Abstract
This review focuses on the treatment options for adult female patients with acne. Acne in adult female patients may start during adolescence and persist or have an onset in adulthood. Acne has various psychosocial effects that impact patients’ quality of life. Treatment of acne in adult women specifically has its challenges due to the considerations of patient preferences, pregnancy, and lactation. Treatments vary widely and treatment should be tailored specifically for each individual woman. We review conventional therapies with high levels of evidence, additional treatments with support from cohort studies and case reports, complementary and/or alternative therapies, and new agents under development for the treatment of patients with acne.
Introduction
Acne vulgaris (AV) is a disease of the pilosebaceous unit that causes noninflammatory lesions (open and closed comedones), inflammatory lesions (papules, pustules, and nodules), and varying degrees of scarring. AV is an extremely common condition with a lifetime prevalence of approximately 85% and occurs mostly during adolescence (Bhate and Williams, 2013). AV can persist into adulthood, with a 50.9% prevalence rate of acne in women ages 20 to 29 years versus 26.3% in women ages 40 to 49 years (Collier et al., 2008). Female patients account for two thirds of visits made to dermatologists for acne, and one third of all dermatology office visits for acne are by women who are older than 25 years (Yentzer et al., 2010).
Acne leads to significant morbidity that is associated with residual scarring and psychological disturbances such as poor self-image, depression, and anxiety, which leads to a negative impact on quality of life (Cunliffe, 1986, Ramos-e-Silva et al., 2015, Shuster et al., 1978). In one epidemiologic study by Yentzer et al. (2010), 8.8% of patients with acne reported depression with women suffering from depression twice as often as men (10.6% vs. 5.3%), but this was unrelated to acne severity.
Pathogenesis
Four key pathogenic processes lead to the formation of acne lesions: alteration of follicular keratinization that leads to comedones; increased and altered sebum production under androgen control; follicular colonization by Propionibacterium acnes; and complex inflammatory mechanisms that involve both innate and acquired immunity (Williams et al., 2012, Zaenglein et al., 2016). Genetics (twin studies Bataille et al., 2002, family history of severe acne Wei et al., 2010), diet (glycemic index Ismail et al., 2012, Kwon et al., 2012, Smith et al., 2007a, Smith et al., 2007b, Smith et al., 2008), including chocolate (Grant and Anderson, 1965, Magin et al., 2005) and dairy consumption (Adebamowo et al., 2006, Adebamowo et al., 2008, Di Landro et al., 2012); and environmental factors (smoking Klaz et al., 2006, Schafer et al., 2001, occlusive cosmetics Plewig et al., 1970, occupational exposures Tucker, 1983) also contribute to the pathogenesis of acne.
The pathogenesis of acne in adult women is particularly complex. Androgens play a major role (Harper, 2008, Lucky et al., 1994, Lucky et al., 1997), as evidenced by the response of acne in adult women to hormonal treatments, especially in the context of hyperandrogenism disorders such as polycystic ovary syndrome (PCOS) and the use of hormone-based therapies such as oral contraceptive and anti-androgen medications in women with normal androgen levels (Lolis et al., 2009). In addition, the lack of acne in androgen-insensitive women (Imperato-McGinley et al., 1993, Thiboutot, 2004) and rising levels of dehydroepiandrosterone sulfate in association with the onset of acne in premenarchal girls and a subset of patients with PCOS also play a major role (Lucky et al., 1994, Chen et al., 2011). Androgens stimulate sebum production via androgen receptors on the sebaceous glands.
Clinical presentation
Acne in women can occur at any age and with varying degrees of severity. Female patients may more frequently develop lesions on the lower third of the face, especially on the chin and jawline (Kamangar and Shinkai, 2012). However, a more recent epidemiologic study by Dreno et al. (2015) suggests that this hormonal distribution may not be the most common clinical presentation of acne in adult women.
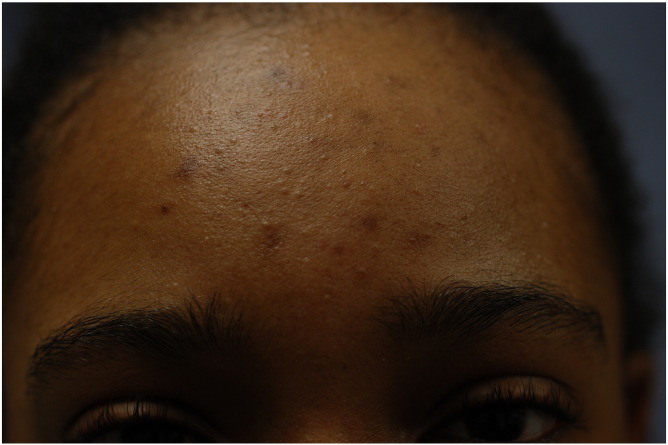
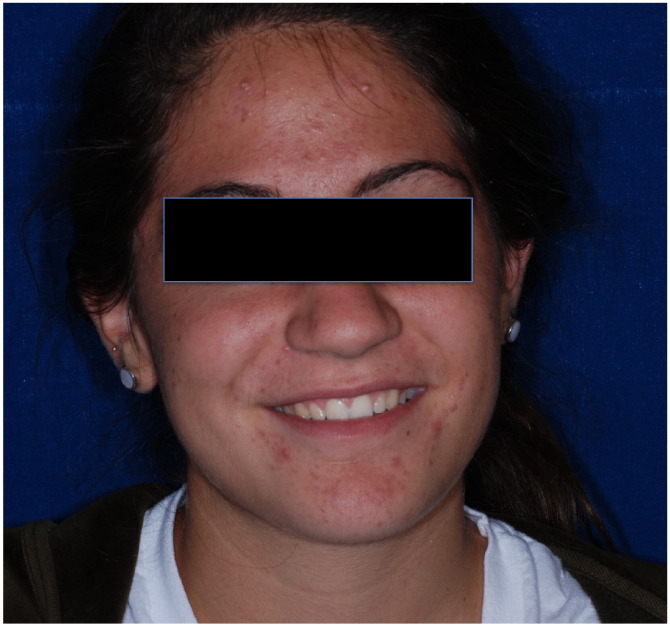
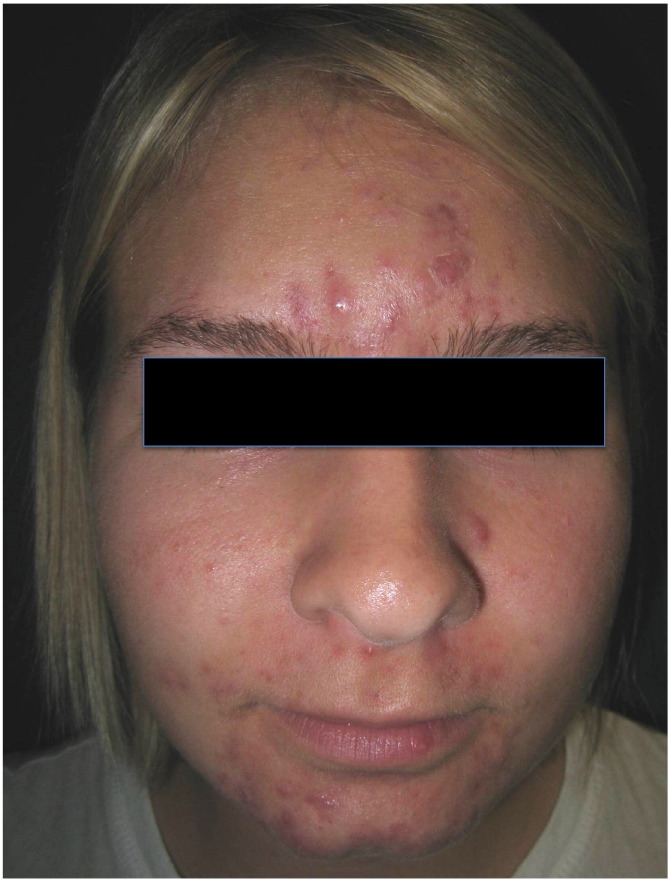
Acne lesions range from comedones (Fig. 1) to papules and pustules (Fig. 2), cysts, and/or nodules (Fig. 3). In one study of postadolescent acne, 85% of patients had mostly comedonal acne (Capitanio et al., 2010) with two subtypes identified as persistent and late-onset acne (Ramos-e-Silva et al., 2015). Persistent acne, which is defined as acne that persists beyond adolescence into adulthood, accounts for 80% of cases in adult female patients (Holzmann and Shakery, 2014). Late-onset acne is defined as acne that begins after the age of 25 years (Holzmann and Shakery, 2014, Ramos-e-Silva et al., 2015). Women with signs of hyperandrogenism such as hirsutism or menstrual irregularities and those with true late-onset acne should be further evaluated for an underlying endocrine disorder such as PCOS.
Figure 1.
Comedones with post-inflammatory hyperpigmentation.
Courtesy of Bethanee Schlosser, MD, PhD.
Figure 2.
Inflammatory papules and pustules.
Courtesy of Bethanee Schlosser, MD, PhD.
Figure 3.
Acne Nodules and Cysts.
Courtesy of Bethanee Schlosser, MD, PhD.
To date, there is no universally agreed-upon grading system for acne, and the grading systems used in clinical trials vary greatly (Zaenglein et al., 2016). Acne grading systems should take into account the type and severity of the acne, number of lesions, anatomic location and the extent of the acne, patient quality of life and other psychosocial metrics, and scarring (Zaenglein et al., 2016). Tan et al. (2013) evaluated 18 global acne grading scales with grading methods that ranged from text descriptions, grades for number and type of lesions, grades for severity, grades for comedonal versus inflammatory acne to the use of standardizing photographs.
Two groups of grading scales exist including those that use quantitative measures such as lesion counts and numeric ranges and those that are based on qualitative descriptions. Quantitative scales include those that specify the number and type of primary acne lesions (Del Rosso et al., 2007, Hayashi et al., 2008, Plewig and Kligman, 1975) and those that assign weights to lesion types to generate a severity index (Doshi et al., 1997, Liden et al., 1980, Michaelsson et al., 1977). Qualitative scales use adjectives such as few, some, and numerous to quantify lesions. There are also scales that solely use photographic templates of varying severity (Burke and Cunliffe, 1984, O'Brien et al., 1998).
Evaluation considerations
The evaluation of any patient with acne should include a thorough medical history and physical examination. Medications and supplement use, social history including tobacco and illicit drug use, menstrual history (i.e., age of menarche, regularity of menses, history of infertility), and prior/current acne treatments must be elucidated (Kamangar and Shinkai, 2012). A complete review of systems should be conducted to seek symptoms of hyperandrogenism or other endocrinology disorders.
Signs and symptoms of hyperandrogenism include acne, hirsutism, seborrhea, androgenetic alopecia, amenorrhea, oligomenorrhea, virilization, clitoromegaly, infertility, polycystic ovaries, increased muscle mass, and decreased breast size (Harper, 2008, Lolis et al., 2009). Of these, hirsutism is the most common manifestation of hyperandrogenism and 70% of women with hirsutism have hyperandrogenism (Lucky, 1995). Hirsutism is highly associated with elevated serum levels of free testosterone. Given that hair removal may obscure a clinician recognition of hirsutism, patients should be asked about the nature and frequency of hair removal practices as well as the locations of hair overgrowth. If patients exhibit signs or symptoms of hyperandrogenism, a thorough endocrinologic work-up should be initiated.
The differential diagnosis of acne in adult female patients is detailed in Table 1, along with distinguishing characteristics. In addition, underlying systemic causes for acne including hyperandrogenism should be assessed. Causes of drug-induced acne are detailed in Table 2.
Table 1.
Differential diagnosis of acne vulgaris
| Disease/condition | Differentiating characteristics |
|---|---|
| Acne keloidalis nuchae | Often seen in black patients; lesions localized to the posterior neck; initially papules and pustules that may progress to confluent keloids |
| Acneiform eruptions | Secondary to systemic medications, topical corticosteroid medications, contrast dye, and cosmetic products; may be abrupt in onset and correlation with exposure; improvement with cessation of exposure (See Table 2 for agents that cause drug-induced acne) |
| Chloracne | Comedones, pustules, and cysts that localize to the post-auricular area, axillae, and groin; history of exposure to halogenated aromatic hydrocarbons; patient may have other systemic manifestations |
| Favre-Racouchot | Open and closed comedones on periorbital and malar areas; no inflammatory lesions; patients are usually older with a history of significant sun exposure |
| Bacterial folliculitis (non-gram-negative) | Erythematous papules and pustules that are follicularly-based; often affects trunk and extremities |
| Gram-negative folliculitis | Frequently occurs in patients with acne who have been on long-term antibiotic medications; pustules and nodules; may also occur in HIV + patients, and after hot tub exposure; lesions may be cultured if acneiform lesions do not respond to typical antibiotic regimen |
| Lupus miliaris disseminatus faciei | Yellow/brown/red smooth papules in the periorbital and eyelid skin; biopsy shows caseating epithelioid granulomas |
| Milia | White keratinaceous cysts; lesions are usually persistent; noninflammatory |
| Periorificial dermatitis | Papules and pustules in the periorificial distribution; often exacerbated by topical corticosteroid use |
| Pyoderma faciale | Rapid onset of erythema, abscesses, cysts, and possible sinus tracts, no comedones |
| Rosacea | Various forms; background erythema with inflammatory papules and pustules often superimposed; environmental factors often can trigger |
| Syringoma | Noninflammatory papules that typically localize to the eyelids and malar cheeks; skin biopsy test results show dilated cysts with tadpole appearance |
| Adenoma sebaceum | Small waxy papules over the medial cheeks, nose, and forehead; multiple lesions associated with tuberous sclerosis; skin biopsy test results show dermal fibrosis and vascular proliferation and dilatation (angiofibromas). Facial angiofibromas are also a feature of multiple endocrine neoplasia type I and, rarely, Birt-Hogg-Dubé syndrome. |
Table 2.
Causative agents of drug-induced acneiform eruptions
| Class of agent | Examples |
|---|---|
| Hormones | |
| Corticosteroids and corticotropin | |
| Androgens and anabolic Steroid medications | |
| Hormonal contraceptive medications | |
| Neuropsychotherapeutic drugs | |
| Tricyclic antidepressant medications | |
| Lithium | |
| Antiepileptic drugs | |
| Aripiprazole | |
| Selective serotonin reuptake inhibitors | |
| Vitamins | |
| Vitamins B1, B6, and B12 | |
| Cytostatic drugs | |
| Dactinomycin (actinomycin D) | |
| Immunomodulating molecules | |
| Cyclosporine | |
| Sirolimus | |
| Antituberculosis drugs | |
| Isoniazid | |
| Rifampin | |
| Ethionamide | |
| Halogens | |
| Iodine | |
| Bromine | |
| Chlorine | |
| Targeted therapies | |
| Epidermal growth factor receptor inhibitors | |
| Multitargeted tyrosine kinase inhibitors | |
| Vascular endothelial growth factor inhibitor | |
| Proteasome inhibitor | |
| Tumor necrosis factor alfa inhibitors | |
| Histone deacetylase inhibitor |
Further testing
Microbiologic testing
P. acnes is thought to be an important pathogen in the development of acne. Routine cultures are not done unless gram-negative folliculitis or Staphylococcus aureus folliculitis are considered in the differential diagnosis (Zaenglein et al., 2016).
Gram-negative folliculitis presents as monomorphic eruptive pustules in the perioral, beard, and neck distribution and typically in the setting of prolonged oral tetracycline use (Zaenglein et al., 2016). Gram-negative folliculitis is caused by gram-negative microbes such as Klebsiella and Serratia and is treated with isotretinoin. Microbe-directed therapy may be considered given the clinical setting and individual patient characteristics. The American Academy of Dermatology (AAD) 2016 working group on the management of acne vulgaris only recommends microbiologic testing for those who exhibit acne-like lesions that are suggestive of gram-negative folliculitis and not otherwise (Zaenglein et al., 2016).
Endocrine testing
The role of androgens in acne is well established. Endocrine testing is only needed in patients who have other signs or symptoms of hyperandrogenism. The most common cause of increased androgens in adult women is PCOS (Lucky, 1983). Clinically, hyperandrogenism can manifest by unwanted hair growth/hirsutism, seborrhea, acne, and/or androgenetic alopecia (Azziz et al., 2009). Significant virilization suggests disorders of severe insulin resistance, androgen-secreting tumors, and androgenic substance abuse (Azziz et al., 2009). A laboratory test panel to screen for PCOS includes free and total testosterone, dehydroepiandrosterone sulfate, androstenedione, luteinizing hormone, and follicle-stimulating hormone (Azziz et al., 2009, Lawrence et al., 1981, Lucky, 1983, Lucky et al., 1983, Lucky et al., 1997, Seirafi et al., 2007, Timpatanapong and Rojanasakul, 1997). The differential diagnosis of PCOS includes thyroid disease, prolactin excess, nonclassical congenital adrenal hyperplasia, and other rare endocrinology disorders (Zaenglein et al., 2016).
Women who are already prescribed oral contraceptive medications and display additional signs of androgen excess should have similar testing done, although oral contraceptive pills may be beneficial to women with clinical and laboratory findings of hyperandrogenism as well as in women without these findings (Zaenglein et al., 2016). An endocrinologist should evaluate patients with abnormal hormone levels. The AAD working group only recommends laboratory evaluations for patients who have acne and additional signs of androgen excess (Zaenglein et al., 2016).
Treatment of acne vulgaris
Table 3 shows the various treatments for patients with AV, along with the strength of recommendations from the AAD working group but modified to include pregnancy and lactation ratings. This review article focuses on topical therapies, systemic antibiotic medications, isotretinoin, and novel therapies under development. We emphasize limiting treatment duration of systemic antibiotic medications in adults with acne and prescribing concomitant and/or maintenance treatment with topical therapy. A more complete discussion on the use of hormonal agents can be found in an article by Trivedi et al. (2017) entitled “A review of hormone-based therapies for adult acne vulgaris in women,” which was also published in the International Journal of Women’s Dermatology. Table 4 shows the AAD working group’s treatment algorithm for the management of AV in adolescents and young adults, which requires an adjustment on the basis of patient risk factors, types and sites of acne lesions, and age.
Table 3.
AAD 2016 Working Group strength of recommendations for the management and treatment of patients with acne vulgarisa
| Recommendation | Strength of recommendationb | Level of evidencec | FDA classification system pregnancy ratingd | Lactation rating |
|
|---|---|---|---|---|---|
| AAP classification system | LactMedd | ||||
| Topical therapies | |||||
| Benzoyl peroxide | A | I, II | C | Not rated | Low risk |
| Topical antibiotic medications (e.g., clindamycin and erythromycin) | A | I, II | B | Compatible | Acceptable |
| Combination of topical antibiotic medications and benzoyl peroxide | A | I | |||
| Topical retinoid medications (e.g., tretinoin, adapalene, and tazarotene) | A | I, II | Tretinoin – C Adapalene - C Tazarotene - X |
Not rated | Low risk |
| Combination of topical retinoid medications and benzoyl peroxide/topical antibiotic medication | A | I, II | |||
| Azelaic acid | A | I | B | Not rated | Low risk |
| Dapsone | A | I, II | C | Compatible | Avoid in G6PD deficiency, newborn/premature infants |
| Salicylic acid | B | II | C | Not rated | Not rated |
| Systemic antibiotic medications | |||||
| Tetracyclines (e.g., tetracycline, doxycycline, and minocycline) | A | I, II | D | Compatible | Short-term use acceptable |
| Macrolides (e.g., azithromycin and erythromycin) | A | I | B | Compatible | Acceptable |
| Trimethoprim (with or without sulfamethoxazole) | B | II | C | Compatible | Avoid in jaundiced, ill, premature infants |
| Limiting treatment duration and concomitant/maintenance topical therapy | A | I, II | |||
| Hormonal agents | |||||
| Combined oral contraceptive medications | A | I | X | Compatible | Avoid < 4 weeks post-partum |
| Spironolactone | B | II, III | C | Compatible | Appears acceptable |
| Flutamide | C | III | D | Not rated | Not rated |
| Oral corticosteroid medications | B | II | C | Compatible | Acceptable |
| Isotretinoin | |||||
| Conventional dosing | A | I, II | X | Not rated | No recommendation made |
| Low-dose treatment for moderate acne | A | I, II | X | Not rated | No recommendation made |
| Monitoring | B | II | |||
| iPledge enrollment and contraception use | A | II | |||
| Novel therapies | |||||
| Minocycline foam | |||||
| Topical nitric oxide-releasing agent | |||||
| Cortexolone 17α-propionate | |||||
AAD, American Academy of Dermatology; AAP, American Academy of Pediatrics; FDA, U.S. Food and Drug Administration; LactMed, Drug and Lactation Database.
Modified from Zaenglein et al., 2016, Table 3, to include pregnancy and lactation ratings (Lowenstein, 2006).
Clinical recommendations from the AAD were developed on the best available evidence tabled in the guideline. The strength of the recommendation was ranked as follows: A. Recommendation based on consistent and good-quality patient-oriented evidence; B. Recommendation based on inconsistent or limited-quality patient-oriented evidence; C. Recommendation based on consensus, opinion, case studies, or disease-oriented evidence.
Evidence was graded using a three-point scale on the basis of the quality of methodology and overall focus of the study as follows: I. Good-quality, patient-oriented evidence; II. Limited-quality, patient-oriented evidence; III. Other evidence including consensus guidelines, opinion, case studies, or disease-oriented evidence.
Produced by the U.S. National Library of Medicine.
Table 4.
American Academy of Dermatology 2016 Working Group treatment algorithm for the management of adolescents and young adults with acne vulgarisa
| Mild | Moderate | Severe | |
|---|---|---|---|
| First-line Treatment | BP or topical retinoid -or- Topical combination therapyb (BP + antibiotic or retinoid + BP or retinoid + BP + antibiotic) |
Topical combination therapyb (BP + antibiotic or retinoid + BP or retinoid + BP + antibiotic) -or- Oral antibiotic + topical retinoid + BP -or- Oral antibiotic + topical retinoid + BP + topical antibiotic |
Oral antibiotic + topical combination therapyb (BP + antibiotic or retinoid + BP or retinoid + BP + antibiotic) -or- Oral isotretinoin |
| Alternative treatment | Add topical retinoid or BP (if not on already) -or- Consider alternate retinoid -or- Consider topical dapsone |
Consider alternate combination therapy -or- Consider change in oral antibiotic -or- Add combined oral contraceptive or oral spironolactone (females) -or- Consider oral isotretinoin |
Consider change in oral antibiotic -or- Add combined oral contraceptive or oral spironolactone (females) -or- Consider oral isotretinoin |
BP, benzoyl peroxide.
Modified from Zaenglein et al., 2016, Figure 1.
Drug may be prescribed as a fixed combination product or as separate component.
The treatment of acne is challenging and often chronic, with high rates of failure and numerous choices. A good therapeutic relationship with the patient is important to establish as well as setting realistic treatment goals. Frequent evaluations (i.e., every 8-12 weeks) are important to enable appropriate monitoring, manage adverse effects, and evaluate for medication compliance (Kamangar and Shinkai, 2012). Patient counseling is critical, especially to establish a time course for medication efficacy and discuss future therapeutic modalities in case of treatment failure or intolerability.
Treatment of acne vulgaris in adult women
The central tenets of acne management as displayed in Table 4 should be followed in the treatment of adult female patients. However, additional considerations exist that should be kept in mind during treatment. Women over the age of 25 years tend to have high rates of treatment failure (Kamangar and Shinkai, 2012). Approximately 80% of women fail multiple courses of systemic antibiotic medications and approximately 30% to 40% fail after a course of isotretinoin (Blasiak et al., 2013, Goulden et al., 1997a, Goulden et al., 1997b, Rademaker, 2016). Suspicion of an underlying endocrinology disorder should be heightened if a recurrence of acne appears shortly after treatment with isotretinoin (Lowenstein, 2006).
Treatment of acne vulgaris during pregnancy and lactation
Women of childbearing potential should also be asked about their plans for reproduction, and treatment should be tailored for safety, whether the patients are actively trying to conceive, pregnant, or lactating (Table 3). Among the physiologic changes of pregnancy is a rise in serum androgen levels (Bozzo et al., 2011), which results in increased sebaceous gland activity and often worsening of the acne. Published information on the effects of acne medications on the developing fetus or breastfeeding infant is very limited (Kong and Tey, 2013). Pregnancy and lactation are often part of the exclusion criteria in clinical trials; therefore, available information on medication-related teratogenicity and effects on lactation are often derived from case reports and animal studies.
The most widely used pregnancy classification is the U.S. Food and Drug Administration (FDA) assessment system, which stratifies drugs into five risk categories (Table 5). However, this classification system has been criticized for its large focus on animal data and frequent classification of new medications as class B (safe in pregnancy; Kong and Tey, 2013) as well as its excessive simplicity and lack of information about the severity and nature of possible side effects on the fetus (Public Affairs Committee of the Teratology Society, 2007). More recently, the FDA released the Pregnancy and Lactation Labeling Rule, which went into effect on June 30, 2015. The most significant change is the abolishment of pregnancy labeling categories for pharmaceuticals (A, B, C, D, and X), which was replaced with individualized narrative summaries for each medication that include “risks of using a drug during pregnancy and lactation, a discussion of the data supporting that summary, and relevant information to help health care providers make prescribing decisions and counsel women about the use of drugs during pregnancy and lactation” (Danesh and Murase, 2015).
Table 5.
Summary of U.S. Food and Drug Administration categories for medication use in pregnancy
| Category | Description |
|---|---|
| A | Controlled studies show no risk. Adequate, well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester of pregnancy. |
| B | No evidence of risk in humans. Adequate, well-controlled studies in pregnant women have not shown an increased risk of fetal abnormalities despite adverse findings in animals. -or- In the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility |
| C | Risk cannot be ruled out. Adequate, well-controlled human studies are lacking and animal studies have shown a risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is administered during pregnancy but the potential benefits may outweigh the potential risk |
| D | Positive evidence of risk. Studies in humans or investigational or post-marketing data have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective. |
| X | Contraindicated in pregnancy. Studies in animals or humans or investigational or post-marketing reports have demonstrated positive evidence of fetal abnormalities or a risk that clearly outweighs any possible benefit to the patient |
| N | No pregnancy category has been assigned |
The most commonly used risk classification systems for lactation are from the American Academy of Pediatrics (AAP), Hale (2010), and the recent Drug and Lactation Database (LactMed) that was produced by the National Library of Medicine. For the purposes of this review, the AAP and LactMed rating systems will be discussed.
The AAP system stratifies drugs into three categories: those that should be used with concern; those with unknown effects but may be of concern; and those that are generally compatible with breastfeeding (American Academy of Pediatrics Committee on Drugs, 2001). The LactMed system is a peer-reviewed database that provides comprehensive information about drugs that may be used in mothers who breastfeed including serum drug levels, adverse effects on infants, and alternative drugs to consider (U.S. National Library of Medicine, 2017).
Given the lack of data, a lack of unified drug classifications for women who are pregnant or lactating, and the serious risk of teratogenicity, clinicians tend to take a conservative approach to treating acne in this group of women. Additionally, primary practitioners and dermatologists have a popular view that acne is a cosmetic issue, which further leads clinicians to choose less effective treatments or even withhold treatment during pregnancy and lactation (Kong and Tey, 2013).
Topical therapies
Topical therapies are considered one of the mainstay treatments for patients with mild-to-moderate acne (Nast et al., 2012). These topical agents are available over the counter and by prescription. More recently, several topical therapy combinations have been developed to treat patients with acne. The absorption of topical therapies is influenced by many factors, including the amount of agent applied, surface area of the application, length of the application time, frequency of the application, application to broken skin/erosions, choice of vehicle used, and thickness of the stratum corneum (Meredith and Ormerod, 2013).
Generally, topical agents are considered safer than oral medications for use in women who are pregnant or lactating because systemic availability of the drug is lower. Some topical medications do not even have a pregnancy category because systemic absorption is generally considered minimal unless use is extensive, intensive, or prolonged (Meredith and Ormerod, 2013).
Commonly used topical treatments for patients with acne include benzoyl peroxide (BP), salicylic acid (SA), antibiotic medications, combination antibiotic medications with BP, retinoid medications, retinoid with BP, retinoid with antibiotic medication, azelaic acid, and sulfone agents (Zaenglein et al., 2016).
Benzoyl peroxide
BP is commonly used to treat patients with acne and is available in a variety of strengths (2.5-10%) and formulations (cream, gel, wash, foam, aqueous gel, leave-on, and wash-off). BP is a comedolytic, keratolytic, anti-inflammatory agent with antimicrobial properties. BP is bactericidal mainly against P. acnes by the production of reactive oxygen radicals and has not developed resistance (Tanghetti, 2008). The addition of BP to antibiotic therapy enhances results and may reduce antibiotic resistance development (Zaenglein et al., 2016). Topical BP in varying formulations may be used 1 to 3 times daily as tolerated.
The use of BP is limited by concentration-dependent irritation, staining and bleaching of fabric, and uncommon contact allergy (Zaenglein et al., 2016). Lower concentrations (2.5-5%), water-based, and wash-off agents may be better tolerated in patients with more sensitive skin (Mills Jr. et al., 1986, Zaenglein et al., 2016).
Some clinicians are reluctant to prescribe BP concurrently with topical tretinoin due to the belief that BP may cause oxidation and degradation of the tretinoin molecule and thereby reduce its effectiveness. However, BP-induced degradation of tretinoin does not apply to all topical tretinoin formulations and multiple studies show the stability of tretinoin concentration and safety when using micronized tretinoin gel (0.05%) in combination with BP (Del Rosso et al., 2010, Gupta et al., 2015, Pariser et al., 2010, Torok and Pillai, 2011).
Salicylic acid
SA is a comedolytic agent that is available over the counter in 0.5 to 2% strengths and in both wash-off and leave-on preparations. SA is generally well tolerated by patients, but its efficacy in acne is limited (Shalita, 1981, Shalita, 1989). BP and SA are the most widely used over-the-counter, topical, acne treatments and are often used in combination. SA may be applied 1 to 3 times daily as tolerated. SA has an FDA pregnancy rating of C.
Topical antibiotic medications
Topical antibiotic medications are thought to accumulate in the follicle and may work through both anti-inflammatory and antibacterial effects (Mills et al., 2002). Due to increasing antibiotic resistance, monotherapy with topical antibiotic medications in the management of acne is not recommended. Topical antibiotic medications are best used in combination with BP (Zaenglein et al., 2016). The main topical antibiotic medications are clindamycin and erythromycin.
Topical clindamycin
Clindamycin is available in a gel, lotion, pledget, or topical solution and has been assigned FDA pregnancy category B. The clindamycin 1% solution or gel is currently the preferred topical antibiotic medication (Padilla et al., 1981). The recommended dosing is an application of a thin layer once daily.
Topical erythromycin
Erythromycin is available as a gel, solution, ointment, pledget, or thin film. Oral and topical erythromycin formulations are both classified as FDA category B. Topical erythromycin is less efficacious in patients with acne than clindamycin because of P. acnes resistance (Becker et al., 1981, Kuhlman and Callen, 1986, Leyden et al., 1987, Mills Jr. et al., 2002, Shahlita et al., 1984). Stable, fixed-combination agents are available with erythromycin 3% plus BP 5%, clindamycin 1% plus BP 5%, and clindamycin 1% plus BP 3.75% (Lookingbill et al., 1997, Pariser et al., 2014, Tschen et al., 2001). Combination agents may enhance compliance with treatment regimens (Zaenglein et al., 2016). Topical erythromycin is usually administered 1 to 2 times daily.
Topical retinoid medications
Topical retinoid medications are vitamin A–derivative prescription agents (Bradford and Montes, 1974, Krishnan, 1976, Lucky et al., 1998, Shalita et al., 1999). Topical retinoid medications are often used as first-line treatment for patients with mild-to-moderate acne, especially when the acne is mainly comedonal. Retinoid therapy is comedolytic and resolves the precursor microcomedone lesion. Retinoid medications are also anti-inflammatory and work in combination with other topical agents for all acne variants (Zaenglein et al., 2016). Topical retinoid treatments are the mainstay in the maintenance of clearance after discontinuation of oral therapy (Zaenglein et al., 2016). The recommended dosing is application of a thin layer once daily.
Three topical retinoid medications are used in the treatment of patients with acne: tretinoin (0.025-0.1% in cream, gel, or microsphere gel vehicles), adapalene (0.1% cream, gel, or lotion and 0.3% gel), and tazarotene (0.05%, 0.1% cream, gel, or foam). Retinoid use is limited by side effects including dryness, peeling, erythema, and irritation, which can be mitigated by reducing the volume used, the frequency of the application, and/or concomitant use of emollients (Pedace and Stoughton, 1971). Generally, therapy is initiated best every other day and then increased to daily as tolerated. The proper amount to use (e.g., pea size) is also important, as is ensuring even distribution with a thin layer and avoiding sensitive areas (e.g., eyelids, perioral area, nasal creases, and mucous membranes). With any of the topical retinoid treatments, higher concentrations are more efficacious but have greater side effects (Christiansen et al., 1974, Cunliffe et al., 1997, Krishnan, 1976). Additionally, topical retinoid medications increase the risk of photosensitivity so sunscreen lotion should be used concurrently.
Generic formulations of tretinoin are typically not photostable and should be applied in the evening. Coadministration of BP with tretinoin also leads to oxidation and inactivation of tretinoin; therefore, these agents should be applied at different times (i.e., BP in the morning, tretinoin at night). The micronized tretinoin formation as well as adapalene and tazarotene do not have similar restrictions.
Available combination agents that contain retinoid include adapalene 0.1% plus BP 2.5% and adapalene 0.3% plus BP 2.5% gels, which are approved for use in patients older than 9 years. In addition, clindamycin phosphate 1.2% plus tretinoin 0.025% gel is approved for patients older than 12 years of age (Dreno et al., 2014, Zouboulis et al., 2000).
There are conflicting reports on the safety of topical retinoid medications in women who are pregnant or lactating. Isolated cases have been reported of congenital malformations that were temporally associated with the use of these agents (Autret et al., 1997, Lipson et al., 1993, Loureiro et al., 2005, Navarre-Belhassen et al., 1998, Panchaud et al., 2012, Selcen et al., 2000). However, a large observational prospective study of 235 women who were exposed to a topical retinoid during their first trimester were compared with 444 women in the control group and no statistically significant differences in the rates of spontaneous abortions and minor or major birth defects were detected (Panchaud et al., 2012). A formal consensus on the safety of topical retinoid medications during pregnancy is lacking (van Hoogdalem, 1998). Additionally, manufacturers advise that these agents should not be used during pregnancy. Tretinoin and adapalene are classified as FDA pregnancy category C but tazarotene is category X. Patients should be counseled on these pregnancy risks when initiating retinoid treatment if they desire pregnancy.
Azelaic acid
Azelaic acid acts as a comedolytic, antimicrobial, and anti-inflammatory agent (Strauss et al., 2007) and is a naturally occurring dicarboxylic acid that is found in whole-grain cereals such as wheat, rye, and barley (Frampton and Wagstaff, 2004). Azelaic acid should be used with caution in patients with sensitive skin due to side effects that include redness, burning, and irritation.
Azelaic acid should also be used with caution in patients with Fitzpatrick skin types IV or greater because of its potential lightening effect (Cunliffe and Holland, 1989, Katsambas et al., 1989, Kircik, 2011). However, because of this side effect, azelaic acid is a useful adjunctive in acne treatment because it aids in the treatment of postinflammatory dyspigmentation.
The dosing recommendation is application of a thin film to the affected areas twice daily. Azelaic acid is categorized as FDA pregnancy class B because animal studies have shown no teratogenicity, but data on humans are not available.
Dapsone
Dapsone is a sulfone agent that is available in a 5% gel and used as a twice-daily agent or 7.5% gel used once daily. Data only show modest-to-moderate efficacy in the reduction of inflammatory acne lesions (Draelos et al., 2007, Lucky et al., 2007). Dapsone has a poorly understood mechanism in the treatment of patients with acne and its ability to kill P. acnes has been studied poorly (Zaenglein et al., 2016). Similar to other topical antibiotic treatments, dapsone is thought to work as an anti-inflammatory agent. The recommended dosing is application of a thin layer twice daily.
Dapsone should be used cautiously in combination with BP because coapplication may cause reversible, orange-brown discoloration of the skin, which can be brushed or washed off. Systemic absorption of topical dapsone is thought to be minimal so baseline glucose-6-phosphate dehydrogenase testing is not required. Topical dapsone is classified as FDA pregnancy category C.
Other topical agents
The following topical agents lack evidence-based data for their use in patients with acne but have been demonstrated to be effective in clinical practice: sodium sulfacetamide (Lebrun, 2004, Tarimci et al., 1997, Thiboutot, 2000), sulfur (Elstein, 1981), resorcinol (Elstein, 1981), aluminum chloride (Hjorth et al., 1985, Hurley and Shelley, 1978), topical zinc (Bojar et al., 1994, Cochran et al., 1985), and niacinamide (Khodaeiani et al., 2013, Shalita et al., 1995).
Systemic antibiotic medications
Oral antibiotic medications are commonly prescribed as second-line therapy for patients with mild-to-moderate acne that is not adequately controlled with topical agents alone and are a mainstay of acne treatment in patients with moderate-to-severe inflammatory acne. Oral antibiotic agents should be used in combination with a topical retinoid and BP if tolerated (Gold et al., 2010, Tan et al., 2014; (Zaenglein et al., 2013).
Given the rise in antibiotic resistance, monotherapy with oral antibiotic medications is strongly discouraged (Moon et al., 2012, Zaenglein et al., 2016). The Centers for Disease Control and Prevention has stressed antibiotic stewardship and limit antibiotic use to the shortest possible duration, ideally 3-4 months (Zaenglein et al., 2016), to reduce the risk of resistance. Concurrent and continued use with a retinoid or retinoid/BP combination can assist in weaning off the systemic antibiotic (Gold et al., 2010, Leyden et al., 2006, Poulin et al., 2011, Tan et al., 2012, Thiboutot et al., 2006).
Limiting systemic antibiotic use may also reduce the risk of inflammatory bowel disease (for tetracyclines; Margolis et al., 2010), pharyngitis (for tetracyclines; Margolis et al., 2012), C. difficile infection (Bartlett et al., 1978, Carroll and Bartlett, 2011), and candida vulvovaginitis; however, studies have shown that these associations are limited. Penicillin, erythromycin, and cephalosporin are thought to have the best safety profile during pregnancy (Hernandez-Diaz et al., 2000).
Of note, there is limited evidence on the administration of antibiotic agents and the potential impact on the effectiveness of oral contraceptive pills (Hoffmann et al., 2015). Although a large epidemiological study that was conducted in the United States showed that there is no association between concomitant antibiotic use and the risk of breakthrough pregnancy among oral contraceptive pill users (Guengerich, 1990), other studies have shown a potential relationship (Back et al., 1980, Bainton, 1986, Fazio, 1991, Skolnick et al., 1976). There are three categories of antibiotic agents that range from those that are likely to reduce the effectiveness of OCPs (rifampin), those that are associated with OCP failure in three or more reported cases (ampicillin, amoxicillin, metronidazole, and tetracycline), and those that were associated with OCP failure in at least one case report (cephalexin, clindamycin, dapsone, erythromycin, griseofulvin, isoniazid, phenoxymethylpenicillin, talampicillin, and trimethoprim; Miller et al., 1994). A conservative approach is the recommend use of a second form of contraception while taking a systemic antibiotic agent (Zhanel et al., 1999), but evidence for this is very limited (Miller et al., 1994).
Tetracycline class
Tetracycline treatments, which include minocycline, doxycycline, and tetracycline, are considered first-line therapy in patients with moderate-to-severe inflammatory acne except in certain circumstances including pregnancy, age < 8 years, or known allergy. Tetracycline agents have notable anti-inflammatory effects (Zaenglein et al., 2016). Pseudotumor cerebri is a rare phenomenon that is associated with the use of tetracycline agents (Zaenglein et al., 2016).
Tetracycline medications including minocycline and doxycycline are classified as FDA pregnancy category D. Tetracycline agents should not be used during pregnancy because use during the second and third trimester is known to cause discoloration of the teeth and bones. However, there is no firm evidence that first-trimester use is associated with major birth defects (Kong and Tey, 2013, Meredith and Ormerod, 2013). Cases of maternal liver toxicity that is associated with the use of tetracycline agents during the third trimester have been reported (Hale and Pomeranz, 2002, Rothman and Pochi, 1988, Wenk et al., 1981). Although there is a theoretical risk of bone and teeth malformation if tetracycline is administered during lactation, low concentrations of neonatal absorption are expected because of its strong binding with calcium ions in breast milk. Tetracycline is generally considered safe for use during breast feeding (Spencer et al., 2001).
Doxycycline
Doxycycline appears to be effective for patients with AV in the 1.7 to 2.4 mg/kg dose range (Leyden et al., 2013), but for practical purposes, doxycycline is usually administered at 50 to 100 mg twice daily for adult patients with acne. Subantimicrobial dosing of doxycycline (i.e., 20 mg twice daily or 40 mg daily) is also effective in patients with moderate inflammatory acne (Moore et al., 2015, Toossi et al., 2008), which further supports tetracycline’s anti-inflammatory properties.
Issues to consider when prescribing doxycycline include the fact that doxycycline is more photosensitizing than minocycline (Zaenglein et al., 2016) and more frequently associated with gastrointestinal disturbances, especially at higher doses (Leyden et al., 2013). To mitigate these side effects, patients should be counseled to use sunscreen lotion and other photoprotective measures to decrease the risk of sunburns, take doxycycline with a meal or a full glass of water, and not take doxycycline less than 1 hour prior to bedtime. Additionally, absorption is decreased with the concomitant intake of iron and calcium. The hyclate version of doxycycline tends to have greater gastrointestinal side effects compared with the monohydrate form. Doxycycline is primarily metabolized by the liver and can be used safely in most patients with renal disease (Zaenglein et al., 2016).
Minocycline
Previously, treatment with minocycline was thought to be superior to doxycycline in reducing P. acnes (Strauss et al., 2007). However, a recent Cochrane review found that minocycline was effective to treat patients with AV but was not superior to other antibiotic medications (Garner et al., 2012). Minocycline has been shown to be safe and effective at dose of 1 mg/kg, but no dose response was found for efficacy (Fleischer Jr. et al., 2006, Zaenglein et al., 2016). For practical purposes, minocycline is generally dosed at 50 to 100 mg twice daily.
Compared with doxycycline, minocycline tends to have lower rates of gastrointestinal side effects but is associated with tinnitus, dizziness, and pigment deposition within the skin, mucous membranes, and teeth. Minocycline-associated pigmentation is more common in patients who take higher doses for longer periods of time (Zaenglein et al., 2016). Rare, serious, immune-mediated events have also been associated with minocycline including drug-induced hypersensitivity syndrome or a drug reaction with eosinophilia and systemic symptoms, drug-induced lupus, and other hypersensitivity reactions (Kermani et al., 2012, Shaughnessy et al., 2010, Smith and Leyden, 2005, Tripathi et al., 2013, Weinstein et al., 2013).
Macrolides
Macrolide medications including erythromycin and azithromycin have been used in the treatment of patients with acne but recently have fallen out of favor as first-line treatment. Macrolide agents are considered alternative therapy when traditional antibiotic medications cannot be used. As with tetracycline, macrolide has some anti-inflammatory properties but the specific mechanism of action in acne is unknown.
The most common side effect is gastrointestinal disturbances (Zaenglein et al., 2016). Macrolide medications occasionally can cause cardiac conduction abnormalities and rarely cause hepatotoxicity (Zaenglein et al., 2016). Macrolide agents as a class have been classified by AAP as safe during lactation (Kong and Tey, 2013).
Erythromycin
Erythromycin is the traditional oral antibiotic medication of choice when a systemic antibiotic treatment is needed for acne while a patient is pregnant (Hale and Pomeranz, 2002, Koren et al., 1998). Due to increasing bacterial resistance, erythromycin should be combined with a topical preparation such as BP (Meredith and Ormerod, 2013). Due to the differences in absorption, 400 mg erythromycin ethyl succinate produces the same serum levels as 250 mg erythromycin base or stearate. For the erythromycin base, dosing ranges from 250 to 500 mg twice daily. For erythromycin ethyl succinate, dosing ranges from 400 to 800 mg twice daily.
Oral erythromycin is classified as FDA pregnancy category B. Erythromycin is more commonly used during pregnancy to treat other infections, which resulted in larger retrospective studies on pregnancy outcomes (Romoren et al., 2012). Although erythromycin is largely considered safe for use during pregnancy, reports of fetal cardiac malformation exist (Kallen et al., 2005) and prolonged use has been associated with hepatotoxicity in 10-15% of pregnant patients (Hale and Pomeranz, 2002, McCormack et al., 1977).
Azithromycin
Azithromycin is an azalide antibiotic agent that is derived from erythromycin (Meredith and Ormerod, 2013) and tends to be better tolerated compared with erythromycin (Kong and Tey, 2013). Azithromycin has been studied in varying doses (from 3 times a week to 4 days a month) with varying efficacy in patients with AV and all trials used pulse-dosing regimens (Antonio et al., 2008, Basta-Juzbasic et al., 2007, Innocenzi et al., 2008, Kus et al., 2005, Maleszka et al., 2011, Parsad et al., 2001, Rafiei and Yaghoobi, 2006, Zaenglein et al., 2016).
Trial doses have included 500 mg once daily for 4 consecutive days per month for 2 consecutive months (Babaeinejad et al., 2011, Parsad et al., 2001), 500 mg once daily for 3 days in the first week followed by 500 mg once weekly until week 10 (Maleszka et al., 2011), or 500 mg once daily for 3 consecutive days each week in month 1 followed by 500 mg once daily for 2 consecutive days each week in month 2 and then 500 mg once daily for 1 day each week in month 3 (Kus et al., 2005). One study from 2005 showed that azithromycin is as effective to treat patients with AV as doxycycline (Kus et al., 2005). A more recent, randomized, controlled trial that compared treatment with azithromycin 3 days per month to daily doxycycline showed the superiority of doxycycline (Ullah et al., 2014). As with erythromycin, azithromycin is classified as FDA pregnancy category B.
Trimethoprim sulfamethoxazole
Trimethoprim sulfamethoxazole (TMP/SMX) can be used to treat patients with AV that is recalcitrant to macrolide and tetracycline. Treatment with TMP/SMX and trimethoprim carries serious risks and is used for other infectious disorders, which makes the risk of development of resistance a greater issue. Although a few case reports espouse the efficacy of TMP/SMX in the treatment of patients with acne, one small double-blind study showed that TMP/SMX was as effective as treatment with oxytetracycline (Jen, 1980).
The side effects of TMP/SMX include gastrointestinal upset, photosensitivity, and drug eruptions, the most severe of which is Stevens-Johnson syndrome/toxic epidermal necrolysis (Firoz et al., 2012, Roujeau et al., 1995). Severe eruptions including Stevens-Johnson syndrome/toxic epidermal necrolysis are more common in patients with HIV.
Rarely, TMP/SMX can cause serious blood dyscrasias such as neutropenia, agranulocytosis, aplastic anemia, and thrombocytopenia, especially if taken as a long-term therapy, which warrants periodic monitoring with a complete blood cell count testing (Zaenglein et al., 2016). According to the AAD working group, TMP/SMX should be restricted to patients who are unable to tolerate tetracycline agents or in patients who are treatment-resistant (Zaenglein et al., 2016). The usual dosing for patients with AV is one double-strength tablet twice daily.
TMPS/SMX is classified as FDA pregnancy category C. The use of TMP/SMX during the first trimester of pregnancy can lead to folic acid deficiency, which may in turn result in neural tube defects, structural malformations of the cardiovascular and urinary system, and cleft lip or palate (Ho and Juurlink, 2011). These risks are increased among women who do not use a multivitamin that contains folic acid (Hernandez-Diaz et al., 2000).
Additionally, exposure during the third trimester of pregnancy is small for gestational age infants as well as associated with hyperbilirubinemia (Ho and Juurlink, 2011). Both AAP and LactMed consider TMP/SMX safe for use during lactation except when the baby is premature, has severe jaundice, or has G6PD deficiency, during which there is a higher risk of kernicterus (Kong and Tey, 2013).
Penicillin and cephalosporin
Penicillin and cephalosporin are well established as safe for use during pregnancy and lactation (Hale and Pomeranz, 2002). However, they are rarely used to treat patients with acne because information with regard to efficacy is sparse. Penicillin and cephalosporin can be used as an alternative to conventional antibiotic medications, especially during pregnancy or with allergies to other classes of antibiotic treatments (Zaenglein et al., 2016). Side effects include risk of hypersensitivity reactions that range from mild drug eruptions to anaphylaxis and gastrointestinal disturbances (i.e., nausea, diarrhea, and abdominal distention and discomfort; Zaenglein et al., 2016). The recommended dosing for amoxicillin is 250 mg twice daily up to 500 mg three times daily.
Cephalosporin has in vitro activity against P. acnes, but as a class, cephalosporin treatment is hydrophilic and thought to poorly penetrate microcomedones in vivo (Mays et al., 2012). A retrospective study of 93 patients showed that cephalexin is effective to treat patients with acne; 78% of patients experienced clinical improvement with a dosing regimen of 500 mg twice daily (Fenner et al., 2008).
Isotretinoin
Isotretionoin is an important nonhormonal and nonantimicrobial treatment option for adult women with acne (Gollnick et al., 2003). Oral isotretinoin is FDA-approved for the treatment of severe recalcitrant AV but can also be used to treat patients with moderate acne that is either treatment-resistant or relapses quickly after discontinuation of oral antibiotic therapy (Agarwal et al., 2011, Akman et al., 2007, Borghi et al., 2011, Choi et al., 2011a, Choi et al., 2011b, Kaymak and Ilter, 2006, Lee et al., 2011). Several studies have shown that isotretinoin effectively decreases sebum production, the number of acne lesions, and acne scarring (Amichai et al., 2006, Chivot and Midoun, 1990, Goldstein et al., 1982, Goulden et al., 1997a, Goulden et al., 1997b, Jones et al., 1983, King et al., 1982, Layton et al., 1993, Lehucher-Ceyrac and Weber-Buisset, 1993, Lester et al., 1985, Peck et al., 1982, Rubinow et al., 1987, Stainforth et al., 1993, Strauss and Stranieri, 1982, Strauss et al., 1984). According to the AAD working group, isotretinoin is also indicated for the treatment of patients with moderate inflammatory acne that is either treatment-resistant or produces physical scarring or significant psychosocial distress (Zaenglein et al., 2016).
Isotretinoin is usually initiated at a starting dose of 0.5 mg/kg/day for the first month and then increased to 1 mg/kg/day as tolerated with a goal of a cumulative dose of between 120 and 150 mg/kg (Goldsmith et al., 2004, Layton et al., 1993, Lehucher-Ceyrac et al., 1999). In very severe cases, lower initial doses in addition to oral corticosteroid medications may be needed. However, more recent studies have demonstrated that higher cumulative doses that exceed 200 mg/kg may be more effective to reduce the rates of acne relapse and retreatment (Coloe et al., 2011, Zeitany et al., 2016).
Low-dose isotretinoin (0.25-0.4 mg/kg/day) and lower cumulative dose regimens may be used to minimize the side effects that are associated with systemic retinoid therapy and thereby lead to improved tolerability and increased patient satisfaction (Agarwal et al., 2011, Akman et al., 2007, Amichai et al., 2006, Choi et al., 2011a, Choi et al., 2011b, De and Kanwar, 2011, Lee et al., 2010, Rademaker, 2010). However, intermittent dosing is not as effective as daily dosing and exhibits higher relapse rates (Agarwal et al., 2011, Akman et al., 2007, Choi et al., 2011a, Choi et al., 2011b, King et al., 1982). Absorption of isotretinoin is increased with fatty foods and isotretinoin is recommended to be taken with meals (Strauss et al., 2001, Webster et al., 2013). The lidose formulation (Absorica) has absorption profiles that are not dependent on fat intake.
In the treatment of adult women, serious considerations should be given to isotretinoin’s potential teratogenicity. In addition, side effects are numerous, including xerosis, cheilitis, xerophthalmia, decreased night vision, vision changes, headache, hepatotoxicity, hypertriglyceridemia, mood changes, bone demineralization, cardiovascular risk factors, possible link to depression/anxiety/mood changes/suicidality, and possible link to inflammatory bowel disease (IBD). With regard to the association of isotretinon with IBD, the position of the AAD is that “current evidence is insufficient to prove either an association of causal relationship between isotretinoin use and IBD” (Zaenglein et al., 2016). The most prevalent side effects of isotretinoin mimic symptoms of hypervitaminosis A (Zaenglein et al., 2016). With standard dosing, these side effects resolve after discontinuation of therapy (Zaenglein et al., 2016).
Data with regard to an evidence-based link between isotretinoin and depression, anxiety, mood changes, or suicidal ideation/suicide is mixed. Although many small case reports and series show that isotretinoin has no negative effect on mood, memory, attention, or executive function (Alhusayen et al., 2013, Bozdag et al., 2009, Chia et al., 2005, Cohen et al., 2007, Etminan et al., 2013, Hull et al., 1991, Jick et al., 2000, Kaymak and Ilter, 2006, Marqueling and Zane, 2007, Nevoralova and Dvorakova, 2013, Ormerod et al., 2012, Rashtak et al., 2014, Rehn et al., 2009, Rubinow et al., 1987, Zaenglein et al., 2016), the FDA presented 40 cases that showed that isotretinoin dechallenge and rechallenge was associated with psychiatric symptoms (U.S. Food and Drug Administration, 2003). In the FDA case series, patients recovered after isotretinoin was discontinued and had a recurrence of symptoms after reinitiating isotretinoin. Of these patients, 75% had no reported prior psychiatric history before isotretinoin therapy and the median time to recovery after discontinuation of isotretinoin was 4.5 days, which is consistent with the half-life of isotretinoin and its metabolites. When patients were rechallenged, the time to onset of the psychiatric symptoms was on average shorter, and 10 patients had persistent psychiatric symptoms after isotretinoin discontinuation. As of December 31, 2001, 140 isotretinoin users worldwide have committed suicide while taking isotretinoin or within a few months of discontinuation of treatment and another 257 patients have been hospitalized for severe depression or attempted suicide (Duenwald, 2002). However, some have argued that the number of reported cases of depression among isotretinoin users is no greater than in the general population (Lamberg, 1998). The AAD working group recommends that prescribing physicians monitor patients for any indication of depressive symptoms and educate patients on the potential risks of treatment with isotretinoin.
Laboratory test result monitoring for patients on isotretinoin varies widely among practitioners. Serum cholesterol, triglycerides, and transaminases are known to increase in some patients who take oral isotretinoin (Bershad et al., 1985, De Marchi et al., 2006, Zech et al., 1983). Routine monitoring of serum lipid profiles and liver function studies are recommended to be done regularly but the interval varies (Bershad et al., 1985, Lammer et al., 1985, Leachman et al., 1999, McElwee et al., 1991, Zech et al., 1983). Some practitioners monitor laboratory test results monthly, but others only check at baseline and after dosing changes.
Hansen et al. (2016) recommend that in healthy patients with normal baseline lipid panel and liver function test results, repeated studies should be performed after 2 months of isotretinoin therapy. If the findings are normal, no further testing may be required. The AAD working group did not find any evidence-based reason to warrant routine monitoring of complete blood cell counts (Zaenglein et al., 2016). Pregnancy testing is required for female patients of childbearing potential at baseline, monthly during therapy, and 1 month after completion of isotretinoin treatment.
The use of oral isotretinoin during pregnancy is absolutely contraindicated (FDA pregnancy category X) due to its known severe teratogenicity including craniofacial, cardiac, and thymic malformations (Lammer et al., 1985). As a result of these serious effects, the manufacturers of oral isotretinoin have developed pregnancy prevention programs where preferably two forms of contraception are recommended (Goodfield et al., 2010). Currently, the United States and United Kingdom require enrollment in these pregnancy prevention programs to receive oral isotretinoin.
In the United States, the FDA established the iPLEDGE program in 2006. Dermatologists should counsel women that they should not become pregnant 1 month before, during, or within 1 month after completion of isotretinoin therapy. However, in a recent survey study of women who were sexually active during isotretinoin therapy, 29% admitted to noncompliance with the iPLEDGE pregnancy prevention requirements (Collins et al., 2014).
Miscellaneous and adjuvant therapies
The following therapies have limited evidence for their efficacy in the treatment of patients with AV. However, these treatments may improve patients’ appearance and be helpful as part of an overall AV treatment regimen. Some of these modalities may be helpful to treat acne scarring as well. These treatments include comedo extraction (Meredith and Ormerod, 2013, Zaenglein et al., 2016), cryotherapy (Fox et al., 2016), electrocauterization (Fox et al., 2016), chemical peels (Dreno et al., 2011, Grover and Reddu, 2003, Ilknur et al., 2010, Kempiak and Uebelhoer, 2008, Levesque et al., 2011, Meredith and Ormerod, 2013, Ramos-e-Silva et al., 2015, Zaenglein et al., 2016; including glycolic acid Atzori et al., 1999, Grover and Reddu, 2003, Ilknur et al., 2010, SA Levesque et al., 2011, and retinoic acid Ramos-e-Silva et al., 2015), microdermabrasion (Karimipour et al., 2010, Kempiak and Uebelhoer, 2008, Lloyd, 2001, Ramos-e-Silva et al., 2015, Tan et al., 2001), laser treatment (Zaenglein et al., 2016; including pulsed dye, potassium titanyl phosphate, fractionated CO2, fractionated and nonfractionated infrared, radiofrequency, and intense pulsed light), photodynamic therapy (Barbaric et al., 2016, Gold et al., 2004, Kong and Tey, 2013, Ma et al., 2013, Nast et al., 2012, Pollock et al., 2004, Ross, 2005, Sakamoto et al., 2010, Wang et al., 2010, Zaenglein et al., 2016 including blue-violet light phototherapy and red light phototherapy), narrow-band ultraviolet B phototherapy (Meredith and Ormerod, 2013, Ross, 2005, Zeichner, 2011), intralesional triamcinolone acetonide (Levine and Rasmussen, 1983, Potter, 1971), surgical techniques (Jacob et al., 2001, Ramos-e-Silva et al., 2015, Sanchez Viera, 2015 including punch excision and subcision), and filler (Jacob et al., 2001, Sanchez Viera, 2015).
Complementary and alternative therapies
Many patients wish to use more natural treatments and may look to herbal and alternative agents for treatment. Although most of these agents are generally well tolerated, there are limited data with regard to efficacy and safety. Additionally, the specific ingredients, concentrations, and potential adulteration with other unwanted chemicals is not well regulated and sometimes cannot be confirmed. These complementary and alternative therapies include tea tree oil (Bassett et al., 1990, Bowe and Shalita, 2008, Enshaieh et al., 2007, Hammer, 2015, Sharquie et al., 2006), niacinamide (Draelos et al., 2006, Fox et al., 2016, Gehring, 2004, Namazi, 2007), ayurvedic compounds (Lalla et al., 2001, Paranjpe and Kulkarni, 1995), barberry extract (Fouladi, 2012), gluconolactone (Hunt and Barnetson, 1992), antioxidant agents (Sardana and Garg, 2010), zinc (Dreno and Blouin, 2008, Gupta et al., 2014, Meredith and Ormerod, 2013, Nast et al., 2012, Sardana and Garg, 2010, Strauss et al., 2007), probiotic treatments (Jung et al., 2013), fish oil (Khayef et al., 2012), green tea (Gaur and Agnihotri, 2014, Mahmood et al., 2010, Zaveri, 2006), basil oil (da Silva et al., 2012), copaiba oil (da Silva et al., 2012), minerals (Gupta et al., 2014, Ma'or et al., 2006, Park et al., 2009), resveratrol (Docherty et al., 2007, Fabbrocini et al., 2011, Simonart, 2012), rose water (rosa damascena; Hajhashemi et al., 2010), seaweed (Capitanio et al., 2012, Choi et al., 2011a, Choi et al., 2011b), taurine bromamine (Marcinkiewicz, 2010, Marcinkiewicz et al., 2006, Marcinkiewicz et al., 2008), dietary modification (Adebamowo et al., 2005, Adebamowo et al., 2006, Bhate and Williams, 2013, Di Landro et al., 2012, Ismail et al., 2012, Strauss et al., 2007, Zaenglein et al., 2016), biofeedback-assisted relaxation (Hughes et al., 1983), cognitive imagery (Hughes et al., 1983), and acupuncture (Kim and Kim, 2012).
Other complementary and alternative medicines (Fox et al., 2016) include Achillea millefolium, amaranth, antimicrobial peptides, arnica, asparagus, bay, benzoin, birch, bittersweet nightshade, black cumin, black walnut, borage, Brewer’s yeast, burdock root, calendula, celandine, chamomile, chaste tree, Commiphora mukul, capaiba oil, coriander, cucumber, duckweed, DuZhong extract, English walnut, Eucalyptus dives, fresh lemon, garlic, geranium, grapefruit seeds, jojoba oil, juniper twig, labrador tea, lemongrass, lemon, neem, oak bark, onion, orange peel, orange, Oregon grape root, patchouli, pea, petitgrain, pine, pomegranate rind extract, poplar, pumpkin, rose myrtle, rhubarb, rosemary, rue, safflower oil, sandalwood, soapwort, Sophora flavescens, specific antibodies, stinging nettle, sunflower oil, Taraxacum officinale, thyme, turmeric, vinegar, vitex, witch hazel, Withania somnifera, and yerba mate extract.
Novel therapies
New therapies to treat patients with AV continue to be developed. The newest agents include minocycline foam, topical nitric oxide-releasing agent, and cortexolone 17α-propionate. Many of these new therapies are in various stages of testing, and although the ultimate efficacy is difficult to predict, preliminary studies show promising results.
Minocycline foam
Oral minocycline has been shown to be effective in the treatment of patients with AV; however, systemic side effects including abnormal mucocutaneous pigmentation and autoimmune reactions may limit its use (Kircik, 2010, Smith and Leyden, 2005). A recent phase 2, prospective, multicenter, randomized, double-blind, dose finding study on topical minocycline 4% foam was conducted and showed a significant reduction from baseline in lesion count versus vehicle at 12 weeks for both inflammatory (71.1% vs. 50.5%; p = 0.0001) and noninflammatory lesions (72.7% vs. 56.5%; p = 0.0197; Shemer et al., 2016). The significant reduction in lesions were observed as early as week 3 and persisted until the end of the study at week 12. Treatment was well tolerated and safe with no drug-related systemic side effects or serious adverse events. Minocycline 4% foam will be further evaluated in phase 3 trials (Shemer et al., 2016).
Topical nitric-oxide
P. acnes is known to induce the production of interleukin-1 cytokines and contribute to the pathogenesis of acne. Nitric oxide (NO) has been shown to have broad-spectrum antimicrobial, wound-healing, and immunomodulatory properties (Friedman and Friedman, 2009, Martinez et al., 2009, Qin et al., 2015). Qin et al. (2015) showed that P. acnes is highly sensitive to NO and human keratinocyte, monocyte, and embryonic zebra fish assays did not reveal cytotoxicity.
In a recent phase 2, randomized, double-blind, placebo-controlled, three-armed study, a topical NO-releasing drug at 1% and 4% had a significantly greater mean percent reduction in noninflammatory lesions and patients who were treated with the 4% concentration had a significantly greater mean percent reduction in inflammatory lesion count at week 12 (Baldwin et al., 2016). Both concentrations were safe and well-tolerated by patients.
Topical cortexolone 17α-propionate 1% cream
Systemic anti-androgens such as spironolactone and combination oral contraceptive medications can be used in the effective treatment of patients with AV (Kong and Tey, 2013, Meredith and Ormerod, 2013, Thiboutot and Chen, 2003, Zaenglein et al., 2016). However, systemic use of anti-androgens is limited to women who wish to conceive or have other endocrine disorders or contraindications (Chen et al., 1995). A topical anti-androgen treatment has not been made available for use to date.
Cortexolone 17α-propionate is a steroidal antiandrogen agent that has strong antiandrogen activity and mild anti-inflammatory properties (Celasco et al., 2004). Cortexolone 17α-propionate competitively inhibits endogenous androgen binding at the human androgen receptor level without inhibiting the skin 5α-reductase (Celasco et al., 2004, Trifu et al., 2011). The agent is quickly metabolized once it penetrates the epidermis and frees inactive cortexolone; therefore, cortexolone 17α-propionate does not have systemic antiandrogenic effects (Trifu et al., 2011).
In a pilot randomized, double-blind, comparative study of cortexolone 17α-propionate versus placebo versus tretinoin 0.05% cream, cortexolone 17α-propionate 1% cream was well tolerated by patients and performed significantly better than placebo with regard to reductions in total lesion count, inflammatory lesion count, and acne severity index. Cortexolone 17α-propionate 1% cream was also clinically more effective than tretinoin 0.05% cream but this difference was not statistically significant.
Conclusions
A myriad of treatment choices is available to treat adult female patients with acne. Treatment options should be tailored to the individual patient with considerations for the patient’s preferences, tolerability of the agent, and psychosocial factors. A relatively limited number of options are available for the management of acne during pregnancy and lactation. However, the level of evidence on the safety of any therapies during pregnancy and lactation is low. Novel agents continue to be developed to treat patients with AV, which will further enhance the clinician’s care of patients with this impactful and prevalent disease.
Footnotes
Sources of support: LaRoche-Posay generously covered the publication costs for this article but did not otherwise affect the content or draft the manuscript.
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- Adebamowo C.A., Spiegelman D., Danby F.W., Frazier A.L., Willett W.C., Holmes M.D. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52(2):207–214. doi: 10.1016/j.jaad.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Adebamowo C.A., Spiegelman D., Berkey C.S., Danby F.W., Rockett H.H., Colditz G.A. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12(4):1. [PubMed] [Google Scholar]
- Adebamowo C.A., Spiegelman D., Berkey C.S., Danby F.W., Rockett H.H., Colditz G.A. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58(5):787–793. doi: 10.1016/j.jaad.2007.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal U.S., Besarwal R.K., Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: A randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77(6):688–694. doi: 10.4103/0378-6323.86482. [DOI] [PubMed] [Google Scholar]
- Akman A., Durusoy C., Senturk M., Koc C.K., Soyturk D., Alpsoy E. Treatment of acne with intermittent and conventional isotretinoin: a randomized, controlled multicenter study. Arch Dermatol Res. 2007;299(10):467–473. doi: 10.1007/s00403-007-0777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhusayen R.O., Juurlink D.N., Mamdani M.M., Morrow R.L., Shear N.H., Dormuth C.R. Isotretinoin use and the risk of inflammatory bowel disease: a population-based cohort study. J Invest Dermatol. 2013;133(4):907–912. doi: 10.1038/jid.2012.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics Committee on Drugs Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108(3):776–789. doi: 10.1542/peds.108.3.776. [DOI] [PubMed] [Google Scholar]
- Amichai B., Shemer A., Grunwald M.H. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54(4):644–646. doi: 10.1016/j.jaad.2005.11.1061. [DOI] [PubMed] [Google Scholar]
- Antonio J.R., Pegas J.R., Cestari T.F., Do Nascimento L.V. Azithromycin pulses in the treatment of inflammatory and pustular acne: efficacy, tolerability and safety. J Dermatolog Treat. 2008;19(4):210–215. doi: 10.1080/09546630701881506. [DOI] [PubMed] [Google Scholar]
- Atzori L., Brundu M.A., Orru A., Biggio P. Glycolic acid peeling in the treatment of acne. J Eur Acad Dermatol Venereol. 1999;12(2):119–122. [PubMed] [Google Scholar]
- Autret E., Berjot M., Jonville-Bera A.P., Aubry M.C., Moraine C. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350(9074):339. doi: 10.1016/S0140-6736(05)63390-9. [DOI] [PubMed] [Google Scholar]
- Azziz R., Carmina E., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H.F., Futterweit W. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Babaeinejad S., Khodaeiani E., Fouladi R.F. Comparison of therapeutic effects of oral doxycycline and azithromycin in patients with moderate acne vulgaris: What is the role of age? J Dermatolog Treat. 2011;22(4):206–210. doi: 10.3109/09546631003762639. [DOI] [PubMed] [Google Scholar]
- Back D.J., Breckenridge A.M., Crawford F.E., Hall J.M., MacIver M., Orme M.L. The effect of rifampicin on the pharmacokinetics of ethynylestradiol in women. Contraception. 1980;21(2):135–143. doi: 10.1016/0010-7824(80)90125-0. [DOI] [PubMed] [Google Scholar]
- Bainton R. Interaction between antibiotic therapy and contraceptive medication. Oral Surg Oral Med Oral Pathol. 1986;61(5):453–455. doi: 10.1016/0030-4220(86)90385-3. [DOI] [PubMed] [Google Scholar]
- Baldwin H., Blanco D., McKeever C., Paz N., Vasquez Y.N., Quiring J. Results of a phase 2 efficacy and safety study with SB204, an investigational topical nitric oxide-releasing drug for the treatment of acne vulgaris. J Clin Aesthet Dermatol. 2016;9(8):12–18. [PMC free article] [PubMed] [Google Scholar]
- Barbaric J., Abbott R., Posadzki P., Car M., Gunn L.H., Layton M.M. Light therapies for acne. Cochrane Database Syst Rev. 2016;9 doi: 10.1002/14651858.CD007917.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J.G., Chang T.W., Gurwith M., Gorbach S.L., Onderdonk A.B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Bassett I.B., Pannowitz D.L., Barnetson R.S. A comparative study of tea-tree oil versus benzoylperoxide in the treatment of acne. Med J Aust. 1990;153(8):455–458. doi: 10.5694/j.1326-5377.1990.tb126150.x. [DOI] [PubMed] [Google Scholar]
- Basta-Juzbasic A., Lipozencic J., Oremovic L., Kotrulja L., Gruber F., Brajac I. A dose-finding study of azithromycin in the treatment of acne vulgaris. Acta Dermatovenerol Croat. 2007;15(3):141–147. [PubMed] [Google Scholar]
- Bataille V., Snieder H., MacGregor A.J., Sasieni P., Spector T.D. The influence of genetics and environmental factors in the pathogenesis of acne: a twin study of acne in women. J Invest Dermatol. 2002;119(6):1317–1322. doi: 10.1046/j.1523-1747.2002.19621.x. [DOI] [PubMed] [Google Scholar]
- Becker L.E., Bergstresser P.R., Whiting D.A., Clendenning W.E., Dobson R.L., Jordan W.P. Topical clindamycin therapy for acne vulgaris. A cooperative clinical study. Arch Dermatol. 1981;117(8):482–485. [PubMed] [Google Scholar]
- Bershad S., Rubinstein A., Paterniti J.R., Le N.A., Poliak S.C., Heller B. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313(16):981–985. doi: 10.1056/NEJM198510173131604. [DOI] [PubMed] [Google Scholar]
- Bhate K., Williams H.C. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–485. doi: 10.1111/bjd.12149. [DOI] [PubMed] [Google Scholar]
- Blasiak R.C., Stamey C.R., Burkhart C.N., Lugo-Somolinos A., Morrell D.S. High-dose isotretinoin treatment and the rate of retrial, relapse, and adverse effects in patients with acne vulgaris. JAMA Dermatol. 2013;149(12):1392–1398. doi: 10.1001/jamadermatol.2013.6746. [DOI] [PubMed] [Google Scholar]
- Bojar R.A., Eady E.A., Jones C.E., Cunliffe W.J., Holland K.T. Inhibition of erythromycin-resistant propionibacteria on the skin of acne patients by topical erythromycin with and without zinc. Br J Dermatol. 1994;130(3):329–336. doi: 10.1111/j.1365-2133.1994.tb02929.x. [DOI] [PubMed] [Google Scholar]
- Borghi A., Mantovani L., Minghetti S., Giari S., Virgili A., Bettoli V. Low-cumulative dose isotretinoin treatment in mild-to-moderate acne: efficacy in achieving stable remission. J Eur Acad Dermatol Venereol. 2011;25(9):1094–1098. doi: 10.1111/j.1468-3083.2010.03933.x. [DOI] [PubMed] [Google Scholar]
- Bowe W.P., Shalita A.R. Effective over-the-counter acne treatments. Semin Cutan Med Surg. 2008;27(3):170–176. doi: 10.1016/j.sder.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Bozdag K.E., Gulseren S., Guven F., Cam B. Evaluation of depressive symptoms in acne patients treated with isotretinoin. J Dermatolog Treat. 2009;20(5):293–296. doi: 10.1080/09546630903164909. [DOI] [PubMed] [Google Scholar]
- Bozzo P., Chua-Gocheco A., Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57(6):665–667. [PMC free article] [PubMed] [Google Scholar]
- Bradford L.G., Montes L.F. Topical application of vitamin A acid in acne vulgaris. South Med J. 1974;67(6):683–687. doi: 10.1097/00007611-197406000-00014. [DOI] [PubMed] [Google Scholar]
- Burke B.M., Cunliffe W.J. The assessment of acne vulgaris--the Leeds technique. Br J Dermatol. 1984;111(1):83–92. doi: 10.1111/j.1365-2133.1984.tb04020.x. [DOI] [PubMed] [Google Scholar]
- Capitanio B., Sinagra J.L., Bordignon V., Cordiali Fei P., Picardo M., Zouboulis C.C. Underestimated clinical features of postadolescent acne. J Am Acad Dermatol. 2010;63(5):782–788. doi: 10.1016/j.jaad.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Capitanio B., Sinagra J.L., Weller R.B., Brown C., Berardesca E. Randomized controlled study of a cosmetic treatment for mild acne. Clin Exp Dermatol. 2012;37(4):346–349. doi: 10.1111/j.1365-2230.2011.04317.x. [DOI] [PubMed] [Google Scholar]
- Carroll K.C., Bartlett J.G. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu Rev Microbiol. 2011;65:501–521. doi: 10.1146/annurev-micro-090110-102824. [DOI] [PubMed] [Google Scholar]
- Celasco G., Moro L., Bozzella R., Ferraboschi P., Bartorelli L., Quattrocchi C. Biological profile of cortexolone 17alpha-propionate (CB-03-01), a new topical and peripherally selective androgen antagonist. Arzneimittelforschung. 2004;54(12):881–886. doi: 10.1055/s-0031-1297043. [DOI] [PubMed] [Google Scholar]
- Chen C., Puy L.A., Simard J., Li X., Singh S.M., Labrie F. Local and systemic reduction by topical finasteride or flutamide of hamster flank organ size and enzyme activity. J Invest Dermatol. 1995;105(5):678–682. doi: 10.1111/1523-1747.ep12324390. [DOI] [PubMed] [Google Scholar]
- Chen M.J., Chen C.D., Yang J.H., Chen C.L., Ho H.N., Yang W.S. High serum dehydroepiandrosterone sulfate is associated with phenotypic acne and a reduced risk of abdominal obesity in women with polycystic ovary syndrome. Hum Reprod. 2011;26(1):227–234. doi: 10.1093/humrep/deq308. [DOI] [PubMed] [Google Scholar]
- Chia C.Y., Lane W., Chibnall J., Allen A., Siegfried E. Isotretinoin therapy and mood changes in adolescents with moderate to severe acne: A cohort study. Arch Dermatol. 2005;141(5):557–560. doi: 10.1001/archderm.141.5.557. [DOI] [PubMed] [Google Scholar]
- Chivot M., Midoun H. Isotretinoin and acne--a study of relapses. Dermatologica. 1990;180(4):240–243. doi: 10.1159/000248038. [DOI] [PubMed] [Google Scholar]
- Choi C.W., Lee D.H., Kim H.S., Kim B.Y., Park K.C., Youn S.W. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25(4):454–461. doi: 10.1111/j.1468-3083.2010.03813.x. [DOI] [PubMed] [Google Scholar]
- Choi J.S., Bae H.J., Kim S.J., Choi I.S. In vitro antibacterial and anti-inflammatory properties of seaweed extracts against acne inducing bacteria, Propionibacterium acnes. J Environ Biol. 2011;32(3):313–318. [PubMed] [Google Scholar]
- Christiansen J.V., Gadborg E., Ludvigsen K., Meier C.H., Norholm A., Pedersen D. Topical tretinoin, vitamin A acid (Airol) in acne vulgaris. A controlled clinical trial. Dermatologica. 1974;148(2):82–89. [PubMed] [Google Scholar]
- Cochran R.J., Tucker S.B., Flannigan S.A. Topical zinc therapy for acne vulgaris. Int J Dermatol. 1985;24(3):188–190. doi: 10.1111/j.1365-4362.1985.tb05756.x. [DOI] [PubMed] [Google Scholar]
- Cohen J., Adams S., Patten S. No association found between patients receiving isotretinoin for acne and the development of depression in a Canadian prospective cohort. Can J Clin Pharmacol. 2007;14(2):e227–33. [PubMed] [Google Scholar]
- Collier C.N., Harper J.C., Cafardi J.A., Cantrell W.C., Wang W., Foster K.W. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58(1):56–59. doi: 10.1016/j.jaad.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Collins M.K., Moreau J.F., Opel D., Swan J., Prevost N., Hastings M. Compliance with pregnancy prevention measures during isotretinoin therapy. J Am Acad Dermatol. 2014;70(1):55–59. doi: 10.1016/j.jaad.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Coloe J., Du H., Morrell D.S. Could higher doses of isotretinoin reduce the frequency of treatment failure in patients with acne? J Am Acad Dermatol. 2011;65(2):422–423. doi: 10.1016/j.jaad.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Cunliffe W.J. Acne and unemployment. Br J Dermatol. 1986;115(3):386. doi: 10.1111/j.1365-2133.1986.tb05757.x. [DOI] [PubMed] [Google Scholar]
- Cunliffe W.J., Holland K.T. Clinical and laboratory studies on treatment with 20% azelaic acid cream for acne. Acta Derm Venereol Suppl (Stockh) 1989;143:31–34. [PubMed] [Google Scholar]
- Cunliffe W.J., Caputo R., Dreno B., Forstrom L., Heenen M., Orfanos C.E. Clinical efficacy and safety comparison of adapalene gel and tretinoin gel in the treatment of acne vulgaris: Europe and U.S. multicenter trials. J Am Acad Dermatol. 1997;36(6 Pt 2):S126–34. doi: 10.1016/s0190-9622(97)70056-2. [DOI] [PubMed] [Google Scholar]
- da Silva A.G., Puziol Pde F., Leitao R.N., Gomes T.R., Scherer R., Martins M.L. Application of the essential oil from copaiba (Copaifera langsdori Desf.) for acne vulgaris: A double-blind, placebo-controlled clinical trial. Altern Med Rev. 2012;17(1):69–75. [PubMed] [Google Scholar]
- Danesh M.J., Murase J.E. The new U.S. Food and Drug Administration pregnancy and lactation labeling rules: their impact on clinical practice. J Am Acad Dermatol. 2015;73(2):310–311. doi: 10.1016/j.jaad.2015.04.026. [DOI] [PubMed] [Google Scholar]
- De D., Kanwar A.J. Combination of low-dose isotretinoin and pulsed oral azithromycin in the management of moderate to severe acne: a preliminary open-label, prospective, non-comparative, single-centre study. Clin Drug Investig. 2011;31(8):599–604. doi: 10.2165/11539570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- De Marchi M.A., Maranhao R.C., Brandizzi L.I., Souza D.R. Effects of isotretinoin on the metabolism of triglyceride-rich lipoproteins and on the lipid profile in patients with acne. Arch Dermatol Res. 2006;297(9):403–408. doi: 10.1007/s00403-006-0638-4. [DOI] [PubMed] [Google Scholar]
- Del Rosso J.Q., Bikowski J.B., Baum E., Smith J., Hawkes S., Benes V. A closer look at truncal acne vulgaris: prevalence, severity, and clinical significance. J Drugs Dermatol. 2007;6(6):597–600. [PubMed] [Google Scholar]
- Del Rosso J.Q., Pillai R., Moore R. Absence of degradation of tretinoin when benzoyl peroxide is combined with an optimized formulation of tretinoin gel (0.05%) J Clin Aesthet Dermatol. 2010;3(10):26–28. [PMC free article] [PubMed] [Google Scholar]
- Di Landro A., Cazzaniga S., Parazzini F., Ingordo V., Cusano F., Atzori L. Family history, body mass index, selected dietary factors, menstrual history, and risk of moderate to severe acne in adolescents and young adults. J Am Acad Dermatol. 2012;67(6):1129–1135. doi: 10.1016/j.jaad.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Docherty J.J., McEwen H.A., Sweet T.J., Bailey E., Booth T.D. Resveratrol inhibition of Propionibacterium acnes. J Antimicrob Chemother. 2007;59(6):1182–1184. doi: 10.1093/jac/dkm099. [DOI] [PubMed] [Google Scholar]
- Doshi A., Zaheer A., Stiller M.J. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36(6):416–418. doi: 10.1046/j.1365-4362.1997.00099.x. [DOI] [PubMed] [Google Scholar]
- Draelos Z.D., Matsubara A., Smiles K. The effect of 2% niacinamide on facial sebum production. J Cosmet Laser Ther. 2006;8(2):96–101. doi: 10.1080/14764170600717704. [DOI] [PubMed] [Google Scholar]
- Draelos Z.D., Carter E., Maloney J.M., Elewski B., Poulin Y., Lynde C. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris. J Am Acad Dermatol. 2007;56(3):439.e1–439.e10. doi: 10.1016/j.jaad.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Dreno B., Blouin E. Acne, pregnant women and zinc salts: a literature review. Ann Dermatol Venereol. 2008;135(1):27–33. doi: 10.1016/j.annder.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Dreno B., Fischer T.C., Perosino E., Poli F., Viera M.S., Rendon M.I. Expert opinion: efficacy of superficial chemical peels in active acne management--what can we learn from the literature today? Evidence-based recommendations. J Eur Acad Dermatol Venereol. 2011;25(6):695–704. doi: 10.1111/j.1468-3083.2010.03852.x. [DOI] [PubMed] [Google Scholar]
- Dreno B., Bettoli V., Ochsendorf F., Layton A.M., Perez M., Dakovic R. Efficacy and safety of clindamycin phosphate 1.2%/tretinoin 0.025% formulation for the treatment of acne vulgaris: pooled analysis of data from three randomised, double-blind, parallel-group, phase III studies. Eur J Dermatol. 2014;24(2):201–209. doi: 10.1684/ejd.2014.2293. [DOI] [PubMed] [Google Scholar]
- Dreno B., Thiboutot D., Layton A.M., Berson D., Perez M., Kang S. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29(6):1096–1106. doi: 10.1111/jdv.12757. [DOI] [PubMed] [Google Scholar]
- Duenwald M. After 20 years, debate over drug persists. New York Times. 2002:F7. [Google Scholar]
- Elstein W. Topical deodorized polysulfides. Broadscope acne therapy. Cutis. 1981;28(4):468–472. [PubMed] [Google Scholar]
- Enshaieh S., Jooya A., Siadat A.H., Iraji F. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: a randomized, double-blind placebo-controlled study. Indian J Dermatol Venereol Leprol. 2007;73(1):22–25. doi: 10.4103/0378-6323.30646. [DOI] [PubMed] [Google Scholar]
- Etminan M., Bird S.T., Delaney J.A., Bressler B., Brophy J.M. Isotretinoin and risk for inflammatory bowel disease: a nested case-control study and meta-analysis of published and unpublished data. JAMA Dermatol. 2013;149(2):216–220. doi: 10.1001/jamadermatol.2013.1344. [DOI] [PubMed] [Google Scholar]
- Fabbrocini G., Staibano S., De Rosa G., Battimiello V., Fardella N., Ilardi G. Resveratrol-containing gel for the treatment of acne vulgaris: a single-blind, vehicle-controlled, pilot study. Am J Clin Dermatol. 2011;12(2):133–141. doi: 10.2165/11530630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Fazio A. Oral contraceptive drug interactions: important considerations. South Med J. 1991;84(8):997–1002. doi: 10.1097/00007611-199108000-00012. [DOI] [PubMed] [Google Scholar]
- Fenner J.A., Wiss K., Levin N.A. Oral cephalexin for acne vulgaris: clinical experience with 93 patients. Pediatr Dermatol. 2008;25(2):179–183. doi: 10.1111/j.1525-1470.2008.00628.x. [DOI] [PubMed] [Google Scholar]
- Firoz B.F., Henning J.S., Zarzabal L.A., Pollock B.H. Toxic epidermal necrolysis: five years of treatment experience from a burn unit. J Am Acad Dermatol. 2012;67(4):630–635. doi: 10.1016/j.jaad.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Fleischer A.B., Jr., Dinehart S., Stough D., Plott R.T. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(4 Suppls):21–31. [PubMed] [Google Scholar]
- Fouladi R.F. Aqueous extract of dried fruit of Berberis vulgaris L. in acne vulgaris, a clinical trial. J Diet Suppl. 2012;9(4):253–261. doi: 10.3109/19390211.2012.726702. [DOI] [PubMed] [Google Scholar]
- Fox L., Csongradi C., Aucamp M., du Plessis J., Gerber M. Treatment modalities for acne. Molecules. 2016:21(8). doi: 10.3390/molecules21081063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton J.E., Wagstaff A.J. Azelaic acid 15% gel: in the treatment of papulopustular rosacea. Am J Clin Dermatol. 2004;5(1):57–64. doi: 10.2165/00128071-200405010-00009. [DOI] [PubMed] [Google Scholar]
- Friedman A., Friedman J. New biomaterials for the sustained release of nitric oxide: past, present and future. Expert Opin Drug Deliv. 2009;6(10):1113–1122. doi: 10.1517/17425240903196743. [DOI] [PubMed] [Google Scholar]
- Garner S.E., Eady A., Bennett C., Newton J.N., Thomas K., Popescu C.M. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database Syst Rev. 2012;(8) doi: 10.1002/14651858.CD002086.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur S., Agnihotri R. Green tea: A novel functional food for the oral health of older adults. Geriatr Gerontol Int. 2014;14(2):238–250. doi: 10.1111/ggi.12194. [DOI] [PubMed] [Google Scholar]
- Gehring W. Nicotinic acid/niacinamide and the skin. J Cosmet Dermatol. 2004;3(2):88–93. doi: 10.1111/j.1473-2130.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- Gold M.H., Bradshaw V.L., Boring M.M., Bridges T.M., Biron J.A., Carter L.N. The use of a novel intense pulsed light and heat source and ALA-PDT in the treatment of moderate to severe inflammatory acne vulgaris. J Drugs Dermatol. 2004;3(6 Suppl):S15–9. [PubMed] [Google Scholar]
- Gold L.S., Cruz A., Eichenfield L., Tan J., Jorizzo J., Kerrouche N. Effective and safe combination therapy for severe acne vulgaris: a randomized, vehicle-controlled, double-blind study of adapalene 0.1%-benzoyl peroxide 2.5% fixed-dose combination gel with doxycycline hyclate 100 mg. Cutis. 2010;85(2):94–104. [PubMed] [Google Scholar]
- Goldsmith L.A., Bolognia J.L., Callen J.P., Chen S.C., Feldman S.R., Lim H.W. American Academy of Dermatology Consensus Conference on the safe and optimal use of isotretinoin: summary and recommendations. J Am Acad Dermatol. 2004;50(6):900–906. doi: 10.1016/j.jaad.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Goldstein J.A., Socha-Szott A., Thomsen R.J., Pochi P.E., Shalita A.R., Strauss J.S. Comparative effect of isotretinoin and etretinate on acne and sebaceous gland secretion. J Am Acad Dermatol. 1982;6(4 Pt 2 Suppl):760–765. doi: 10.1016/s0190-9622(82)70066-0. [DOI] [PubMed] [Google Scholar]
- Gollnick H., Cunliffe W., Berson D., Dreno B., Finlay A., Leyden J.J. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(1 Suppl):S1–37. doi: 10.1067/mjd.2003.618. [DOI] [PubMed] [Google Scholar]
- Goodfield M.J., Cox N.H., Bowser A., McMillan J.C., Millard L.G., Simpson N.B. Advice on the safe introduction and continued use of isotretinoin in acne in the U.K. 2010. Br J Dermatol. 2010;162(6):1172–1179. doi: 10.1111/j.1365-2133.2010.09836.x. [DOI] [PubMed] [Google Scholar]
- Goulden V., Clark S.M., Cunliffe W.J. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997;136(1):66–70. [PubMed] [Google Scholar]
- Goulden V., Clark S.M., McGeown C., Cunliffe W.J. Treatment of acne with intermittent isotretinoin. Br J Dermatol. 1997;137(1):106–108. [PubMed] [Google Scholar]
- Grant J.D., Anderson P.C. Chocolate as a cause of acne: a dissenting view. Mo Med. 1965;62:459–460. [PubMed] [Google Scholar]
- Grover C., Reddu B.S. The therapeutic value of glycolic acid peels in dermatology. Indian J Dermatol Venereol Leprol. 2003;69(2):148–150. [PubMed] [Google Scholar]
- Guengerich F.P. Inhibition of oral contraceptive steroid-metabolizing enzymes by steroids and drugs. Am J Obstet Gynecol. 1990;163(6 Pt 2):2159–2163. doi: 10.1016/0002-9378(90)90557-n. [DOI] [PubMed] [Google Scholar]
- Gupta M., Mahajan V.K., Mehta K.S., Chauhan P.S. Zinc therapy in dermatology: A review. Dermatol Res Pract. 2014;2014:709152. doi: 10.1155/2014/709152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Singh S., Kotla N.G., Webster T.J. Formulation and evaluation of a topical niosomal gel containing a combination of benzoyl peroxide and tretinoin for antiacne activity. Int J Nanomedicine. 2015;10:171–182. doi: 10.2147/IJN.S70449. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hajhashemi V., Ghannadi A., Hajiloo M. Analgesic and anti-inflammatory effects of rosa damascena hydroalcoholic extract and its essential oil in animal models. Iran J Pharm Res. 2010;9(2):163–168. [PMC free article] [PubMed] [Google Scholar]
- Hale T. 14th ed. Hale Publishing; Amarillo: 2010. Medications and mothers' milk. [Google Scholar]
- Hale E.K., Pomeranz M.K. Dermatologic agents during pregnancy and lactation: An update and clinical review. Int J Dermatol. 2002;41(4):197–203. doi: 10.1046/j.1365-4362.2002.01464.x. [DOI] [PubMed] [Google Scholar]
- Hammer K.A. Treatment of acne with tea tree oil (melaleuca) products: a review of efficacy, tolerability and potential modes of action. Int J Antimicrob Agents. 2015;45(2):106–110. doi: 10.1016/j.ijantimicag.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Hansen T.J., Lucking S., Miller J.J., Kirby J.S., Thiboutot D.M., Zaenglein A.L. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75(2):323–328. doi: 10.1016/j.jaad.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Harper J.C. Evaluating hyperandrogenism: a challenge in acne management. J Drugs Dermatol. 2008;7(6):527–530. [PubMed] [Google Scholar]
- Hayashi N., Akamatsu H., Kawashima M. Establishment of grading criteria for acne severity. J Dermatol. 2008;35(5):255–260. doi: 10.1111/j.1346-8138.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Diaz S., Werler M.M., Walker A.M., Mitchell A.A. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343(22):1608–1614. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- Hjorth N., Storm D., Dela K. Topical anhydrous aluminum chloride formulation in the treatment of acne vulgaris: a double-blind study. Cutis. 1985;35(5):499–500. [PubMed] [Google Scholar]
- Ho J.M., Juurlink D.N. Considerations when prescribing trimethoprim-sulfamethoxazole. CMAJ. 2011;183(16):1851–1858. doi: 10.1503/cmaj.111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K., George A., Heschl L., Leifheit A.K., Maier M. Oral contraceptives and antibiotics. A cross-sectional study about patients' knowledge in general practice. Reprod Health. 2015;12:43. doi: 10.1186/s12978-015-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann R., Shakery K. Postadolescent acne in females. Skin Pharmacol Physiol. 2014;27(Suppl. 1):3–8. doi: 10.1159/000354887. [DOI] [PubMed] [Google Scholar]
- Hughes H., Brown B.W., Lawlis G.F., Fulton J.E., Jr. Treatment of acne vulgaris by biofeedback relaxation and cognitive imagery. J Psychosom Res. 1983;27(3):185–191. doi: 10.1016/0022-3999(83)90021-1. [DOI] [PubMed] [Google Scholar]
- Hull S.M., Cunliffe W.J., Hughes B.R. Treatment of the depressed and dysmorphophobic acne patient. Clin Exp Dermatol. 1991;16(3):210–211. doi: 10.1111/j.1365-2230.1991.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Hunt M.J., Barnetson R.S. A comparative study of gluconolactone versus benzoyl peroxide in the treatment of acne. Australas J Dermatol. 1992;33(3):131–134. doi: 10.1111/j.1440-0960.1992.tb00100.x. [DOI] [PubMed] [Google Scholar]
- Hurley H.J., Shelley W.B. Special topical approach to the treatment of acne. Suppression of sweating with aluminum chloride in an anhydrous formulation. Cutis. 1978;22(6):696–703. [PubMed] [Google Scholar]
- Ilknur T., Demirtasoglu M., Bicak M.U., Ozkan S. Glycolic acid peels versus amino fruit acid peels for acne. J Cosmet Laser Ther. 2010;12(5):242–245. doi: 10.3109/14764172.2010.514919. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J., Gautier T., Cai L.Q., Yee B., Epstein J., Pochi P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol Metab. 1993;76(2):524–528. doi: 10.1210/jcem.76.2.8381804. [DOI] [PubMed] [Google Scholar]
- Innocenzi D., Skroza N., Ruggiero A., Concetta Potenza M., Proietti I. Moderate acne vulgaris: efficacy, tolerance and compliance of oral azithromycin thrice weekly for. Acta Dermatovenerol Croat. 2008;16(1):13–18. [PubMed] [Google Scholar]
- Ismail N.H., Manaf Z.A., Azizan N.Z. High glycemic load diet, milk and ice cream consumption are related to acne vulgaris in Malaysian young adults: a case control study. BMC Dermatol. 2012;12:13. doi: 10.1186/1471-5945-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C.I., Dover J.S., Kaminer M.S. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45(1):109–117. doi: 10.1067/mjd.2001.113451. [DOI] [PubMed] [Google Scholar]
- Jen I. A comparison of low dosage trimethoprim/sulfamethoxazole with oxytetracycline in acne vulgaris. Cutis. 1980;26(1):106–108. [PubMed] [Google Scholar]
- Jick S.S., Kremers H.M., Vasilakis-Scaramozza C. Isotretinoin use and risk of depression, psychotic symptoms, suicide, and attempted suicide. Arch Dermatol. 2000;136(10):1231–1236. doi: 10.1001/archderm.136.10.1231. [DOI] [PubMed] [Google Scholar]
- Jones D.H., King K., Miller A.J., Cunliffe W.J. A dose-response study of I3-cis-retinoic acid in acne vulgaris. Br J Dermatol. 1983;108(3):333–343. doi: 10.1111/j.1365-2133.1983.tb03973.x. [DOI] [PubMed] [Google Scholar]
- Jung G.W., Tse J.E., Guiha I., Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg. 2013;17(2):114–122. doi: 10.2310/7750.2012.12026. [DOI] [PubMed] [Google Scholar]
- Kallen B.A., Otterblad Olausson P., Danielsson B.R. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20(2):209–214. doi: 10.1016/j.reprotox.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Kamangar F., Shinkai K. Acne in the adult female patient: a practical approach. Int J Dermatol. 2012;51(10):1162–1174. doi: 10.1111/j.1365-4632.2012.05519.x. [DOI] [PubMed] [Google Scholar]
- Karimipour D.J., Karimipour G., Orringer J.S. Microdermabrasion: an evidence-based review. Plast Reconstr Surg. 2010;125(1):372–377. doi: 10.1097/PRS.0b013e3181c2a583. [DOI] [PubMed] [Google Scholar]
- Katsambas A., Graupe K., Stratigos J. Clinical studies of 20% azelaic acid cream in the treatment of acne vulgaris. Comparison with vehicle and topical tretinoin. Acta Derm Venereol Suppl (Stockh) 1989;143:35–39. [PubMed] [Google Scholar]
- Kaymak Y., Ilter N. The effectiveness of intermittent isotretinoin treatment in mild or moderate acne. J Eur Acad Dermatol Venereol. 2006;20(10):12–60. doi: 10.1111/j.1468-3083.2006.01784.x. [DOI] [PubMed] [Google Scholar]
- Kempiak S.J., Uebelhoer N. Superficial chemical peels and microdermabrasion for acne vulgaris. Semin Cutan Med Surg. 2008;27(3):212–220. doi: 10.1016/j.sder.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Kermani T.A., Ham E.K., Camilleri M.J., Warrington K.J. Polyarteritis nodosa-like vasculitis in association with minocycline use: a single-center case series. Semin Arthritis Rheum. 2012;42:213–221. doi: 10.1016/j.semarthrit.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Khayef G., Young J., Burns-Whitmore B., Spalding T. Effects of fish oil supplementation on inflammatory acne. Lipids Health Dis. 2012;11:165. doi: 10.1186/1476-511X-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaeiani E., Fouladi R.F., Amirnia M., Saeidi M., Karimi E.R. Topical 4% nicotinamide vs. 1% clindamycin in moderate inflammatory acne vulgaris. Int J Dermatol. 2013;52(8):999–1004. doi: 10.1111/ijd.12002. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Kim Y.B. Anti-inflammatory effect of Keigai-rengyo-to extract and acupuncture in male patients with acne vulgaris: a randomized controlled pilot trial. J Altern Complement Med. 2012;18(5):501–508. doi: 10.1089/acm.2011.0218. [DOI] [PubMed] [Google Scholar]
- King K., Jones D.H., Daltrey D.C., Cunliffe W.J. A double-blind study of the effects of 13-cis-retinoic acid on acne, sebum excretion rate and microbial population. Br J Dermatol. 1982;107(5):583–590. doi: 10.1111/j.1365-2133.1982.tb00410.x. [DOI] [PubMed] [Google Scholar]
- Kircik L.H. Doxycycline and minocycline for the management of acne: a review of efficacy and safety with emphasis on clinical implications. J Drugs Dermatol. 2010;9(11):1407–1411. [PubMed] [Google Scholar]
- Kircik L.H. Efficacy and safety of azelaic acid (AzA) gel 15% in the treatment of post-inflammatory hyperpigmentation and acne: a 16-week, baseline-controlled study. J Drugs Dermatol. 2011;10(6):586–590. [PubMed] [Google Scholar]
- Klaz I., Kochba I., Shohat T., Zarka S., Brenner S. Severe acne vulgaris and tobacco smoking in young men. J Invest Dermatol. 2006;126(8):1749–1752. doi: 10.1038/sj.jid.5700326. [DOI] [PubMed] [Google Scholar]
- Kong Y.L., Tey H.L. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73(8):779–787. doi: 10.1007/s40265-013-0060-0. [DOI] [PubMed] [Google Scholar]
- Koren G., Pastuszak A., Ito S. Drugs in pregnancy. N Engl J Med. 1998;338(16):1128–1137. doi: 10.1056/NEJM199804163381607. [DOI] [PubMed] [Google Scholar]
- Krishnan G. Comparison of two concentrations of tretinoin solution in the topical treatment of acne vulgaris. Practitioner. 1976;216(1291):106–109. [PubMed] [Google Scholar]
- Kuhlman D.S., Callen J.P. A comparison of clindamycin phosphate 1 percent topical lotion and placebo in the treatment of acne vulgaris. Cutis. 1986;38(3):203–206. [PubMed] [Google Scholar]
- Kus S., Yucelten D., Aytug A. Comparison of efficacy of azithromycin vs. doxycycline in the treatment of acne vulgaris. Clin Exp Dermatol. 2005;30(3):215–220. doi: 10.1111/j.1365-2230.2005.01769.x. [DOI] [PubMed] [Google Scholar]
- Kwon H.H., Yoon J.Y., Hong J.S., Jung J.Y., Park M.S., Suh D.H. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92(3):241–246. doi: 10.2340/00015555-1346. [DOI] [PubMed] [Google Scholar]
- Lalla J.K., Nandedkar S.Y., Paranjape M.H., Talreja N.B. Clinical trials of ayurvedic formulations in the treatment of acne vulgaris. J Ethnopharmacol. 2001;78(1):99–102. doi: 10.1016/s0378-8741(01)00323-3. [DOI] [PubMed] [Google Scholar]
- Lamberg L. Acne drug depression warnings highlight need for expert care. JAMA. 1998;279(14):1057. [PubMed] [Google Scholar]
- Lammer E.J., Chen D.T., Hoar R.M., Agnish N.D., Benke P.J., Braun J.T. Retinoic acid embryopathy. N Engl J Med. 1985;313(14):837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Lawrence D.M., Katz M., Robinson T.W., Newman M.C., McGarrigle H.H., Shaw M. Reduced sex hormone binding globulin and derived free testosterone levels in women with severe acne. Clin Endocrinol (Oxf) 1981;15(1):87–91. doi: 10.1111/j.1365-2265.1981.tb02752.x. [DOI] [PubMed] [Google Scholar]
- Layton A.M., Knaggs H., Taylor J., Cunliffe W.J. Isotretinoin for acne vulgaris--10 years later: A safe and successful treatment. Br J Dermatol. 1993;129(3):292–296. doi: 10.1111/j.1365-2133.1993.tb11849.x. [DOI] [PubMed] [Google Scholar]
- Leachman S.A., Insogna K.L., Katz L., Ellison A., Milstone L.M. Bone densities in patients receiving isotretinoin for cystic acne. Arch Dermatol. 1999;135(8):961–965. doi: 10.1001/archderm.135.8.961. [DOI] [PubMed] [Google Scholar]
- Lebrun C.M. Rosac cream with sunscreens (sodium sulfacetamide 10% and sulfur 5%) Skinmed. 2004;3(2):92. doi: 10.1111/j.1540-9740.2004.03465.x. [DOI] [PubMed] [Google Scholar]
- Lee J.J., Feng L., Reshef D.S., Sabichi A.L., Williams B., Rinsurongkawong W. Mortality in the randomized, controlled lung intergroup trial of isotretinoin. Cancer Prev Res (Phila) 2010;3(6):738–744. doi: 10.1158/1940-6207.CAPR-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.W., Yoo K.H., Park K.Y., Han T.Y., Li K., Seo S.J. Effectiveness of conventional, low-dose and intermittent oral isotretinoin in the treatment of acne: a randomized, controlled comparative study. Br J Dermatol. 2011;164(6):1369–1375. doi: 10.1111/j.1365-2133.2010.10152.x. [DOI] [PubMed] [Google Scholar]
- Lehucher-Ceyrac D., Weber-Buisset M.J. Isotretinoin and acne in practice: A prospective analysis of 188 cases over 9 years. Dermatology. 1993;186(2):123–128. doi: 10.1159/000247322. [DOI] [PubMed] [Google Scholar]
- Lehucher-Ceyrac D., de La Salmoniere P., Chastang C., Morel P. Predictive factors for failure of isotretinoin treatment in acne patients: results from a cohort of 237 patients. Dermatology. 1999;198(3):278–283. doi: 10.1159/000018130. [DOI] [PubMed] [Google Scholar]
- Lester R.S., Schachter G.D., Light M.J. Isotretinoin and tetracycline in the management of severe nodulocystic acne. Int J Dermatol. 1985;24(4):252–257. doi: 10.1111/j.1365-4362.1985.tb05775.x. [DOI] [PubMed] [Google Scholar]
- Levesque A., Hamzavi I., Seite S., Rougier A., Bissonnette R. Randomized trial comparing a chemical peel containing a lipophilic hydroxy acid derivative of salicylic acid with a salicylic acid peel in subjects with comedonal acne. J Cosmet Dermatol. 2011;10(3):174–178. doi: 10.1111/j.1473-2165.2011.00566.x. [DOI] [PubMed] [Google Scholar]
- Levine R.M., Rasmussen J.E. Intralesional corticosteroids in the treatment of nodulocystic acne. Arch Dermatol. 1983;119(6):480–481. [PubMed] [Google Scholar]
- Leyden J.J., Shalita A.R., Saatjian G.D., Sefton J. Erythromycin 2% gel in comparison with clindamycin phosphate 1% solution in acne vulgaris. J Am Acad Dermatol. 1987;16(4):822–827. doi: 10.1016/s0190-9622(87)70107-8. [DOI] [PubMed] [Google Scholar]
- Leyden J., Thiboutot D.M., Shalita A.R., Webster G., Washenik K., Strober B.E. Comparison of tazarotene and minocycline maintenance therapies in acne vulgaris: a multicenter, double-blind, randomized, parallel-group study. Arch Dermatol. 2006;142(5):605–612. doi: 10.1001/archderm.142.5.605. [DOI] [PubMed] [Google Scholar]
- Leyden J.J., Bruce S., Lee C.S., Ling M., Sheth P.B., Stewart D.M. A randomized, phase 2, dose-ranging study in the treatment of moderate to severe inflammatory facial acne vulgaris with doxycycline calcium. J Drugs Dermatol. 2013;12(6):658–663. [PubMed] [Google Scholar]
- Liden S., Goransson K., Odsell L. Clinical evaluation in acne. Acta Derm Venereol Suppl (Stockh) 1980;(Suppl. 89):47–52. [PubMed] [Google Scholar]
- Lipson A.H., Collins F., Webster W.S. Multiple congenital defects associated with maternal use of topical tretinoin. Lancet. 1993;341(8856):1352–1353. doi: 10.1016/0140-6736(93)90868-h. [DOI] [PubMed] [Google Scholar]
- Lloyd J.R. The use of microdermabrasion for acne: a pilot study. Dermatol Surg. 2001;27(4):329–331. doi: 10.1046/j.1524-4725.2001.00313.x. [DOI] [PubMed] [Google Scholar]
- Lolis M.S., Bowe W.P., Shalita A.R. Acne and systemic disease. Med Clin North Am. 2009;93(6):1161–1181. doi: 10.1016/j.mcna.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Lookingbill D.P., Chalker D.K., Lindholm J.S., Katz H.I., Kempers S.E., Huerter C.J. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37(4):590–595. doi: 10.1016/s0190-9622(97)70177-4. [DOI] [PubMed] [Google Scholar]
- Loureiro K.D., Kao K.K., Jones K.L., Alvarado S., Chavez C., Dick L. Minor malformations characteristic of the retinoic acid embryopathy and other birth outcomes in children of women exposed to topical tretinoin during early pregnancy. Am J Med Genet A. 2005;136(2):117–121. doi: 10.1002/ajmg.a.30744. [DOI] [PubMed] [Google Scholar]
- Lowenstein E.J. Diagnosis and management of the dermatologic manifestations of the polycystic ovary syndrome. Dermatol Ther. 2006;19:210–223. doi: 10.1111/j.1529-8019.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- Lucky A.W. Endocrine aspects of acne. Pediatr Clin North Am. 1983;30(3):495–499. doi: 10.1016/s0031-3955(16)34397-8. [DOI] [PubMed] [Google Scholar]
- Lucky A.W. Hormonal correlates of acne and hirsutism. Am J Med. 1995;98(1a):89s–94s. doi: 10.1016/s0002-9343(99)80064-3. [DOI] [PubMed] [Google Scholar]
- Lucky A.W., McGuire J., Rosenfield R.L., Lucky P.A., Rich B.H. Plasma androgens in women with acne vulgaris. J Invest Dermatol. 1983;81(1):70–74. doi: 10.1111/1523-1747.ep12539043. [DOI] [PubMed] [Google Scholar]
- Lucky A.W., Biro F.M., Huster G.A., Leach A.D., Morrison J.A., Ratterman J. Acne vulgaris in premenarchal girls. An early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130(3):308–314. doi: 10.1001/archderm.130.3.308. [DOI] [PubMed] [Google Scholar]
- Lucky A.W., Biro F.M., Simbartl L.A., Morrison J.A., Sorg N.W. Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. J Pediatr. 1997;130(1):30–39. doi: 10.1016/s0022-3476(97)70307-x. [DOI] [PubMed] [Google Scholar]
- Lucky A.W., Cullen S.I., Funicella T., Jarratt M.T., Jones T., Reddick M.E. Double-blind, vehicle-controlled, multicenter comparison of two 0.025% tretinoin creams in patients with acne vulgaris. J Am Acad Dermatol. 1998;38(4):S24–30. doi: 10.1016/s0190-9622(98)70142-2. [DOI] [PubMed] [Google Scholar]
- Lucky A.W., Maloney J.M., Roberts J., Taylor S., Jones T., Ling M. Dapsone gel 5% for the treatment of acne vulgaris: Safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6(10):981–987. [PubMed] [Google Scholar]
- Ma L., Xiang L.H., Yu B., Yin R., Chen L., Wu Y. Low-dose topical 5-aminolevulinic acid photodynamic therapy in the treatment of different severity of acne vulgaris. Photodiagnosis Photodyn Ther. 2013;10(4):583–590. doi: 10.1016/j.pdpdt.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Magin P., Pond D., Smith W., Watson A. A systematic review of the evidence for 'myths and misconceptions' in acne management: diet, face-washing and sunlight. Fam Pract. 2005;22(1):62–70. doi: 10.1093/fampra/cmh715. [DOI] [PubMed] [Google Scholar]
- Mahmood T., Akhtar N., Khan B.A., Khan H.M., Saeed T. Outcomes of 3% green tea emulsion on skin sebum production in male volunteers. Bosn J Basic Med Sci. 2010;10(3):260–264. doi: 10.17305/bjbms.2010.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleszka R., Turek-Urasinska K., Oremus M., Vukovic J., Barsic B. Pulsed azithromycin treatment is as effective and safe as 2-week-longer daily doxycycline treatment of acne vulgaris: a randomized, double-blind, noninferiority study. Skinmed. 2011;9(2):86–94. [PubMed] [Google Scholar]
- Ma'or Z., Henis Y., Alon Y., Orlov E., Sorensen K.B., Oren A. Antimicrobial properties of Dead Sea black mineral mud. Int J Dermatol. 2006;45(5):504–511. doi: 10.1111/j.1365-4632.2005.02621.x. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz J. Taurine bromamine (TauBr)--its role in immunity and new perspectives for clinical use. J Biomed Sci. 2010;17(Suppl. 1):S3. doi: 10.1186/1423-0127-17-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz J., Biedron R., Bialecka A., Kasprowicz A., Mak M., Targosz M. Susceptibility of Propionibacterium acnes and Staphylococcus epidermidis to killing by MPO-halide system products. Implication for taurine bromamine as a new candidate for topical therapy in treating acne vulgaris. Arch Immunol Ther Exp (Warsz) 2006;54(1):61–68. doi: 10.1007/s00005-006-0007-1. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz J., Wojas-Pelc A., Walczewska M., Lipko-Godlewska S., Jachowicz R., Maciejewska A. Topical taurine bromamine, a new candidate in the treatment of moderate inflammatory acne vulgaris: a pilot study. Eur J Dermatol. 2008;18(4):433–439. doi: 10.1684/ejd.2008.0460. [DOI] [PubMed] [Google Scholar]
- Margolis D.J., Fanelli M., Hoffstad O., Lewis J.D. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105(12):2610–2616. doi: 10.1038/ajg.2010.303. [DOI] [PubMed] [Google Scholar]
- Margolis D.J., Fanelli M., Kupperman E., Papadopoulos M., Metlay J.P., Xie S.X. Association of pharyngitis with oral antibiotic use for the treatment of acne: a cross-sectional and prospective cohort study. Arch Dermatol. 2012;148(3):326–332. doi: 10.1001/archdermatol.2011.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqueling A.L., Zane L.T. Depression and suicidal behavior in acne patients treated with isotretinoin: a systematic review. Semin Cutan Med Surg. 2007;26(4):210–220. doi: 10.1016/j.sder.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Martinez L.R., Han G., Chacko M., Chacko M., Mihu M.R., Jacobson M. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol. 2009;129(10):2463–2469. doi: 10.1038/jid.2009.95. [DOI] [PubMed] [Google Scholar]
- Mays R.M., Gordon R.A., Wilson J.M., Silapunt S. New antibiotic therapies for acne and rosacea. Dermatol Ther. 2012;25(1):23–37. doi: 10.1111/j.1529-8019.2012.01497.x. [DOI] [PubMed] [Google Scholar]
- McCormack W.M., George H., Donner A., Kodgis L.F., Alpert S., Lowe E.W. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12(5):630–635. doi: 10.1128/aac.12.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee N.E., Schumacher M.C., Johnson S.C., Weir T.W., Greene S.L., Scotvold M.J. An observational study of isotretinoin recipients treated for acne in a health maintenance organization. Arch Dermatol. 1991;127(3):341–346. [PubMed] [Google Scholar]
- Meredith F.M., Ormerod A.D. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14(5):351–358. doi: 10.1007/s40257-013-0041-9. [DOI] [PubMed] [Google Scholar]
- Michaelsson G., Juhlin L., Vahlquist A. Effects of oral zinc and vitamin A in acne. Arch Dermatol. 1977;113(1):31–36. doi: 10.1001/archderm.1977.01640010033003. [DOI] [PubMed] [Google Scholar]
- Miller D.M., Helms S.E., Brodell R.T. A practical approach to antibiotic treatment in women taking oral contraceptives. J Am Acad Dermatol. 1994;30(6):1008–1011. doi: 10.1016/s0190-9622(94)70127-x. [DOI] [PubMed] [Google Scholar]
- Mills O.H., Jr., Kligman A.M., Pochi P., Comite H. Comparing 2.5%, 5%, and 10% benzoyl peroxide on inflammatory acne vulgaris. Int J Dermatol. 1986;25(10):664–667. doi: 10.1111/j.1365-4362.1986.tb04534.x. [DOI] [PubMed] [Google Scholar]
- Mills O., Jr., Thornsberry C., Cardin C.W., Smiles K.A., Leyden J.J. Bacterial resistance and therapeutic outcome following three months of topical acne therapy with 2% erythromycin gel versus its vehicle. Acta Derm Venereol. 2002;82(4):260–265. doi: 10.1080/000155502320323216. [DOI] [PubMed] [Google Scholar]
- Moon S.H., Roh H.S., Kim Y.H., Kim J.E., Ko J.Y., Ro Y.S. Antibiotic resistance of microbial strains isolated from Korean acne patients. J Dermatol. 2012;39(10):833–837. doi: 10.1111/j.1346-8138.2012.01626.x. [DOI] [PubMed] [Google Scholar]
- Moore A., Ling M., Bucko A., Manna V., Rueda M.J. Efficacy and safety of subantimicrobial dose, modified-release doxycycline 40 mg versus doxycycline 100 mg versus placebo for the treatment of inflammatory lesions in moderate and severe acne: a randomized, double-blinded, controlled study. J Drugs Dermatol. 2015;14(6):581–586. [PubMed] [Google Scholar]
- Namazi M.R. Nicotinamide in dermatology: a capsule summary. Int J Dermatol. 2007;46(12):1229–1231. doi: 10.1111/j.1365-4632.2007.03519.x. [DOI] [PubMed] [Google Scholar]
- Nast A., Dreno B., Bettoli V., Degitz K., Erdmann R., Finlay A.Y. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(Suppl. 1):1–29. doi: 10.1111/j.1468-3083.2011.04374.x. [DOI] [PubMed] [Google Scholar]
- Navarre-Belhassen C., Blanchet P., Hillaire-Buys D., Sarda P., Blayac J.P. Multiple congenital malformations associated with topical tretinoin. Ann Pharmacother. 1998;32(4):505–506. doi: 10.1345/aph.17138. [DOI] [PubMed] [Google Scholar]
- Nevoralova Z., Dvorakova D. Mood changes, depression and suicide risk during isotretinoin treatment: A prospective study. Int J Dermatol. 2013;52(2):163–168. doi: 10.1111/j.1365-4632.2011.05334.x. [DOI] [PubMed] [Google Scholar]
- O'Brien S.C., Lewis J.B., Cunliffe W.J. The Leeds revised acne grading system. J Dermatolog Treat. 1998;9:215–220. [Google Scholar]
- Ormerod A.D., Thind C.K., Rice S.A., Reid I.C., Williams J.H., McCaffery P.J. Influence of isotretinoin on hippocampal-based learning in human subjects. Psychopharmacology (Berl) 2012;221(4):667–674. doi: 10.1007/s00213-011-2611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla R.S., McCabe J.M., Becker L.E. Topical tetracycline hydrochloride vs. topical clindamycin phosphate in the treatment of acne: a comparative study. Int J Dermatol. 1981;20(6):445–448. doi: 10.1111/j.1365-4362.1981.tb02013.x. [DOI] [PubMed] [Google Scholar]
- Panchaud A., Csajka C., Merlob P., Schaefer C., Berlin M., De Santis M. Pregnancy outcome following exposure to topical retinoids: a multicenter prospective study. J Clin Pharmacol. 2012;52(12):1844–1851. doi: 10.1177/0091270011429566. [DOI] [PubMed] [Google Scholar]
- Paranjpe P., Kulkarni P.H. Comparative efficacy of four Ayurvedic formulations in the treatment of acne vulgaris: a double-blind randomised placebo-controlled clinical evaluation. J Ethnopharmacol. 1995;49(3):127–132. doi: 10.1016/0378-8741(95)01309-1. [DOI] [PubMed] [Google Scholar]
- Pariser D., Bucko A., Fried R., Jarratt M.T., Kempers S., Kircik L. Tretinoin gel microsphere pump 0.04% plus 5% benzoyl peroxide wash for treatment of acne vulgaris: morning/morning regimen is as effective and safe as morning/evening regimen. J Drugs Dermatol. 2010;9(7):805–813. [PubMed] [Google Scholar]
- Pariser D.M., Rich P., Cook-Bolden F.E., Korotzer A. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once-daily treatment of moderate to severe acne vulgaris. J Drugs Dermatol. 2014;13(9):1083–1089. [PubMed] [Google Scholar]
- Park S.K., Lee C.W., Lee M.Y. Antibacterial effects of minerals from ores indigenous to Korea. J Environ Biol. 2009;30(1):151–154. [PubMed] [Google Scholar]
- Parsad D., Pandhi R., Nagpal R., Negi K.S. Azithromycin monthly pulse vs daily doxycycline in the treatment of acne vulgaris. J Dermatol. 2001;28(1):1–4. doi: 10.1111/j.1346-8138.2001.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Peck G.L., Olsen T.G., Butkus D., Pandya M., Arnaud-Battandier J., Gross E.G. Isotretinoin versus placebo in the treatment of cystic acne. A randomized double-blind study. J Am Acad Dermatol. 1982;6(4 Pt 2 Suppl):735–745. doi: 10.1016/s0190-9622(82)70063-5. [DOI] [PubMed] [Google Scholar]
- Pedace F.J., Stoughton R. Topical retinoic acid in acne vulgaris. Br J Dermatol. 1971;84(5):465–469. doi: 10.1111/j.1365-2133.1971.tb02533.x. [DOI] [PubMed] [Google Scholar]
- Plewig G., Kligman A.M. Springer-Verlag; New York, NY: 1975. Acne: Morphogenesis and treatment. [Google Scholar]
- Plewig G., Fulton J.E., Kligman A.M. Pomade acne. Arch Dermatol. 1970;101(5):580–584. [PubMed] [Google Scholar]
- Pollock B., Turner D., Stringer M.R., Bojar R.A., Goulden V., Stables G.I. Topical aminolaevulinic acid-photodynamic therapy for the treatment of acne vulgaris: A study of clinical efficacy and mechanism of action. Br J Dermatol. 2004;151(3):616–622. doi: 10.1111/j.1365-2133.2004.06110.x. [DOI] [PubMed] [Google Scholar]
- Potter R.A. Intralesional triamcinolone and adrenal suppression in acne vulgaris. J Invest Dermatol. 1971;57(6):364–370. doi: 10.1111/1523-1747.ep12292717. [DOI] [PubMed] [Google Scholar]
- Poulin Y., Sanchez N.P., Bucko A., Fowler J., Jarratt M., Kempers S. A 6-month maintenance therapy with adapalene-benzoyl peroxide gel prevents relapse and continuously improves efficacy among patients with severe acne vulgaris: results of a randomized controlled trial. Br J Dermatol. 2011;164(6):1376–1382. doi: 10.1111/j.1365-2133.2011.10344.x. [DOI] [PubMed] [Google Scholar]
- Public Affairs Committee of the Teratology Society Teratology public affairs committee position paper: Pregnancy labeling for prescription drugs: Ten years later. Birth Defects Res A Clin Mol Teratol. 2007;79:627–630. doi: 10.1002/bdra.20389. [DOI] [PubMed] [Google Scholar]
- Qin M., Landriscina A., Rosen J.M., Wei G., Kao S., Olcott W. Nitric oxide-releasing nanoparticles prevent propionibacterium acnes-induced inflammation by both clearing the organism and inhibiting microbial stimulation of the innate immune response. J Invest Dermatol. 2015;135(11):2723–2731. doi: 10.1038/jid.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker M. Adverse effects of isotretinoin: a retrospective review of 1743 patients started on isotretinoin. Australas J Dermatol. 2010;51(4):248–253. doi: 10.1111/j.1440-0960.2010.00657.x. [DOI] [PubMed] [Google Scholar]
- Rademaker M. Making sense of the effects of the cumulative dose of isotretinoin in acne vulgaris. Int J Dermatol. 2016;55(5):518–523. doi: 10.1111/ijd.12942. [DOI] [PubMed] [Google Scholar]
- Rafiei R., Yaghoobi R. Azithromycin versus tetracycline in the treatment of acne vulgaris. J Dermatolog Treat. 2006;17(4):217–221. doi: 10.1080/09546630600866459. [DOI] [PubMed] [Google Scholar]
- Ramos-e-Silva M., Ramos-e-Silva S., Carneiro S. Acne in women. Br J Dermatol. 2015;172:20–26. doi: 10.1111/bjd.13638. [DOI] [PubMed] [Google Scholar]
- Rashtak S., Khaleghi S., Pittelkow M.R., Larson J.J., Lahr B.D., Murray J.A. Isotretinoin exposure and risk of inflammatory bowel disease. JAMA Dermatol. 2014;150(12):1322–1326. doi: 10.1001/jamadermatol.2014.1540. [DOI] [PubMed] [Google Scholar]
- Rehn L.M., Meririnne E., Hook-Nikanne J., Isometsa E., Henriksson M. Depressive symptoms and suicidal ideation during isotretinoin treatment: a 12-week follow-up study of male Finnish military conscripts. J Eur Acad Dermatol Venereol. 2009;23(11):1294–1297. doi: 10.1111/j.1468-3083.2009.03313.x. [DOI] [PubMed] [Google Scholar]
- Romoren M., Lindbaek M., Nordeng H. Pregnancy outcome after gestational exposure to erythromycin - a population-based register study from Norway. Br J Clin Pharmacol. 2012;74(6):1053–1062. doi: 10.1111/j.1365-2125.2012.04286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E.V. Optical treatments for acne. Dermatol Ther. 2005;18(3):253–266. doi: 10.1111/j.1529-8019.2005.05024.x. [DOI] [PubMed] [Google Scholar]
- Rothman K.F., Pochi P.E. Use of oral and topical agents for acne in pregnancy. J Am Acad Dermatol. 1988;19(3):431–442. doi: 10.1016/s0190-9622(88)70195-4. [DOI] [PubMed] [Google Scholar]
- Roujeau J.C., Kelly J.P., Naldi L., Rzany B., Stern R.S., Anderson T. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med. 1995;333(24):1600–1607. doi: 10.1056/NEJM199512143332404. [DOI] [PubMed] [Google Scholar]
- Rubinow D.R., Peck G.L., Squillace K.M., Gantt G.G. Reduced anxiety and depression in cystic acne patients after successful treatment with oral isotretinoin. J Am Acad Dermatol. 1987;17(1):25–32. doi: 10.1016/s0190-9622(87)70166-2. [DOI] [PubMed] [Google Scholar]
- Sakamoto F.H., Torezan L., Anderson R.R. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part II. Understanding parameters for acne treatment with photodynamic therapy. J Am Acad Dermatol. 2010;63(2):195–211. doi: 10.1016/j.jaad.2009.09.057. quiz 211–92. [DOI] [PubMed] [Google Scholar]
- Sanchez Viera M. Management of acne scars: fulfilling our duty of care for patients. Br J Dermatol. 2015;172(Suppl. 1):47–51. doi: 10.1111/bjd.13650. [DOI] [PubMed] [Google Scholar]
- Sardana K., Garg V.K. An observational study of methionine-bound zinc with antioxidants for mild to moderate acne vulgaris. Dermatol Ther. 2010;23(4):411–418. doi: 10.1111/j.1529-8019.2010.01342.x. [DOI] [PubMed] [Google Scholar]
- Schafer T., Nienhaus A., Vieluf D., Berger J., Ring J. Epidemiology of acne in the general population: The risk of smoking. Br J Dermatol. 2001;145(1):100–104. doi: 10.1046/j.1365-2133.2001.04290.x. [DOI] [PubMed] [Google Scholar]
- Seirafi H., Farnaghi F., Vasheghani-Farahani A., Alirezaie N.S., Esfahanian F., Firooz A. Assessment of androgens in women with adult-onset acne. Int J Dermatol. 2007;46(11):1188–1191. doi: 10.1111/j.1365-4632.2007.03411.x. [DOI] [PubMed] [Google Scholar]
- Selcen D., Seidman S., Nigro M.A. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22(4):218–220. doi: 10.1016/s0387-7604(00)00104-2. [DOI] [PubMed] [Google Scholar]
- Shahlita A.R., Smith E.B., Bauer E. Topical erythromycin v clindamycin therapy for acne. A multicenter, double-blind comparison. Arch Dermatol. 1984;120(3):351–355. doi: 10.1001/archderm.120.3.351. [DOI] [PubMed] [Google Scholar]
- Shalita A.R. Treatment of mild and moderate acne vulgaris with salicylic acid in an alcohol-detergent vehicle. Cutis. 1981;28(5):556–558. 561. [PubMed] [Google Scholar]
- Shalita A.R. Comparison of a salicylic acid cleanser and a benzoyl peroxide wash in the treatment of acne vulgaris. Clin Ther. 1989;11(2):264–267. [PubMed] [Google Scholar]
- Shalita A.R., Smith J.G., Parish L.C., Sofman M.S., Chalker D.K. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. Int J Dermatol. 1995;34(6):434–437. doi: 10.1111/j.1365-4362.1995.tb04449.x. [DOI] [PubMed] [Google Scholar]
- Shalita A.R., Chalker D.K., Griffith R.F., Herbert A.A., Hickman J.G., Maloney J.M. Tazarotene gel is safe and effective in the treatment of acne vulgaris: a multicenter, double-blind, vehicle-controlled study. Cutis. 1999;63(6):349–354. [PubMed] [Google Scholar]
- Sharquie K.E., Al-Turfi I.A., Al-Shimary W.M. Treatment of acne vulgaris with 2% topical tea lotion. Saudi Med J. 2006;27(1):83–85. [PubMed] [Google Scholar]
- Shaughnessy K.K., Bouchard S.M., Mohr M.R., Herre J.M., Salkey K.S. Minocycline-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome with persistent myocarditis. J Am Acad Dermatol. 2010;62(2):315–318. doi: 10.1016/j.jaad.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Shemer A., Shiri J., Mashiah J., Farhi R., Gupta A.K. Topical minocycline foam for moderate to severe acne vulgaris: phase 2 randomized double-blind, vehicle-controlled study results. J Am Acad Dermatol. 2016;74(6):1251–1252. doi: 10.1016/j.jaad.2015.09.065. [DOI] [PubMed] [Google Scholar]
- Shuster S., Fisher G.H., Harris E., Binnell D. The effect of skin disease on self image [proceedings] Br J Dermatol. 1978;99(Suppl. 16):18–19. [PubMed] [Google Scholar]
- Simonart T. Newer approaches to the treatment of acne vulgaris. Am J Clin Dermatol. 2012;13(6):357–364. doi: 10.2165/11632500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Skolnick J.L., Stoler B.S., Katz D.B., Anderson W.H. Rifampin, oral contraceptives, and pregnancy. JAMA. 1976;236(12):1382. [PubMed] [Google Scholar]
- Smith K., Leyden J.J. Safety of doxycycline and minocycline: A systematic review. Clin Ther. 2005;27(9):1329–1342. doi: 10.1016/j.clinthera.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Smith R.N., Mann N.J., Braue A., Makelainen H., Varigos G.A. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86(1):107–115. doi: 10.1093/ajcn/86.1.107. [DOI] [PubMed] [Google Scholar]
- Smith R.N., Mann N.J., Braue A., Makelainen H., Varigos G.A. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57(2):247–256. doi: 10.1016/j.jaad.2007.01.046. [DOI] [PubMed] [Google Scholar]
- Smith R., Mann N., Makelainen H., Roper J., Braue A., Varigos G. A pilot study to determine the short-term effects of a low glycemic load diet on hormonal markers of acne: A nonrandomized, parallel, controlled feeding trial. Mol Nutr Food Res. 2008;52(6):718–726. doi: 10.1002/mnfr.200700307. [DOI] [PubMed] [Google Scholar]
- Spencer J.P., Gonzalez L.S., III, Barnhart D.J. Medications in the breast-feeding mother. Am Fam Physician. 2001;64(1):119–126. [PubMed] [Google Scholar]
- Stainforth J.M., Layton A.M., Taylor J.P., Cunliffe W.J. Isotretinoin for the treatment of acne vulgaris: which factors may predict the need for more than one course? Br J Dermatol. 1993;129(3):297–301. doi: 10.1111/j.1365-2133.1993.tb11850.x. [DOI] [PubMed] [Google Scholar]
- Strauss J.S., Stranieri A.M. Changes in long-term sebum production from isotretinoin therapy. J Am Acad Dermatol. 1982;6(4 Pt 2 Suppl):751–756. doi: 10.1016/s0190-9622(82)80055-8. [DOI] [PubMed] [Google Scholar]
- Strauss J.S., Rapini R.P., Shalita A.R., Konecky E., Pochi P.E., Comite H. Isotretinoin therapy for acne: Results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10(3):490–496. doi: 10.1016/s0190-9622(84)80100-0. [DOI] [PubMed] [Google Scholar]
- Strauss J.S., Leyden J.J., Lucky A.W., Lookingbill D.P., Drake L.A., Hanifin J.M. A randomized trial of the efficacy of a new micronized formulation versus a standard formulation of isotretinoin in patients with severe recalcitrant nodular acne. J Am Acad Dermatol. 2001;45(2):187–195. doi: 10.1067/mjd.2001.115965. [DOI] [PubMed] [Google Scholar]
- Strauss J.S., Krowchuk D.P., Leyden J.J., Lucky A.W., Shalita A.R., Siegfried E.C. Guidelines of care for acne vulgaris management. J Am Acad Dermatol. 2007;56(4):651–663. doi: 10.1016/j.jaad.2006.08.048. [DOI] [PubMed] [Google Scholar]
- Tan M.H., Spencer J.M., Pires L.M., Ajmeri J., Skover G. The evaluation of aluminum oxide crystal microdermabrasion for photodamage. Dermatol Surg. 2001;27(11):943–949. doi: 10.1046/j.1524-4725.2001.01120.x. [DOI] [PubMed] [Google Scholar]
- Tan J., Stein Gold L., Schlessinger J., Brodell R., Jones T., Cruz A. Short-term combination therapy and long-term relapse prevention in the treatment of severe acne vulgaris. J Drugs Dermatol. 2012;11(2):174–180. [PubMed] [Google Scholar]
- Tan J.K., Jones E., Allen E., Pripotnev S., Raza A., Wolfe B. Evaluation of essential clinical components and features of current acne global grading scales. J Am Acad Dermatol. 2013;69(5):754–761. doi: 10.1016/j.jaad.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Tan J., Humphrey S., Vender R., Barankin B., Gooderham M., Kerrouche N. A treatment for severe nodular acne: a randomized investigator-blinded, controlled, noninferiority trial comparing fixed-dose adapalene/benzoyl peroxide plus doxycycline vs. oral isotretinoin. Br J Dermatol. 2014;171(6):1508–1516. doi: 10.1111/bjd.13191. [DOI] [PubMed] [Google Scholar]
- Tanghetti E. The evolution of benzoyl peroxide therapy. Cutis. 2008;82(5 Suppl):5–11. [PubMed] [Google Scholar]
- Tarimci N., Sener S., Kilinc T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22(4):301. doi: 10.1046/j.1365-2710.1997.9975099.x. [DOI] [PubMed] [Google Scholar]
- Thiboutot D. New treatments and therapeutic strategies for acne. Arch Fam Med. 2000;9:179–187. doi: 10.1001/archfami.9.2.179. [DOI] [PubMed] [Google Scholar]
- Thiboutot D. Acne: hormonal concepts and therapy. Clin Dermatol. 2004;22(5):419–428. doi: 10.1016/j.clindermatol.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Thiboutot D., Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206(1):57–67. doi: 10.1159/000067823. [DOI] [PubMed] [Google Scholar]
- Thiboutot D.M., Shalita A.R., Yamauchi P.S., Dawson C., Kerrouche N., Arsonnaud S. Adapalene gel, 0.1%, as maintenance therapy for acne vulgaris: A randomized, controlled, investigator-blind follow-up of a recent combination study. Arch Dermatol. 2006;142(5):597–602. doi: 10.1001/archderm.142.5.597. [DOI] [PubMed] [Google Scholar]
- Timpatanapong P., Rojanasakul A. Hormonal profiles and prevalence of polycystic ovary syndrome in women with acne. J Dermatol. 1997;24(4):223–229. doi: 10.1111/j.1346-8138.1997.tb02778.x. [DOI] [PubMed] [Google Scholar]
- Toossi P., Farshchian M., Malekzad F., Mohtasham N., Kimyai-Asadi A. Subantimicrobial-dose doxycycline in the treatment of moderate facial acne. J Drugs Dermatol. 2008;7(12):1149–1152. [PubMed] [Google Scholar]
- Torok H.M., Pillai R. Safety and efficacy of micronized tretinoin gel (0.05%) in treating adolescent acne. J Drugs Dermatol. 2011;10(6):647–652. [PubMed] [Google Scholar]
- Trifu V., Tiplica G.S., Naumescu E., Zalupca L., Moro L., Celasco G. Cortexolone 17alpha-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165(1):177–183. doi: 10.1111/j.1365-2133.2011.10332.x. [DOI] [PubMed] [Google Scholar]
- Tripathi S.V., Gustafson C.J., Huang K.E., Feldman S.R. Side effects of common acne treatments. Expert Opin Drug Saf. 2013;12(1):39–51. doi: 10.1517/14740338.2013.740456. [DOI] [PubMed] [Google Scholar]
- Trivedi M.K., Shinkai K., Murase J.E. A review of hormone-based therapies to treat adult acne vulgaris in women. Int J Womens Dermatol. 2017;3:44–52. doi: 10.1016/j.ijwd.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschen E.H., Katz H.I., Jones T.M., Monroe E.W., Kraus S.J., Connolly M.A. A combination benzoyl peroxide and clindamycin topical gel compared with benzoyl peroxide, clindamycin phosphate, and vehicle in the treatment of acne vulgaris. Cutis. 2001;67(2):165–169. [PubMed] [Google Scholar]
- Tucker S.B. Occupational tropical acne. Cutis. 1983;31(1):79–81. [PubMed] [Google Scholar]
- U.S. Food and Drug Administration Transcripts from the Dermatologic Drugs Advisory Committee Meeting [Internet] 2003. http://www.fda.gov/ohrms/dockets/ac/accutane.htm [accessed November 12, 2016]. Available from:
- U.S. National Library of Medicine LactMed: A new NLM database on drugs and lactation [Internet] 2017. https://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm [accessed November 12, 2016]. Available from.
- Ullah G., Noor S.M., Bhatti Z., Ahmad M., Bangash A.R. Comparison of oral azithromycin with oral doxycycline in the treatment of acne vulgaris. J Ayub Med Coll Abbottabad. 2014;26(1):64–67. [PubMed] [Google Scholar]
- van Hoogdalem E.J. Transdermal absorption of topical anti-acne agents in man; review of clinical pharmacokinetic data. J Eur Acad Dermatol Venereol. 1998;11(Suppl. 1):S13–S19. doi: 10.1111/j.1468-3083.1998.tb00902.x. [discussion S28–19] [DOI] [PubMed] [Google Scholar]
- Wang X.L., Wang H.W., Zhang L.L., Guo M.X., Huang Z. Topical ALA PDT for the treatment of severe acne vulgaris. Photodiagnosis Photodyn Ther. 2010;7(1):33–38. doi: 10.1016/j.pdpdt.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Webster G.F., Leyden J.J., Gross J.A. Comparative pharmacokinetic profiles of a novel isotretinoin formulation (isotretinoin-Lidose) and the innovator isotretinoin formulation: a randomized, 4-treatment, crossover study. J Am Acad Dermatol. 2013;69(5):762–767. doi: 10.1016/j.jaad.2013.05.036. [DOI] [PubMed] [Google Scholar]
- Wei B., Pang Y., Zhu H., Qu L., Xiao T., Wei H.C. The epidemiology of adolescent acne in North East China. J Eur Acad Dermatol Venereol. 2010;24(8):953–957. doi: 10.1111/j.1468-3083.2010.03590.x. [DOI] [PubMed] [Google Scholar]
- Weinstein M., Laxer R., Debosz J., Somers G. Doxycycline-induced cutaneous inflammation with systemic symptoms in a patient with acne vulgaris. J Cutan Med Surg. 2013;17(4):283–286. doi: 10.2310/7750.2013.12085. [DOI] [PubMed] [Google Scholar]
- Wenk R.E., Gebhardt F.C., Bhagavan B.S., Lustgarten J.A., McCarthy E.F. Tetracycline-associated fatty liver of pregnancy, including possible pregnancy risk after chronic dermatologic use of tetracycline. J Reprod Med. 1981;26(3):135–141. [PubMed] [Google Scholar]
- Williams H.C., Dellavalle R.P., Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–372. doi: 10.1016/S0140-6736(11)60321-8. [DOI] [PubMed] [Google Scholar]
- Yentzer B.A., Hick J., Reese E.L., Uhas A., Feldman S.R., Balkrishnan R. Acne vulgaris in the United States: a descriptive epidemiology. Cutis. 2010;86(2):94–99. [PubMed] [Google Scholar]
- Zaenglein A.L., Shamban A., Webster G., Del Rosso J., Dover J.S., Swinyer L. A phase IV, open-label study evaluating the use of triple-combination therapy with minocycline HCl extended-release tablets, a topical antibiotic/retinoid preparation and benzoyl peroxide in patients with moderate to severe acne vulgaris. J Drugs Dermatol. 2013;12(6):619–625. [PubMed] [Google Scholar]
- Zaenglein A.L., Pathy A.L., Schlosser B.J., Alikhan A., Baldwin H.E., Berson D.S. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- Zaveri N.T. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78(18):2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Zech L.A., Gross E.G., Peck G.L., Brewer H.B. Changes in plasma cholesterol and triglyceride levels after treatment with oral isotretinoin. A prospective study. Arch Dermatol. 1983;119(12):987–993. [PubMed] [Google Scholar]
- Zeichner J.A. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147(5):537–539. doi: 10.1001/archdermatol.2011.96. [DOI] [PubMed] [Google Scholar]
- Zeitany A.E., Bowers E.V., Morrell D.S. High-dose isotretinoin has lower impact on wallets: a cost analysis of dosing approaches. J Am Acad Dermatol. 2016;74(1):174–176. doi: 10.1016/j.jaad.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Zhanel G.G., Siemens S., Slayter K., Mandell L. Antibiotic and oral contraceptive drug interactions: is there a need for concern? Can J Infect Dis. 1999;10(6):429–433. doi: 10.1155/1999/539376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis C.C., Derumeaux L., Decroix J., Maciejewska-Udziela B., Cambazard F., Stuhlert A. A multicentre, single-blind, randomized comparison of a fixed clindamycin phosphate/tretinoin gel formulation (Velac) applied once daily and a clindamycin lotion formulation (Dalacin T) applied twice daily in the topical treatment of acne vulgaris. Br J Dermatol. 2000;143(3):498–505. doi: 10.1111/j.1365-2133.2000.03701.x. [DOI] [PubMed] [Google Scholar]