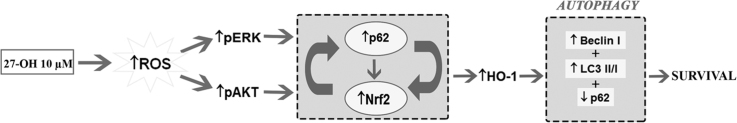
Abstract
Autophagy has been shown to be stimulated in advanced atherosclerotic plaques by metabolic stress, inflammation and oxidized lipids. The lack of published studies addressing the potential stimulation of pro-survival autophagy by oxysterols, a family of cholesterol oxidation products, has prompted our study. Thus, the goal of the current study is to elucidate the molecular mechanism of the autophagy induced by 27-hydroxycholesterol (27-OH), that is one of the most abundant oxysterols in advanced atherosclerotic lesions, and to assess whether the pro-oxidant effect of the oxysterol is involved in the given response. Here we showed that 27-OH, in a low micromolar range, activates a pro-survival autophagic response in terms of increased LC3 II/LC3 I ratio and Beclin 1, that depends on the up-regulation of extracellular signal-regulated kinase (ERK) and phosphoinositide 3-kinase (PI3K)/Akt pathways as a potential result of an intracellular reactive oxygen species increase provoked by the oxysterol in human promonocytic U937 cells. Moreover, 27-OH induced autophagy is dependent on the relation between nuclear factor erythroid 2 p45-related factor 2 (Nrf2)-dependent antioxidant response and p62. The data obtained highlight the involvement of cholesterol oxidation products in the pathogenesis of oxidative stress related chronic diseases like atherosclerosis. Therefore, deeply understanding the complex mechanism and generating synthetic or natural molecules targeting this survival mechanism might be very promising tools in the prevention of such diseases.
Abbreviations: 27-OH, 27-hydroxycholesterol; 7-K, 7-ketocholesterol; AMPK, AMP-activated protein kinase; ARE, antioxidant response element; DPI, diphenyleneiodonium chloride; ERK, extracellular signal-regulated kinase; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate fluorochrome; HNE, 4-hydroxy-trans-2-nonenal; HO-1, heme oxygenase-1; JNK, c-Jun NH2-terminal kinase; Keap1, Kelch-like ECH-associated protein 1; MAPKs, mitogen-activated protein kinases; MEK, mitogen-activated protein kinase ERK kinase; mTOR, mammalian target of rapamycin; NAC, N-acetyl cysteine; Nrf2, nuclear factor erythroid 2 p45-related factor 2; oxLDLs, oxidized low density lipoproteins; PAGE, SDS-polyacrylamide gel electrophoresis; SMCs, smooth muscle cells; PI3K, phosphoinositide 3-kinase; PKCs, protein kinases C; PVDF, polyvinylidene difluoride; ROS, reactive oxygen species; RT-PCR, reverse transcription–polymerase chain reaction; siRNA, small interfering RNA
Keywords: Oxysterols, 27-hydroxycholesterol, Autophagy, ROS, Survival signaling
Graphical abstract
A hypothetical scheme for 27-hydroxycholesterol (27-OH) involvement in autophagy modulated survival signaling.
Highlights
-
•
Low concentrations of 27-OH activates autophagy in human promonocytic cells.
-
•
Pharmacological inhibition of ERK or Akt pathways prevents autophagy.
-
•
Nrf2/p62 pathway affects the autophagy response induced by 27-OH.
1. Introduction
Cholesterol oxidation products have been shown to modulate several signaling pathways, thus biochemical effects of these compounds are varied from their strong pro-inflammatory, pro-apoptotic and pro-fibrogenic properties [1]. Among them, scientist are increasingly drawing attention to oxysterols, a family of 27-carbon molecules originated from cholesterol oxidation, that appear significantly involved in the progression of inflammatory-based chronic pathologies, such as atherosclerosis, neurodegenerative diseases, diabetes mellitus and cancer [2], [3], [4], [5]. The oxysterol 27-hydroxycholesterol (27-OH), one of the most represented oxysterols in the peripheral blood of healthy individuals, is enzymatically produced by 27-hydroxylase (CYP27A1) [6]. Nowadays, 27-OH has been shown to act as a competitive ligand for liver X receptor [7], [8] and a selective estrogen receptor [9], thus it appears to modulate intracellular signals related to cancer cell growth and atherosclerosis progression [10], [11].
It is now well accepted that certain oxysterols, including 27-OH, modulate not only the pro-apoptotic and pro-inflammatory response but also cell survival pathways in various cell types [12], [13]. In particular, they trigger signaling transduction pathways either pro-apoptotic or anti-apoptotic, depending on cell types, oxysterol concentration and exposure time [14], [15]. Moreover, this dual effect exerted by oxysterols appeared to be modulated via a mechanism involving reactive oxygen species (ROS) [16], [17], [18]. Very recently, our group provided a clear evidence of modulation of survival signaling with a low micromolar concentration (10 µM) of 27-OH in U937 human promonocytic cells through extracellular signal-regulated kinase (ERK) and the phosphoinositide 3-kinase (PI3K)/Akt pathways [19]. Moreover, the marked up-regulation of the ERK/Akt axis was, in turn, able to quench the oxidative imbalance exerted by the oxysterol itself through the induction of nuclear factor erythroid 2 p45-related factor 2 (Nrf2) antioxidant defense system.[20]. Namely, a significant induction of Nrf2 and synthesis of Nrf2 target enzymes, including heme oxygenase-1 (HO-1) and NAD(P)H:quinone oxireductase, appeared to be dependent on the activation of ERK and Akt pathways and fundamentally responsible for the observed oxysterol-induced pro-survival response, at low concentration (10 µM), in promonocytic cells. However, relatively high concentration of 27-OH (100 µM) did not exert any survival effect in U937 cells while a constant pro-oxidant effect induced by the oxysterol was demonstrated.
Macroautophagy (generally referred to as autophagy) breaks down protein aggregates and damaged organelles in order to maintenance cellular recycling during stress conditions. Autophagy is a multi-step physiological process that starts with sequestration of the cell material to be eliminated within double-membrane vesicles, known as autophagosomes; then, these autophagosomes fuse with lysosomes for subsequent degradation [21]. It is generally accepted that even if autophagy serves as a cell survival mechanism, under certain stress conditions its excessive enhancement may lead to cell death [22]. Accumulating data point to a critical role for autophagy at intersection of life and death of cancer cells, while a cross-talk exists between autophagy and classical apoptosis. It is now well established that autophagy appears regulated by ROS [23]. Hence, the redox regulation of autophagy has been observed in various pathological conditions but how autophagy induction affects these processes remains incompletely understood. Recent studies have highlighted the important role of oxidized lipids, including 4-hydroxy-trans-2-nonenal (HNE) and oxysterols, in the regulation of autophagy in atherosclerosis. Additional work is needed to elucidate the up-stream signal transduction pathways responsible for the effects of oxysterols on autophagy modulation [24], [25].
The Kelch-like ECH-associated protein 1 (Keap1)-Nrf2 signaling pathway functions as one of the key regulator of the cellular defense system against oxidative stress [26]. Nrf2 is a redox-sensitive transcription factor, that is sequestered in the cytoplam by binding to Keap1; however, under oxidative stress, Nrf2 dissociates from Keap1 and translocates into the nucleus where it binds to antioxidant response element (ARE) sequences, codifying for antioxidant enzymes [27], [28]. A growing body of evidence has demonstrated the interplay between the Keap1/Nrf2 system and selective autophagy mainly relied on the p62/SQSTM1 protein (hereafter referred to as p62), which is an autophagy substrate and also a receptor for degradation of ubiquitinated protein aggregates, in turn associating with both LC3 and ubiquitin [29], [30]. Evidence suggests that p62 interacts with the Nrf2-binding site of Keap1 and competes with Nrf2 for the interaction with Keap1 which results in transcriptional activation of Nrf2 and its target gene expression [30], [31]. Moreover, Nrf2 regulates p62 expression by binding directly to ARE binding motif of the p62 promoter, suggesting a positive feedback loop between these two proteins [32].
To investigate in depth the molecular mechanism of Nrf2-modulated 27-OH induced survival signaling [19], [20], in the present paper we aimed to elucidate the potential stimulation of pro-survival autophagy by low micromolar concentration of 27-OH and to explore the relevance between Nrf2 pathway and selective autophagy, with regard to the modulation of p62 protein. The obtained data showed that 27-OH up-regulated autophagic proteins including LC3 and Beclin 1 in a ROS-dependent manner in human promonocytic cells. Indeed, autophagy seems involved in 27-OH-induced Nrf2 modulated survival signaling.
2. Materials and methods
2.1. Cell culture and treatments
The human promonocytic cell line U937 was cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin/streptomycin (Pan Biotech GmbH, Aidenbach, Germany) at 37 °C with 5% CO2. For treatments, cells were dispensed at 1 × 106/ml and made quiescent through overnight incubation in serum-free medium; they were then placed in RPMI-1640 medium with 2% FBS and treated with 27-OH (Steraloids Inc., Newport, RI, USA) dissolved in ethanol. In some experiments, cells were pre-treated with PD98059 (40 μM), a selective inhibitor of mitogen-activated protein kinase ERK kinase 1/2 (MEK1/2), or with LY294002 (25 μM), a selective inhibitor of PI3K (Calbiochem, EMD Millipore Corporation, Billerico, MA, USA), or with N-acetylcysteine (NAC) (100 μM), an antioxidant compound (Sigma, Darmstadt, Germany), or with diphenyleneiodonium chloride (DPI) (50 μM), an inhibitor of NADPH oxidase (Sigma, St. Louis, MO, USA), or with Bafilomycin A1 (10 nM), an inhibitor of late phase of autophagy (Sigma) or with Torin 1 (250 nM), an inhibitor of mammalian target of rapamycin 1/2 (mTOR1/2) (Tocris Bioscience, UK). Moreover, some cells were incubated with an antibody against Nrf2 (Santa Cruz Biotechnology Inc., Denver, CO, USA). Final concentrations and incubation times for all experiments are reported in the figure legends.
2.2. Protein extraction and immunoblotting
The human promonocytic U937 cells were treated as indicated and harvested by centrifugation at 300g for 30 s. Following resuspension in 1 ml of ice-cold PBS and transfer to 1.5 ml microfuge tubes, cells were spun at 2640g for 30 s. The pellet was lysed by incubation for 30 min in 200 μl of cold cell lysis buffer containing 50 mM Tris/HCl (pH 8), 150 mM NaCl, 1 mM phenylmethanesulfonyl, protease and phosphatase inhibitor cocktails (Roche, F. Hoffman-La Roche Ltd., Basel, Switzerland), and 1% Nonidet P-40 (v/v) (Sigma). After centrifugation at 2640g for 10 min, the supernatant containing the total protein extract was removed and stored at − 80 °C. Protein concentrations were determined by the BSA protein assay (Bio-Rad, Munich, Germany).
Following the lysis of cells, 30 μg of whole cell lysates were mixed with loading buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromophenol blue, 0.125 M Tris/HCl pH:6.8) (Sigma), denatured at 95 °C for 5 min, and separated on 10–15% SDS-polyacrylamide gel electrophoresis (PAGE), followed by blotting onto polyvinylidene difluoride (PVDF) membranes (Amersham Pharmacia Biotech, Freiburg, Germany). The membranes were blocked with 5% blocking reagent (Amersham) in PBS-Tween 20 and incubated with appropriate primary antibodies (anti-Nrf2, anti-Beclin 1, anti-p62, anti-LC3I and anti-LC3 II, and anti-β-actin) and horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology Inc., Beverly, MA) in 5% blocking reagent. For some experiments, to analyze the levels of p62 50 μg of total proteins were immunoprecipitated with 4 μl of anti-p62 primary antibody (Cell Signaling Technology Inc.), purified on Protein A Sepharose resin (GE Healthcare Europe, Milan, Italy), separated on 10% PAGE, and then electroblotted as described above. The membranes were immunoblotted overnight at 4 °C with an anti-p62 (1:1500), and then with an anti-rabbit secondary antibody (1:2000) (Cell Signaling Technology Inc.). After the required washes with PBS 1×-Tween 20, proteins were finally analyzed using an enhanced chemiluminescence detection system and exposed to Hyperfilm-ECL (Amersham Pharmacia Biotech). Immunoblots were visualized using X-ray film and quantified by densitometry using ImageJ software. All experiments were repeated at least three times.
2.3. siRNA transfection
Small interfering RNA (siRNA) was used for transient gene knock-down studies. The siRNAs used were Hs_BECN1_1 (Beclin 1), NFE2L2 s9493 (Nrf2), and siRNA #1 for the negative control (scramble siRNA) (Life Technologies, Thermo Fisher Scientific, Monza, Italy). Negative control corresponds to a siRNA with nonspecific sequence. Transfection of Beclin 1 and Nrf-2 specific siRNAs and scramble siRNAs were performed following the manufacturer's instructions. Briefly, 50 nM of siRNAs were mixed with 25 μl of transfection reagent solution (NeoFX, Life Technologies) and left at room temperature for 10 min in RPMI medium with 1% FBS and without antibiotics. After 48 h of reverse transfection, cells were harvested, incubated with 27-OH and assays were carried out to evaluate cell death, protein levels and autophagy induction. The transfection efficiency validated by quantitative reverse transcription-polymerase chain reaction (RT-PCR), was approximately 71% for Beclin 1 and Nrf2 (data not shown).
2.4. Cell death, viability and proliferation assay
Apoptotic cell death was determined by an Annexin V affinity assay. U937 cells seeded in 12-well plates were treated as indicated, transferred to flow cytometry tubes, and harvested by centrifugation at 300 g for 5 min. The cells were then resuspended in 1 ml of cold PBS and again centrifuged at 300g for 5 min. After removal of supernatant, the cells were incubated in Annexin V buffer (140 mM Hepes, 10 mM NaCl, 2.5 mM CaCl2, pH 7.4) (Sigma) containing 1% (v/v) Annexin V conjugated with fluorescein isothiocyanate fluorochrome (FITC) (Alexis Biochemicals, Enzo Life Sciences, Inc., Farmingdale, NY) for 15 min in the dark. Cells were analyzed by FACS (FACS Canto, Becton Dickinson, Franklin Lakes, NJ).
In order to detect cell viability and proliferation, exposure to 27-OH was determined by WST-1 (Cell Proliferation Reagent) following the manufacturer's instructions (Roche Diagnostics, GmbH, Penzberg, Germany). Briefly, cells in 96-well plates were treated as indicated and 10 μl of WST-1 reagent was added to each well, after which the plates were incubated for 4 h at 37 °C. Absorbance was measured with a microtiter plate reader (Bio-Rad, CA, USA) at a test wavelength of 450 nm and a reference wavelength of 655 nm.
2.5. Statistical analyses
All the illustrated results represent one of at least three independent experiments with similar outcomes. All data are presented as means ± standard deviation (S.D.). Statistical significance of the results was analyzed by the Student's t-tail test and *P < 0.05, **P < 0.01 and ***P < 0.001 were considered statistically significant.
3. Results
3.1. Induction of autophagy by low concentrations of 27-OH in U937 cells
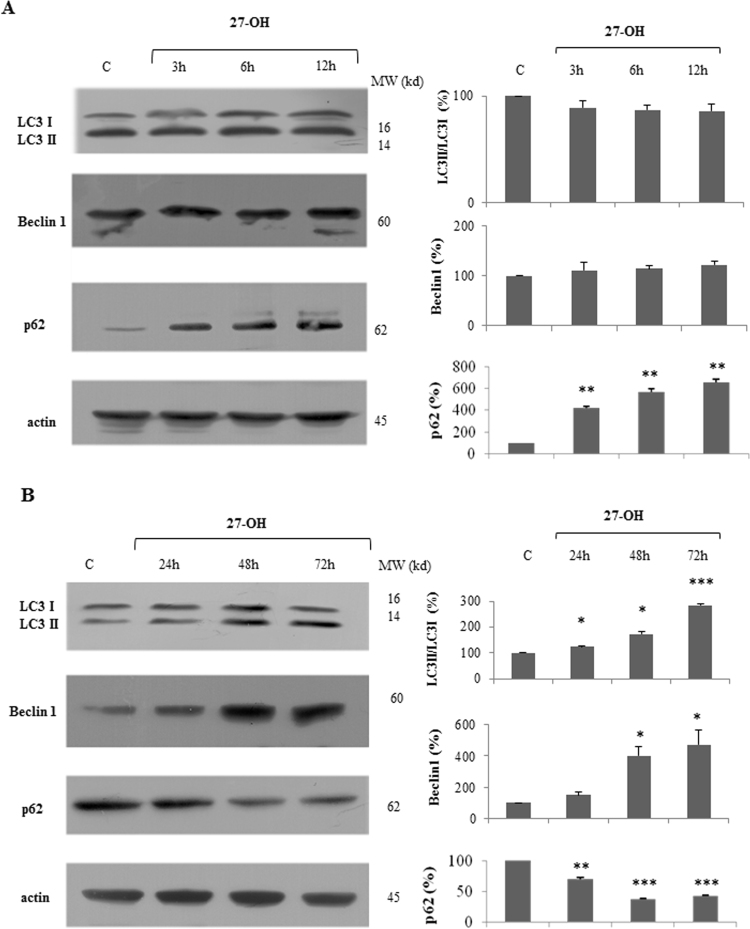
To determine whether autophagy could be induced by low concentration (10 μM) of 27-OH in U937 cells, we investigated the protein levels of some autophagy-specific markers: LC3, Beclin 1 and p62 by immunoblot analysis at different time points. As shown in Fig. 1A, the single addition of 10 μM 27-OH to U937 cells did not show any significant modulation of both the total cellular levels of Beclin 1 and the ratio of LC3 II/LC3 I between 3 and 12 h. Consistently, p62 protein expression was significantly increased in response to low dose of 27-OH in a time-dependent manner. Furthermore, at different time points (24, 48 and 72 h), we observed that LC3 II/LC3 I ratio and Beclin 1 were significantly increased especially after 48 h cell treatment (Fig. 1B). Moreover, the down-regulation of p62 protein levels demonstrated an increasing autophagic flux in promonocytic cells starting from 24 h cell treatment.
Fig. 1.
27-hydroxycholesterol (27-OH) induces autophagy in U937 cells. Protein levels of LC3 I and LC3 II, Beclin 1 and p62 in U937 cells treated with 10 μM 27-OH for 3, 6 and 12 h (A) and for 24, 48 and 72 h (B) were analyzed by Western blotting. Untreated cells were taken as controls. One blot representative of three experiments is shown. Histograms represent the mean values ± S.D. of three independent experiments; LC3 I, LC3 II, Beclin 1 and p62 band intensities were quantified using ImageJ software; densitometric measurements were normalized against the corresponding actin levels and expressed as percentage of control value; *p < 0.05, **p < 0.01 and ***p < 0.001 vs. control group.
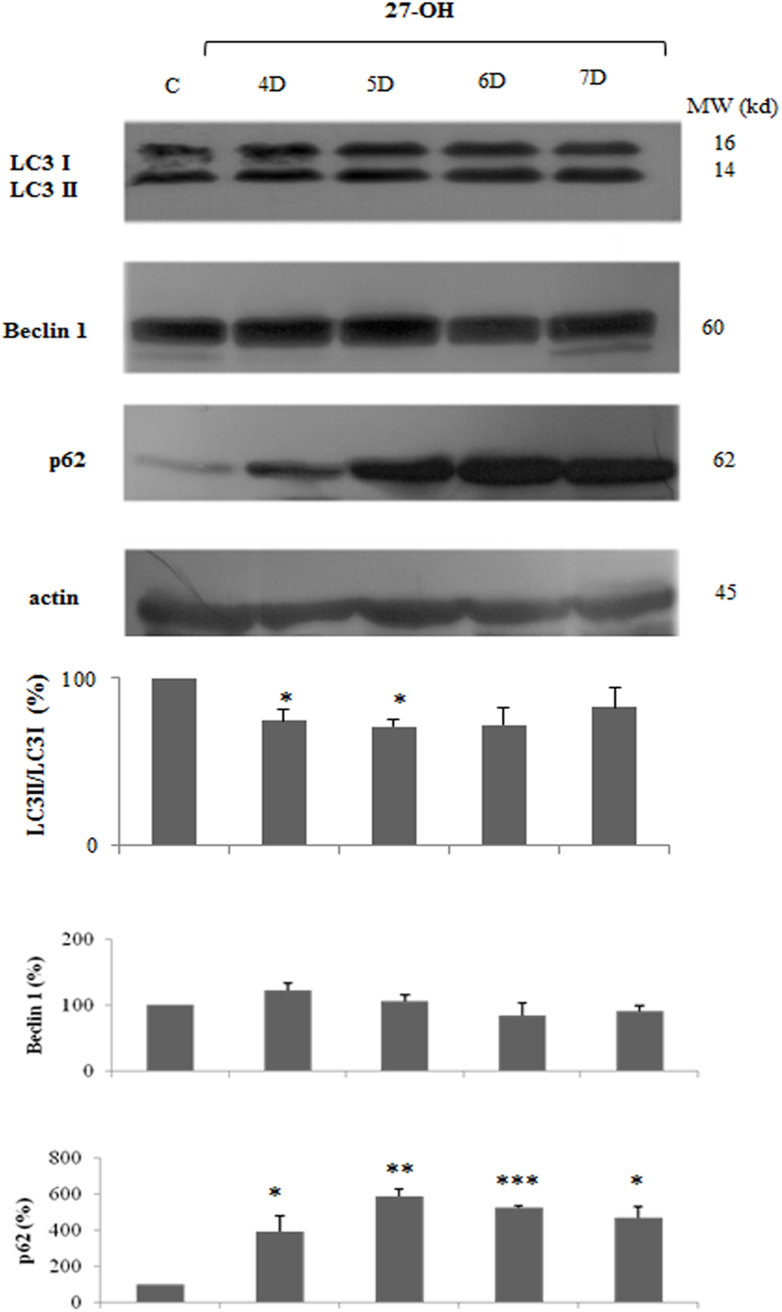
We further investigated the autophagic markers between 4 days and 1 week: 27-OH did not stimulate but, on the contrary, it down-regulated LC3 II/LC3 I ratio and the total levels of Beclin 1, while p62 protein levels were significantly up-regulated till 1 week (Supplementary Fig. 1). These results demonstrated that a low dose of 27-OH promoted cellular autophagic process in U937 cells only between 24 and 72 h. In our previous report [19], in the time frame corresponding to the pro-apoptotic effect of 27-OH, i.e. between 4 days and 1 week after cell treatment, autophagy induction was down-regulated. This finding suggest that the autophagic response may protect cells against apoptosis, thus favoring cell survival.
3.2. Impact of ERK and PI3K/Akt signaling on 27-OH induced autophagy
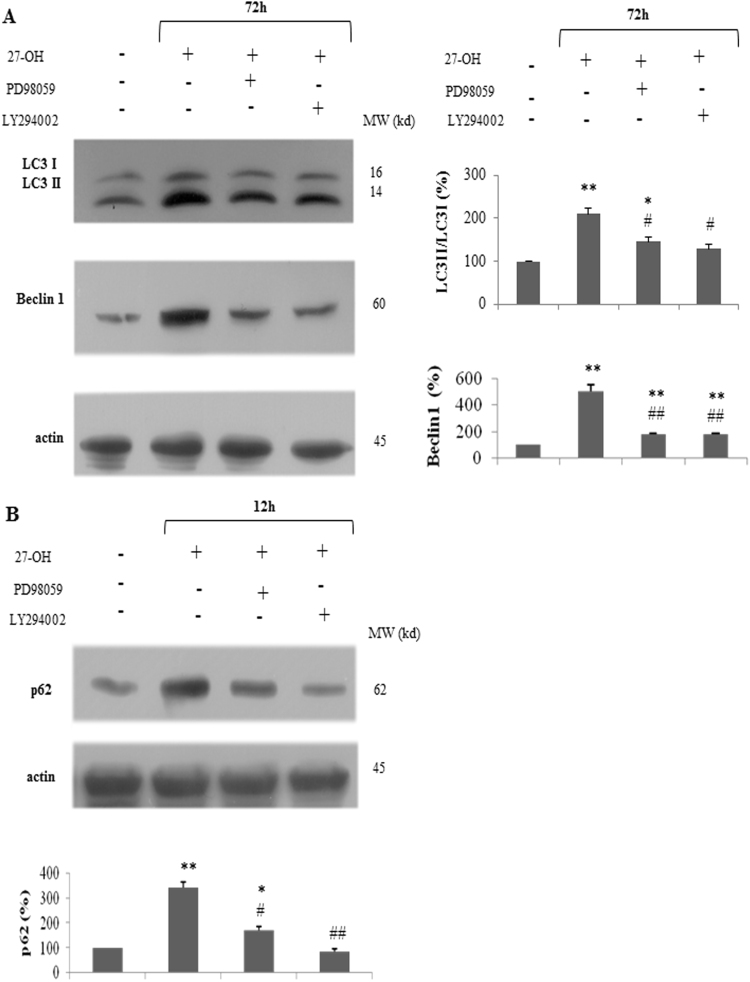
Previous studies showed that ERK and Akt play role in autophagy regulation [33], [34], [35], [36]. To investigate whether ERK and PI3K/Akt signaling pathways were involved in 27-OH-mediated autophagy in U937 cells, a series of immunoblotting experiments employing selective inhibitors of these kinases were performed. Firstly, U937 cell aliquots were pre-treated with the MEK/ERK inhibitor PD98059 (40 μM) or with the PI3K/Akt inhibitor LY294002 (25 μM) in the presence of 10 μM 27-OH. As the Fig. 2A shows, 40 μM PD98059 significantly reduced LC3 II/LC3 I ratio and Beclin 1 protein levels after 72 h treatment with 27-OH. As expected, cell pre-incubation with 25 μM LY294002 effectively prevented the 27-OH-mediated up-regulation of LC3 II levels and Beclin 1 protein levels. Further, as reported in Fig. 2B, the effect of these selective inhibitors was also determined for p62 protein after 12 h treatment with 27-OH. It has been clearly demonstrated that pre-incubation with ERK/Akt inhibitors resulted in a net prevention of the 27-OH-induced p62 expression observed after 12 h treatment with the oxysterol. Taken together, these data suggest that ERK and PI3K/Akt survival pathways are involved in 27-OH induced autophagy and stimulate p62 synthesis in U937 promonocytic cells.
Fig. 2.
Inhibition of MEK/ERK and PI3K/Akt signaling pathways down-regulates autophagy. U937 cells were either treated with 10 μM 27-hydroxycholesterol (27-OH) alone or pre-incubated with PD98059 (40 μM) or LY294002 (25 μM) 45 min before 27-OH treatment. Untreated cells were taken as controls. LC3 I and LC3 II, Beclin 1 (A) and p62 (B) protein levels were analyzed by Western blotting. One blot representative of three experiments is shown. Histograms represent the mean values ± S.D. of three independent experiments; LC3 I, LC3 II, Beclin 1 and p62 band intensities were quantified using ImageJ software; densitometric measurements were normalized against the corresponding actin levels and expressed as percentage of control value; *p < 0.05 and **p < 0.01 vs. control group; #p < 0.05 and ##p < 0.01vs. 27-OH.
3.3. Involvement of autophagy in 27-OH induced survival response
As we demonstrated that 27-OH-induced redox-modulated survival signaling [19], we further explored the role of autophagy in delaying the apoptotic cell death.
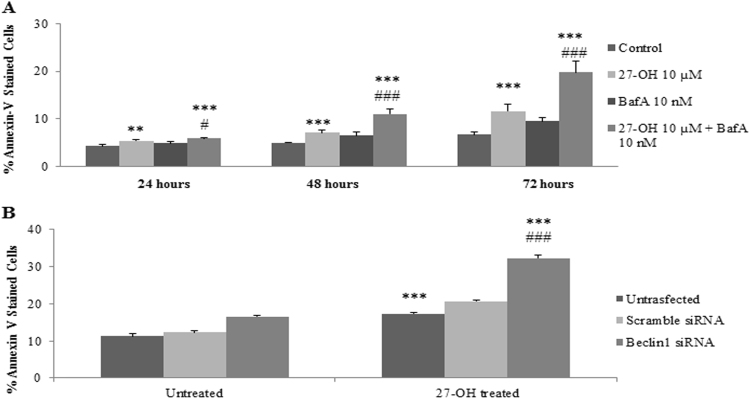
To ascertain whether autophagy is involved in 27-OH induced survival response in U937 cells, autophagy inhibition related experiments were carried out. Flow cytometric analysis showed that in aliquots of U937 cells pre-incubated with a non cytotoxic concentration (10 nM) of Bafilomycin A1, a well-known inhibitor of autophagosomal lysosome degradation, the number of Annexin V positive cells markedly increased compared to cells treated with 27-OH alone (Fig. 3A). Indeed, the percentage of apoptotic cells was about 11% after 48 h treatment with 10 nM Bafilomycin A1 plus 10 μM 27-OH, which is approximately the percentage of Annexin V positive cells induced by 27-OH alone after 72 h cell treatment.
Fig. 3.
Inhibition of autophagy anticipates the apoptotic effect of 27-hydroxycholesterol (27-OH). (A) Pharmacological inhibition of autophagy: U937 cells were either treated with 27-OH (10 μM) alone or preincubated with Bafilomycin A1 (10 nM) 1 h before 27-OH treatment. Untreated cells were taken as controls. (B) Molecular inhibition of autophagy: percentage of Annexin V-stained cells after scramble siRNA or Beclin 1 siRNA transfection followed by 27-OH treatment. Untreated and unstransfected cells were taken as controls. FACS analysis was performed by harvesting and FITC-Annexin V staining the cells, to analyze the effect of autophagy modulation on cell death response. Histograms represent the mean values ± S.D. of four independent experiments; **p < 0.01 and ***p < 0.001 vs. untreated control group; #p < 0.05 and ###p < 0.001 vs. 27-OH.
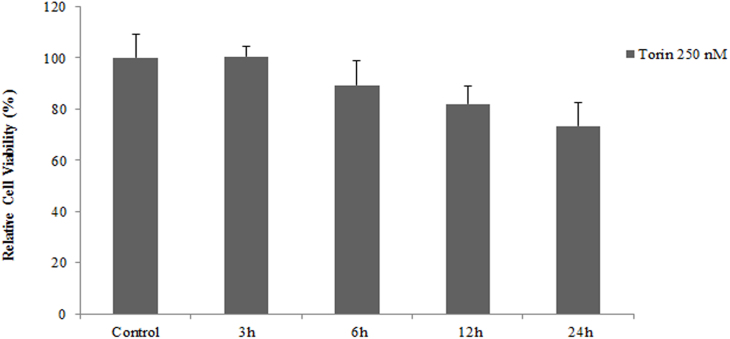
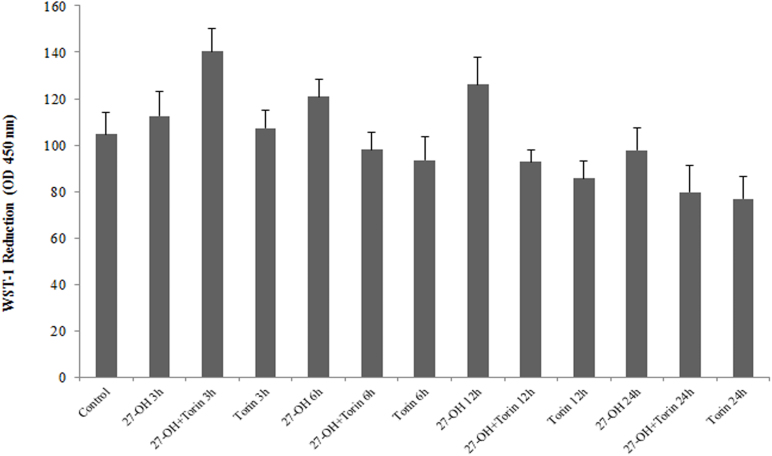
To validate these observations, the actual role of autophagy in the 27-OH-mediated survival was further studied by knocking-down Beclin 1 expression. The expression of Beclin 1 protein was remarkably suppressed in U937 cells transfected with Beclin 1 siRNA when compared to control group (data not shown). Accordingly, cell transfection with specific Beclin 1 siRNA determined a sharp increase in the number of apoptotic cells after 24 h incubation with 10 μM 27-OH (Fig. 3B). Namely, the percentage of apoptotic cells nearly doubled from 17% in the absence of siRNA to 32% in the presence of Beclin 1 siRNA. In addition to these results, using Torin 1, mTOR inhibitor at noncytotoxic concentration of 250 nM (Supplementary Fig. 2) we observed significant induction of cell viability and proliferation versus the control after 3 h cell treatment as measured by WST-1 assay (Supplementary Fig. 3). These results support that prevention of autophagy-dependent survival signaling accelerated the apoptosis induced by the low micromolar concentration of the oxysterol.
3.4. Dependence of 27-OH induced autophagy on the intracellular rise of ROS
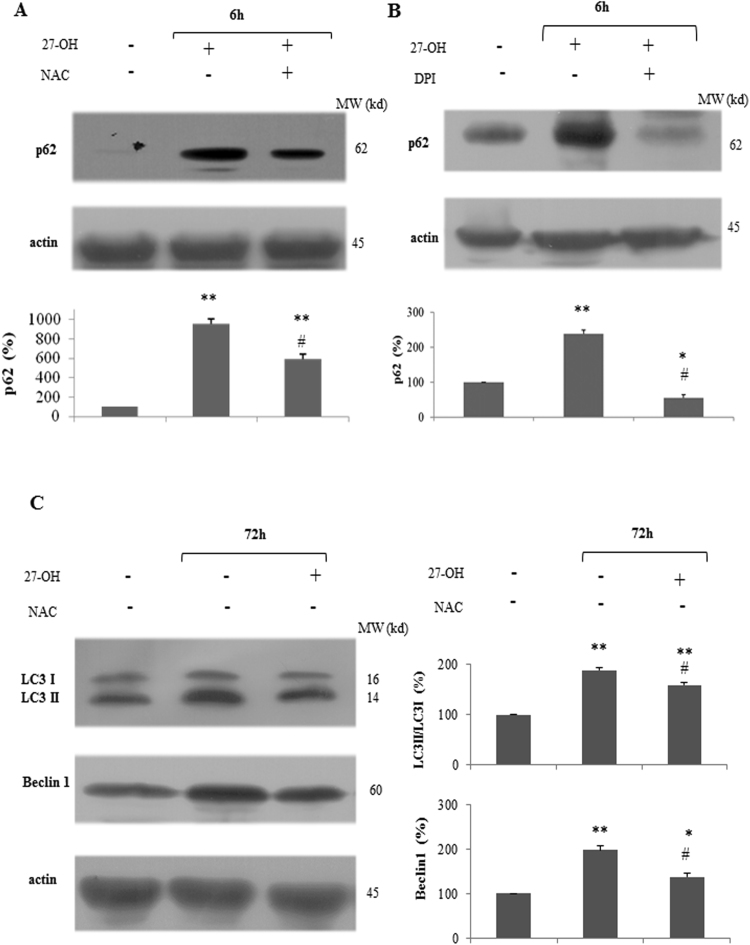
Accumulated evidence shows that ROS modulate the autophagy pathways and the interplay between ROS and autophagy signaling plays a role in diverse pathological conditions [37], [38]. Based on the above results and literature, we wondered whether 27-OH induced autophagy and p62 up-regulation could be mediated by intracellular generation of ROS. In our previous report we showed an increase of ROS cellular levels operated by 27-OH through the derangement of mitochondrial membrane potential and activation of NADPH oxidase type 2 (Nox-2) [19]. In the light of these findings, to investigate whether Nox-2 sourced ROS could play a critical role in mediating 27-OH-induced autophagy, U937 cells were pre-treated with the antioxidant NAC or DPI, a NADPH oxidase inhibitor, prior to 6 h challenge with 10 μM 27-OH, and p62 protein levels were analyzed by immunoblotting (Fig. 4A,B). We found that 27-OH-dependent increase of p62 protein levels was significantly inhibited by 100 μM NAC. Moreover, as shown in Fig. 4B, cell pre-treatment (45 min) with 50 μM DPI was able to nearly suppress the induction of p62 after 6 h 27-OH treatment. Further, we examined the effect of ROS up-regulation on LC3 II/LC3 I ratio and cell Beclin 1 content. As shown in Fig. 4C, pre-incubation of cells with the antioxidant NAC partially prevented 27-OH-dependent increase of LC3 II accumulation and total Beclin 1 after 72 h oxysterol treatment.
Fig. 4.
Modulation of autophagy induction by N-acetyl cysteine (NAC) and diphenyleneiodonium chloride (DPI). Effect of NAC or DPI on 27-OH-dependent p62 induction: cells were pre-incubated (1 h) with 100 μM NAC (A) or 50 μM DPI (B) and then treated with 27-OH (10 μM) for 6 h. Effect of NAC on 27-OH-dependent autophagy induction: cells were pre-incubated (1 h) with 100 μM NAC and then treated with 10 μM 27-OH for 72 h (C). Untreated cells were taken as controls. Protein levels of p62, LC3 I and LC3 II, and Beclin 1 were analyzed by Western blotting. One blot representative of three experiments is shown. Histograms represent the mean values ± S.D. of three independent experiments; LC3 I, LC3 II, Beclin 1, and p62 band intensities were quantified using ImageJ software; densitometric measurements were normalized against the corresponding actin levels and expressed as percentage of control value *p < 0.05 and **p < 0.01 vs. control group; #p < 0.05 vs. 27-OH.
3.5. Impact of autophagy on 27-OH induced p62 and Nrf2 activation
Recently, several studies suggest that Nrf2 pathway protects cells from oxidative stress via the induction of autophagy [39], [40]. Noteworthy, induction of autophagic adaptor p62 by oxidative stress is mediated by Nrf2, and p62 contributes to the activation of Nrf2 through creating a positive feed-back loop [31], [32], [41]. Our previous study has ascertained that Nrf2-ARE antioxidant pathway has a crucial role in 27-OH-induced survival signaling in human promonocytic cells to protect cells from pro-oxidant stimuli [20].
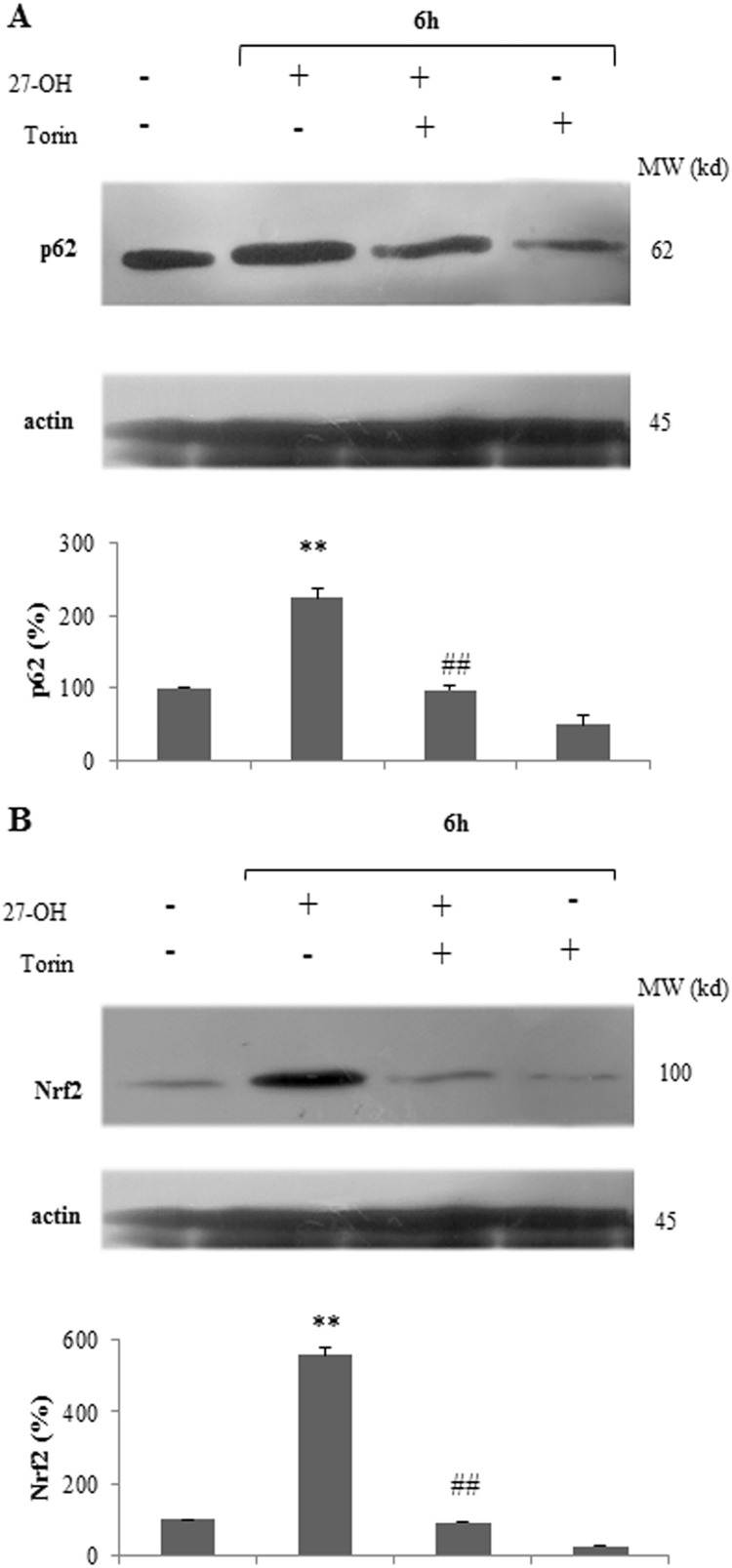
Based on our previous findings and published reports, we investigated whether autophagy contributes to 27-OH exerted induction of Nrf2 in U937 promonocytic cells. Firstly, U937 cells were pre-treated with the mTOR inhibitor Torin 1 (250 nM) in the presence or absence of 10 μM 27-OH. In order to examine whether 27-OH-mediated p62 expression would be affected by addition of Torin 1 which can induce autophagy, the Western blotting was utilized (Fig. 5A). The obtained results revealed that induction of autophagy by Torin 1 in oxysterol-treated cells afforded to prevent 27-OH induced up-regulation of total cell level of p62. Statistical analysis of data obtained after 6 h treatment showed that p62 synthesis was almost halved in Torin 1 plus 27-OH treated cells in comparison to cells treated with 27-OH alone. Further, as shown in Fig. 5B, induction of autophagy signaling with Torin 1 markedly prevented the 27-OH-mediated up-regulation of Nrf2 protein content, as monitored after 6 h oxysterol treatment. Statistical analysis showed that total cell level of Nrf2 was significantly decreased in combined treated cells as to cells treated with the oxysterol alone.
Fig. 5.
27-Hydroxycholesterol (27-OH) induced Nrf2 in U937 cells is mediated by autophagy. U937 cells were either treated with 27-OH (10 μM) alone or pre-incubated with Torin 1 (250 nM) before the 27-OH treatment. Untreated cells were taken as controls. (A) p62 and (B) Nrf2 protein levels were analyzed by Western blotting. One blot representative of three experiments is shown. Histograms represent the mean values ± S.D. of three independent experiments; p62 and Nrf2 band intensities were quantified using ImageJ software; densitometric measurements were normalized against the corresponding actin levels and expressed as percentage of control value *p < 0.05 and **p < 0.01 vs. control group; #p < 0.05 and ##p < 0.01 vs. 27-OH.
Taken together, these results suggest that the relevance of Nrf2 pathway and autophagy contributes to redox regulated 27-OH induced survival response in U937 promonocytic cells incubated in the presence of low micromolar amount of oxysterol.
3.6. The relation between Nrf2/p62 and autophagy modulated by 27-OH
The protein p62 directly interacts with the Nrf2- binding site of Keap1, which results in constitutive activation of Nrf2 and consequently in Nrf2 target gene expression, including antioxidant enzyme genes [32]. Thus, up-regulation of p62 is due to autophagy dysfunction that leads to transcriptional activation of the Nrf2. In turn, Nrf2 regulates p62 expression through direct binding to ARE binding motif of the p62 promoter.
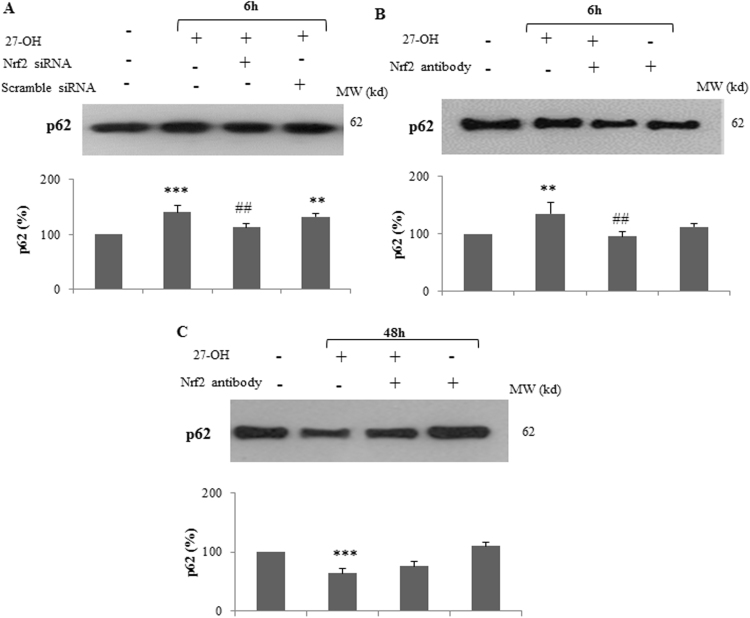
Based on these evidence, the potential involvement of Nrf2 in p62 regulation by 27-OH was investigated. Cells were transfected for 48 h with Nrf2 siRNA and then incubated with 10 μM 27-OH for 6 h that, in agreement to the data shown in Fig. 1A, is the time at which p62 is significantly up-regulated by 27-OH. Following Nrf2 gene knock-down, p62 protein levels are significantly lower than in cells treated with 27-OH alone (Fig. 6A). To support this hypothesis, the activity of Nrf2 was blocked co-treating the U937 cells with a specific antibody anti-Nrf2 and 27-OH (10 μM). Blocking Nrf2 activation negatively affects p62 synthesis (Fig. 6B).
Fig. 6.
The relation between p62 and Nrf2 leading to authophagy. U937 cells were transfected with a specific Nrf2 siRNA for 48 h and then incubated with 27-OH (10 μM) for 6 h (A). Other cells were incubated with 27-OH (10 μM) in the presence of a specific antibody against Nrf2 for 6 h (B) or 48 h (C). Untreated cells were taken as controls. Each blot is representative of three experiments. Histograms represent mean values ± S.D. of three experiments and they are expressed as percentage of control value. **p < 0.01 and ***p < 0.001 vs. untreated control group; ##p < 0.01 vs. 27-OH.
Since activation of Nrf2 positively regulates the autophagy signaling pathway by up-regulating its target gene HO-1 and consequently LC3 [42], an event that leads to the reduction of p62 levels as evidence that autophagy has been activated.
Cells were incubated with anti-Nrf2 antibody and 27-OH (10 μM) for 48 h to verify whether Nrf2 is involved in the promotion of authophagy reversing the effect of 27-OH on p62 levels reported in Fig. 1B. As expected, blocking Nrf2 with a selective antibody, the degradation of p62 was markedly prevented (Fig. 6C). Cell incubation with the anti-Nrf2 antibody alone did not affect the levels of p62 compared with untreated cells (Fig. 6B,C).
4. Discussion
Oxysterols are quantitatively relevant components of oxidized low density lipoproteins (oxLDLs) and their multifaceted biochemical properties were well-characterized in vascular cells [2], [3], [17]. 27-OH is one of the most represented oxysterols in the human circulation and also in atherosclerotic lesions [43]. On behalf of its important role in pathophysiology, 27-OH has been established as a good ligand of nuclear receptors that leads to modulate cell viability, immunological response and metabolism [7], [8]. In this relation, eliciting survival and functional signals, modulated by 27-OH, might contribute to expand the knowledge about the molecular mechanisms of the pathogenesis of several oxysterol-related diseases.
Autophagy is a dynamic process in which long lived proteins and cellular organelles are removed by autophagosomes. It has become accepted that although autophagy has been regarded as cell survival mechanism, under certain conditions, excessive autophagy may lead to a non-apoptotic type of cell death [22]. Despite the increasing interest in understanding the mechanism of autophagy, there is limited information on how cellular signaling pathways regulate this complex process. It is now well established that autophagy is stimulated in advanced atherosclerotic plaques by inflammation and oxidized lipids where progression of atherosclerosis is characterized by formation of these plaques [44]. The protective role of autophagy in atherosclerosis involves the removal of damaged organelles by autophagy in response to mild oxidative stress which contributes to cellular recovery [45]. While the effects of autophagy on pathological processes including atherosclerosis are complex, further studies are required to distinguish the relation between oxysterols and autophagy.
In the current study, we examined the mechanism by which 27-OH induces autophagy using U937 promonocytic cells. We demonstrated that the ROS-mediated induction of autophagy by low micromolar concentration of 27-OH is primary responsible for the observed oxysterol-induced pro-survival response and stimulated expression of antioxidant Nrf2. Moreover, the critical role of Nrf2 in controlling autophagy via p62 in response to oxidative stress was observed. To the best of our knowledge, this is the first demonstration of the stimulation of pro-survival autophagy by 27-OH at low micromolar concentrations in promonocytic cells. In consistent with our findings, Martinet et al. showed that 7-ketocholesterol (7-K) stimulated autophagy in human vascular smooth muscle cells (SMCs) in terms of myelin figure formation, LC3 processing and also intense protein ubiquitination [24]. In contrast, in another study using SMCs, high concentrations of 7-K treatment caused autophagic cell death via autophagic vesicle formation with LC3 processing. This autophagic response induced by 7-K attenuated SMC apoptosis induced by low concentrations of lipophilic statins by suppressing caspase activation [46]. This different outcome of the autophagic response may be due to the relatively greater cytotoxicity of 7-K than 27-OH.
As summarized in Fig. 1, autophagy up-regulation in terms of Beclin 1 induction, increase of LC3 II and p62 down-regulation were observed between 24 and 72 h of cell incubation, this coinciding with the end of the period 27-OH caused slight reduction of cell viability and proliferation as previously reported [19]. Moreover, the decrease of autophagy induction, as marked by the induction of p62 between 4 up to 7 days oxysterol treatment was consistent with the interval considered the pro-apoptotoic effect of 10 μM 27-OH was demonstrated, suggesting that oxysterol activates cytoprotective autophagy which provides sustained cellular survival (Supplementary Fig. 1).
In addition, as depicted in Fig. 3, blockade of autophagy subsequently enhanced the apoptosis in U937 promonocytic cells. Using chemical and molecular approaches, Bafilomycin A1 and Beclin 1 siRNA administration respectively, we found that induction of autophagy was obligatory for low concentrations of 27-OH-induced survival response, most likely an adaptive survival strategy that recycles aggregates and eliminates damaged organelles, to protect against the apoptotic cell death. This observation is consistent with previous work showing that 7-K induces NADPH oxidase-mediated autophagy as a cellular protective response that extenuated endoplasmic reticulum stress/apoptosis pathway induced by 7-K in human aortic SMCs [47]. An additional study shows that autophagy induction by 7-oxysterols, mixed in an atheroma-relevant proportion reduced 7-oxysterols induced cell death by reducing lysosomal membrane permeabilization where induced autophagy may have a cytoprotective effect to limit necrotic core formation in atheroma progression [48]. On the other hand, treatment of 158 N murine oligodendrocytes with a relatively high concentration (50 μM) of 7-K was shown to trigger a type of death termed oxyapoptophagy with the concomitent induction of autophagy and apoptosis [49]. Not only oxysterols but also low levels of oxLDLs, were demonstrated to activate autophagic markers in vascular cell lines whereas high concentrations of oxLDLs induce apoptosis by modulating oxLDL scavenger receptor 1 [50]. It has to be pointed out that the outcome of an autophagic response depends on oxidant concentration and exposure time. In fact, these oxidized lipids are able to trigger pro-survival autophagy upon treatment of cells only with low amount.
In the last years, it has been accepted that serine/threonine kinases involving AMP-activated protein kinase (AMPK), mTOR, protein kinases C (PKCs), Akt and mitogen-activated protein kinases (MAPKs), such as ERK, p38 and c-Jun NH2-terminal kinase (JNK), have regulatory role in various steps of autophagy [51]. Indeed, these kinases either positively or negatively regulate autophagic response depending on the inducer and the cellular context [15]. In a recent study it has been shown that HNE, a lipid peroxidation-derived aldehyde, stimulated pro-survival autophagic response interms of LC3 II formation in a JNK-dependent mechanism in rat aortic SMCs [52]. Our results demonstrate that ERK and Akt phosphorylation is required for autophagy induction and also p62 up-regulation in 27-OH-treated U937 cells. Indeed, as shown in Fig. 2, pharmacological inhibition of these pathways by the MEK/ERK inhibitor PD98059 and the PI3K/Akt inhibitor LY294002 respectively, prevented LC3 II formation and decreased Beclin 1 and p62 expression in response to oxysterol treatment. These metabolic inhibitors were used at the relevant concentrations reported in the literature and also tested as not being cytotoxic in the experimental model adopted [19]. Accumulating research now interest in modulating autophagy for cancer therapy. Of note, MEK/ERK and PI3K/Akt pathways are activated in several cancers and contribute to cancer progression [53], [54]. Therefore, targeting these pathways to regulate autophagy could be a promising tool for cancer therapy to maintain autophagic response at sufficient levels.
Accumulating data suggest that ROS trigger autophagy but, in turn, autophagy reduces ROS levels [23]. Our results are in agreement because 27-OH mediated autophagy induction interms of LC3 II formation and Beclin 1 protein expression was suppressed by treating the promonocytic cells with the antioxidant NAC (Fig. 4C). Consistently, 7-K was shown to be involved in pro-survival autophagic response, specifically Beclin 1 transcription that regulated by a mitochondrial enzyme, proline oxidase-dependent ROS in oxLDL challenged cancer cells [55]. Another critical observation was that cell supplementation with either NAC or DPI prevents the induction of p62 expression by 27-OH (Fig. 4A,B). Several recent reports establish a role for p62 in delivering oxidized proteins to autophagosomes for the removal of these protein aggregates [23]. Moreover, Mathew et al. indicated p62 as a new critical player in cancer showing that impairment in autophagy leads to p62 accumulation, thereby promoting tumorigenesis [56]. Notably, a feed-forward loop between ROS and p62 contributes to this process; in particular, excessive ROS induces p62 expression while, in turn, enforced p62 expression induces ROS production as part of an amplifying loop, thereby promoting genome instability. Besides the demonstration that induction of autophagic response in 27-OH treated cells was ROS-dependent, of note autophagy modulation by chemical inhibitor and inducers in turn was able to regulate ROS levels.
As mentioned in the Introduction, the redox-sensitive transcription factor Nrf2 and autophagy are both involved in oxidative stress response to antagonize cellular stressing signals by promoting a series of antioxidant programs. In fact, as shown in Fig. 5, both p62 and Nrf2 synthesis as we expected were abrogated by mTOR inhibitor Torin 1. It can be suggested that, in response to oxidative stress, autophagy and Nrf2 would be in negative interaction with each other, in particular if Nrf2 antioxidant pathways is suppressed, the further activation of autophagy is required to reduce ROS accumulation to ensure adaptive cell survival. Consistently, suppression of autophagy at particular stages with different inhibitors enhanced Nrf2 expression and nuclear translocation upon ROS stimulation in pancreatic cancer cells [57]. Certainly all results obtained by using inhibitors should be taken with caution. Hence, all our data concerning the modulation of Nrf2 via autophagy are fully consistent among them and further indirectly validated by using both Nrf2 siRNA and antibody (Fig. 6).
Taken together, these data suggest that autophagic responses induced by 27-OH could be associated, in part, to Nrf2 antioxidant response which stimulates autophagy via p62 protein and promotes cell survival during oxidative stress. Emerging studies pointed that understanding the relationship between the Nrf2 pathway and autophagy will advance our knowledge of the progression of oxidative stress-related diseases. Namely, Nrf2 pathway is induced in response to pro-oxidant stimuli that up-regulated p62 expression which can further promote autophagy to protect cells from oxidative damage.
To summarize, our data support the hypothesis that in 27-OH treated cells induction of autophagy by low concentrations of the oxysterol is required for the cell survival, as well as for the induction of cytoprotective responses, i.e. stimulation of Nrf2 antioxidant response (graphical abstract). Notably, autophagy induced by 27-OH is, in part, mediated by oxidative stress and that an increase in intracellular ROS is quenched by activated survival signals including redox-sensitive Nrf2. Both mitochondrial depolarization and Nox-2 activity contribute to the pro-oxidant effect of the oxysterol. Moreover, 27-OH-induced MEK/ERK and PI3K/Akt pathways play regulatory role in oxysterol-mediated pro-survival autophagy. In the current study, we speculated that ROS plays a key role in autophagy and in the activation of Nrf2 pathway. In this connection, 27-OH leads to p62 accumulation thus activating Nrf2 pathway, while the oxysterol pro-oxidant effect activates Nrf2 pathway against the intracellular ROS generation. It can be suggested that autophagy and Nrf2 activation might function as a adaptive survival response and resistance mechanism against the previously established pro-apoptotic action of 27-OH [19] to delay macrophage apoptosis that favor growth and destabilization of advanced atherosclerotic plaques [58]. Further studies are needed to assess the interplay between autophagy and apoptosis in our proposed model. Since both Nrf2 and autophagy contribute to the chemoresistance, and the interplay between cell death pathways and autophagy has important pathophysiological consequences, developing therapeutic interventions to target these crosstalks are exciting prospects for future studies.
Acknowledgements
Authors wish to thank Sabanci University (Turkey), Tubitak (Cost Eu-Ros, 113Z463), and the University of Turin (Italy) for supporting this work. Beyza Vurusaner is supported by Sabanci University Post-doctoral research scholarship.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.05.010.
Contributor Information
Beyza Vurusaner, Email: beyzav@sabanciuniv.edu.
Simona Gargiulo, Email: simona.gargiulo@unito.it.
Gabriella Testa, Email: gabriella.testa@unito.it.
Paola Gamba, Email: paola.gamba@unito.it.
Gabriella Leonarduzzi, Email: gabriella.leonarduzzi@unito.it.
Giuseppe Poli, Email: giuseppe.poli@unito.it.
Huveyda Basaga, Email: huveyda@sabanciuniv.edu.
Appendix A. Supplementary material
Fig. S1.
No up-regulation of autophagic proteins in U937 cells treated with 27-hydroxycholesterol (27-OH) for 4 up to 7 days.
Fig. S2.
Effect of Torin 1 (250 nM) on U937 cell viability.
Fig. S3.
Induction of autophagy accelerated the survival effect of 27-hydroxycholesterol (27-OH).
References
- 1.Sottero B., Gamba P., Gargiulo S., Leonarduzzi G., Poli G. Cholesterol oxidation products and disease: an emerging topic of interest in medicinal chemistry. Curr. Med. Chem. 2009;16:685–705. doi: 10.2174/092986709787458353. [DOI] [PubMed] [Google Scholar]
- 2.Leonarduzzi G., Sottero B., Poli G. Oxidized products of cholesterol: dietary and metabolic origin, and proatherosclerotic effects (review) J. Nutr. Biochem. 2002;13:700–710. doi: 10.1016/s0955-2863(02)00222-x. [DOI] [PubMed] [Google Scholar]
- 3.Poli G., Biasi F., Leonarduzzi G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013;1:125–130. doi: 10.1016/j.redox.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown A.J., Watts G.F., Burnett J.R., Dean R.T., Jessup W. Sterol 27-hydroxylase acts on 7-ketocholesterol in human atherosclerotic lesions and macrophages in culture. J. Biol. Chem. 2000;275:27627–27633. doi: 10.1074/jbc.M004060200. [DOI] [PubMed] [Google Scholar]
- 5.Gamba P., Testa G., Sottero B., Gargiulo S., Poli G., Leonarduzzi G. The link between altered cholesterol metabolism and Alzheimer's disease. Ann. N.Y. Acad. Sci. 2012;1259:54–64. doi: 10.1111/j.1749-6632.2012.06513.x. [DOI] [PubMed] [Google Scholar]
- 6.Honda A., Yamashita K., Hara T., Ikegami T., Miyazaki T., Shirai M., Xu G., Numazawa M., Matsuzaki Y. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J. Lipid Res. 2009;50:350–357. doi: 10.1194/jlr.D800040-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Janowski B.A., Grogan M.J., Jones S.A., Wisely G.B., Kliewer S.A., Corey E.J., Mangelsdorf D.J. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. USA. 1999;96:266–271. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janowski B.A., Willy P.J., Devi T.R., Falck J.R., Mangelsdorf D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 9.Umetani M., Domoto H., Gormley A.K., Yuhanna I.S., Cummins C.L., Javitt N.B., Korach K.S., Shaul P.W., Mangelsdorf D.J. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat. Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 10.Gargiulo S., Gamba P., Testa G., Rossin D., Biasi F., Poli G., Leonarduzzi G. Relation between TLR4/NF-κB signaling pathway activation by 27-hydroxycholesterol and 4-hydroxynonenal, and atherosclerotic plaque instability. Aging Cell. 2015;14:569–581. doi: 10.1111/acel.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson E.R., Wardell S.E., Jasper J.S., Park S., Suchindran S., Howe M.K., Carver N.J., Pillai R.V., Sullivan P.M., Sondhi V., Umetani M., Geradts J., McDonnell D.P. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemaire-Ewing S., Prunet C., Montange T., Vejux A., Berthier A., Bessède G., Corcos L., Gambert P., Néel D., Lizard G. Comparison of the cytotoxic, pro-oxidant and pro-inflammatory characteristics of different oxysterols. Cell Biol. Toxicol. 2005;21:97–114. doi: 10.1007/s10565-005-0141-2. [DOI] [PubMed] [Google Scholar]
- 13.Lizard G., Miguet C., Besséde G., Monier S., Gueldry S., Neel D., Gambert P. Impairment with various antioxidants of the loss of mitochondrial transmembrane potential and of the cytosolic release of cytochrome c occurring during 7-ketocholesterol-induced apoptosis. Free Radic. Biol. Med. 2000;28:743–753. doi: 10.1016/s0891-5849(00)00163-5. [DOI] [PubMed] [Google Scholar]
- 14.Leonarduzzi G., Poli G., Sottero B., Biasi F. Activation of the mitochondrial pathway of apoptosis by oxysterols. Front. Biosci. 2007;12:791–799. doi: 10.2741/2102. [DOI] [PubMed] [Google Scholar]
- 15.Vurusaner B., Leonarduzzi G., Gamba P., Poli G., Basaga H. Oxysterols and mechanisms of survival signaling. Mol. Asp. Med. 2016;49:8–22. doi: 10.1016/j.mam.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Trevisi L., Bertoldo A., Agnoletto L., Poggiani C., Cusinato F., Luciani S. Antiapoptotic and proliferative effects of low concentrations of 7??-Hydroxycholesterol in human endothelial cells via ERK activation. J. Vasc. Res. 2009;47:241–251. doi: 10.1159/000255967. [DOI] [PubMed] [Google Scholar]
- 17.Riendeau V., Garenc C. Effect of 27-hydroxycholesterol on survival and death of human macrophages and vascular smooth muscle cells. Free Radic. Res. 2009;43:1019–1028. doi: 10.1080/10715760903040610. [DOI] [PubMed] [Google Scholar]
- 18.Berthier A., Lemaire-Ewing S., Prunet C., Montange T., Vejux A., Pais De Barros J.P., Monier S., Gambert P., Lizard G., Néel D. 7-Ketocholesterol-induced apoptosis: involvement of several pro-apoptotic but also anti-apoptotic calcium-dependent transduction pathways. FEBS J. 2005;272:3093–3104. doi: 10.1111/j.1742-4658.2005.04723.x. [DOI] [PubMed] [Google Scholar]
- 19.Vurusaner B., Gamba P., Testa G., Gargiulo S., Biasi F., Zerbinati C., Iuliano L., Leonarduzzi G., Basaga H., Poli G. Survival signaling elicited by 27-hydroxycholesterol through the combined modulation of cellular redox state and ERK/Akt phosphorylation. Free Radic. Biol. Med. 2014;77:376–385. doi: 10.1016/j.freeradbiomed.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Vurusaner B., Gamba P., Gargiulo S., Testa G., Staurenghi E., Leonarduzzi G., Poli G., Basaga H. Nrf2 antioxidant defense is involved in survival signaling elicited by 27-hydroxycholesterol in human promonocytic cells. Free Radic. Biol. Med. 2015 doi: 10.1016/j.freeradbiomed.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 21.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galluzzi L., Aaronson S.A., Abrams J., Alnemri E.S., Andrews D.W., Baehrecke E.H., Bazan N.G., Blagosklonny M.V., Blomgren K., Borner C., Bredesen D.E., Brenner C., Castedo M., Cidlowski J.A., Ciechanover A., Cohen G.M., De Laurenzi V., De Maria R., Deshmukh M., Dynlacht B.D., El-Deiry W.S., Flavell R.A., Fulda S., Garrido C., Golstein P., Gougeon M.-L., Green D.R., Gronemeyer H., Hajnóczky G., Hardwick J.M. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 2009;16:1093–1107. doi: 10.1038/cdd.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherz-Shouval R., Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem. Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Martinet W., De Bie M., Schrijvers D.M., De Meyer G.R.Y., Herman A.G., Kockx M.M. 7-ketocholesterol induces protein ubiquitination, myelin figure formation, and light chain 3 processing in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2004;24:2296–2301. doi: 10.1161/01.ATV.0000146266.65820.a1. [DOI] [PubMed] [Google Scholar]
- 25.Nowicki M., Zabirnyk O., Duerrschmidt N., Borlak J., Spanel-Borowski K. No upregulation of lectin-like oxidized low-density lipoprotein receptor-1 in serum-deprived EA.hy926 endothelial cells under oxLDL exposure, but increase in autophagy. Eur. J. Cell Biol. 2007;86:605–616. doi: 10.1016/j.ejcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen T., Sherratt P.J., Pickett C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003;43:233–260. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 27.Motohashi H., Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen T., Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279–296. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichimura Y., Waguri S., Sou Y.S., Kageyama S., Hasegawa J., Ishimura R., Saito T., Yang Y., Kouno T., Fukutomi T., Hoshii T., Hirao A., Takagi K., Mizushima T., Motohashi H., Lee M.S., Yoshimori T., Tanaka K., Yamamoto M., Komatsu M. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol. Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Lau A., Wang X.-J., Zhao F., Villeneuve N.F., Wu T., Jiang T., Sun Z., White E., Zhang D.D. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol. Cell. Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain A., Lamark T., Sjøttem E., Larsen K.B., Awuh J.A., Øvervatn A., McMahon M., Hayes J.D., Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J., Whiteman M.W., Lian H., Wang G., Singh A., Huang D., Denmark T. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J. Biol. Chem. 2009;284:21412–21424. doi: 10.1074/jbc.M109.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinojima N., Yokoyama T., Kondo Y., Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3:635–637. doi: 10.4161/auto.4916. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J.-H., Horbinski C., Guo F., Watkins S., Uchiyama Y., Chu C.T. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am. J. Pathol. 2007;170:75–86. doi: 10.2353/ajpath.2007.060524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang R.C., Wei Y., An Z., Zou Z., Xiao G., Bhagat G., White M., Reichelt J., Levine B., Altomare D.A., Testa J.R., Engelman J.A., Luo J., Cantley L.C., Degtyarev M., Levine B., Kroemer G., Boehm J.S., Thoreen C.C., Mizushima N., Yoshimori T., Levine B., Obenauer J.C., Cantley L.C., Yaffe M.B., Xue Y., Manning B.D., Cantley L.C., Paraiso K.H., Wang M.Y. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Sci. Am. Assoc. Adv. Sci. 2012;338:956–959. doi: 10.1126/science.1225967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dewaele M., Maes H., Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy. 2010;6:838–854. doi: 10.4161/auto.6.7.12113. [DOI] [PubMed] [Google Scholar]
- 38.Scherz-Shouval R., Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Zhu L., Barret E.C., Xu Y., Liu Z., Manoharan A. Regulation of cigarette smoke (CS)-induced autophagy by Nrf2. PLoS One. 2013;8:e55695. doi: 10.1371/journal.pone.0055695. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.He Y., Li S., Zhang W., Dai W., Cui T., Wang G., Gao T., Li C. Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci. Rep. 2017;7:42394. doi: 10.1038/srep42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.-S., Ueno I., Sakamoto A., Tong K.I., Kim M., Nishito Y., Iemura S., Natsume T., Ueno T., Kominami E., Motohashi H., Tanaka K., Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 42.Zhao M., Zhu P., Fujino M., Nishio Y., Chen J., Ito H., Takahashi K., Nakajima M., Tanaka T., Zhao L., Zhuang J., Li X. 5-Aminolevulinic acid with sodium ferrous citrate induces autophagy and protects cardiomyocytes from hypoxia-induced cellular injury through MAPK-Nrf-2-HO-1 signaling cascade. Biochem. Biophys. Res. Commun. 2016;479:663–669. doi: 10.1016/j.bbrc.2016.09.156. [DOI] [PubMed] [Google Scholar]
- 43.Khatib S., Vaya J. Oxysterols and symptomatic versus asymptomatic human atherosclerotic plaque. Biochem. Biophys. Res. Commun. 2014;446:709–713. doi: 10.1016/j.bbrc.2013.12.116. [DOI] [PubMed] [Google Scholar]
- 44.Martinet W., De Meyer G.R.Y. Autophagy in atherosclerosis: a cell survival and death phenomenon with therapeutic potential. Circ. Res. 2009;104:304–317. doi: 10.1161/CIRCRESAHA.108.188318. [DOI] [PubMed] [Google Scholar]
- 45.R. Kiffin, U. Bandyopadhyay, A.M. Cuervo Oxidative stress and autophagy. Antioxid. Redox Signal., vol. 8, pp. 152–162. [DOI] [PubMed]
- 46.Martinet W., Schrijvers D.M., Timmermans J.-P., Bult H. Interactions between cell death induced by statins and 7-ketocholesterol in rabbit aorta smooth muscle cells. Br. J. Pharmacol. 2008;154:1236–1246. doi: 10.1038/bjp.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He C., Zhu H., Zhang W., Okon I., Wang Q., Li H., Le Y.-Z., Xie Z. 7-Ketocholesterol induces autophagy in vascular smooth muscle cells through Nox4 and Atg4B. Am. J. Pathol. Am. Soc. Investig. Pathol. 2013;183:626–637. doi: 10.1016/j.ajpath.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan X.-M., Sultana N., Siraj N., Ward L.J., Ghafouri B., Li W. Autophagy induction protects against 7-oxysterol-induced cell death via lysosomal pathway and oxidative stress. J. Cell Death. 2016;9:1–7. doi: 10.4137/JCD.S37841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nury T., Zarrouk A., Vejux A., Doria M., Riedinger J.M., Delage-Mourroux R., Lizard G. Induction of oxiapoptophagy, a mixed mode of cell death associated with oxidative stress, apoptosis and autophagy, on 7-ketocholesterol-treated 158N murine oligodendrocytes: impairment by α-tocopherol. Biochem. Biophys. Res. Commun. 2013;446:714–719. doi: 10.1016/j.bbrc.2013.11.081. [DOI] [PubMed] [Google Scholar]
- 50.Ding Z., Wang X., Schnackenberg L., Khaidakov M., Liu S., Singla S., Dai Y., Mehta J.L. Regulation of autophagy and apoptosis in response to ox-LDL in vascular smooth muscle cells, and the modulatory effects of the microRNA hsa-let-7 g. Int. J. Cardiol. 2013;168:1378–1385. doi: 10.1016/j.ijcard.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 51.Suffixidharan S., Jain K., Basu A. Regulation of autophagy by kinases. Cancers. 2011;3:2630–2654. doi: 10.3390/cancers3022630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haberzettl P., Hill B.G. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 2013;1:56–64. doi: 10.1016/j.redox.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Wong E.W.T., Chang F., Lehmann B., Terrian D.M., Milella M., Tafuri A., Stivala F., Libra M., Basecke J., Evangelisti C., Martelli A.M., Franklin R.A. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. (NIH Public Access) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Luca A., Maiello M.R., D’Alessio A., Pergameno M., Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin. Ther. Targets. 2012;16(Suppl 2):S17–S27. doi: 10.1517/14728222.2011.639361. [DOI] [PubMed] [Google Scholar]
- 55.Zabirnyk O., Liu W., Khalil S., Sharma A., Phang J.M. Oxidized low-density lipoproteins upregulate proline oxidase to initiate ROS-dependent autophagy. Carcinogenesis. 2010;31:446–454. doi: 10.1093/carcin/bgp299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathew R., Karp C.M., Beaudoin B., Vuong N., Chen G., Chen H.-Y., Bray K., Reddy A., Bhanot G., Gelinas C., Dipaola R.S., Karantza-Wadsworth V., White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L., Li J., Ma J., Chen X., Chen K., Jiang Z., Zong L., Yu S., Li X., Xu Q., Lei J., Duan W., Li W., Shan T., Ma Q., Shen X., Zhang L., Li J., Ma J., Chen X., Chen K., Jiang Z., Zong L., Yu S., Li X., Xu Q., Lei J., Duan W., Li W., Shan T. The relevance of Nrf2 pathway and autophagy in pancreatic cancer cells upon stimulation of reactive oxygen species. Oxid. Med. Cell. Longev. 2016;2016:1–11. doi: 10.1155/2016/3897250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinet W., Schrijvers D.M., De Meyer G.R.Y. Molecular and cellular mechanisms of macrophage survival in atherosclerosis. Basic Res. Cardiol. 2012;107:297. doi: 10.1007/s00395-012-0297-x. [DOI] [PubMed] [Google Scholar]