Abstract
Background
Alopecia areata is a relapsing hair disorder characterized by a sudden hairloss and has a considerable impact on patient’s quality of life. The goal of this study was to determine quality of life among patients with mild and severe forms of alopecia areata and compare the two groups.
Methods
During one year, 176 patients (96 mild, 80 severe) were selected and asked to complete Dermatology Life Quality Index (DLQI) questionnaires.
Results
Our study revealed that the severe group was predominantly female and had higher amount of unemployment, more prolonged disease duration, unstable disease course and facial involvement. The mean DLQI scores in the severe and mild groups were 10.7 ± 7.5 and 5.4 ± 6.8, respectively which was significantly different and severe group had higher DLQI scores and more quality of life impairment. As well, there was a significant association between total DLQI scores and acute stress during last 6 months.
Conclusions
Our study confirms that alopecia areata considerably impacts quality of life and this is more pronounced in patients with severe disease and those who had acute stress recently.
Keyword: Alopecia areata, quality of life, DLQI
Introduction
Alopecia areata (AA) is a relapsing, non-scaring and immune-mediated form of hair loss that occurs in all ethnic groups, ages, and both sexes, with an estimated lifetime risk of 1.7% among the general population (Villasante Fricke and Miteva, 2015).
Some authors reported an increased prevalence of psychiatric disorders in AA, especially anxiety and depression, others concluded that these comorbidities are less important (Gulec et al., 2004). Another controversial issue is that whether stressful life events are able to trigger episodes of AA. However it can be assumed that even if stressful life events play a minimal role in the triggering of AA, anxiety and depression due to this chronic disorder could negatively affect disease course via stress mediators (Matzer et al., 2011).
It has been described that the disease has a considerable impact on patient’s quality of life (QoL). This is due to the visibility of this disorder, which affects patient’s appearance, emotional health and social communications (Fabbrocini et al., 2013, Ruiz-Doblado et al., 2003). So, QoL determination has become increasingly important in assessing AA severity and impacts (Dubois et al., 2010).
There are now several dermatology-specific questionnaires such as Skindex (Finlay and Khan, 1994), Dermatology Life Quality Index (DLQI) (Finlay and Khan, 1994), Dermatology Quality of Life Scales (Morgan et al., 1997), and Dermatology Specific Quality of Life (Anderson and Rajagopalan, 1997).
The goal of this study was to determine QoL among patients with mild and severe forms of AA and compare the two groups. In this way, we used DLQI questionnaire that is the most cited tool due to its high degree of reliability, applicability and reproducibility (Morgan et al., 1997).
Materials and methods
The study was approved by the Ethic Committee of Tehran University of Medical sciences.
From October 2013 until October 2014 from 200 patients with definite diagnosis of AA, 80 severe (alopecia totalis, universalis and ophyasis) and 96 mild (less than 25% of scalp hairloss) AA patients over the age of 16 who were randomly selected from the outpatient clinic of Razi Hospital accepted to participate in the study and were asked to complete questionnaires.
The questionnaires consisted of two parts: the first part which was fulfilled by physician, including questions about gender, age, family history of AA, disease duration, educational state, acute stress during last 6 months, disease course (course of the disease was defined as “unstable” if there was alternation of worsening and improvement phases in the last 2 years, and “stable” otherwise), facial hairloss, occupation (currently working and currently not working) and other illnesses (thyroid disease, diabetes mellitus, atopy); the second part included questions measuring DLQI.
DLQI, which was introduced by Finlay and Khan (1994), is a self-explanatory survey which consists of ten questions. The DLQI is calculated by summing the score of each question resulting in a possible score of 0 to 30. The higher the score, the more QoL is impaired. The valid Persian version was used for measuring patient’s QoL (Aghaei et al., 2004).
The questions can be classified under 6 headings items: symptoms and feelings (questions 1- 2), daily activities (questions 3- 4), leisure (questions 5- 6), and personal relationships (questions 8- 9) each item with maximum score 6; work and school (question 7), and treatment (question10) each item with maximum score 3.
In order to help the clinical interpretation of the DLQI scores a banding system has been validated. According to this system, DLQI scores 0-1 = no affect at all, 2-5 = small effect, 6-10 = moderate effect, 11-20 = very large effect and DLQI score of 21-30 = extremely large effect on patient’s life.
For statistical analysis the SPSS version 16 was used. Continuous variables were described using Mean, Standard Deviation (SD). Categorical variables were reported as frequencies and percent. For comparison of continuous variables, we used independent samples t-test for normally distributed data and non-parametric Mann-Whitney U test for variables showing skewed distribution. For qualitative variables the Chi-square test was used for each contingency table. The correlation between continuous variables was assessed using Pearson’s and Spearman’s correlation coefficients. Variables with P-value < 0.2 were included in multivariable linear regression model to adjust the effect of potentially confounding factors. In all analyses two-tailed P < 0.05 was considered statistically significant.
Results
One hundred seventy-six patients participated in this cross-sectional study. Ninety six (54.5%) mild AA patients (84 men and 12 women) with mean age of 31.2 years (19 to 63 year) and 80 (45.5%) severe AA patients (29 men and 51women) with mean age of 31.6 (16 to 62 year) were included. The mean AA duration was 10.17 ± 25.4 months in mild group (1 to 240 months) and 77.7 ± 82.5 in severe group (2 to 324 months) which was significantly different (P < 0.001).
Disease course was stable in 78 subjects in mild group, but it was stable in 41 and unstable in 39 subjects of severe group (Table 1). So, disease course was significantly unstable in the severe group (P < 0.001).
Table 1.
Basic information of mild and severe alopecia areata patients
| Mild AA N = 96 N (%) |
Severe AA N = 80 N (%) |
P-value1 | |
|---|---|---|---|
| Sex | |||
| Male | 84(87.5%) | 29(36.3%) | |
| Female | 12(12.5%) | 51(63.8%) | < 0.001 |
| Family history | |||
| Yes | 15(15.6%) | 8(10%) | |
| No | 81(84.4%) | 72(90%) | 0.27 |
| Acute stress2 | |||
| Yes | 55(57.3%) | 48(60%) | |
| No | 41(42.7%) | 32(40%) | 0.71 |
| Educational level | |||
| Under diploma3 | 27(28.1%) | 30(37.5%) | |
| ≥ diploma | 69(71.9%) | 50(62.5%) | 0.18 |
| Occupational status | |||
| Not working | 19(19.8%) | 47(58.8%) | |
| Working | 77(80.2%) | 33(41.3%) | < 0.001 |
| Other disease4 | |||
| Yes | 3(3.1%) | 16(19%) | |
| No | 93(96.9%) | 64(81%) | 0.001 |
| Disease course5 | |||
| Stable | 78(81.3%) | 41(51.3%) | |
| Unstable | 18(18.8%) | 39(48.8%) | < 0.001 |
| Face involvement6 | |||
| Yes | 61(63.5%) | 63(78.8%) | |
| No | 35(36.5%) | 17(21.3%) | 0.03 |
| Age7 | 31.21 ± 8.2 | 31.6 ± 10.07 | 0.78 |
| Disease duration7, 8 | 10.1 ± 25.3 | 77.7 ± 82.5 | < 0.001 |
Chi-square test was used for categorical variables and independent samples T-test for continuous variables.
Acute stress during last 6 months
diploma = high school degree
Autoimmune thyroid disease, diabetes mellitus, atopy
Course of the disease was defined as “unstable” if there was alternation of worsening and improvement phases in the last 2 years, and “stable” otherwise.
Eyebrow/eyelash/beard area
Mean ± SD.
Logarithm of duration was used for analysis.
There were also significant differences between two groups from the viewpoint of sex, occupational status, facial involvement and other associated disorders (Table 1), revealing that severe group was predominantly female (P < 0.001) and had higher amount of unemployment (P < 0.001) and facial involvement (P = 0.03), as well, frequency of thyroid disease, diabetes mellitus and atopy was significantly higher (P = 0.001).
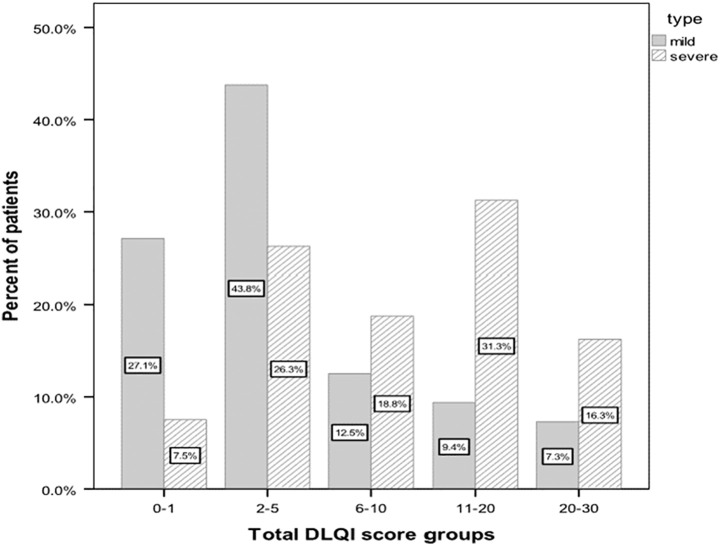
The total DLQI scores of all participants were between 0 and 28 and the mean was 7.9 ± 7.6 (Table 2). The distribution of patients according to banding system of DLQI was shown (Figure 1). The mean DLQI scores in the severe and mild groups were 10.7 ± 7.5 and 5.4 ± 6.8 respectively, which was statistically significant (P < 0.001). The mean score in the male group was 6.8 ± 7.4 and in the female group was 9.6 ± 7.6 which was statistically different (P = 0.02). There was a significant association between total DLQI scores and disease duration (P < 0.001) and acute stress during last 6 months (P = 0.02).
Table 2.
Associations between DLQI dimension scores and total scores, and basic characteristics in alopecia areata patients
| Symptom and feelings | Daily activities | leisure | Personal relationships | Work and school | treatment | Total score | |
|---|---|---|---|---|---|---|---|
| Type | |||||||
| Mild | 1.6 ± 1.2 | 0.77 ± 1.4 | 0.88 ± 1.6 | 1.05 ± 1.7 | 0.54 ± 1.09 | 0.63 ± 0.9 | 5.4 ± 6.8 |
| Severe | 2.6 ± 1.6 | 1.6 ± 1.7 | 2.5 ± 2.2 | 1.6 ± 1.8 | 1.01 ± 1.3 | 1.1 ± 1.1 | 10.7 ± 7.5 |
| P 1 | < 0.001 | 0.001 | < 0.001 | 0.03 | 0.01 | 0.001 | < 0.001 |
| Sex | |||||||
| Male | 1.8 ± 1.4 | 1 ± 1.5 | 1.3 ± 2.04 | 1.1 ± 1.7 | 0.8 ± 1.2 | 0.69 ± 0.92 | 6.8 ± 7.4 |
| Female | 2.5 ± 1.5 | 1.4 ± 1.8 | 2.09 ± 2.1 | 1.5 ± 1.9 | 0.66 ± 1.1 | 1.2 ± 1.1 | 9.6 ± 7.6 |
| P | 0.002 | 0.086 | 0.03 | 0.17 | 0.47 | 0.003 | 0.02 |
| Age 2(rho) | -0.08 | -0.02 | -0.07 | 0.03 | -0.03 | 0.05 | -0.02 |
| P | 0.25 | 0.75 | 0.33 | 0.64 | 0.65 | 0.47 | 0.70 |
| Disease duration2 | 0.2 | 0.25 | 0.32 | 0.1 | 0.16 | 0.17 | 0.28 |
| P | 0.008 | 0.01 | < 0.001 | 0.16 | 0.03 | 0.02 | < 0.001 |
| Family history | |||||||
| Yes | 2.1 ± 1.3 | 1.3 ± 1.9 | 1.9 ± 2.4 | 1.5 ± 2.01 | 0.56 ± 1.1 | 1 ± 0.95 | 8.7 ± 8.7 |
| No | 2.1 ± 1.5 | 1.1 ± 1.6 | 1.5 ± 2.07 | 1.2 ± 1.7 | 0.78 ± 1.2 | 0.86 ± 1.07 | 7.7 ± 7.4 |
| P | 0.93 | 0.58 | 0.49 | 0.57 | 0.42 | 0.55 | 0.56 |
| Acute stress3 | |||||||
| Yes | 2.3 ± 1.5 | 1.3 ± 1.8 | 1.8 ± 2.2 | 1.5 ± 1.8 | 0.83 ± 1.2 | 0.96 ± 1.08 | 8.9 ± 7.8 |
| No | 1.7 ± 1.3 | 0.87 ± 1.4 | 1.2 ± 1.8 | 1.06 ± 1.8 | 0.64 ± 1.2 | 0.78 ± 1.01 | 6.3 ± 6.9 |
| P | 0.008 | 0.04 | 0.04 | 0.11 | 0.31 | 0.26 | 0.02 |
| Educational level | |||||||
| Under diploma | 2.3 ± 1.6 | 1.2 ± 1.7 | 1.6 ± 2.09 | 1.5 ± 1.9 | 0.91 ± 1.3 | 0.84 ± 1.1 | 8.6 ± 7.9 |
| ≥ diploma | 2.0 ± 1.4 | 1.1 ± 1.6 | 1.6 ± 2.1 | 1.2 ± 1.7 | 0.68 ± 1.1 | 0.90 ± 1.0 | 7.5 ± 7.4 |
| P | 0.20 | 0.54 | 0.87 | 0.19 | 0.26 | 0.70 | 0.38 |
| Occupational status | |||||||
| Not working | 2.4 ± 1.5 | 1.4 ± 1.8 | 2 ± 2.1 | 1.3 ± 1.8 | 0.66 ± 1.1 | 1.1 ± 1.1 | 9.05 ± 7.7 |
| Working | 1.9 ± 1.4 | 1.01 ± 1.5 | 1.4 ± 2.06 | 1.3 ± 1.8 | 0.80 ± 1.2 | 0.70 ± 0.97 | 7.1 ± 7.4 |
| P | 0.04 | 0.14 | 0.07 | 0.90 | 0.46 | 0.004 | 0.11 |
| Other disease4, 5 | |||||||
| Yes | 2.0 ± 1.4 | 1 ± 1.3 | 1.3 ± 1.6 | 1 ± 1.5 | 0.22 ± 0.42 | 0.83 ± 0.98 | 6.3 ± 3.7 |
| No | 2.1 ± 1.5 | 1.1 ± 1.6 | 1.7 ± 2.1 | 1.3 ± 1.8 | 0.82 ± 1.2 | 0.87 ± 1.05 | 7.9 ± 7.8 |
| P | 0.81 | 0.91 | 0.99 | 0.45 | 0.17 | 0.97 | 0.71 |
| Disease course6 | |||||||
| Stable | 1.9 ± 1.4 | 1.01 ± 1.7 | 1.4 ± 2.09 | 1.3 ± 1.9 | 0.68 ± 1.1 | 0.78 ± 1 | 7.1 ± 7.7 |
| Unstable | 2.4 ± 1.5 | 1.4 ± 1.5 | 2.05 ± 2.1 | 1.3 ± 1.6 | 0.89 ± 1.3 | 1.08 ± 1.1 | 9.4 ± 7.1 |
| P | 0.02 | 0.07 | 0.08 | 0.96 | 0.30 | 0.08 | 0.06 |
| Face involvement7 | |||||||
| Yes | 2.05 ± 1.4 | 1.2 ± 1.7 | 1.7 ± 2.1 | 1.3 ± 1.8 | 0.81 ± 1.2 | 0.91 ± 1.09 | 8.2 ± 7.6 |
| No | 2.2 ± 1.5 | 0.86 ± 1.4 | 1.3 ± 2.0 | 1.1 ± 1.7 | 0.61 ± 1.1 | 0.80 ± 0.97 | 7.06 ± 7.3 |
| P | 0.48 | 0.08 | 0.28 | 0.53 | 0.33 | 0.52 | 0.35 |
Chi-square test was used for categorical variables and independent samples T-test for continuous variables.
Spearman’s correlation coefficient was used.
Acute stress during last 6 months.
Mann-Whitney U test was used.
Autoimmune thyroid disease, diabetes mellitus, atopy
Course of the disease was defined as “unstable” if there was alternation of worsening and improvement phases in the last 2 years, and “stable” otherwise.
Eyebrow/eyelash/beard area
Figure 1.
Distribution of patients according to banding system of DLQI.
The total DLQI scores of patients did not differ from each other according to their age, family history of AA, educational level, occupation status, other associated diseases, disease course and facial involvement (P values > 0.05).
Table 3 shows that after adjustment in multivariable analysis, only suffering from severe type of disease and having acute stress during last 6 months significantly increased the total scores of DLQI.
Table 3.
Multivariable regression analysis of DLQL determinants in patients with alopecia areata.
| Variables | Coefficient | P values | 95% confidence interval |
|---|---|---|---|
| Type (severe vs mild) | 4.98 | 0.001 | 2.14 to 7.82 |
| Sex (male vs female) | 0.357 | 0.844 | -3.22 to 3.93 |
| Disease duration | 0.004 | 0.650 | -0.014 to 0.023 |
| Acute stress (yes vs no) | 2.47 | 0.025 | 0.31 to 4.63 |
| Occupational status (not working vs working) | -0.104 | 0.950 | -3.36 to 3.15 |
| Disease course (unstable vs stable) | 0.505 | 0.678 | -1.89 to 2.90 |
Discussion
AA is a chronic and relapsing disorder which seriously affects patient’s QoL by interfering with personal relationships and self-perception (Fabbrocini et al., 2013). The correlation between AA and psychological disorders is reciprocal; on one side psychiatric disorders can be considered as a trigger for initiation or exacerbation of AA (Brajac et al., 2003, Gulec et al., 2004) and on the other side the disease itself, through its negative impacts on patient’s QoL, causes psychological problems (Picardi et al., 2003, Reid et al., 2010)
This study highlights the impact of AA on patient’s QoL especially severe cases and the importance of psychological evaluation of these patients.
Our findings revealed that the rates of unemployment, unstable disease course, facial involvement and associated disorders were higher in the severe group. They were more frequently female and had more prolonged disease duration. Also, according to the review of Alzolibani et al. (2012) men seem to be more severely affected by AA than women. This can be explained by limited patients number in our study, referral bias and the fact that women more than men are seeking medical help for AA. Another fact is that, women in this part of the world tend to wear clothing usually covering their hair, mild forms of AA may easily be disregarded by the patients. It could explain why women seek for medical advice only when it involves facial hair (eyebrows or eyelashes) or severe forms of the disease.
The mean total DLQI score was 7.9 ± 7.6. The mean score in severe group was 10.7 ± 7.5 which showed moderate to very large effect of AA on patient’s life based on banding system of DLQI. Among this group, total scores in 38 (47%) of patients were between 11 and 30 which showed very large to extremely large effect and the most affected dimensions were symptom and feelings and leisure. On the other hand, the mean score in the mild group was 5.4 ± 6.8 with moderate effect on patient’s life and 68 (71%) of this group had total scores between 0 and 5 which showed no or small effect and the most altered dimension was symptom and feelings. The total DLQI score, all the heading items, and QoL impairment, were significantly higher in the severe group compared to mild group.
The total DLQI score in the study of Ghajarzadeh et al. (2011) was 6.4 ± 5.5 which is near to our finding, and the score of patients with less than 25% of involvement was 5.9 ± 4 similar to our mild group. According to the study of Al-Mutairi and Eldin (2011) total DLQI score in 300 extensive AA patients was 13.54 which are similar to our findings.
Various skin diseases have been recognized as having a detrimental effect on the patient’s QoL. In the study of Ghajarzadeh et al. (2012) the mean DLQI score of AA patients was 6.4 ± 5.5 which is close to our score, but they found higher DLQI scores in psoriasis compared to AA and vitiligo. In a study by Wong and Baba (2012) the mean DLQI score of patients with vitiligo was 6.4 ± 5.1 which is less than our patients՚ score. These findings suggest that life is impaired in AA at the same or even higher level than other disfiguring disorders like vitiligo.
Apart from clinical severity, other variables that associated with higher DLQI scores were sex and disease duration in bivariate analysis. Female patients had higher total DLQI scores and more impairment in symptom and feelings, leisure and treatment parameters. Also, in multivariable analysis, there was not any significant association between sex and disease duration with total DLQI scores. Unlike to our findings, Al-Mutairi and Eldin (2011) revealed that sex and duration of illness did not impact total DLQI scores.
We hypothesized that unstable disease course can be associated with more QoL impairment, but surprisingly difference between total scores in stable and unstable groups was not statistically significant.
Acute stress within the last 6 months was correlated with superior QoL impairment in total score and its parameters (symptom and feelings, daily activities and leisure). However, this can be related to the nature of the stress itself not only the impact of AA on patients. We hypothesized that face involvement should be with greater QoL impairment, but our finding revealed that facial hair loss did not affect patient’s QoL based on DLQI.
Conclusion
To sum up, this study confirms our initial hypothesis that AA considerably impairs QoL and this is more pronounced in patients with severe AA and acute stress within the last 6 months.
No funding source.
Authors declare no conflict of interest.
Acknowledgments
Acknowledgements
The authors thank the patients, who so kindly agreed to participate in this study.
References
- Aghaei S., Sodaifi M., Jafari P. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8. doi: 10.1186/1471-5945-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mutairi N., Eldin O.N. Clinical profile and impact on quality of life: Seven years experience with patients of alopecia areata. Indian J Dermatol Venereol Leprol. 2011;77:489–493. doi: 10.4103/0378-6323.82411. [DOI] [PubMed] [Google Scholar]
- Alzolibani A.A., Zari S., Ahmed A.A. Epidemiologic and genetic characteristics of alopecia areata (part 2) Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:15–19. [PubMed] [Google Scholar]
- Anderson R.T., Rajagopalan R. Development and validation of a quality of life instrument for cutaneous diseases. J Am Acad Dermatol. 1997;37:41–50. doi: 10.1016/s0190-9622(97)70210-x. [DOI] [PubMed] [Google Scholar]
- Brajac I., Tkalcic M., Dragojević D.M., Gruber F. Roles of stress, stress perception and trait-anxiety in the onset and course of Alopecia areata. J Dermatol. 2003;30:871–878. doi: 10.1111/j.1346-8138.2003.tb00341.x. [DOI] [PubMed] [Google Scholar]
- Dubois M., Baumstarck-Barrau K., Gaudy-Marqueste C. Quality of life in alopecia areata: a study of 60 cases. J Invest Dermatol. 2010;130:2830–2833. doi: 10.1038/jid.2010.232. [DOI] [PubMed] [Google Scholar]
- Fabbrocini G., Panariello L., De Vita V. Quality of life in alopecia areata: a disease-specific questionnaire. J Eur Acad Dermatol Venereol. 2013;27:e276–e281. doi: 10.1111/j.1468-3083.2012.04629.x. [DOI] [PubMed] [Google Scholar]
- Finlay A.Y., Khan G.K. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Ghajarzadeh M., Ghiasi M., Kheirkhah S. Depression and quality of life in Iranian patients with Alopecia Areata. Iran J Dermatol. 2011;14:140–143. [Google Scholar]
- Ghajarzadeh M., Ghiasi M., Kheirkhah S. Associations between skin diseases and quality of life: a comparison of psoriasis, vitiligo, and alopecia areata. Acta Med Iran. 2012;50:511–515. [PubMed] [Google Scholar]
- Gulec A.T., Tanriverdi N., Duru C. The role of psychological factors in alopecia areata and the impact of the disease on the quality of life. Int J Dermatol. 2004;43:352–356. doi: 10.1111/j.1365-4632.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- Matzer F., Egger J.W., Kopera D. Psychosocial stress and coping in alopecia areata: a questionnaire survey and qualitative study among 45 patients. Acta Derm Venereol. 2011;91:318–327. doi: 10.2340/00015555-1031. [DOI] [PubMed] [Google Scholar]
- Morgan M., McCreedy R., Simpson J., Hay R.J. Dermatology quality of life scales – a measure of the impact of skin diseases. Br J Dermatol. 1997;136:202–206. [PubMed] [Google Scholar]
- Picardi A., Pasquini P., Cattaruzza M.S. Psychosomatic factors in first-onset Alopecia areata. Psychosomatics. 2003;44:374–381. doi: 10.1176/appi.psy.44.5.374. [DOI] [PubMed] [Google Scholar]
- Reid E.E., Haley A.C., Borovicka J.H. Clinical severity does not reliably predict quality of life in women with alopecia areata, telogen effluvium, or androgenic alopecia. J Am Acad Dermatol. 2010;66:e97–102. doi: 10.1016/j.jaad.2010.11.042. [DOI] [PubMed] [Google Scholar]
- Ruiz-Doblado S., Carrizosa A., García-Hernández M.J. Alopecia areata: psychiatric comorbidity and adjustment to illness. Int J Dermatol. 2003;42:434–437. doi: 10.1046/j.1365-4362.2003.01340.x. [DOI] [PubMed] [Google Scholar]
- Villasante Fricke A.C., Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;24:397–403. doi: 10.2147/CCID.S53985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.M., Baba R. Quality of life among Malaysian patients with vitiligo. Int J Dermatol. 2012;51:158–161. doi: 10.1111/j.1365-4632.2011.04932.x. [DOI] [PubMed] [Google Scholar]