Abstract
Rationale
The promising clinical benefits of delivering human mesenchymal stem cells (hMSCs) for treating heart disease warrant a better understanding of underlying mechanisms of action. hMSC exosomes increase myocardial contractility; however, the exosomal cargo responsible for these effects remains unresolved.
Objective
This study aims to identify lead cardioactive hMSC exosomal microRNAs to provide a mechanistic basis for optimizing future stem cell-based cardiotherapies.
Methods and Results
Integrating systems biology and human engineered cardiac tissue (hECT) technologies, partial least squares regression analysis of exosomal microRNA profiling data predicted microRNA-21-5p (miR-21-5p) levels positively correlate with contractile force and calcium handling gene expression responses in hECTs treated with conditioned media from multiple cell types. Furthermore, miR-21-5p levels were significantly elevated in hECTs treated with the exosome-enriched fraction of the hMSC secretome (hMSC-exo) versus untreated controls. This motivated experimentally testing the human-specific role of miR-21-5p in hMSC-exo–mediated increases of cardiac tissue contractility. Treating hECTs with miR-21-5p alone was sufficient to recapitulate effects observed with hMSC-exo on hECT developed force and expression of associated calcium handling genes (eg, SERCA2a and L-type calcium channel). Conversely, knockdown of miR-21-5p in hMSCs significantly diminished exosomal procontractile and associated calcium handling gene expression effects on hECTs. Western blots supported miR-21-5p effects on calcium handling gene expression at the protein level, corresponding to significantly increased calcium transient amplitude and decreased decay time constant in comparison to miR-scramble control. Mechanistically, cotreating with miR-21-5p and LY294002, a PI3K inhibitor, suppressed these effects. Finally, mathematical simulations predicted the translational capacity for miR-21-5p treatment to restore calcium handling in mature ischemic adult human cardiomyocytes.
Conclusions
miR-21-5p plays a key role in hMSC-exo–mediated effects on cardiac contractility and calcium handling, likely via PI3K signaling. These findings may open new avenues of research to harness the role of miR-21-5p in optimizing future stem cell-based cardiotherapies.
Keywords: exosomes, microRNAs, myocardium, systems biology, tissue engineering
Human bone marrow-derived mesenchymal stem cells are an emerging and promising approach to treat ischemic and nonischemic cardiomyopathies.1, 2 To date, phase II clinical trials of human mesenchymal stem cell (hMSC) delivery have supported the conduct of phase III trials.3–5 Despite this advancement, there remains a need to enhance and refine stem cell-based treatment strategies for myocardial diseases through rational design. It is therefore of great interest to better understand underlying hMSC mechanisms of action to optimize future stem cell-based cardiotherapies.
Acting predominantly through paracrine signaling mechanisms, hMSCs have well-recognized immunomodulatory, proangiogenic, and antifibrotic effects on diseased myocardium.1 Additionally, hMSC paracrine factors can modulate cardiac excitation–contraction coupling and contractility.6–8 We recently demonstrated the exosomal fraction of the hMSC secretome—taken up by both SIRPα+/CD90− human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) and SIRPα−/CD90+ hPSC-fibroblasts comprising our human engineered cardiac tissues (hECTs)6—to be largely responsible for hMSC paracrine-mediated increases in hECT contractility and associated calcium handling gene expression.6 Although exosomal microRNAs (miRs) are widely thought to mediate intercellular communications and gene regulation,9–11 the specific exosomal miRs responsible for hMSC-mediated cardioactivity remain largely unresolved.
Advancements in systems biology have facilitated associating complex networks of biological inputs and functional outputs via partial least squares regression (PLSR).9, 11 This method has shown promise for predicting cardioactive antifibrotic and proangiogenic exosomal miR cargo released by cardiac progenitor cells, using classic tube formation and fibrosis assays as functional outputs.9, 11 However, in the context of human cardiac contractile function, other assays are necessary; to this end, our custom multi-hECT platform has proven valuable as a contractility assay to test paracrine potency.6, 7
In this study, we hypothesized that integrating systems biology and tissue engineering approaches would help identify lead cardioactive hMSC exosomal miRs responsible for increasing human cardiac tissue contractility. We first predicted miR-21-5p as a lead cardioactive hMSC exosomal miR via PLSR. Next, we experimentally tested the human-specific role of miR-21-5p in hMSC exosomal effects on hECT contractile function, as well as cardiomyocyte calcium handling. Finally, we investigate the underlying mechanism of miR-21-5p-mediated effects on cardiomyocyte calcium handling and contractility and predicted how these findings may translate to ischemic human adult cardiomyocytes. Altogether, we demonstrate a key role of miR-21-5p in hMSC exosomal treatments to increase hECT contractility likely via PI3K signaling, suggesting a specific molecular mediator to exploit for optimizing future stem cell-based cardiotherapies.
Methods
All data, methods, and study materials are available on request by contacting Joshua Mayourian (joshua.mayourianicahn.mssm.edu) or the corresponding author.
An expanded methods section is available in the Online Data Supplement.
Partial Least Squares Regression
PLSR was performed with the nonlinear iterative partial least squares algorithm, as described elsewhere.9, 11 Similar to our previous work,6 predicted direct activation was performed via the Ingenuity Pathway Analysis (IPA, Qiagen) Molecule Activity Predictor.
hECT Contractility Assay
hECTs were created from hPSC-CMs and type-I collagen using methods previously described.6 Baseline functional analysis was performed on culture day 5, followed by a single treatment with either (1) serum-free defined media (SFDM), (2) fresh hMSC conditioned SFDM media, (3) exosome-depleted hMSC conditioned SFDM media, (4) hMSC exosome-enriched SFDM (exosome-enriched fraction of the hMSC secretome [hMSC-exo]), (5) SFDM supplemented with 2 nmol/L of naked human miR mimic negative control (miR-scr-con; Thermo Fisher Scientific), (6) SFDM supplemented with 2 nmol/L of naked human miR-21-5p mimic (Thermo Fisher Scientific), (7) exosome-enriched fraction from naked human miR inhibitor negative control (Thermo Fisher Scientific) treated hMSCs (hMSC-exo:miR-scr-con), or (8) exosome-enriched fraction from miR-21-5p inhibitor (Thermo Fisher Scientific) treated hMSCs (hMSC-exo:miR-21-5p-KD). Following 5 days of post-treatment functional analysis (culture day 10), hECTs were flash-frozen for real-time quantitative polymerase chain reaction (qRT-PCR). All primers used can be found in the Online Data Supplement.
Western Blots of Lysate From hPSC-CM Monolayers
hPSC-CM monolayers in 6-well tissue culture plates were used for Western blots as described previously.12 hPSC-CM monolayers were treated for 48 hours with either 10 nmol/L miR-scr-con or 10 nmol/L miR-21-5p. See Online Table I for specific characteristics of antibodies used for Western blots.
Calcium Transient Measurements
Calcium transients were performed on hPSC-CMs using the IonOptix Calcium Imaging System (Westwood, MA) as described previously.12 hPSC-CMs were treated for 48 hours with either (1) 10 nmol/L miR-scr-con without LY294002, (2) 10 nmol/L miR-21-5p without LY294002, (3) 10 nmol/L miR-scr-con with 10 µmol/L LY294002, or (4) 10 nmol/L miR-21-5p with 10 µmol/L LY294002.
Single-Cell Cardiomyocyte Electrophysiology Models
Multiple single-cell cardiomyocyte electrophysiological models were used in this study, including the Paci et al13 human induced pluripotent stem cell-derived ventricular-like cardiomyocyte model, ten Tusscher et al14 human healthy cardiomyocyte model, and Weiss et al15 human ischemic cardiomyocyte model. All single-cell models were numerically integrated with MATLAB’s (The MathWorks, Natick MA) stiff ordinary differential equation solver (ode15s) until steady state was achieved.
Experimental Calibration of miR-21-5p Effects on Single-Cell Cardiomyocyte Electrophysiology
Effects of miR-21-5p were simulated using experimentally calibrated6, 16, 17 adjustments to the baseline L-type calcium channel (LTCC) maximum permeability (GLCa) and SERCA2a (sarcoendoplasmic reticulum calcium-ATPase) maximum uptake activity (Vmaxup) parameters in the single-cell cardiomyocyte models described above.
We experimentally calibrated the effects of hMSC miR-21-5p on GLCa and Vmaxup using original calcium transient experimental data found herein and a modified version of methods described previously.6, 16, 17 GLCa and Vmaxup were chosen because LTCC and SERCA2a mRNA levels are modulated by miR-21-5p, as demonstrated herein. Briefly, we constructed a population of 1000 models of the single-cell Paci et al13 human induced pluripotent stem cell-derived ventricularlike cardiomyocyte electrophysiology model by randomly assigning empirically relevant parameter values to characterize changes in maximal fluxes because of miR-21-5p:
| (1) |
| (2) |
where G′LCa and V′maxup represent the updated values of maximum LTCC permeability and SERCA2a maximum uptake activity because of miR-21-5p effects, respectively, and ΔGLCa and ΔVmaxup represent fold changes in baseline LTCC maximum permeability and SERCA2a maximum uptake activity parameters because of miR-21-5p effects, respectively. Motivated by empirical data herein, ΔVmaxup was randomly assigned between 1 and 4, whereas ΔGLCa was randomly assigned between 1 and 2.
Our model calibration algorithm determined whether a given set of model parameters should be added to an accepted population based on comparison of simulated outputs to the following metrics from experimental data within this study (1) calcium transient amplitude ([Ca2+]i amplitude); and (2) decay time constant (τCa). Modified from Prinz et al,18 bounds on allowed output variability were set to 1 SD around mean values. Select accepted parameter sets were input into the Weiss et al15 ischemic human cardiomyocyte model.
Statistical Analyses
Descriptive statistics are presented as mean±SEM, with comparative statistical methods described in the corresponding figure legend. P values <0.05 were considered statistically significant.
Results
Lead Candidate Cardioactive hMSC Exosomal miRs Predicted via PLSR
To predict key cardioactive hMSC exosomal miR cargo, PLSR was used to form relationships between the relative abundance of specific exosomal miRs from a set of parent cells releasing exosomes and resultant hECT contractile force responses as well as relevant calcium handling and apoptotic gene expression. More specifically, we sought to predict miRs that may increase SERCA2a and LTCC expression, decrease the BAX/BCL2 expression ratio, and increase contractile force in our hECT system similar to hMSC-exo treatment in our previous study.6
In this analysis, the parent cells included hMSCs, human adult cardiac fibroblasts, and human foreskin fibroblasts (hFFs) with previously established6, 7 parent cell-dependent paracrine effects on hECT contractility. More specifically, conditioned media from hMSCs6 and human adult cardiac fibroblasts,7 but not hFFs,7 significantly increased hECT contractile function, although mediated through distinct mechanisms.9, 10 Although hMSC exosomes are the main contributors to increasing contractility via mechanisms described above, human adult cardiac fibroblast soluble factors (eg, transforming growth factor-β) are largely responsible for increasing contractility via hypertrophy, as well as sodium and potassium channel remodeling.7, 19, 20
Hierarchical clustering of published exosomal miR profiling data representative of these parent cells (obtained from National Institutes of Health/National Center for Biotechnology Information GEO-series GSE71241, GSE76175, and Pope et al21 for hMSC, rat adult cardiac fibroblast (rACF), and hFF, respectively; rACF was used because of lack of available human adult cardiac fibroblast data) demonstrates grouping primarily by cell type (Figure 1A). Notably, several of the top 20 cardiac-related hMSC exosomal miRs were differentially expressed in the other fibroblast cell types (Figure 1A), motivating the systematic PLSR method to predict key cardioactive miRs.
Figure 1. Predicting lead cardioactive human mesenchymal stem cell (hMSC) exosomal microRNAs (miRs) via partial least squares regression (PLSR).
A, Heatmap and hierarchical clustering of expression of top 20 cardiac-related hMSC exosomal miRs compared with their expression in human foreskin fibroblasts (hFFs) and rat adult cardiac fibroblasts (rACFs). Expression levels represented as fold change (FC) relative to the average for hFF. Select miRs experimentally investigated are bolded and in blue. Grey boxes indicate data not available. B, PLSR score plot suggests cell type-dependent separation mainly across principal component (PC) 1 (x axis), not 2 (y axis). C, Correlation loading plot from PLSR suggests several PI3K/Akt-related (blue) and PI3K/Akt-unrelated (grey) miRs from (A) covary with human engineered cardiac tissue (hECT) developed force (DF), L-type calcium channel (LTCC) gene expression, and SERCA2a (sarcoendoplasmic reticulum calcium-ATPase) gene expression. D, Expression of miR-22-3p, miR-21-5p, and miR-181b-5p in hECTs 5 days after exosome-enriched fraction of the hMSC secretome (hMSC-exo) treatment relative to serum-free defined media (SFDM)-treated controls. *P<0.05, P values from unpaired t tests (n=4). E, Ingenuity Pathway Analysis (IPA, Qiagen) predictions suggest miR-21-5p positively regulates miR-181b-5p (top), but not vice versa (bottom). BAX indicates BCL2-associated × protein; and BCL2, B-cell lymphoma 2.
PLSR was performed on the expression of the top 20 cardiac-related hMSC exosomal miRs across the above parent cells (as well as SFDM control), matched to hECT developed force (DF) as well as calcium handling (ie, SERCA2a and LTCC) and apoptotic (ie, BAX/BCL2) gene expression responses. In agreement with the hierarchical clustering (Figure 1A), the PLSR score plot (Figure 1B) shows parent cell type-dependent clustering: fibroblast parent cells (ie, rACFs and hFFs) were grouped near the origin along the negative x axis with SFDM control, whereas hMSC parent cells uniquely clustered toward the positive x axis (Figure 1B).
In the correlation loading plot, expression of both calcium handling genes—SERCA2a and LTCC—clustered on the positive x axis (Figure 1C), whereas the expression of the apoptotic BAX/BCL2 ratio was on the negative x axis. DF was uniquely along the positive y axis, also being in the positive x direction. This is consistent with the parent cell distribution in the score plot, as (1) the hMSC exosomal groups were unique to the right of the x axis (effectively corresponding to our previous empirical findings6), (2) SFDM and hFF exosomal groups were near the origin (both previously found to have null effects on contractility and associated gene expression7), and (3) the rACF exosomal group was also near the origin, as our model inputs are specific to exosomal miRs (ie, neglect soluble factors) and rACF effects on contractility are largely soluble factor (eg, transforming growth factor-β) dependent.19, 20
Altogether, given our focus on cardioactive hMSC-exo miRs, we sought miRs that (1) cluster to the right of the positive x axis, thus positively correlating with SERCA2a and LTCC gene expression and negatively correlating with BAX/BCL2 gene expression ratio, (2) cluster with DF, and (3) are known to modulate myocyte PI3K/Akt signaling, a pathway previously identified to be involved in hMSC paracrine-mediated increases in cardiac contractility.6 The correlation loading plot shows several miRs that meet these criteria in the upper right-hand quadrant (Figure 1C); these include several miRs that modulate myocyte PI3K/Akt signaling (Figure 1C, blue dots; grey dots denote miRs not known to modulate myocyte PI3K/Akt signaling) including miR-21-5p,22 miR-22-3p,23 and miR-181b-5p.24
Experimentally, we found that treatment of hECTs with hMSC-exo led to significantly increased miR-21-5p expression relative to SFDM control, whereas miR-22-3p expression remained unchanged (Figure 1D). In addition, treating hECTs with fresh hMSC conditioned media (containing exosomes and soluble factors) had significantly greater miR-21-5p expression relative to exosome-depleted hMSC conditioned media (Online Figure I). Interestingly, although miR-181b-5p abundance was relatively low in hMSC exosomes (Figure 1A) and is only slightly positive of the x axis in the correlation loading plot, hECTs treated with hMSC-exo also led to significantly increased miR-181b-5p expression relative to control. IPA predictions suggest miR-21-5p positively regulates miR-181b-5p (Figure 1E, top), but not vice versa (Figure 1E, bottom). Indeed, experimentally treating hECTs with miR-21-5p significantly increased expression of miR-181b-5p, but not miR-22-3p, versus miR-scr-con treated hECT controls (Figure 2A).
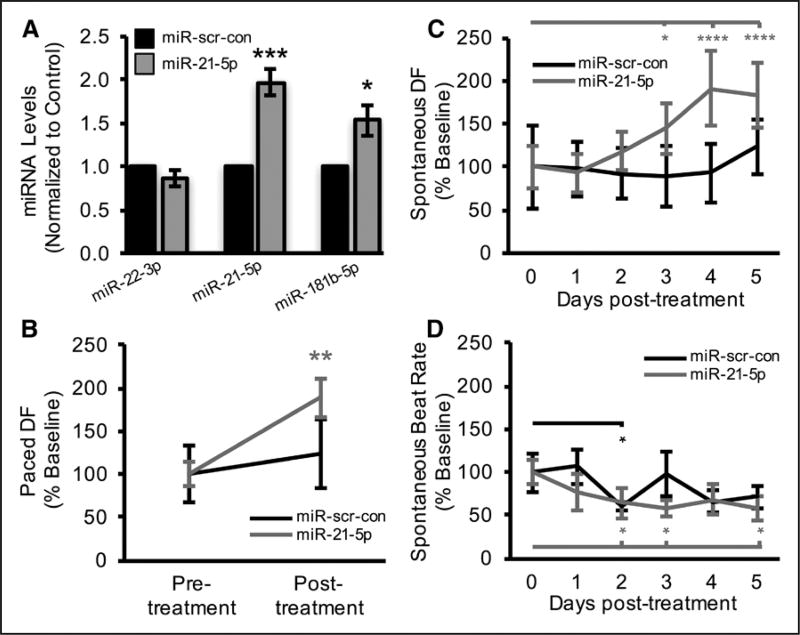
Figure 2. MicroRNA-21-5p (miR-21-5p) effects on human engineered cardiac tissue (hECT) contractility.
A, Expression of miR-22-3p, miR-21-5p, and miR-181b-5p in hECTs 5 days after miR-21-5p treatment relative to miR-scr-con. P values from unpaired t tests (n=3–5). B, Five days after miR-21-5p treatment, hECT developed force (DF) was significantly increased during 0.5 Hz pacing, whereas miR-scr-con negative controls had no significant effect. P values from repeated measures ANOVA followed by Bonferroni multiple comparisons test (n=5). Daily measurements of (C) DF and (D) beat rate in unpaced hECTs during spontaneous beating. P values from repeated measures ANOVA followed by Dunnett multiple comparisons test (n=4–5). In all panels, *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001.
Altogether, this motivated directly testing the human-specific role of miR-21-5p—the only top 5 most abundant hMSC exosomal miR across at least 3 independent studies7, 25–27—in increasing hECT contractile function. To investigate this in our hECT bioreactor system, we examined the cardioactive effects of miR-21-5p alone, as well as the effects of exosomes released from miR-21-5p knockdown hMSCs.
miR-21-5p Effects on hECT Contractile Function
Successful delivery of miR-21-5p (Figure 2A) significantly increased 0.5 Hz-paced hECT DF 5 days post-treatment relative to pretreatment baseline (Figure 2B), comparable to effects of hMSC-exo treatment.6 To examine the time dependence of treatment effects, daily monitoring of spontaneously beating hECTs showed that miR-21-5p treatment led to a significant increase in spontaneous DF on days 3 to 5 posttreatment relative to day 0 pretreatment (Figure 2C); these findings were likely not confounded by the force–frequency relationship, as there was no significant difference in beat rate between miR treatment groups despite a significant effect of time (Figure 2D).
Knockdown of miR-21-5p in hMSCs Diminishes Exosomal Effects on hECT Contractile Function
With just a modest (<40%; see Discussion and Detailed Methods in Online Data Supplement for explanation) yet statistically significant decrease in miR-21-5p, but not miR-181b-5p, expression in hMSC parent cells (Online Figure II), delivery of hMSC-exo:miR-21-5p-KD significantly decreased miR-21-5p and miR-181-5p expression in hECTs in comparison to the hMSC-exo:miR-scr-con experimental group (Figure 3A). This again suggests miR-21-5p delivery is mediating changes in miR-181b expression in hECTs during hMSC-exo treatment. More importantly, hMSC-exo:miR-21-5p-KD significantly diminished the procontractile effects that were observed in the hMSC-exo:miR-scr-con experimental group during pacing post-treatment (Figure 3B). In support of our previous findings,6 treating hECTs with hMSC-exo:miR-scr-con significantly increased DF during 0.5 Hz pacing 5 days post-treatment relative to pretreatment baseline (Figure 3B).
Figure 3. Knockdown of microRNA-21-5p (miR-21-5p) in human mesenchymal stem cells (hMSCs) diminishes procontractile effects of exosomes on human engineered cardiac tissue (hECT) contractility.
A, Expression of miR-22-3p, miR-21-5p, and miR-181b-5p in hECTs 5 days after exosome-enriched fraction of the hMSC secretome (hMSC-exo):miR-21-5p-KD treatment relative to hMSC-exo:miR-scr-con. P values from unpaired t tests (n=3). B, Five days after hMSC-exo:miR-scr-con treatment significantly increased hECT developed force (DF) during 0.5 Hz pacing in comparison to hMSC-exo:miR-21-5p-KD, which had no significant effect. P values from repeated measures ANOVA followed by Bonferroni multiple comparisons test (n=4). Daily measurements of (C) DF and (D) beat rate in unpaced hECTs during spontaneous beating. P values from repeated measures ANOVA followed by Dunnett multiple comparisons test (n=4). In all panels, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. # denotes statistical significance between groups at a given time point, #P<0.05, ##P<0.01.
To examine the time dependence of these treatment effects, daily monitoring of spontaneously beating hECTs showed that hMSC-exo:miR-scr-con treatment, but not hMSC-exo:miR-21-5p-KD, led to significant increases in spontaneous DF on days 3 to 5 post-treatment relative to day 0 pretreatment (Figure 3C). Although the spontaneous beat rate of the hMSC-exo:miR-21-5p-KD-treated hECTs was significantly higher than hMSC-exo:miR-scr-con on days 2 and 4 (Figure 3D), spontaneous beat rates reached a maximum of ≈0.5 Hz in both groups and there was no statistical difference on days 3 and 5, making it unlikely these findings were largely confounded by the force–frequency relationship that is relatively flat for hECTs in this low frequency range.28
miR-21-5p Increases Calcium Handling Gene Expression
The hECT functional measurements in Figures 2 and 3 were corroborated by molecular characterization using prospective qRT-PCR of cardiac-specific, calcium handling, and apoptotic genes. Revealing a gene expression profile remarkably similar to our prior observations with hMSC-exo treatment6 (Online Figure III), miR-21-5p supplementation significantly increased hECT mRNA levels of LTCC and SERCA2a (Figure 4A) and decreased the α/β myosin heavy chain ratio (Figure 4A). In addition, analogous to our prior observations with hMSC-exo treatment6 (Online Figure III), miR-21-5p modestly yet significantly decreased the BAX/BCL2 ratio (Figure 4B), a marker of possible antiapoptotic effects.29
Figure 4. Molecular characterization of microRNA-21-5p (miR-21-5p) effects on human engineered cardiac tissues (hECTs) and human pluripotent stem cell-derived cardiomyocyte (hPSC-CM) monolayers.
hECTs treated with miR-scr-con vs miR-21-5p (A and B) and hMSC-exo:miR-scr-con vs hMSC-exo:miR-21-5p-KD (C and D) were snap-frozen for qRT-PCR on day 5 post-treatment, where expression of (A and C) cardiac-specific, calcium handling, and (B and D) apoptotic genes were studied. P values from 1-way ANOVA with post hoc Tukey test (n=3–5). E, Western blot of hPSC-CM monolayers treated for 48 h with miR-21-5p or miR-scr-con. In all panels, *P<0.05, **P<0.01, ***P<0.001. BAX indicates BCL2-associated × protein; BCL2, B-cell lymphoma 2; Casp, caspase; cTnT, cardiac troponin-T; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LTCC, L-type calcium channel; MHC, myosin heavy chain; SERCA2a, sarcoendoplasmic reticulum calcium-ATPase; and WCL, whole-cell lysate.
When depleting hMSCs of miR-21-5p (Online Figure II), delivery of hMSC-exo:miR-21-5p-KD significantly decreased mRNA levels of LTCC and SERCA2a in hECTs in comparison to hMSC-exo:miR-scr-con treatment (Figure 4C). However, there were no significant effects on the α/β myosin heavy chain ratio (Figure 4C) nor the BAX/BCL2 ratio (Figure 4D). Altogether, both hECT experiments in Figure 4A and 4C support miR-21-5p altering expression of calcium handling genes; however, the effects on apoptotic gene expression are relatively unclear (Figure 4B and 4D).
To better understand these findings at the protein level, Western blots were performed on hPSC-CM monolayers treated with miR-scr-con or miR-21-5p (Figure 4E). Although there was no discernible effect on nonspecific LTCC protein levels, miR-21-5p increased cardiomyocyte-specific SERCA2a protein levels independent of nonspecific (ie, GAPDH and α-actinin) or myocyte-specific (ie, cTnT) loading controls, leading to an ≈2-fold to 3-fold increase in comparison to miR-scr-con treatment (Figure 4E). In comparison, miR-21-5p had no discernable effect on BAX/BCL2 protein level (Figure 4E). Altogether, these findings motivate further investigation into the mechanism by which miR-21-5p increases calcium handling and thereby contractility.
miR-21-5p Increases hPSC-CM Calcium Transients Likely via the PI3K Signaling Cascade
To mechanistically corroborate our hECT findings, we examined the effects of miR-21-5p on hPSC-CM calcium transients with or without the PI3K inhibitor LY294002; for sample calcium transients of each experimental group (Figure 5A). In the absence of LY294002, treatment with miR-21-5p significantly increased calcium transient amplitude (Figure 5B) in comparison to miR-scr-con; in the presence of LY294002, these effects were abolished (Figure 5C). In addition, in the absence of LY294002, treating hPSC-CMs with miR-21-5p decreased the calcium transient decay time constant (Figure 5D) relative to miR-scr-con; in the presence of LY294002, these effects were modestly attenuated (Figure 5E).
Figure 5. MicroRNA-21-5p (miR-21-5p) increases human pluripotent stem cell-derived cardiomyocyte (hPSC-CM) calcium transients likely via the PI3K signaling cascade.
A, Sample calcium transients from hPSC-CMs treated with miR-21-5 or miR-scr-con with or without LY294002 (LY), a PI3K inhibitor. B and C, Calcium transient amplitude of hPSC-CMs treated with miR-21-5p or miR-scr-con (B) without or (C) with LY. D and E, Calcium transient decay time constant (τCa) of hPSC-CMs treated with miR-21-5p or miR-scr-con (D) without or (E) with LY. For each panel, unpaired student t tests were performed between respective miR-scr-con and miR-21-5p experimental groups (n=5–6 per condition). *P<0.05, **P<0.01.
Predicting the Effects of miR-21-5p in Ischemic Adult Human Cardiomyocytes
To corroborate our experimental findings on miR-21-5p effects on LTCC and SERCA2a expression and to predict how miR-21-5p effects on immature hPSC-CM calcium transients may translate to mature ischemic adult human cardiomyocytes, we adopted established computational methods6, 16, 17 by closely matching simulation outputs to corresponding experimental recordings (Figure 6). To do so, we generated a large initial population of 1000 model variants with empirically relevant (based on Western blot data in Figure 4E) randomly chosen parameter sets to increase maximum LTCC and SERCA2a flux constants in a human induced pluripotent stem cell-derived cardiomyocyte electrophysiology model13 by 1-fold to 2-fold and 1-fold to 4-fold changes, respectively. The initial population was then filtered to retain (Figure 6A, white dots) only select models that were consistent (ie, within±1 SD) with 2 calcium transient metrics from Figure 5, amplitude and decay time constant ([Ca2+]i amplitude and τCa, respectively).
Figure 6. Experimentally calibrating microRNA-21-5p (miR-21-5p) effects on human cardiomyocyte calcium transients.
miR-21-5p effects on immature human cardiomyocyte L-type calcium channel (LTCC) and SERCA2a (sarcoendoplasmic reticulum calcium-ATPase) activity were experimentally calibrated. A, Scatter plots of the initial population (grey dots) filtered (white dots) to be within 1 SD (boxed region) of calcium transient decay time constant (τCa), and calcium transient amplitude ([Ca2+]i amplitude) metrics from Figure 5. B, Accepted sets of calibrated model parameters. C and D, Histograms illustrating distributions of the output simulation metrics—(C) [Ca2+]i amplitude and (D) τCa—resulting from the accepted population of calibrated models. E, Ten select accepted model parameters were subsequently input into ischemic adult human cardiomyocyte models to predict miR-21-5p effects on calcium transients (grey) in comparison to healthy (black line) and ischemic (dotted black line) adult human cardiomyocytes. ΔGLCa and ΔVmaxup denote fold changes in LTCC and SERCA2a maximal flux constants, respectively, because of miR-21-5p effects.
This calibration process reduced the initial population of 1000 model variants to 535 accepted model parameter sets (Figure 6B). The histograms in Figure 6C and 6D illustrate the distribution of output simulation metrics [Ca2+]i amplitude and τCa, respectively, resulting from the range of accepted model parameters. Figure 6B further shows the distribution of the 535 accepted ΔGLCa and ΔVmaxup parameter sets used to experimentally calibrate effects of miR-21-5p on calcium handling. In agreement with our Western blot findings (Figure 4E), 10 of 535 accepted models have no discernable (ie, <1%) changes in LTCC activity, suggesting increased SERCA2a activity alone was sufficient to recapitulate the effects on calcium transients from Figure 5. On the other hand, all of the accepted models required discernable changes in SERCA2a activity. Interestingly, the same 10 accepted models with no discernable changes in LTCC (ie, along the x axis) ranged from ≈1.5-fold to 3-fold changes in SERCA2a, which also theoretically agrees with our Western blot findings in Figure 4E. These 10 select models that are in agreement with our empirical findings were therefore chosen as the finalized set of accepted models of miR-21-5p effects on calcium handling.
Next, using the 10 finalized accepted models described above, we predicted miR-21-5p effects on adult human ischemic cardiomyocytes. As expected, in the absence of miR-21-5p, adult human ischemic cardiomyocytes were simulated to have diminished calcium handling in comparison to adult human healthy cardiomyocytes (Figure 6E). However, in the presence of miR-21-5p, adult human ischemic cardiomyocytes were predicted to restore or even exceed healthy cell calcium transients.
Discussion
The molecular mediators of myocardial contractility in hMSC-based cardiotherapies remain largely unresolved, hindering efforts to maximize therapeutic efficacy. In this study, we used an integrated systems biology and tissue engineering approach to identify key cardioactive exosomal miRs. First, we provide bioinformatics and experimental data supporting miR-21-5p as a lead cardioactive exosomal miR in therapeutic hMSC-based paracrine signaling (Figure 1). Next, using our hECT system, we provide the first (to our knowledge) human-specific experimental data revealing (1) miR-21-5p effects are remarkably similar to hMSC-exo effects on hECT contractile function (Figure 2), and (2) knockdown of miR-21-5p in hMSCs diminishes exosomal procontractile effects (Figure 3). The effects of miR-21-5p on calcium handling and contractility are supported at both the mRNA and protein levels (Figure 4). Finally, we mechanistically support miR-21-5p increases calcium handling and thereby contractility via the PI3K signaling cascade (Figure 5), and use a computational model to predict how these findings may translate to adult human cardiomyocytes (Figure 6). Based on these findings, we provide a schematic of our working hypothesis as to how hMSC-exo increases cardiac contractile function (Figure 7).
Figure 7. Working hypothesis for human mesenchymal stem cell (hMSC) paracrine-mediated increases in cardiac contractility.
hMSCs (parent cells) release exosomes (exosome-enriched fraction of the hMSC secretome [hMSC-exo]) that are taken up by recipient human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs). hMSC-exo delivery of microRNA-21-5p (miR-21-5p) increases hPSC-CM calcium handling gene expression, calcium handling, and thus contractility via the PI3K signaling cascade.
PLSR for Predicting Lead Cardioactive Exosomal miRs
Bioinformatics has proven successful for investigating proangiogenic, antifibrotic, and immunomodulatory paracrine mechanisms of stem cell therapies.30 However, in the context of stem cell paracrine effects on cardiac contractility and calcium handling, in silico analysis is a relatively nascent approach.11 Building on previous stem cell applications of PLSR,9, 11 we demonstrate the utility of using hECT functional and gene expression responses in the PLSR cue-signal-response paradigm to identify key hMSC exosomal miRs responsible for increasing human cardiac contractility.
In addition to the 98.3% predictability achieved with this trained PLSR model, the clustering of parent cell groups in the score plot further provided confidence in the predictive power of this methodology. Like our previous study, hMSC parent cell groups positively correlated with DF as well as LTCC and SERCA2a expression, whereas it negatively correlated with the BAX/BCL2 expression ratio.6 As expected, hFF and SFDM parent cell groups had weak correlations with these responses. Finally, rACF groups were also near the origin, consistent with the established theory that these parent cells affect contractility largely via soluble factor (eg, transforming growth factor-β) mechanisms.19, 20
Clustering with miR-21-5p, both miR-22-3p and miR-181b-5p were among the lead PLSR-predicted miRs that correlated with hECT twitch force in the upper right-hand quadrant (ie, positive x axis and positive y axis). Interestingly, these 3 clustering miRs affect common pathways (ie, cardiomyocyte PI3K/Akt signaling22–24) and are each established oncomiRs.31–33 To further support this, these 3 oncomiRs negatively correlate with the apoptotic BAX/BCL2 expression ratio (Figure 1C). The similarity between these miRs supports the validity of this method, while also providing insight into the previously hypothesized role of cardiac-specific oncogenes for cardiotherapeutics.34
Role of miR-21-5p in hMSC Exosome-Mediated Increases in Cardiac Contractility
It is first important to note that miR-21 guide (ie, miR-21-5p) and passenger (ie, miR-21-3p) strands each have distinct cardioactive effects,10, 35, 36 which may obscure findings when overexpressing or deleting premiR-21. For the purpose of our study, because of (1) the high abundance of miR-21-5p (not miR-21-3p) in hMSC exosomes (Figure 1A), (2) the predicted correlation of miR-21-5p with DF and calcium handling gene expression (Figure 1C), (3) the experimental validation of increased miR-21-5p expression in hMSC-exo treated hECTs (Figure 1D), and (4) our focus on identifying key exosomal cargo for hMSC therapeutics, we focused the current study on the miR-21 guide strand miR-21-5p.
The effects of miR-21 on calcium handling have been previously examined in a nonhuman cardiomyocyte setting. For example, miR-21 has been previously shown to bind to phospholamban strongly37 or activate endothelial nitric oxide synthase,38 both of which increase SERCA2a activity.8, 37 Herein, we demonstrate through miR-21-5p delivery and knockdown experiments that miR-21-5p increases human-specific cardiac contraction and modulates LTCC and SERCA2a calcium handling gene expression. These findings are further supported at the protein level via Western blots. Next, we mechanistically support these effects via the PI3K signaling cascade. These findings support our previous findings that hMSC-exo increases hECT contractility,6 while also providing novel insight into specific cargo contributing to these effects with a likely mechanism of action.
Robustness of hMSC Exosome and miR-21-5p Effects Across Human Stem Cell Lines
In this study, we confirm that hMSC-exo procontractile effects occur across 2 hPSC lines. In our previous study, hMSC-exo increased contractility in hECTs comprised of human embryonic stem cell-derived cardiomyocytes.6 In this study, the hMSC-exo:miR-scr-con increases contractility in hECTs comprised of human cardiomyocytes derived from induced pluripotent stems reprogrammed from a healthy volunteer’s dermal fibroblasts (Figure 3).
Furthermore, we confirm that miR-21-5p effects occur across the same 2 hPSC lines. For example, in Figure 2, we show that hECTs comprised of human embryonic stem cell-derived cardiomyocytes treated with miR-21-5p increase contractility, whereas in Figure 3, we show that knockdown of miR-21-5p in hMSCs abolished procontractile effects of exosomes on hECTs comprised of human induced pluripotent stem cell-derived cardiomyocytes. In addition, in Figure 4, we confirm miR-21-5p effects at the mRNA and protein level using both human embryonic and induced pluripotent stem cell-derived cardiomyocytes. Finally, in Figure 5, we yet again confirm miR-21-5p effects on calcium transients in human induced pluripotent stem cell-derived cardiomyocytes. Altogether, this demonstrates the robustness of hMSC-exo and miR-21-5p effects on derived human cardiomyocytes across hPSC lines.
Kinetics of hMSC-exo and miR-21-5p Effects on hECT Contractility
In comparison to nearly instantaneous β-adrenergic signaling mechanisms,39 paracrine mechanisms are expected to be slower and longer-lasting. In our recent study, hMSC-exo effects were evident at least 5 days post-treatment; however, until this current study, we had not yet ascertained the time dependence of treatment effects. In this study, we better understand hMSC exosome (Figure 3), as well as miR-21-5p (Figure 2), time-dependent treatment effects on hECTs. Both hMSC exosomes and miR-21-5p begin to increase hECT contractility ≈72 hours post-treatment (Figures 2 and 3). These effects persist for at least 5 days post-treatment. Unfortunately, the multiday kinetics of miR-21-5p and hMSC exosomes would likely make it unfeasible to study these responses in short-term ex vivo contractility assays, such as the Langendorff isolated heart model, for which maximum viability is 24 hours.40 Therefore, to extend our findings from hECTs to another relevant system, we developed a mathematical model to predict how the results on hPSC-CMs might translate to mature ischemic human cardiomyocytes (Figure 6).
Human-Specific Effects of miR-21-5p on Cardiac Tissue Remodeling
It is important to note that the activity of miR-21 is not limited to myocytes, as it has been shown to also alter cardiac tissue-level remodeling; this is clearly an important consideration for optimizing cardiotherapeutics. Interestingly, the cardiac remodeling effects of miR-21 have been inconclusive among murine studies. For example, one group found that miR-21 contributes to adverse cardiac remodeling by targeting specific nonmyocyte cardiac cell populations,35, 36 whereas another group reported a null contribution of miR-21 to stress-induced cardiac remodeling.41 In yet another study, miR-21 overexpression was cardioprotective in the setting of ischemia.10
In our hECTs comprised of ≈4:1 human stem cell-derived myocytes to fibroblasts (Online Figure IV), treatment with miR-21-5p leads to increased expression of the transforming growth factor-β signaling pathway, proangiogenic vascular endothelial growth factor-α, and angiopoietin-1, and hypertrophic atrial natriuretic factor and brain natriuretic peptide (Online Figure V). Although not addressing the conflicting data previously described, these findings nevertheless (1) are consistent with gene expression effects of hMSC exosomes on hECTs (Online Figure V), (2) are consistent with various findings established on hMSCs,42–45 (3) complement previous in vitro and in vivo work specifically on miR-21-5p46, 47 and its role in hMSC exosome-mediated effects on cardiac tissue remodeling,46 and (4) underscore the need to further investigate how miR-21-5p—alone or in the context of hMSC exosomes—affects remodeling in human-specific cardiac tissue.
Limitations and Future Directions
Several limitations of the study should be noted. First, we acknowledge that although this is (to our knowledge) the first study of miR-21-5p effects on engineered human cardiac tissue, the simplified hECT model system does not fully represent native human myocardium. Nevertheless, the controlled biocomplexity enables isolation of hMSC-mediated effects on myocyte contractility that may be obscured by other processes in vivo, and provides insight into cardiac tissue remodeling and twitch force not possible in traditional 2-dimensional monolayer assays.
Second, we recognize that hPSC-CMs have immature calcium handling, not representative of adult myocytes. To address this, we developed a mathematical model that predicted how miR-21-5p effects on calcium handling may translate to mature human cardiomyocytes. Given that miR-21-5p can increase SERCA2a protein levels and thereby calcium handling and contraction, our findings may even be able to build on recent efforts to improve hPSC-CM maturation48 by utilizing miR-21-5p or other synthetic miRs as a conditioning media supplement.
Third, we note the incomplete knockdown of miR-21-5p in hMSCs (Online Figure II). This was likely a consequence of culturing hMSCs in SFDM for 5 days after the initial 2 days of miR-21-5p inhibitor treatment; we used this experimental design to be consistent with our previous successful methodology to treat hECTs with hMSC exosomes.6 Nevertheless, even with roughly a 40% knockdown achieved, we still observed diminished effects on hECT contractility and associated calcium handling gene expression (Figures 3 and 4, respectively), showing the potent cardioactive effects of miR-21-5p in hMSC exosomes. Further knockdown may have provided further insight into the role of miR-21-5p on BAX/BCL2 and α/β myosin heavy chain gene expression.
Fourth, we acknowledge that hECTs were treated for a maximum of 5 days before molecular characterization to be consistent with our previous study.6 Although the time-dependent data on the effects of hMSC-exo and miR-21-5p helped justify focusing on the 5-day time point for these studies, it will be necessary to perform extended time course and dose-response experiments in follow-up studies examining the translational potential of these findings.
Finally, multiple paracrine factors may also impact hMSC-mediated effects on human myocardial contractility. For example, the incomplete miR-21-5p knockdown efficiency may have contributed to null effects on the BAX/BCL2 and α/β myosin heavy chain gene expression ratios; alternatively, this may reflect overlapping or compensatory roles of other hMSC exosomal miRs or proteins. Nonetheless, the remarkable similarity between miR-21-5p and intact hMSC-exo effects on hECTs, the diminished cardioactivity of exosomes when knocking down miR-21-5p in hMSCs, and the known abundance and role of miR-21-5p in hMSCs7 and other cardiotherapeutic stem cell exosomes (eg, cardiac progenitor cells)49 emphasizes the need to study miR-21-5p in the context of stem cell-based or even synthetic miR-based50 cardiotherapies.
Conclusions
In summary, this study integrates systems biology analysis and hECT technology to predict and experimentally validate exosomal miRs responsible for hMSC paracrine-mediated increases in myocardial contractility and expression of associated calcium handling genes. miR-21-5p is identified as a lead cardioactive hMSC exosomal miR; treatment with miR-21-5p nearly reproduced the effects of hMSC-exo on hECT contractile function and underlying molecular characteristics, whereas knockdown of miR-21-5p in hMSCs diminished exosomal procontractile effects on hECTs. The miR-21-5p modulation of calcium handling gene expression (eg, SERCA2a) was supported at the protein level. Mechanistically, miR-21-5p increases calcium handling and thereby contractility likely through the PI3K signaling cascade. Altogether, these findings help elucidate the human-specific cardioactive role of miR-21-5p, which could be used to maximize the efficacy of future stem cell-based therapies for heart failure.
Supplementary Material
Novelty and Significance.
What Is Known?
Human mesenchymal stem cells (hMSCs) are an emerging and promising cardiotherapeutic approach.
hMSC paracrine factors can modulate cardiac excitation–contraction coupling and thereby contractility.
Exosomes are primarily responsible for hMSC paracrine-mediated increases in human engineered cardiac tissue contractility and associated calcium handling gene expression; however, the exosomal cargo responsible for these effects remains unresolved.
What New Information Does This Article Contribute?
Bioinformatics data predicting microRNA-21-5p (miR-21-5p) as a lead cardioactive exosomal miR in therapeutic hMSC-based paracrine signaling.
Experimental human engineered cardiac tissue data demonstrating exosomal miR-21-5p mediates hMSC paracrine effects on human engineered cardiac tissue contractility.
Mechanistic support of miR-21-5p increasing cardiac calcium handling and thereby contractility via the PI3K signaling cascade.
The molecular modulators of myocardial contractility in hMSC-based cardiotherapies remain unresolved, hindering efforts to maximize therapeutic efficacy. In this study, we used an integrated systems biology and tissue engineering approach to identify key cardioactive exosomal miRs. Bioinformatics and experimental data support miR-21-5p as a lead cardioactive hMSC exosomal miR. Indeed, delivery of miR-21-5p recapitulates effects of hMSC exosome treatment on human engineered cardiac tissue contractile function, whereas knocking down miR-21-5p in hMSCs diminishes exosomal enhancement of contractility. The effects of miR-21-5p on calcium handling and contractility are supported at both the mRNA and protein levels. Mechanistically, miR-21-5p increases calcium handling and thereby contractility likely via the PI3K signaling cascade. Finally, we modified a computational model of excitation–contraction coupling to predict the translational capacity for miR-21-5p treatment to restore calcium handling in adult human ischemic cardiomyocytes. These findings support harnessing the role of exosomal miRs, such as miR-21-5p, in optimizing future stem cell-based cardiotherapies.
Acknowledgments
We thank Kasoorelope Oguntuyo for technical assistance and the Pluripotent Stem Cell Facility at the Icahn School of Medicine at Mount Sinai as a validated source of H7 human embryonic stem cells and related reagents.
Sources of Funding
This study was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) 1F30HL134283-01A1 (J. Mayourian); American Heart Association 15POST25090116 (D.K. Ceholski); Canadian Institutes of Health Research postdoctoral fellowship (P.A. Gorski); NIH grants R01 HL107110, R01 HL094849, R01 HL110737, R01 HL084275, and 5UM HL113460 (J.M. Hare); grants from the Starr Foundation, the Marcus Foundation, and the Soffer Family Foundation (J.M. Hare); NIH R01HL124187 and American Heart Association 17GRNT33460554 (S. Sahoo); NIH R01HL119046, R01HL129814, R01HL128072, R01HL131404, and R01HL135093 (R.J. Hajjar); and NIH/NHLBI R01HL132226 (K.D. Costa).
J.M. Hare is the Chief Scientific Officer, a compensated consultant, and advisory board member for Longeveron and holds equity in Longeveron. He is also the coinventor of intellectual property licensed to Longeveron. He also discloses a relationship with Vestion Inc that includes equity, board membership, and consulting. Vestion Inc and Longeveron did not play any role in the design or conduct of this study. K.D. Costa discloses his role as scientific cofounder and Chief Scientific Officer of NovoHeart Ltd. NovoHeart did not play any role in the design or conduct of this study.
Nonstandard Abbreviations and Acronyms
- DF
developed force
- hECT
human engineered cardiac tissue
- hFF
human foreskin fibroblast
- hMSC
human mesenchymal stem cell
- hMSC-exo
exosome-enriched fraction of the hMSC secretome
- hPSC-CM
human pluripotent stem cell-derived cardiomyocyte
- LTCC
L-type calcium channel
- miR
microRNA
- PLSR
partial least squares regression
- rACF
rat adult cardiac fibroblast
- SERCA2a
sarcoendoplasmic reticulum calcium-ATPase
- SFDM
serum-free defined media
Footnotes
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.118.312420/-/DC1.
Disclosures
The other authors report no conflicts.
References
- 1.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–1430. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hare JM, DiFede DL, Rieger AC, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J Am Coll Cardiol. 2017;69:526–537. doi: 10.1016/j.jacc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, Hahn A, Fichtner S, Schaefer A, Arseniev L, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30:2978–2984. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- 5.Fisher SA, Doree C, Mathur A, Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circ Res. 2015;116:1361–1377. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- 6.Mayourian J, Cashman TJ, Ceholski DK, Johnson BV, Sachs D, Kaji DA, Sahoo S, Hare JM, Hajjar RJ, Sobie EA, Costa KD. Experimental and computational insight into human mesenchymal stem cell paracrine signaling and heterocellular coupling effects on cardiac contractility and arrhythmogenicity. Circ Res. 2017;121:411–423. doi: 10.1161/CIRCRESAHA.117.310796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayourian J, Ceholski DK, Gonzalez DM, Cashman TJ, Sahoo S, Hajjar RJ, Costa KD. Physiologic, pathologic, and therapeutic paracrine modulation of cardiac excitation-contraction coupling. Circ Res. 2018;122:167–183. doi: 10.1161/CIRCRESAHA.117.311589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSantiago J, Bare DJ, Semenov I, Minshall RD, Geenen DL, Wolska BM, Banach K. Excitation-contraction coupling in ventricular myocytes is enhanced by paracrine signaling from mesenchymal stem cells. J Mol Cell Cardiol. 2012;52:1249–1256. doi: 10.1016/j.yjmcc.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116:255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang C, Batkai S, Dangwal S, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal U, George A, Bhutani S, Ghosh-Choudhary S, Maxwell JT, Brown ME, Mehta Y, Platt MO, Liang Y, Sahoo S, Davis ME. Experimental, systems, and computational approaches to understanding the MicroRNA-mediated reparative potential of cardiac progenitor cell-derived exosomes from pediatric patients. Circ Res. 2017;120:701–712. doi: 10.1161/CIRCRESAHA.116.309935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceholski DK, Turnbull IC, Pothula V, Lecce L, Jarrah AA, Kho C, Lee A, Hadri L, Costa KD, Hajjar RJ, Tarzami ST. CXCR4 and CXCR7 play distinct roles in cardiac lineage specification and pharmacologic β-adrenergic response. Stem Cell Res. 2017;23:77–86. doi: 10.1016/j.scr.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paci M, Hyttinen J, Aalto-Setälä K, Severi S. Computational models of ventricular- and atrial-like human induced pluripotent stem cell derived cardiomyocytes. Ann Biomed Eng. 2013;41:2334–2348. doi: 10.1007/s10439-013-0833-3. [DOI] [PubMed] [Google Scholar]
- 14.ten Tusscher KH, Panfilov AV. Alternans and spiral breakup in a human ventricular tissue model. Am J Physiol Heart Circ Physiol. 2006;291:H1088–H1100. doi: 10.1152/ajpheart.00109.2006. [DOI] [PubMed] [Google Scholar]
- 15.Weiss DL, Ifland M, Sachse FB, Seemann G, Dössel O. Modeling of cardiac ischemia in human myocytes and tissue including spatiotemporal electrophysiological variations. Biomed Tech (Berl) 2009;54:107–125. doi: 10.1515/BMT.2009.016. [DOI] [PubMed] [Google Scholar]
- 16.Britton OJ, Bueno-Orovio A, Van Ammel K, Lu HR, Towart R, Gallacher DJ, Rodriguez B. Experimentally calibrated population of models predicts and explains intersubject variability in cardiac cellular electrophysiology. Proc Natl Acad Sci USA. 2013;110:E2098–E2105. doi: 10.1073/pnas.1304382110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X, Bueno-Orovio A, Orini M, Hanson B, Hayward M, Taggart P, Lambiase PD, Burrage K, Rodriguez B. In vivo and in silico investigation into mechanisms of frequency dependence of repolarization alternans in human ventricular cardiomyocytes. Circ Res. 2016;118:266–278. doi: 10.1161/CIRCRESAHA.115.307836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. doi: 10.1038/nn1352. [DOI] [PubMed] [Google Scholar]
- 19.Cartledge JE, Kane C, Dias P, Tesfom M, Clarke L, Mckee B, Al Ayoubi S, Chester A, Yacoub MH, Camelliti P, Terracciano CM. Functional crosstalk between cardiac fibroblasts and adult cardiomyocytes by soluble mediators. Cardiovasc Res. 2015;105:260–270. doi: 10.1093/cvr/cvu264. [DOI] [PubMed] [Google Scholar]
- 20.Liau B, Jackman CP, Li Y, Bursac N. Developmental stage-dependent effects of cardiac fibroblasts on function of stem cell-derived engineered cardiac tissues. Sci Rep. 2017;7:42290. doi: 10.1038/srep42290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pope SM, Lasser C. Toxoplasma gondii infection of fibroblasts causes the production of exosome-like vesicles containing a unique array of mRNA and miRNA transcripts compared to serum starvation. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem. 2010;285:20281–20290. doi: 10.1074/jbc.M110.109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu XD, Song XW, Li Q, Wang GK, Jing Q, Qin YW. Attenuation of microRNA-22 derepressed PTEN to effectively protect rat cardiomyocytes from hypertrophy. J Cell Physiol. 2012;227:1391–1398. doi: 10.1002/jcp.22852. [DOI] [PubMed] [Google Scholar]
- 24.Li TJ, Chen YL, Gua CJ, Xue SJ, Ma SM, Li XD. MicroRNA 181b promotes vascular smooth muscle cells proliferation through activation of PI3K and MAPK pathways. Int J Clin Exp Pathol. 2015;8:10375–10384. [PMC free article] [PubMed] [Google Scholar]
- 25.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y, Ochi M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589:1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 28.Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee JJ, Xie C, Senyei G, Gordon RE, Li RA, Akar FG, Hajjar RJ, Hulot JS, Costa KD. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J. 2014;28:644–654. doi: 10.1096/fj.13-228007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Poeta G, Venditti A, Del Principe MI, Maurillo L, Buccisano F, Tamburini A, Cox MC, Franchi A, Bruno A, Mazzone C, Panetta P, Suppo G, Masi M, Amadori S. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML) Blood. 2003;101:2125–2131. doi: 10.1182/blood-2002-06-1714. [DOI] [PubMed] [Google Scholar]
- 30.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Uzair-Ur-Rehman, Guo Y, et al. miR-181b functions as an oncomiR in colorectal cancer by targeting PDCD4. Protein Cell. 2016;7:722–734. doi: 10.1007/s13238-016-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan LX, Wu QN, Zhang Y, Li YY, Liao DZ, Hou JH, Fu J, Zeng MS, Yun JP, Wu QL, Zeng YX, Shao JY. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res. 2011;13:R2. doi: 10.1186/bcr2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budd WT, Seashols-Williams SJ, Clark GC, Weaver D, Calvert V, Petricoin E, Dragoescu EA, O’Hanlon K, Zehner ZE. Dual action of miR-125b as a tumor suppressor and oncomiR-22 promotes prostate cancer tumorigenesis. PLoS One. 2015;10:e0142373. doi: 10.1371/journal.pone.0142373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cottage CT, Bailey B, Fischer KM, Avitabile D, Avitable D, Collins B, Tuck S, Quijada P, Gude N, Alvarez R, Muraski J, Sussman MA. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res. 2010;106:891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 36.Ramanujam D, Sassi Y, Laggerbauer B, Engelhardt S. Viral vector-based targeting of miR-21 in cardiac nonmyocyte cells reduces pathologic remodeling of the heart. Mol Ther. 2016;24:1939–1948. doi: 10.1038/mt.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soller KJ, Yang J, Veglia G, Bowser MT. Reversal of phospholamban inhibition of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) using short, protein-interacting RNAs and oligonucleotide analogs. J Biol Chem. 2016;291:21510–21518. doi: 10.1074/jbc.M116.738807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiao S, Olson JM, Paterson M, Yan Y, Zaja I, Liu Y, Riess ML, Kersten JR, Liang M, Warltier DC, Bosnjak ZJ, Ge ZD. MicroRNA-21 mediates isoflurane-induced cardioprotection against ischemia-reperfusion injury via Akt/nitric oxide synthase/mitochondrial permeability transition pore pathway. Anesthesiology. 2015;123:786–798. doi: 10.1097/ALN.0000000000000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eschenhagen T. Beta-adrenergic signaling in heart failure-adapt or die. Nat Med. 2008;14:485–487. doi: 10.1038/nm0508-485. [DOI] [PubMed] [Google Scholar]
- 40.Wiechert S, El-Armouche A, Rau T, Zimmermann WH, Eschenhagen T. 24-h Langendorff-perfused neonatal rat heart used to study the impact of adenoviral gene transfer. Am J Physiol Heart Circ Physiol. 2003;285:H907–H914. doi: 10.1152/ajpheart.00856.2002. [DOI] [PubMed] [Google Scholar]
- 41.Patrick DM, Montgomery RL, Qi X, Obad S, Kauppinen S, Hill JA, van Rooij E, Olson EN. Stress-dependent cardiac remodeling occurs in the absence of microRNA-21 in mice. J Clin Invest. 2010;120:3912–3916. doi: 10.1172/JCI43604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552–570. doi: 10.4252/wjsc.v6.i5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F. Extracellular vesicles in angiogenesis. Circ Res. 2017;120:1658–1673. doi: 10.1161/CIRCRESAHA.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu RX, Chen X, Chen JH, Han Y, Han BM. Mesenchymal stem cells promote cardiomyocyte hypertrophy in vitro through hypoxia-induced paracrine mechanisms. Clin Exp Pharmacol Physiol. 2009;36:176–180. doi: 10.1111/j.1440-1681.2008.05041.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang K, Jiang Z, Webster KA, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal MicroRNA-21. Stem Cells Transl Med. 2017;6:209–222. doi: 10.5966/sctm.2015-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS One. 2011;6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K, Dahl CP, Fiane A, Tønnessen T, Kryshtal DO, Louch WE, Knollmann BC. Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res. 2017;121:1323–1330. doi: 10.1161/CIRCRESAHA.117.311920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, Jiang L, Feng J, Yu XY. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016;7:e2277. doi: 10.1038/cddis.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesizza P, Prosdocimo G, Martinelli V, Sinagra G, Zacchigna S, Giacca M. Single-dose intracardiac injection of pro-regenerative MicroRNAs improves cardiac function after myocardial infarction. Circ Res. 2017;120:1298–1304. doi: 10.1161/CIRCRESAHA.116.309589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.