Abstract
Profilin (PFN) is an ubiquitous, low-Mr, actin-binding protein involved in the organization of the cytoskeleton of eukaryotes including higher plants. PFNs are encoded by a multigene family in Arabidopsis. We have analyzed in vivo functions of Arabidopsis PFN by generating transgenic plants carrying a 35S-PFN-1 or 35S-antisense PFN-1 transgene. Etiolated seedlings underexpressing PFN (PFN-U) displayed an overall dwarf phenotype with short hypocotyls whose lengths were 20% to 25% that of wild type (WT) at low temperatures. Light-grown PFN-U plants were smaller in stature and flowered early. Compared with equivalent cells in WT, most cells in PFN-U hypocotyls and roots were shorter, but more isodiametric, and microscopic observations of etiolated PFN-U hypocotyls revealed a rough epidermal surface. In contrast, light-grown seedlings overexpressing PFN had longer roots and root hair although etiolated seedlings overexpressing PFN were either the same size or slightly longer than WT seedlings. Transgenic seedlings harboring a PFN-1-GUS transgene directed expression in root and root hair and in a ring of cells at the elongating zone of the root tip. As the seedlings matured PFN-1-GUS was mainly expressed in the vascular bundles of cotyledons and leaves. Our results show that Arabidopsis PFNs play a role in cell elongation, cell shape maintenance, polarized growth of root hair, and unexpectedly, in determination of flowering time.
Actin is the major constituent protein of microfilaments in eukaryotic cells. The polymerization and depolymerization of actin filaments are highly regulated, spatially and temporally, to provide cells with the ability to rapidly remodel cytoskeleton in response to endogenous cues or external signals. In animal cells actin filaments are involved in cell locomotion and cell shape changes, whereas in plants it has been implicated in cytoplasmic streaming, cytokinesis, cell expansion, and development (Williamson, 1993; Meagher and Williamson, 1994; Meagher et al., 1999).
The dynamic rearrangement of actin filaments in cells are brought about by a number of actin-binding proteins. Profilin (PFN) is a low-Mr, actin monomer-binding protein that is ubiquitously present in organisms ranging from amoebae and fungi through to higher plants and mammals. Apart from actin binding, PFN also binds to phosphatidylionositol 4,5-bisphosphate (Sohn et al., 1995), poly-l-Pro (Bjorkegren et al., 1993; Gibbon et al., 1998), a Pro-rich protein called vasodialator-stimulated phosphoprotein (Haffner et al., 1995; Reinhard et al., 1995), formin homology domain-containing proteins (Frazier and Field, 1997; Kamei et al., 1998), Arp2/3 complex (Mullins et al., 1998; Loisel et al., 1999), and annexins (Alvarez-Martinez et al., 1996, 1997). Available evidence suggests that PFN is a multifunctional protein (Haarer et al., 1990) that exerts positive and negative regulatory effects on actin polymerization (Theriot and Mitchison, 1993) and, in certain cases it may be involved in signal transduction (Sohn and Goldschmidt-Clermont, 1994).
In vitro studies showed that PFN can facilitate actin polymerization at the barb ends by lowering the critical concentration and promote nucleotide exchange on G-actin (for review, see Pantaloni and Carlier, 1993; Theriot and Mitchison, 1993; Sohn and Goldschmidt-Clermont, 1994). The latter property is clearly not essential for the positive regulatory effect of PFNs because Arabidopsis PFNs lack this property, yet they are still able to promote actin assembly (Perelroizen et al., 1996).
PFN mutations affected multiple actin-dependent processes in Drosophila (Verheyen and Cooley, 1994), and blocked cell budding in Saccharomyces cerevisiae (Haarer et al., 1990) and cytokinesis in Schizosaccharomyces pombe (Balasubramanian et al., 1994). In vivo studies on the effects of PFNs on actin filaments have yielded mixed results. Microinjection of PFN into animal (Cao et al., 1992) or plant (Staiger et al., 1994; Gibbon et al., 1998) cells depolymerized actin, and deletion of both PFN genes from Dictyostelium discoideum resulted in a 60% to 70% increase in F-actin content at the rim below the plasma membrane (Haugwitz et al., 1994). By contrast, PFN overexpression in stably transfected cells increased actin polymerization at the cell periphery by prolonging the half-life of cortical actin filament (Finkel et al., 1994).
PFNs have been identified and characterized from several plant species including maize and tomato pollen (Staiger et al., 1993; Yu et al., 1998), tobacco (Mittermann et al., 1995), leaves and root nodules of Phaseolus vulgaris (Vidali et al., 1995; Guillen et al., 1999), and Arabidopsis (Christensen et al., 1996; Huang et al., 1996). Plant PFNs have been shown to bind plant and animal actin in vitro (Valenta et al., 1993; Giehl et al., 1994; Ruhlandt et al., 1995; Rothkegel et al., 1996). Moreover, Arabidopsis PFNs can complement the S. cerevisiae PFN deletion mutant and the S. pombe cdc3–124 PFN mutation (Christensen et al., 1996) and maize PFNs can rescue the aberrant phenotype of PFN-deficient Dictyostelium cells (Karakesisoglou et al., 1996). Kovar et al. (2000) have recently identified two functionally distinct classes of PFNs class I and class II, of which class II PFNs showed higher affinity to poly-l-Pro, sequestered more monomeric actin and disrupted the actin architecture more rapidly when compared with the class I PFNs. Despite these advances on the ability of plant PFNs to function in heterologous systems, the roles of PFNs in whole plant development have not yet been investigated (for review, see Staiger et al., 1997).
In our present study we investigated the in vivo functions of Arabidopsis PFNs (Christensen et al., 1996) genes by analyzing transgenic Arabidopsis plants expressing 35S-PFN-1 and 35S-antisense PFN-1. Our results suggest that Arabidopsis PFNs play a role in cell elongation, cell shape maintenance, polarized growth of root hair, and flowering time.
RESULTS
Analysis of PFN-Overexpressing (PFN-O) and -Underexpressing (PFN-U) Transgenic Plants
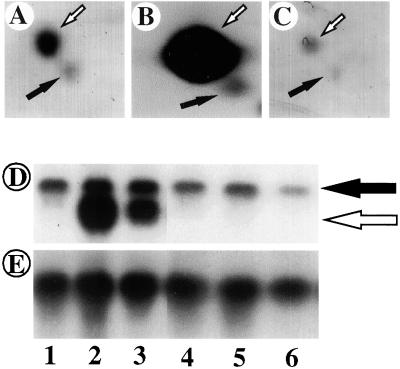
We previously reported the identification and molecular analysis of four Arabidopsis PFN genes including PFN-1 (Christensen et al., 1996). Huang et al. (1996) also characterized, independently, four Arabidopsis PFN (called PRF) genes, with their PRF-1 being identical to our PFN-1 (Christensen et al., 1996). To investigate the in vivo functions of PFN-1 we generated homozygous transgenic lines of Arabidopsis containing either 35S-PFN-1 or 35S-antisense PFN-1. Several lines of transgenic plants were analyzed by protein and RNA gel blots. Figure 1 shows that 35S-PFN-1 transgenic plants (hereafter called PFN-O) overexpressed PFN mRNA (Fig. 1D) and proteins (Fig. 1B), whereas the 35S-antisense PFN-1 transgenic lines (hereafter called PFN-U) expressed reduced levels of PFN mRNA (Fig. 1D) and proteins (Fig. 1C) as compared with wild type (WT) controls. We identified at least two lines of PFN-O (lines 3 and 6) that showed 20 times or higher PFN-1 RNA and protein levels and at least three lines of PFN-U (lines 1, 3, and 6) with a 25% to 50% reduction in PFN-1 RNA and protein levels. The reduction of the expression levels of both PFN isoforms (Fig. 1C) suggests that the antisense effect was not restricted to PFN-1 only. This is not surprising in view of the high sequence homology of the PFN gene members. Because the phenotypes of the two lines of PFN-O were similar, as were the three lines of PFN-U, detailed analyses were done with PFN-O-3 and PFN-U-6.
Figure 1.
Expression of PFN proteins and transcript in WT and transgenic seedlings. Proteins were separated by isoelectric focusing followed by SDS-PAGE. Affinity antibodies against PFN were used for the immunostaining. In A, B, and C, the white arrow indicates PFN-1 and the black arrow represents other members of the PFN family. RNA gel blots were hybridized to an in vitro transcribed antisense PFN-1 RNA. A, WT, 100 μg. B, PFN-O-3, 100 μg. C, PFN-U-6, 100 μg. D, PFN transcript levels in WT and transgenic plants. Each lane contained 30 μg RNA. Black arrow, Endogenous PFN mRNA; white arrow, transgenic PFN-1 mRNA. Lane 1, WT; lane 2, PFN-O-3; lane 3, PFN-O-6; lane 4, PFN-U-1; lane 5, PFN-U-3; lane 6, PFN-U-6. E, 18S rRNA levels in WT and transgenic plants. RNA gel blot from D was stripped and rehybridized with 18S rDNA lanes 1 through 6 same as D.
Hypocotyl Regions of Etiolated PFN-U Seedlings Show Severe Phenotype
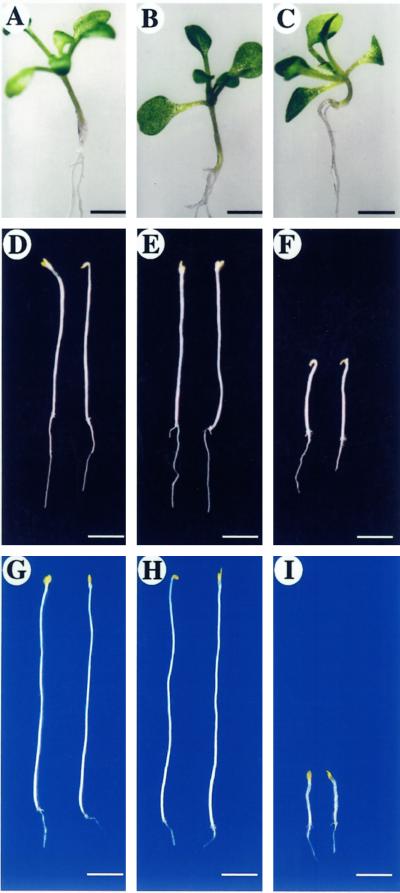
We compared PFN-U and PFN-O plants with WT plants under different growth conditions. No marked morphological phenotype was observed with seedlings grown in white light for either 5 (data not shown) or 10 d (Fig. 2, A–C). PFN-U seedlings on the average were 20% shorter (Fig. 2C) than WT seedlings (Fig. 2A), whereas PFN-O seedlings (Fig. 2B) were not significantly different from WT seedlings. This was perhaps not surprising because hypocotyl cell elongation is inhibited by light and under this condition PFN levels may not be limiting for cell elongation. To investigate this possibility we germinated seedlings in the dark for 5 (data not shown) or 10 d (Fig. 2, D–F). Etiolated PFN-U seedlings showed an obvious dwarf phenotype that became more pronounced 10 d after germination (Fig. 2F). Hypocotyl length of PFN-U seedlings (Fig. 2F) was about 40% that of WT seedlings (Fig. 2D), whereas PFN-O seedlings (Fig. 2E) showed no difference in hypocotyl lengths or were slightly longer. To see whether the difference in hypocotyl length between WT and transgenic plants could be enhanced, seedlings were germinated in the dark at 4°C for 28 d. Under this condition, PFN-O seedlings were slightly longer (Fig. 2H) than WT seedlings (Fig. 2G), whereas PFN-U seedlings (Fig. 2I) showed a drastic decrease in hypocotyl length. These results confirm that PFN plays a role in hypocotyl elongation.
Figure 2.
Phenotypes of PFN-O and PFN-U transgenic seedlings. A, WT, 10 d in white light at 22°C. B, PFN-O-3, 10 d in white light at 22°C. C, PFN-U-6, 10 d in white light at 22°C. D, WT, 10 d in dark at 22°C; average hypocotyl length = 1.36 ± 0.6 cm (n = 20). E, PFN-O-3, 10 d in dark at 22°C; average hypocotyl length = 1.40 ± 1.7 cm (n = 40). F, PFN-U-6, 10 d in dark at 22°C; average hypocotyl length = 0.54 ± 0.1 cm (n = 40). G, WT, 28 d in dark at 4°C. H, PFN-O-3, 28 d in dark at 4°C. I, PFN-U-6, 28 d in dark at 4°C. Bars in A, B, and C = 0.2 cm; bars in D, E, and F = 0.5 cm; bars in G, H, and I = 0.2 cm.
Genetic and physiological studies have also established a role for hormones in hypocotyl elongation. Auxins and gibberellic acid (GA) are known to promote hypocotyl elongation (Davies, 1995) and GA-deficient or -insensitive mutants (Finkelstein and Zeevaart, 1994), as well as auxin-resistant mutants (Estelle and Klee, 1994) show dwarf hypocotyls in the dark (Lincoln et al., 1990; Desnos et al., 1996). To rule out the possibility that the short hypocotyl phenotype of PFN-U plants was due to a GA or auxin deficiency, we treated the seedlings with appropriate concentrations of GA and indole acetic acid. These treatments did not restore the PFN-U seedlings to the WT size (data not shown), suggesting that the PFN-U phenotype is not due to a GA or auxin deficiency nor a to block in the signaling pathway of these hormones.
We also analyzed several organs of transgenic seedlings and mature plants for possible phenotypic changes as compared with WT counterparts. To this end we measured the lengths of main root, root hair (to be discussed later), trichomes, hypocotyl lengths of etiolated seedlings, siliques, and petiole (Table I). With the exception of trichomes all these cell types/organs were shorter in PFN-U plants and the same or slightly longer in PFN-O plants as compared with WT.
Table I.
Morphological alterations in PFN-O and PFN-U plants
| Plant Organ/Tissue | WT | PFN-O | PFN-U |
|---|---|---|---|
| Etiolated hypocotyl length (cm) | 1.36 ± 0.16 (n = 20) | 1.40 ± 0.17 (n = 40) | 0.54 ± 0.1 (n = 20) |
| Petiole length (cm) | 1.11 ± 0.07 (n = 20) | 1.06 ± 0.07 (n = 40) | 0.52 ± 0.07 (n = 40) |
| Silique length (cm) | 1.17 ± 0.12 (n = 15) | 1.16 ± 0.10 (n = 15) | 0.67 ± 0.14 (n = 14) |
| Root length (cm) | 6.3 ± 0.6 (n = 15) | 10 ± 1 (n = 15) | N.D. |
| Root hair length (mm) | 0.45 ± 0.08 (n = 36) | 0.8 ± 0.12 (n = 31) | N.D. |
The petiole length of the biggest rosette leaf from 21-d-old plants was measured. The lengths of root and root hair were determined using 5-d-old seedlings grown on Murashige and Skoog medium under 16-h light/8-h dark cycles at 22°C. N.D., Not determined.
PFNs Play a Role in Cell Elongation and Cell Shape Maintenance
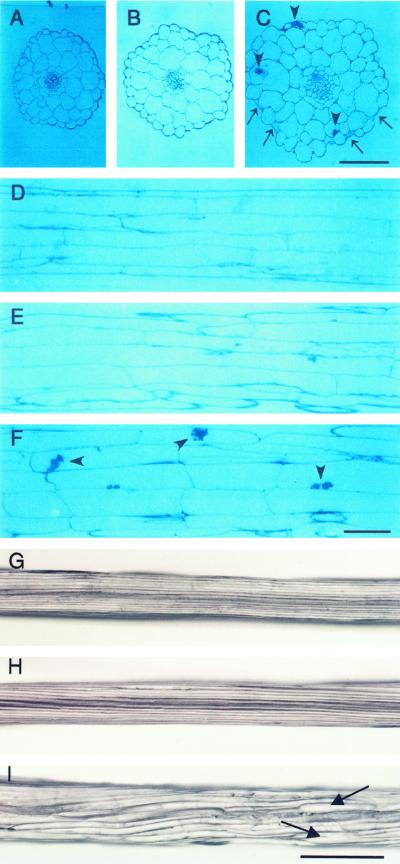
To investigate the effects of over or underexpression of PFN on cell elongation and cell shape, we analyzed hypocotyls of etiolated seedlings by light microscopy (Fig. 3, G–I). WT hypocotyls were straight and had smooth surfaces (Fig. 3G), as were PFN-O seedlings (Fig. 3H). By contrast PFN-U hypocotyls (Fig. 3I) were clearly thicker than those of PFN-O and WT; moreover, they had rough surfaces with dots at several places and some epidermal cells were swollen (Fig. 3C).
Figure 3.
Hypocotyl phenotypes of transgenic seedlings. Seedlings were grown for 10 d in the dark and sections were made through the mid-portions of the hypocotyls. A, WT, cross section. B, PFN-O-3, cross section. C, PFN-U-6, cross section. D, WT, longitudinal section. Average length of cortical cells = 567 ± 131 μm; average width of cortical cells = 27.45 ± 7.23 μm; n = 80. E, PFN-O-3, longitudinal section. Average length of cortical cells = 569 ± 147 μm; average width of cortical cells = 32.75 ± 7.59 μm; n = 80. F, PFN-U-6, longitudinal section. Average length of cortical cells = 266 ± 71 μm; average width of cortical cells = 39.77 ± 10.27 μm; n = 80. G, WT, whole hypocotyl; width = 172.7 ± 12.0 μm (n = 5). H, PFN-O-3, whole hypocotyl; width = 205.3 ± 3.5 μm (n = 5). I, PFN-U-6, whole hypocotyl; width = 250.8 ± 27.0 μm (n = 5). Arrows in C point to the collapsed epidermal cells and arrowheads in C and F indicate some structures in the epidermal and cortex cells. Arrows in I show the swollen region of A, B, and C; same magnification. Bar in C = 0.5 mm; D, E, and F, same magnification; bar in F = 0.1 mm; G, H, and I, same magnification; bar in I = 0.1 mm.
Analysis of longitudinal sections of hypocotyl regions revealed that WT cortical cells were long and straight (Fig. 3D), whereas those of PFN-U showed about a 2-fold reduction in length when compared with WT cells with some of the cells showing condensed cell matrix (Fig. 3F; Table I). On the other hand PFN-U cells were about 1.5 times wider than the corresponding WT cortical cells. This result indicates that the reduction in hypocotyl lengths of PFN-U can be largely accounted for by the reduction in cell length. No obvious difference between cortical cells of PFN-O and those of WT (Fig. 3E) was found.
WT hypocotyl contains a single tier of uniform epidermal cells and two layers of cortical cells (Fig. 3A). The arrangement of epidermal and cortical cells was similar in WT and PFN-O seedlings and the cells were also similar in size (Fig. 3B). The epidermal cells of PFN-U hypocotyls were not uniform; some cells were smaller, whereas others showed uncontrolled swelling. A few epidermal cells were at the verge of collapsing and condensed cell wall materials were found in some epidermal and cortex cells (Fig. 3C).
Electron Microscopic Analyses
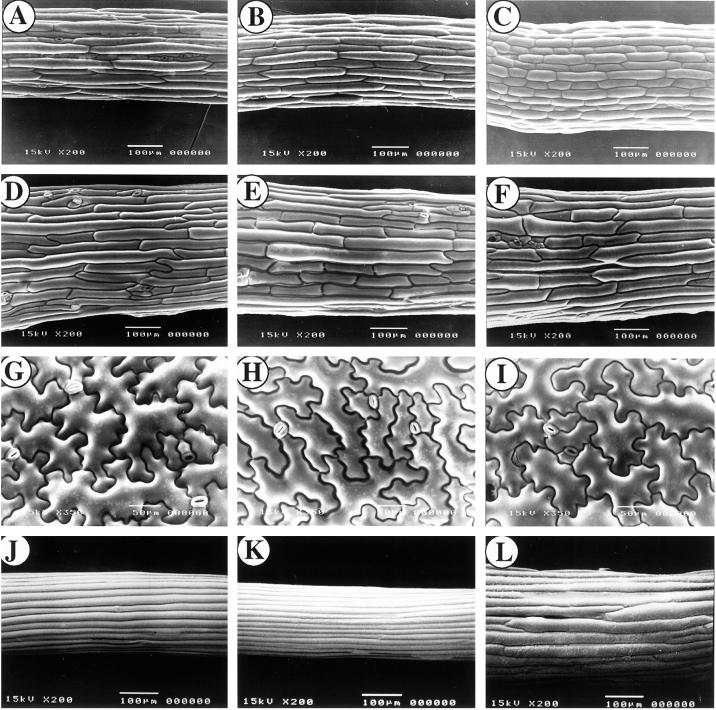
To confirm the rough surface of the PFN-U seedlings (Fig. 3I) we performed cryo-scanning electron microscopic (SEM) analysis of hypocotyl regions of WT and transgenic seedlings grown in light (Fig. 4, A–I) or dark (Fig. 4, J–L) for 10 d 22°C. Under both conditions WT and PFN-O hypocotyls showed smooth epidermal surfaces with no significant difference between them (Fig. 4, A, B, J, and K). PFN-U hypocotyls contained shorter and wider cells in light (Fig. 4C) or dark (Fig. 4L) and the shape of epidermal cells were altered resulting in rough surfaces (Fig. 4L). PFN-O cotyledon petiole (Fig. 4E) and epidermis (Fig. 4H) also showed no significant difference from the corresponding organ/tissue of WT (Fig. 4, D and G). Similar results were found with PFN-U plants (Fig. 4, F and I).
Figure 4.
SEM analysis of hypocotyl, petiole, and cotyledon surfaces of WT and transgenic seedlings. Hypocotyl and petiole specimens were prepared from the mid-portions of 10-d-old light- and dark-grown seedlings. A, Hypocotyl of light-grown WT. B, Hypocotyl of light-grown PFN-O-3. C, Hypocotyl of light-grown PFN-U-6. D, Cotyledon petiole of light-grown WT. E, Cotyledon petiole of light-grown PFN-O-3. F, Cotyledon petiole of light-grown PFN-U-6. G, Cotyledon surface of light-grown WT. H, Cotyledon surface of light-grown PFN-O-3. I, Cotyledon surface of light-grown PFN-U-6. J, Hypocotyl of etiolated WT. K, Hypocotyl of etiolated PFN-O-3. L, Hypocotyl of etiolated PFN-U-6. Bars = 100 μm.
Transmission electron microscopic analyses of hypocotyl epidermal cell walls of WT and transgenic seedlings did not show any noticeable difference in thickness. Whereas PFN-O cells were similar to WT cells with respect to cell wall structure, PFN-U cells showed a thicker cuticular layer and regions of the cell wall accumulated condensed materials. These epidermal cells contained some vesicular structures, which eventually may have been deposited on the cell walls to form the regions with condensed materials. The cortical cell walls of PFN-O seedlings sometimes showed unusual structures when compared with the WT and the PFN-U seedlings showed many vesicles containing electron dense materials near the cell wall (data not shown).
Flowering Time
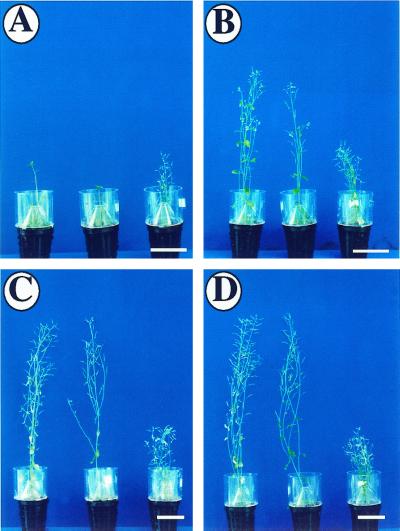
PFN-U transgenic plants were smaller in stature compared with PFN-O and WT plants (Fig. 5, A –D) and they flowered at least 12 d earlier than WT (Table II). At the time of flowering PFN-U plants had 40% fewer number of leaves as compared with WT (Table II). On the other hand, PFN-O plants had a similar stature as WT plants and there was no significant difference in the flowering time and the leaf number between the two at the time of flowering. PFN-O and PFN-U plants had similar numbers of flowers as WT and the flower structures were normal except that the PFN-U flowers were smaller in size. The flowers of PFN-O and PFN-U plants were fertile and set seeds with no apparent abnormality. The early flowering phenotype of PFN-U plants suggests the involvement of PFN, directly or indirectly, in the regulation of flowering time.
Figure 5.
Phenotypes of mature PFN-O and PFN-U plants grown in the light. Seedlings were grown for 20 d in Petri plates and then transferred into soil. A, 15 d after transfer. B, 25 d after transfer. C, 35 d after transfer. D, 45 d after transfer. For A through D, left, WT; middle, PFN-O-3; right, PFN-U-6. Bars = 4 cm.
Table II.
Flowering time of WT and transgenic plants
| Feature | WT | PFN-O | PFN-U |
|---|---|---|---|
| Flowering time (days) | 40 ± 3 (n = 15) | 38 ± 3 (n = 30) | 28 ± 3 (n = 45) |
| Leaf number at flowering | 22 ± 3 (n = 15) | 21 ± 3 (n = 30) | 14 ± 3 (n = 45) |
See “Materials and Methods” for details.
Expression of PFN-1 in the Elongation Zone of the Roots, Root Hairs, and Vascular Bundles
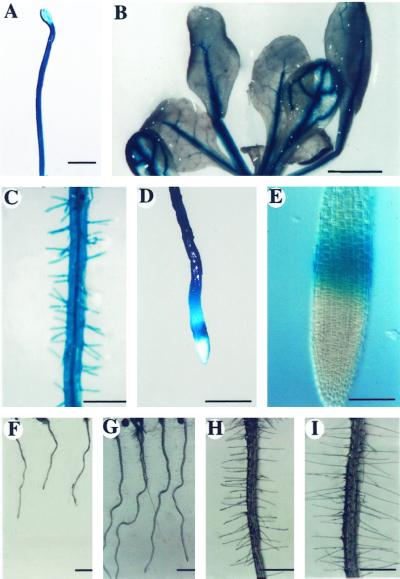
To analyze the expression profile of the PFN-1 we generated homozygous T3 transgenic seedlings carrying a PFN-1-GUS (GUS, β-glucuronidase) transgene. Figure 6A shows that PFN-1-GUS was expressed in hypocotyls and roots of 10-d-old etiolated seedlings. In 10-d-old light-grown seedlings GUS expression was restricted to the vascular bundles of hypocotyls and cotyledons, as well as root and root hair (data not shown). Twenty-day-old light-grown transgenic plants expressed PFN-1-GUS in the vascular bundles of cotyledons and leaves (Fig. 6B), and in root and root hair (Fig. 6C). It is notable that there was no expression in the root tip except in a ring of cells in the elongation zone (Fig. 6, D and E).
Figure 6.
GUS staining pattern of PFN-1-GUS transgenic seedlings and root phenotypes of PFN-O seedlings. A, GUS expression in etiolated seedlings. B, GUS expression pattern in 20-d-old light-grown seedlings. C, GUS activity in the root and root hairs of 20-d-old light-grown seedlings. D and E, GUS staining of the root elongation zone of 20-d-old light-grown seedlings. F, Roots of 5-d-old light-grown WT seedlings. G, Roots of 5-d-old light-grown PFN-O-3 seedlings. H, Root hairs of 5-d-old light-grown WT seedlings. I, Root hairs of 5-d-old light-grown PFN-O-3 seedlings. Bar in A = 0.2 mm; B = 0.2 mm; C = 0.05 mm; D = 0.05 mm; E = 0.01 mm; F and G = 0.2 mm; H and I = 0.05 mm.
The expression of PFN-1 in the root and root hair prompted us to investigate possible phenotypic changes in the root and root hair of PFN-O (Fig. 6, F and I) and PFN-U seedlings (data not shown). Five-day-old PFN-U seedlings showed shorter root and root hair (data not shown), but the most obvious changes were obtained with PFN-O transgenic plants. Roots of PFN-O plants were at least 50% longer (Fig. 6G) than WT root (Fig. 6F) and PFN-O root hairs were at least two times longer (Fig. 6I) than WT root hairs (Fig. 6H; Table I).
Actin Staining Patterns
Overexpression or underexpression of PFN has been shown to alter actin cytoskeletal arrangement in S. pombe, D. discoideum, and CHO cells lines (Balasubramanian et al., 1994; Finkel et al., 1994; Haugwitz et al., 1994). Moreover, microinjection of PFN into Tradescantia stamen hair cells depolymerized actin microfilaments and arrested cytoplasmic streaming (Staiger et al., 1994; Gibbon et al., 1997, 1998). Consideration of these results prompted us to analyze actin cytoskeleton arrangements in PFN-O and PFN-U seedlings and compare them with WT. Because these experiments were done before the availability of the green fluorescent protein-talin fusion gene (Kost et al., 1998) we standardized a protocol for phalloidin staining of actin microfilament in etiolated Arabidopsis seedlings. Petiole and cotyledonary cells of 10-d-old etiolated seedlings of WT and transgenic plants were subjected to such analysis. It is surprising that we did not find any significant difference in the cortical actin architecture of WT, PFN-O, and PFN-U cells (data not shown).
DISCUSSION
In this paper we have analyzed the in vivo function of Arabidopsis PFN by changing its expression level in transgenic plants using overexpression and antisense strategies. Arabidopsis PFNs are encoded by a multigene family with eight to 10 closely related members (based on Southern hybridizations) designated as PFNs by Christensen et al. (1996) and PRFs by Huang et al. (1996). So far five PFN family members have been characterized: PFN-1, PFN-2, and PFN-3 of Christensen et al. (1996) correspond to PRF-1, PRF-2, and PRF-4 of Huang et al. (1996), respectively. PFN-4 of Christensen et al. (1996) was not reported by Huang et al. (1996), and conversely, PRF-3 of Huang et al. (1996) was not isolated by Christensen et al. (1996). These five PFN (or PRF) family members are highly homologous with respect to nucleotide and amino acid sequences. Under our hybridization conditions, the PFN-1 probe likely recognized transcripts from all gene members. Therefore, the reduction of PFN transcript levels in PFN-U plants probably affected all PFN gene members. This notion is further supported by two-dimensional gel analysis (Fig. 1A), where we observed two major isoforms of PFN in vegetative tissues (10-d-old etiolated or light-grown seedlings) that are products of the gene members. Both isoforms reacted with our polyclonal antibodies raised against PFN1 and 2 and were reduced in PFN-U plants. Based on their pIs, the major spot likely contains PFN-1 (pI = 4.54; Christensen et al., 1996) and PRF-3 (pI = 4.55; Huang et al., 1996), whereas the minor spot likely contains PFN-2 (pI = 4.78; Christensen et al., 1996; Huang et al., 1996). Note that PFN-1 and PFN-2 are expressed in vegetative tissues (Christensen et al., 1996).
PFN Levels Are Important for Cell Elongation
In PFN-U transgenic plants, the expression levels of PFN transcript transcript is reduced by 25% to 50% as compared with WT. Detailed analysis was carried out with PFN-U-6, which showed a 50% reduction in PFN protein levels. PFN-U-6 transgenic plants showed a pronounced dwarfed phenotype in seedling, as well as in adult plants. When grown in the dark to promote hypocotyl elongation, PFN-U hypocotyl length was only about 40% that of WT. A more dramatic reduction in hypocotyl lengths was observed at 4°C, which presumably limited cytoskeletal functions. Taken together, these results demonstrate the critical importance of PFN levels in regulating cell elongation.
PFN has been shown to be involved in cell division and its deletion resulted in cells with clearly impaired cytokinesis in Dictyostelium (Haugwitz et al., 1994) and S. pombe (Balasubramanian et al., 1994), abnormal regulation of mitosis in Drosophila (Verheyen and Cooley, 1994), and multinucleation in S. cerevisiae (Haarer et al., 1990). To determine whether the dwarf phenotype of PFN-U plants is due to such cell division problems we analyzed the entire or sections of hypocotyls of etiolated PFN-U seedlings by light and SEM. Our results show that the difference in hypocotyl lengths between PFN-U and WT seedlings can be largely accounted for by the difference in cell length (Fig. 3, D and F; Table I), indicating no significant difference in cell number. Although not investigated in the same details, the difference in length of the other plant organs, e.g. petiole and silique, is likely attributable to a reduction in the cell length of these organs of PFN-U transgenic plants as well. Thus PFN expression levels in cells appear to be rate limiting and critical for cell elongation, and reduction in expression levels by 50% resulted in an elongation defect with no apparent impact on cell division. This notwithstanding, there was no detectable change in the cortical actin cytoskeleton of PFN-U transgenic plants (data not shown). We note that PFN deletions in Dictyostelium (Haugwitz et al., 1994) and in yeast (Haarer et al., 1990) were not lethal, but produced severe phenotypes. By contrast, PFN deletion in Drosophila resulted in death during late embryonic development (Verheyen and Cooley, 1994) and similar deletion in mice arrested embryo development at the 100-cell stage. It is possible that a severe reduction of Arabidopsis PFN protein levels (e.g. greater than 50%) may also arrest development, but in this case no transgenic plants would be recovered.
We found that overexpression of PFN-1 did not appear to have any significant effect on the morphology of aerial organs in general, except that PFN-O plants have longer roots and root hairs (Fig. 6; Table I). The latter finding is perhaps not surprising because PFN-1 is expressed in the root elongation zone and root hairs (Fig. 6, C–E), indicating that this protein functions as a rate-limiting factor in these cell types to promote cell elongation and root hair extension. Like elongating pollen tubes, the growth of root hairs is restricted to the tip by polarized secretion and it is likely that PFN-1 somehow promotes this process. As in the PFN-U plants, the cortical actin cytoskeleton in PFN-O hypocotyl cells appeared to be indistinguishable from that of WT (data not shown), even though PFN-1 levels was increased by about 20 times in these plants. In animal tissue cultured cells, microinjection of PFN only reduced the centrally located actin filaments with no effect on cortical actin filaments (Cao et al., 1992).
Staiger and coworkers investigated the functions of maize pollen PFNs in vivo by microinjecting purified proteins into Tradescantia stamen hair cells (Staiger et al., 1994; Gibbon et al., 1997, 1998). They found that injection of PFN clearly caused a reduction in F-actin, which was correlated with a disruption of cytoplasmic streaming and a translocation of the nucleus from the cell center to the periphery. In our experiments the transgenic PFN-O plants expressed PFN-1 at levels 20 times higher than that in WT; nevertheless, these plants displayed apparently normal cortical actin filaments. Several factors could explain the discrepancy between our results and those of Gibbon et al. (1997, 1998). The overexpression of PFN-1 was constitutive in transgenic PFN-O plants, which could have resulted in a physiological adaptation of the plants. Although we have not observed any significant changes in the actin and actin depolymerizing factor expression levels in these plants (data not shown), we cannot rule out changes in other actin-binding proteins that may provide a compensatory effect on the F-actin architecture. Gibbon et al. (1997) found that 1 h after injection, many injected hair cells were able to recover from the effects caused by PFNs, indicating that F-actin filaments can be reconstituted in the presence of excess PFNs even in this type of transient assay. It is, therefore, not surprising that no apparent effect on cortical actin filaments was seen in the PFN-O transgenic plants.
Cell Shape Maintenance
Cross sections of PFN-U hypocotyls showed swollen epidermal, cortical, and endodermal cells (Fig. 3C). These morphological characteristics were reminiscent of those reported for the procuste-1 mutant (Desnos et al., 1996), which has a hypocotyl elongation defect. Alterations of actin cytoskeleton by cytochalasin treatment (Wernicke and Jung, 1992; Baskin and Bivens, 1995) caused cell swelling and induced irregular-shaped cells. In addition, enlarged spherical cells have been reported in yeast (Haarer et al., 1990) and in Dictyostelium (Haugwitz et al., 1994) that have mutations in or underexpressing actin-associated proteins such as PFN. Therefore, it seems possible that the cell shape changes observed in PFN-U plants are due to changes in actin cytoskeleton and perturbations in polarized secretion.
A defect in secretion is also consistent with our observation that some PFN-U epidermal cells were bigger than the others, whereas other epidermal cells were at the verge of collapsing, presumably due to weakened cell wall structures (Fig. 3, C and F). The cellulose microfibrils, the components of cell walls, are deposited in an orderly fashion and they are generally believed to control the cell shape (Green, 1987). The use of cellulose biosynthesis inhibitor 2, 6, dichloro benzonitrile (Delmer, 1987) caused short and deformed hypocotyl phenotype in WT Arabidopsis plants, reminiscent of the prc mutant and PFN-U seedling phenotype.
Effect on Flowering Time
An unexpected finding was that PFN-U plants flowered earlier than WT and PFN-O plants, suggesting that a reduction in PFN levels decreased flowering time. It is unclear at present whether this is a direct or an indirect effect. In other eukaryotic systems, a role for PFN in cell signaling (Sohn and Goldschmidt-Clermont, 1994) has been proposed, in addition to its role in actin cytoskeletal dynamics. Although the results here implicate PFN in the determination of flowering time in Arabidopsis, the precise mechanism remains to be clarified by future experiments.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The Arabidopsis C24 ecotype was used for this study. For growth in the dark, plates were wrapped with three layers of aluminum foil and kept vertically in a tissue culture room at 22°C. For cold treatment, the wrapped plates were kept vertically in a refrigerator for 4 weeks. For light growth, plates were exposed to 16 h of light and 8 h of darkness at 22°C. After 3 weeks seedlings were potted in soil and transferred to a growth chamber maintained with 16 h of light and 8 h of darkness at 22°C and a humidity of 75%.
Vector Constructions and Plant Transformations
The coding region of PFN-1 cDNA (Christensen et al., 1996) was cloned downstream of a 35S promoter in the pVIP40 vector (van der Krol and Chua, 1993) in the sense and the antisense orientation. The PFN-1-GUS fusion construct contained 1.0 kb of the PFN-1 5′-upstream region in the pVIP40 vector. Transformation was performed according to Valvekens et al. (1988). T3 transgenic lines were generated and used to obtain homozygous T4 seeds. Two independent lines (nos. 3 and 6) for PFN-1 PFN-O and three lines (nos. 1, 3, and 6) for PFN-U were used in this study.
RNA Analysis
Total RNA was extracted from 10-d-old dark- or light-grown seedlings of WT and transgenic plants using an RNA isolation kit (Qiagen, Valencia, CA), RNAs were separated by electrophoresis on 1.2% (w/v) agarose gels, and samples were blotted on to a nylon membrane that was then used for hybridizations. Radiolabeled antisense strand of PFN-1 was transcribed from the PFN-1 cDNA cloned in pBlueScript (Christensen et al., 1996) and used as a probe for the hybridizations. Arabidopsis actin-7 cDNA and ADF-1 cDNA (Carlier et al., 1997) were used as probes. 18S rRNA was used as a loading control.
Antibody Preparation, Two-Dimensional Gel Electrophoresis, and Western-Blot Analyses
PFN-1 expressed in Escherichia coli (Christensen et al., 1996) was used to immunize rabbits. IgG purified by affinity chromatography on a Protein A Sepharose CL-4B (Pharmacia, Piscataway, NJ) column was used for western-blot analyses. Proteins were extracted from 10-d-old (light- or dark-grown) seedlings grown at 22°C or 4-week-old etiolated seedlings grown at 4°C. The plant materials were extracted on ice using a mortar and a pestle with 50 mm Tris [tris(hydroxymethyl)-aminomethane]-Cl (pH 8.0), 0.5 mm CaCl2, 0.5% (w/v) NP40, 0.5 mm β-mercaptoethanol, aprotinin (1 μg/mL), and leupeptin (1 μg/mL). The extracts were centrifuged for 10 min at 4°C and the supernatant was collected. Protein concentrations were determined by Bradford's method and 10 μg was used for two-dimensional gel electrophoresis (Hochstrasser et al., 1988). After electrophoresis, proteins were transferred onto nitrocellulose membranes. Membranes were incubated with 3% (w/v) non-fat milk in phosphate-buffered saline (PBS) buffer containing 0.05% (w/v) Tween 20 for 1 h and washed several times with PBS buffer followed by incubation with anti-PFN-1 IgG overnight. The membranes were then washed with PBS and then with alkaline phosphatase conjugated anti-rabbit IgG (Promega, Madison, WI) for 1 h. After several washes the spots were developed following the manufacturer's instructions.
Cell Length and Diameter Measurements
Longitudinal and cross sections of 10-d-old etiolated WT and transgenic seedlings were photographed under a light microscope. The length and diameter of epidermal and cortex cells were measured on enlarged photographs.
Flowering Time and Leaf Number
WT and transgenic plants were grown with 16 h of light and 8 h of darkness at 22°C and flowering time was scored as the number of days from the time when the plates were placed in a tissue culture room to the time of flowering. Leaf number was scored as the number of leaves on the rosette (excluding cotyledon) and on the inflorescence stem at the time of opening of the first flower. Three weeks after germination about 15 randomly selected plants from WT and each of the two independent lines of PFN-O and the three independent lines of PFN-U transgenic plants were transferred to soil.
GUS Assay
GUS assay was performed using 5-bromo-4-chloro-3-indoyl glucronide (Jefferson, 1987) as a substrate (Toriyama et al., 1991).
Footnotes
This work was supported by a grant from the National Science and Technology Board, Singapore. Work done at Rockefeller University was supported in part by the Department of Energy (grant no. 94ER20143 to N.H.C.).
LITERATURE CITED
- Alvarez-Martinez MT, Mani JC, Porte F, Faivre-Sarrailh C, Liautard JP, Sri Widada J. Characterization of the interaction between annexin I and profilin. Eur J Biochem. 1996;238:777–784. doi: 10.1111/j.1432-1033.1996.0777w.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Martinez MT, Porte F, Liautard JP, Sri Widada J. Effects of profilin-annexin I association on some properties of both profilin and annexin I: modification of the inhibitory activity of profilin on actin polymerization and inhibition of the self association of annexin I and its interactions with liposomes. Biochim Biophys Acta. 1997;1339:331–340. doi: 10.1016/s0167-4838(97)00018-6. [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, Hirani BR, Burke JD, Gould KL. The Schizosaccaromyces pombe cdc3+ gene encodes a profilin essential for cytokinesis. J Cell Biol. 1994;125:1289–1301. doi: 10.1083/jcb.125.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin TI, Bivens NJ. Stimulation of radial expansion in Arabidopsis roots by inhibitors of actomyosin and vesicle secretion but not by various inhibitors of metabolism. Planta. 1995;197:514–521. doi: 10.1007/BF00196673. [DOI] [PubMed] [Google Scholar]
- Bjorkegren C, Rozycki M, Schutt CE, Lindberg U, Karlsson R. Mutagenesis of human profilin locates its poly (L-proline)-binding site to a hydrophobic patch of aromatic amino acids. FEBS Lett. 1993;333:123–126. doi: 10.1016/0014-5793(93)80388-b. [DOI] [PubMed] [Google Scholar]
- Cao LG, Babcock GG, Rubenstein PA, Wang YL. Effects of profilin and profilactin on actin structure and function in living cells. J Cell Biol. 1992;117:1023–1029. doi: 10.1083/jcb.117.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/Çofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–1323. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen HEM, Ramachandran S, Tan CT, Surana U, Dong CH, Chua NH. Arabidopsis profilins are functionally similar to yeast profilins: identification of a vascular bundle-specific profilin and a pollen-specific profilin. Plant J. 1996;10:269–279. doi: 10.1046/j.1365-313x.1996.10020269.x. [DOI] [PubMed] [Google Scholar]
- Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. [Google Scholar]
- Delmer DP. Cellulose biosynthesis. Annu Rev Plant Physiol. 1987;38:259–290. [Google Scholar]
- Desnos T, Orbovic V, Bellini C, Kronenberger J, Caboche M, Trass J, Hofte H. Procuste1 mutants identify two distinct genetic pathways controlling hypocotyl cell elongation, respectively in dark- and light-grown Arabidopsis. Development. 1996;122:683–693. doi: 10.1242/dev.122.2.683. [DOI] [PubMed] [Google Scholar]
- Estelle M, Klee HJ. Auxin and cytokinin in Arabidopsis. In: Meyerowitz EM, Sommerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 555–578. [Google Scholar]
- Finkel T, Theriot JA, Dise KR, Tomaselli GF, Goldschmidt-Clermont PJ. Dynamic actin structures stabilized by profilins. Proc Natl Acad Sci USA. 1994;91:1510–1514. doi: 10.1073/pnas.91.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Zeevaart IAD. Gibberellin and abscisic acid biosynthesis and response. In: Meyerowitz EM, Sommerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 523–553. [Google Scholar]
- Frazier JA, Field CM. Actin cytoskeleton: are FH proteins local organizers? Curr Biol. 1997;7:R414–417. doi: 10.1016/s0960-9822(06)00205-3. [DOI] [PubMed] [Google Scholar]
- Gibbon BC, Ren H, Staiger CJ. Characterization of maize (Zea mays) pollen profilin function in vitro and in live cells. Biochem J. 1997;327:909–915. doi: 10.1042/bj3270909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon BC, Zonia LE, Kovar DR, Hussey PJ, Staiger CJ. Pollen profilin function depends on interaction with proline-rich motifs. Plant Cell. 1998;10:981–993. doi: 10.1105/tpc.10.6.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl K, Valenta R, Rothkegel M, Ronsiek M, Mannherz HG, Jockusch BM. Interaction of plant profilin with mammalian actin. Eur J Biochem. 1994;226:681–689. doi: 10.1111/j.1432-1033.1994.tb20096.x. [DOI] [PubMed] [Google Scholar]
- Green PB. Inheritance of pattern: analysis from phenotype to gene. Am Zoo. 1987;27:657–673. [Google Scholar]
- Guillen G, Valdes-Lpoez V, Noguez R, Oliveares J, Rodriguez-Zapata LC, Perez H, Viali L, Villanueva MA, Sanchez F. Profilins in Phaseolus vulgaris is encoded by two genes (only one expressed in root nodules) but multiple isoforms are generated in vivo by phosphorylation on tyrosine residues. Plant J. 1999;19:497–508. doi: 10.1046/j.1365-313x.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- Haarer BK, Lillie SH, Adams AEM, Magdolen V, Bandlow W, Brown SS. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J Cell Biol. 1990;110:105–114. doi: 10.1083/jcb.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner C, Jarchau T, Reinhard M, Hoppe J, Lohmann SM, Walter U. Molecular cloning, structural analysis and functional expression of the proline-rich focal adhesion and microfilament-associated protein VASP. EMBO J. 1995;14:19–27. doi: 10.1002/j.1460-2075.1995.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugwitz M, Noegel AA, Karakesisoglou J, Schleider M. Dictyostelium amoebae that lack G-actin-sequestering profilins show defects in F-actin content, cytokinesis, and development. Cell. 1994;79:303–314. doi: 10.1016/0092-8674(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Hochstrasser DF, Harrington MG, Hochstrasser AC, Miller MJ, Merril CR. Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem. 1988;173:424–435. doi: 10.1016/0003-2697(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Huang S, McDowell JM, Weise MJ, Meagher RB. The Arabidopsis profilin gene family. Plant Physiol. 1996;111:115–126. doi: 10.1104/pp.111.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- Kamei T, Tanaka K, Hihara T, Umikawa M, Imamura H, Kikyo M, Ozaki K, Takai Y. Interaction of Bnr1p with a novel Src homology 3 domain-containing Hof1p: implication in cytokinesis in Saccharomyces cerevisiae. J Biol Chem. 1998;273:28341–28345. doi: 10.1074/jbc.273.43.28341. [DOI] [PubMed] [Google Scholar]
- Karakesisoglou I, Schleicher M, Gibbon BC, Staiger CJ. Plant profilins rescue the aberrant phenotype of profilin-deficient Dictyostelium cells. Cell Motil Cytoskelet. 1996;34:36–47. doi: 10.1002/(SICI)1097-0169(1996)34:1<36::AID-CM4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kost B, Spielhofer P, Chua NH. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 1998;16:393–401. doi: 10.1046/j.1365-313x.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- Kovar DR, Drobak BK, Staiger CJ. Maize profilin isoforms are functionally distinct. Plant Cell. 2000;12:583–598. doi: 10.1105/tpc.12.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel TP, Boujemaa R, Pantaloni D, Carlier MF. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401:104–112. doi: 10.1038/44183. [DOI] [PubMed] [Google Scholar]
- Meagher RB, McKinney EC, Vitale AV. The evolution of new structures: clues from plant cytoskeletal genes. Trends Genet. 1999;15:278–284. doi: 10.1016/s0168-9525(99)01759-x. [DOI] [PubMed] [Google Scholar]
- Meagher RB, Williamson RE. The plant cytoskeleton. In: Meyerowitz EM, Sommerville CR, editors. Arabidopsis. The Plant Cytoskeleton. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 1049–1084. [Google Scholar]
- Mittermann I, Swoboda I, Pierson E, Eller N, Kraft D, Valenta R, Heberle-Bors E. Molecular cloning and characterization of profilin from tobacco (Nicotiana tabacum): increased profilin expression during pollen maturation. Plant Mol Biol. 1995;27:137–146. doi: 10.1007/BF00019185. [DOI] [PubMed] [Google Scholar]
- Mullins RD, Kelleher JF, Xu J, Pollard TD. Arp2/3 complex from Acanthamoeba binds profilin and cross-links actin filaments. Mol Biol Cell. 1998;9:841–852. doi: 10.1091/mbc.9.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaloni D, Carlier MF. How profilin promotes actin filament assembly in the presence of thymosin β4. Cell. 1993;75:1007–1014. doi: 10.1016/0092-8674(93)90544-z. [DOI] [PubMed] [Google Scholar]
- Perelroizen I, Didry D, Christensen H, Chua NH, Carlier MF. Role of nucleotide exchange and hydrolysis in the function of profilin in actin assembly. J Biol Chem. 1996;271:12302–12309. doi: 10.1074/jbc.271.21.12302. [DOI] [PubMed] [Google Scholar]
- Reinhard M, Giehl K, Abel K, Haffner C, Jarchau T, Hoppe V, Jockusch BM, Walter U. The proline-rich focal adhesion and microfilament protein VASP is a ligand for PFNs. EMBO J. 1995;14:1583–1589. doi: 10.1002/j.1460-2075.1995.tb07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkegel M, Mayboroda O, Rohde M, Wucherrpfennig C, Valenta R, Jockusch BM. Plant and animal profilins are functionally equivalent and stabilize microfilaments in living animal cells. J Cell Sci. 1996;109:83–90. doi: 10.1242/jcs.109.1.83. [DOI] [PubMed] [Google Scholar]
- Ruhlandt G, Lange U, Grolig F. Profilins purified from higher plants bind to actin from cardiac muscle and to actin from green algae. EMBO J. 1995;14:1583–1589. doi: 10.1093/oxfordjournals.pcp.a078668. [DOI] [PubMed] [Google Scholar]
- Sohn RH, Chen J, Koblan KS, Bray PF, Goldschmidt-Clermont PJ. Localization of a binding site for phosphatidylinositol 4, 5-bisphosphate on human profilin. J Biol Chem. 1995;270:21114–21120. doi: 10.1074/jbc.270.36.21114. [DOI] [PubMed] [Google Scholar]
- Sohn RH, Goldschmidt-Clermont PJ. Profilin: at the crossroads of signal transduction and the actin cytoskeleton. BioEssays. 1994;16:465–472. doi: 10.1002/bies.950160705. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Gibbon BC, Kovar DR, Zonia LE. PFN and actin depolymerizing factor: modulators of actin organization in plants. Trends Plant Sci. 1997;2:275–281. [Google Scholar]
- Staiger CJ, Goodbody KC, Hussey PJ, Valenta R, Drobak BJ, Lloyd CW. The profilin multigene family of maize: differential expression of three isoforms. Plant J. 1993;4:631–641. doi: 10.1046/j.1365-313x.1993.04040631.x. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Yuan M, Valenta R, Shaw PJ, Warn RM, Lloyd CW. Microinjected profilin affects cytoplasmic streaming in plant cells by rapidly depolymerizing actin microfilaments. Curr Biol. 1994;4:215–219. doi: 10.1016/s0960-9822(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Theriot JA, Mitchison TJ. The three faces of profilin. Cell. 1993;75:835–838. doi: 10.1016/0092-8674(93)90527-w. [DOI] [PubMed] [Google Scholar]
- Toriyama K, Thorsness MK, Nasarallah ME, Nasarallah JB. A Brassica S locus gene promoter directs sporophytic expression in the anther tapetum of transgenic Arabidopsis. Dev Biol. 1991;143:427–431. doi: 10.1016/0012-1606(91)90094-j. [DOI] [PubMed] [Google Scholar]
- Valenta R, Ferreira F, Grote M, Swoboda I, Vrtala S, Duchene M, Deviller P, Meagher RM, McKinney E, Heberle-Bors E, Kraft D, Scheiner O. Identification of profilin as an actin-binding protein in higher plants. J Biol Chem. 1993;268:22777–22781. [PubMed] [Google Scholar]
- Valvekens D, Van Montagu M, van-Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A, Chua NH. Flower development in petunia. Plant Cell. 1993;5:1195–1203. doi: 10.1105/tpc.5.10.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen EM, Cooley L. Profilin mutations disrupts multiple actin-dependent processes during Drosophila development. Development. 1994;120:717–728. doi: 10.1242/dev.120.4.717. [DOI] [PubMed] [Google Scholar]
- Vidali L, Perez HE, Lopez VV, Noguez R, Zamudio F, Sanchez F. Purification, characterization, and cDNA cloning of profilin from Phaseolus vulgaris. Plant Physiol. 1995;108:115–123. doi: 10.1104/pp.108.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke W, Jung G. Role of cytoskeleton in cell shaping of developing mesophyll of wheat (Triticum aestivum L.) Eur J Cell Biol. 1992;57:88–94. [PubMed] [Google Scholar]
- Williamson RE. Organelle movements. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:181–202. [Google Scholar]
- Yu LX, Nasrallah J, Valenta R, Parthasarathy MV. Molecular cloning and mRNA localization of tomato pollen profilin. Plant Mol Biol. 1998;36:699–707. doi: 10.1023/a:1005971327353. [DOI] [PubMed] [Google Scholar]