anxiety disorders have also been reported in smaller studies of patients with AA by Colon et al. (1991) using the structured psychiatric Diagnostic Interview Schedule (a structured interview administered by nonclinician interviewers to diagnose the major psychiatric disorders in accordance with the DSM-IV in a reliable and valid fashion) and the DLQI, Beck Depression Inventory (a 21-question, multiple-choice, self-reported inventory that helps measure the severity of depression), and the SF-36 questionnaire (Ghajarzadeh et al., 2012).
Shi et al. (2013) analyzed data from 532 patients with AA (73% women) from the National Alopecia Areata Registry. The registry includes three validated instrument tools to assess patients’ QoL including the DLQI, Skindex-16 (a single-page version of the Skindex-29 that accurately measures how much patients are bothered by their skin condition), and a brief version of the Fear of Negative Evaluation Scale (a 12-item version of the Fear of Negative Evaluation Scale that is applied to many areas of research in personality and social psychology). The mean QoL scores were compared with those of healthy controls and patients with other skin diseases. Overall, the results showed that greater than 50% of patients experienced a poorer QoL and risk factors for a poorer QoL when including patient age between 20 to 50 years, female sex, changes in physical appearance as a result of hair loss, and feeling that hair loss led to a change in social status or job status (Shi et al., 2013).
More recently, Liu et al. (2016) did a systematic review of all published studies of QoL in patients with AA. The researchers examined 11 studies with more than 1986 patients and four measuring tools including the DLQI, Skindex-16, SF-36, and the Pediatric Quality of Life Inventory Parent and Child Versions (which evaluate physical function, psychological function, and social function in many disorders with scores that range from 0 [poorer QoL] to 100 [high QoL]; Liu et al., 2016). The conclusions from this review showed that patients with AA consistently had lower QoL scores with lower scores that correlated with greater scalp involvement. Although patients with AA had QoL scores that were comparable with those of patients with psoriasis or atopic dermatitis, their overall emotional wellbeing was at times poorer compared with that of the control patients with atopic dermatitis or psoriasis (Liu et al., 2016).
Rencz et al. (2016) conducted a meta-analysis of 2530 patients (using more than 14 QoL measuring tools including the DLQI and SF-36 as the most common tools) and demonstrated that AA significantly reduced patients’ QoL across emotional, mental health, and vitality domains. Similar to other studies, the degree of scalp involvement and a history of either anxiety or depression resulted in a poorer QoL. Interestingly, this meta-analysis showed that wearing wigs had a positive impact on the QoL of patients with AA (Rencz et al., 2016).
Very few studies have discussed QoL in patients with primary cicatricial (scarring) alopecia. In one study, 44 women with scarring (19 patients; mean age: 46.5 years) and nonscarring (25 patients; mean age: 39 years) alopecia were included to evaluate the psychosocial impact of hair loss (Katoulis et al., 2015). In the group of patients with scarring alopecia, the diagnoses included LPP, DLE, FFA, and folliculitis decalvans. In the nonscarring alopecia group, the diagnoses included FPHL, telogen effluvium, and AA. Four QoL tools were used for each patient. In addition to the DLQI, patients were evaluated for anxiety and depression using the Hospital Anxiety and Depression Scale (a self-reported, 14-item rating scale that measures depression and anxiety), and self-esteem using the Rosenberg Self-Esteem Scale. For loneliness/social isolation, the researchers used the University of California, Los Angeles Loneliness Scale. The results demonstrated that the psychological impact (mainly anxiety and depression) was more significant in patients with scarring alopecia compared with patients with nonscarring alopecia. This may be due to the fact that patients with scarring alopecia have associated symptomatology (i.e., itching, dysesthesias, and pain) along with hair loss, a poor response to therapy, hair loss that is often irreversible, and thus a poorer prognosis (Katoulis et al., 2015).
Another pilot study examined the QoL of 50 black women with scarring alopecia in South Africa (ages 21-79 years). The most prevalent type was LPP and FFA was the most common pattern (Dlova et al., 2016). This cross-sectional study occurred over a 6-month period and utilized the Alopecia Quality of Life Indicators (A-QLI) questionnaire that was developed by Fabbrocini et al. (2013). This questionnaire gathers demographic and general clinical data on subjects, such as age, ethnicity, duration of disease, diagnosis, and percentage of scalp affected. The A-QLI also includes 24 questions on subjective symptoms, objective signs, and social/sexual relationship concerns, and scores range from 0 to 100 with 0 denoting a positive impact on QoL and 100 a negative impact on QoL.
On the basis of a calculated A-QLI score of 67.7 with the patients in this study, the results showed a negative impact on QoL in patients with scarring alopecia and particularly in younger patients. Among 74% of patients, the QLI score was greater than 50, which correlated with significant impairment of QoL including anxiety and low self-esteem. This study demonstrates that hair loss is a global concern and various ethnic groups can be affected (Dlova et al., 2016).
Chiang et al. (2015) conducted a study of 105 patients with primary cicatricial alopecia using the DLQI, Hospital Anxiety and Depression Scale, and revised Illness Perception Questionnaire, which identifies specific groups of patients’ beliefs, positive and negative, about their illness including whether patients believe that their symptoms are related to their illness, beliefs about the duration and course of illness over time, perceived consequences of living with the illness, belief whether the illness is controllable by medications, and whether patients understand their illness. Each entity is graded on a 5-point Likert scale. For the first three entities on symptoms, duration, and consequences, a higher score reflects a longer duration and more negative consequences in relation to the disease. For controllability and understanding of the disease, higher scores indicate that patients do understand their disease and it is controllable by medication (Chiang et al., 2015).
Similar to previous studies, anxiety and depression were experienced among patients with alopecia and higher disease activity had a significant correlation with depression. In addition, this study demonstrated that patients perceived their primary cicatricial alopecia as a chronic condition with emotional impairment and that they had little control over the condition and its treatment (Chiang et al., 2015).
Conclusion
Alopecia has been shown in multiple studies to have a psychosocial impact in both men and women; however, the impact may be more severe and devastating in women. The psychological burden of hair loss in women is significant and should not be overlooked. Both scarring and nonscarring forms of alopecia have been shown to have a negative impact on QoL. Early recognition and treatment of hair loss in women is imperative and recommendations should include medical and cosmetic treatments, camouflage techniques, and participation in alopecia support groups.
References
- Afifi L., Maranda E.L., Zarei M., Delcanto G.M., Falto-Aizpurua L., Kluijfhout W.P. Low-level laser therapy as a treatment for androgenetic alopecia. Lasers Surg Med. 2017;49(1):27–39. doi: 10.1002/lsm.22512. [DOI] [PubMed] [Google Scholar]
- Al-Mutairi N., Eldin O.N. Clinical profile and impact on quality of life: seven years experience with patients of alopecia areata. Indian J Dermatol Venereol Leprol. 2011;77(4):489–493. doi: 10.4103/0378-6323.82411. [DOI] [PubMed] [Google Scholar]
- Avci P., Gupta G.K., Clark J., Wikonkal N., Hamblin M.R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014;46(2):144–151. doi: 10.1002/lsm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume-Peytavi U., Kunte C., Krisp A., Garcia Bartels N., Ellwanger U., Hoffmann R. Comparison of the efficacy and safety of topical minoxidil and topical alfatradiol in the treatment of androgenetic alopecia in women. J Dtsch Dermatol Ges. 2007;5(5):391–395. doi: 10.1111/j.1610-0387.2007.06295.x. [DOI] [PubMed] [Google Scholar]
- Callender V.D., Lawson C.N., Onwudiwe O.C. Hair transplantation in the surgical treatment of central centrifugal cicatricial alopecia. Dermatol Surg. 2014;40(10):1125–1131. doi: 10.1097/DSS.0000000000000127. [DOI] [PubMed] [Google Scholar]
- Cash T.F. The psychosocial consequences of androgenetic alopecia: a review of the research literature. Br J Dermatol. 1999;141(3):398–405. doi: 10.1046/j.1365-2133.1999.03030.x. [DOI] [PubMed] [Google Scholar]
- Cash T.F., Price V.H., Savin R.C. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol. 1993;29(4):568–575. doi: 10.1016/0190-9622(93)70223-g. [DOI] [PubMed] [Google Scholar]
- Chiang Y.Z., Bundy C., Griffiths C.E., Paus R., Harries M.J. The role of beliefs: lessons from a pilot study on illness perception, psychological distress and quality of life in patients with primary cicatricial alopecia. Br J Dermatol. 2015;172(1):130–137. doi: 10.1111/bjd.13259. [DOI] [PubMed] [Google Scholar]
- Colon E.A., Popkin M.K., Callies A.L., Dessert N.J., Hordinsky M.K. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32(3):245–251. doi: 10.1016/0010-440x(91)90045-e. [DOI] [PubMed] [Google Scholar]
- Dlova N.C., Fabbrocini G., Lauro C., Spano M., Tosti A., Hift R.H. Quality of life in South African Black women with alopecia: a pilot study. Int J Dermatol. 2016;55(8):875–881. doi: 10.1111/ijd.13042. [DOI] [PubMed] [Google Scholar]
- Esmat S.M., Hegazy R.A., Gawdat H.I., Abdel Hay R.M., Allam R.S., El Naggar R. Low level light-minoxidil 5% combination versus either therapeutic modality alone in management of female patterned hair loss: a randomized controlled study. Lasers Surg Med. 2017;49(9):835–843. doi: 10.1002/lsm.22684. [DOI] [PubMed] [Google Scholar]
- Fabbrocini G., Panariello L., De Vita V., Vincenzi C., Lauro C., Nappo D. Quality of life in alopecia areata: a disease-specific questionnaire. J Eur Acad Dermatol Venereol. 2013;27(3):e276–81. doi: 10.1111/j.1468-3083.2012.04629.x. [DOI] [PubMed] [Google Scholar]
- Famenini S., Slaught C., Duan L., Goh C. Demographics of women with female pattern hair loss and the effectiveness of spironolactone therapy. J Am Acad Dermatol. 2015;73(4):705–706. doi: 10.1016/j.jaad.2015.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati A., Rinaldi F., Maestroni L. Trichologic consultation and personality disorders. G Ital Dermatol Venereol. 1993;128:101–108. [Google Scholar]
- Ghajarzadeh M., Ghiasi M., Kheirkhah S. Associations between skin diseases and quality of life: a comparison of psoriasis, vitiligo, and alopecia areata. Acta Med Iran. 2012;50(7):511–515. [PubMed] [Google Scholar]
- Hamilton J.B. Patterned loss of hair in man: types and incidence. Ann N Y Acad Sci. 1951;53(3):708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- Herskovitz I., Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11(4):e9860. doi: 10.5812/ijem.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic S., Peric J., Maksimovic N., Cirkovic A., Marinkovic J., Jankovic J. Quality of life in patients with alopecia areata: a hospital-based cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30(5):840–846. doi: 10.1111/jdv.13520. [DOI] [PubMed] [Google Scholar]
- Katoulis A.C., Christodoulou C., Liakou A.I., Kouris A., Korkoliakou P., Kaloudi E. Quality of life and psychosocial impact of scarring and non-scarring alopecia in women. J Dtsch Dermatol Ges. 2015;13(2):137–142. doi: 10.1111/ddg.12548. [DOI] [PubMed] [Google Scholar]
- Kelly Y., Blanco A., Tosti A. Androgenetic alopecia: An update of treatment options. Drugs. 2016;76(14):1349–1364. doi: 10.1007/s40265-016-0629-5. [DOI] [PubMed] [Google Scholar]
- Laird M.E., Lo Sicco K.I., Reed M.L., Brinster N.K. Platelet-rich plasma for the treatment of female pattern hair loss: a patient survey [e-pub ahead of print] Dermatol Surg. 2017 doi: 10.1097/DSS.0000000000001109. [DOI] [PubMed] [Google Scholar]
- Liu L.Y., King B.A., Craiglow B.G. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75(4):806–812.e3. doi: 10.1016/j.jaad.2016.04.035. [DOI] [PubMed] [Google Scholar]
- Maffei C., Fossati A., Rinaldi F., Riva E. Personality disorders and psychopathologic symptoms in patients with androgenetic alopecia. Arch Dermatol. 1994;130(7):868–872. [PubMed] [Google Scholar]
- Olsen E.A. The midline part: an important physical clue to the clinical diagnosis of androgenetic alopecia in women. J Am Acad Dermatol. 1999;40(1):106–109. doi: 10.1016/s0190-9622(99)70539-6. [DOI] [PubMed] [Google Scholar]
- Olsen E.A. Female pattern hair loss. J Am Acad Dermatol. 2001;45(3 Suppl):S70–80. doi: 10.1067/mjd.2001.117426. [DOI] [PubMed] [Google Scholar]
- Olsen E.A., Bergfeld W.F., Cotsarelis G., Price V.H., Shapiro J., Sinclair R. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on cicatricial alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48(1):103–110. doi: 10.1067/mjd.2003.68. [DOI] [PubMed] [Google Scholar]
- Puig C.J., Reese R., Peters M. Double-blind, placebo-controlled pilot study on the use of platelet-rich plasma in women with female androgenetic alopecia. Dermatol Surg. 2016;42(11):1243–1247. doi: 10.1097/DSS.0000000000000883. [DOI] [PubMed] [Google Scholar]
- Rencz F., Gulacsi L., Pentek M., Wikonkal N., Baji P., Brodszky V. Alopecia areata and health-related quality of life: a systematic review and meta-analysis. Br J Dermatol. 2016;175(3):561–571. doi: 10.1111/bjd.14497. [DOI] [PubMed] [Google Scholar]
- Rogers N.E. Hair transplantation update. Semin Cutan Med Surg. 2015;34(2):89–94. doi: 10.12788/j.sder.2015.0131. [DOI] [PubMed] [Google Scholar]
- Sancak E.B., Oguz S., Akbulut T., Uludag A., Akbas A., Kurt O. Female sexual dysfunction in androgenetic alopecia: case-control study. Can Urol Assoc J. 2016;10(7-8):E251–6. doi: 10.5489/cuaj.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweiger E.S., Boychenko O., Bernstein R.M. Update on the pathogenesis, genetics and medical treatment of patterned hair loss. J Drugs Dermatol. 2010;9(11):1412–1419. [PubMed] [Google Scholar]
- Shi Q., Duvic M., Osei J.S., Hordinsky M.K., Norris D.A., Price V.H. Health-related quality of life (HRQoL) in alopecia areata patients-a secondary analysis of the National Alopecia Areata Registry Data. J Investig Dermatol Symp Proc. 2013;16(1):S49–50. doi: 10.1038/jidsymp.2013.18. [DOI] [PubMed] [Google Scholar]
- Van Der Donk J., Hunfeld J.A., Passchier J., Knegt-Junk K.J., Nieboer C. Quality of life and maladjustment associated with hair loss in women with alopecia androgenetica. Soc Sci Med. 1994;38(1):159–163. doi: 10.1016/0277-9536(94)90311-5. [DOI] [PubMed] [Google Scholar]
- van der Donk J., Passchier J., Knegt-Junk C., van der Wegen-Keijser M.H., Nieboer C., Stolz E. Psychological characteristics of women with androgenetic alopecia: a controlled study. Br J Dermatol. 1991;125(3):248–252. doi: 10.1111/j.1365-2133.1991.tb14749.x. [DOI] [PubMed] [Google Scholar]
- Williamson D., Gonzalez M., Finlay A.Y. The effect of hair loss on quality of life. J Eur Acad Dermatol Venereol. 2001;15(2):137–139. doi: 10.1046/j.1468-3083.2001.00229.x. [DOI] [PubMed] [Google Scholar]
- Zhuang X.S., Zheng Y.Y., Xu J.J., Fan W.X. Quality of life in women with female pattern hair loss and the impact of topical minoxidil treatment on quality of life in these patients. Exp Ther Med. 2013;6(2):542–546. doi: 10.3892/etm.2013.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]