Abstract
The tau fibrillar structures from the brain of an Alzheimer’s patient have a core with C-shaped motif of third and fourth repeat domains (R3-R4). Our simulations indicated that the C-shaped motif is only stable for the R3-R4, while R1-R2 tends to be linear in shape. These two structural motifs appear in the most stable K18 protofilament. Heparin can further stabilize the C-shaped R3-R4 motif, but not other repeats.
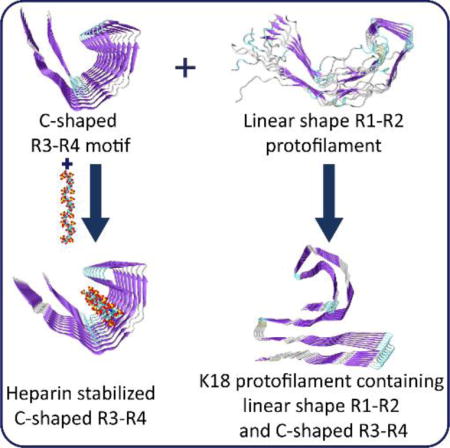
TOC image
Among the four bi-repeat protofilaments, only R3-R4 can maintain C-shaped structure that can be stabilized by heparins, while R1-R2 tends to be linear shape, and these two structural motifs appear in the most stable K18 protofilament.
One of the hallmarks of Alzheimer disease is amyloid deposit formed by amyloid-β (Aβ) plaques and tau protein tangles.1 Aβ plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating tau aggregation,2 possibly through a “stretching-and-packing” cross-seeding mechanism.3 Both Aβ and tau can trigger neuroinflammation and lead to neuro-degeneration.4 Interest is growing in tau as a potential drug target, by blocking tau aggregation.5 Two major types of isoforms exist for tau proteins, with either three (R1, R3 and R4) or four (R1~R4) microtubule binding repeats (MBR). K18 is one of the truncated constructs of tau, consisting of four repeats. During tau aggregation, the MBR domain becomes structured and forms a protease-resistant core. Studies of tau amyloids are complicated because of potential structural differences between filaments in different tau-related diseases6. Structural variations in tau filaments have also been observed in vitro and could be further modulated by polyanionic amyloid inducers such as heparin.7, 8 Previous molecular dynamic (MD) simulations and EPR experiments reported linear straight or β-turn-β U-shaped motifs for the four repeats.9–12 An electron microscopy study showed that tau filament cores consist of a double helical stack of C-shaped subunits.13 Recently, the atomic details of repeats R3-R4 plus 10 ten extra C-terminal residues (residues 306~378) of filament cores were revealed by high resolution Cyro-EM14, provided important insights into the polymorphic tau protein tangles. However, whether other bi-repeat combinations R1-R2, R1-R3 and R2-R3 have a preference to adopt a C-shaped structure remains elusive. Since in AD all isoforms are incorporated into amyloid core, the structural compatibility of different repeats is one of keys to understand prion-like properties of tau protein aggregation.
Here we examine the stabilities of the octameric C-shaped structural motifs (Fig. S1A ESI†) formed by different bi-repeat combinations with or without heparins and different conformations of K18 protofilaments. All-atom explicit-solvent MD simulations were performed for twenty-four various tau protofilaments and filaments (see Methods ESI†).
We first examined the stabilities of all possible bi-repeat (Fig. 1A and Fig. S1B-C ESI†) C-shaped motifs (R1-R2 (residues 244~315), R2-R3 (residues 275~346), R1-R3 (residues 244~274 and 306~346), and R3-R4 (residues 306~378)) in both paired helical filaments (PHFs) and straight filaments (SFs). According to the recent Cryo-EM work, a filament consists of two protofilaments.14 The protofilament system includes five different bi-repeat combinations: PHF34, PHF12, PHF13, PHF23 and SF34 (Fig. 1A and Fig. S1B). The filament system includes four different PHF and SF filaments: 2PHF34, 2PHF12, 2PHF13, 2PHF23, 2SF34, 2SF12, 2SF13, and 2SF23 (Fig. 1A and Fig. S1C ESI†). The structures of R3-R4 protofilaments and filaments were taken from cryo-EM coordinates, and other combinations were constructed using the R3-R4 structures as templates.
Figure 1.
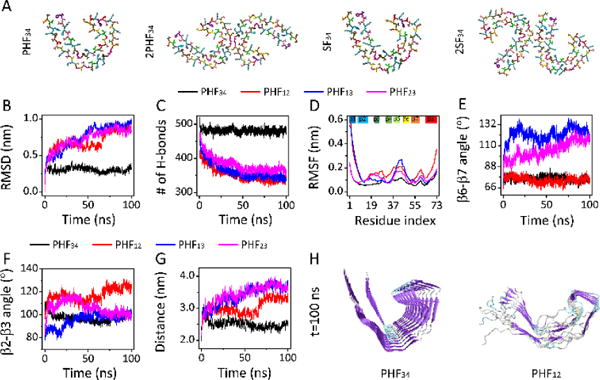
Only R3-R4 PHF/SF protofilaments and filaments can maintain C-shaped structure. (A) Initial structure of a single chain in protofilaments and of two chains in filaments for the R3-R4 combination. The trajectories of the RMSD (B) and number of hydrogen bond (H-bond) (C) for four PHF protofilaments. (D) The RMSF of four PHF protofilaments. Time evolution of β6-β7 angle (E) and β2-β3 angle (F) for all protofilaments, and Q351-I371 distance in PHF34, Q288-I308 distance in PHF12, and T319-V339 distance in PHF13 and PHF23 (G). (H) The snapshots of PFH34 and PHF12 at t=100 ns.
The stability of all PHF protofilaments are examined by probing the trajectories of RMSD and hydrogen bond (H-bond) number, and the RMSF (Fig. 1B-D). The RMSD shows that PHF34 structure stabilizes quickly around 0.30 nm, while PHF12, PHF13 and PHF23 continue to deviate from their initial structures, with their RMSDs reaching to respectively, 0.93 nm, 0.95 nm and 0.83 nm at t= 100 ns (Fig. 1B). Consistently, H-bond number of PHF34 fluctuates around 480, while that of other three protofilaments decreased rapidly (Fig. 1C). The RMSF values show that almost all β regions (Fig. S1B ESI†) in PHF34 exhibit the highest rigidity (Fig. 1D). The high structural stability of PHF34 can also be seen from the residue-based β-sheet probability (Fig. S2A ESI†). We characterize the C-shaped structure by monitoring the β2-β3/β6-β7 angles and a distance between two representative residues in β6 and β8 (i.e. Q351-I371 in PHF34, Q288-I308 in PHF12, and T319-V339 in PHF13 and PHF23) (Fig. 1E-G). The β2-β3 and β6-β7 angles in PHF34 stay respectively around 100° and 73° throughout simulations. But the β6-β7 angles in PHF13 and PHF23 increase to 116° and 118° at t=100 ns, implying that structures in β6 and β7 regions of PHF13 and PHF23 tend to be extended. In PHF12, though the β6-β7 angle (75°) stays close to that in PHF34, the β2-β3 angle is much larger than 100°, indicating that PHF12 has a strong tendency to be straight (Fig. 1H). The β-sheet distance confirmed the instability of the C-shaped PHF12, PHF13 and PHF23. Although the value of the β2-β3 angle in PHF34, PHF13, and in PHF23 at t=100 ns are same, the distance between β6 and β8 in PHF13 and PHF23 is much larger than that in PHF34 system. The distance increase in the former two systems is mainly caused by the twisting of β1-β2 plane relative to β3 plane (Fig. S2C ESI†). PHF23 is prone to be V-shaped, and PHF13 has a preference to be U-shaped, as seen from the final snapshot (Fig. S2C ESI†). These results indicate that PHF34 can maintain its C-shaped structure well, while the other three are not compatible with the C-shape structure. Similar structural stability is observed for SF34. (Fig. S3 ESI†) Note that β8 is not in tau repeats domain but have a large interface with β1~β2, and it helps the shape formation of the four in the order of PHF12 > PHF34 > PHF13 > PHF23 (Supplementary Materials).
To understand why the other three protofilaments cannot be stabilized in a C-shaped structure, we examine the inter-chain and intra-chain pairwise residue contact probability (Fig. S4 ESI†). The contact pattern of PHF34 reflects that the interactions between the C-terminal residues of one chain and the N-terminal residues of the nearby chain helps to stabilize the C-shaped structure (Fig. S4A ESI†). Other three bi-repeat protofilaments have weak interactions. In the PHF12, the interactions in β1, β3, β7 and the β8 C-terminal regions are reduced. In the PHF13, the regions with weakened interactions change to β1, β3, β5 and β7. In PHF23, β3, β5 and β7 are the weak interaction regions. Intra-chain interactions are also important in maintaining the C-shaped structure. PHF34 has the strongest intra-chain interactions (Fig. S4B ESI†). In the PHF34, the interactions among K353, S356 and D358 residues stabilize the β6-β7 angle and the interactions among C322, L325, I328, P364 and G367 can stabilize the β2-β3 angle (Fig. S4C ESI†), while in other systems, the two angles cannot be simultaneously stabilized around their initial values. The lack of intra-chain interactions between P301 and G304 explains the extension of PHF12 (Fig. S4B and Fig.1H). The β6-β7 angle is stabilized by the salt-bridges K353/D358 in PHF34 and K290/D295 in PHF12 (Fig. S4D ESI†). However, these two salt-bridges are missing in the other two systems. The destabilization of the C-shaped structure of PHF13 and PHF23 can be seen in Fig. S2C.
The structural differences between MBR have a great effect on interaction patterns among the four protofilaments (Fig. S5 ESI†). In the PHF34 system, the hydrophobic residues of the hexapeptide V306~K311 in β1 and T373~F378 in β8 form a face-to-face packing interface. However, similar interactions in both PHF12 and PHF13 were missing due to the reduced number of hydrophobic residues in β1. In addition, three consecutive β-breaking prolines (P247, P249, and P251) destroy the β-sheet structure of β1 and the N-terminal region of β2, and the β-sheet structure of C-terminal regions of β8 in PHF12 is also disrupted by P312. In PHF34, there is a right-angle turn accomplished through G323 and G326 on the outer side of the C-shaped protofilament, while in the other three systems, the reduced number of Glycine is not sufficient for keeping the turn of the C-shaped structure. In the PHF34 system, a triangle-shaped hydrophobic cluster formed by L325 in the turn region, I328 in β3 and V363 in β7. However, in PHF12, the cluster disappears and lead to an extended turn conformation. In the PHF13 and PHF23 systems, there is only one hydrophobic residue in the hydrophobic cluster region, which greatly reduces the stability of that region. The β4~β6 regions in PHF34 system form a β-helix structure. Two-residue (E342 and K343) and three-residue (K347, D348 and R349) β-arc corners facilitate the triangular β-helix geometry, whereas, residues in the corresponding β-arc position are not favorable for β-arc formation. In addition, hydrophobic (V339, L344, V350, and I354) and aromatic (F346) residues stabilize the interior of the β-helix geometry in PHF34. However, aromatic residues are missing in all the other systems. Moreover, the β-breaking residue P312 in PHF34 is located between β1 and β2, while proline residues are missing at the corresponding position in PHF23, which renders the β1 and β2 to combine into a longer β-strand. And this makes the whole structure of PHF23 look like a V shape. (Fig. S2 ESI†).
The dimerization of protofilaments cannot help stabilizing other repeat combinations in the C-shaped structure, but is favourable for R3-R4, indicated by MD simulations of octameric 2PHF and 2SF filaments (a tetramer for each protofilament) in four bi-repeat combinations. (Fig. S6B ESI†) In cryoEM-derived 2PHF34, the interface is formed by the anti-parallel stacking of residues P332~Q336. The corresponding residues in the interface are P270~K274 for both PHF12 and PHF13, and P301~S305 for PHF23. Fig. S7A shows the contact probabilities of eight interface residues of all 2PHF combinations. Overall, the protofilament interfaces of 2PHF34 and 2PHF23 are more stable than those of 2PHF12 and 2PHF13. In the 2PHF12 and 2PHF13, the interface interactions are weak and the two protofilaments tend to separate (Fig. S7B ESI†). Both protofilaments in the 2PHF34 system retain the C-shaped structure well, while all others have conformational deviations from the C-shaped structure. In cryoEM-derived 2SF34, the two protofilaments pack asymmetrically between residues K321~S324 in one protofilament and V313~K317 in the other. To compare with 2PHF, we also choose eight residues in each protofilament (K317~S324 and D314~K321) to analyze the interface stability. The contact probability maps and the last frame snapshots in Fig. S7 and Fig. S8 show that compared to 2PHF34, 2SF34 has weak interface interactions involving residue pairs L315-K321 L315-C322 and L315-G323. In the SF34 protofilament interface, consistent with Cryo-EM, the main interactions are strong VdW interactions between L315-K321 L315-C322 and L315-G323 (Fig. S8A ESI†). In the 2SF12 system, the two protofilaments separate. The interface contact residues in 2SF13 and 2SF23 are respectively L253-K259, L253-I260, L253-G261 and N255-K259, and L284-K290, L284-C291, L284-G292 and N286-K290, like those in 2SF34. (Fig. S8A ESI†) More results and discussion the contributions of dimerization to the shape formation be found in Supplementary Materials.
Full-length tau quickly aggregates when anionic cofactors are present.15 In in vitro experiments, heparin is often used to induce and accelerate tau filament formation.16–18 In vivo, it has been reported that tau and heparin coexist in neurons with overt neurofibrillary lesion.19 However, how heparin promotes tau fibrillation is unclear. Also, whether it is a component of tau filaments is open to debate.20, 21 The C-shaped structural feature of tau filaments suggests that heparin could fit into the groove of the protofilament to stabilize its structure (Fig. 2). The length of hexameric heparin is comparable to the length of the groove of octameric PHF protofilaments. Thus, we simulated the tau protofilament-heparin complex (PHF-HP) to examine if heparin can stabilize the C-shaped core.
Figure 2.
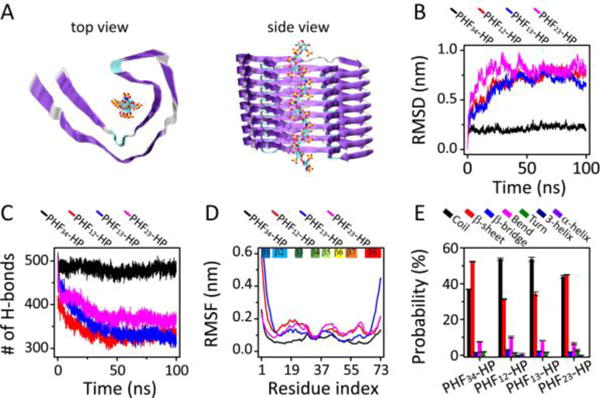
The stabilization effect of heparin on four PHF combinations. (A) Initial structures of PHF-HP protofilament with side view on the left and main view on the right. Trajectories of (B) RMSD and (C) hydrogen bond number of protofilaments. (D) The RMSF of PHF-HP combinations. (E) The probabilities of each type of secondary structures.
Fig. 2 indicates that, among all PHF-HP systems simulated, PHF34-HP is the most stable one. Even though heparin reduces structural flexibilities of all bi-repeat combinations, only PHF34-HP system maintains its C-shaped structure (Fig. S9A ESI†). Even though the RMSD of PHF13 decreases from 0.90 nm to 0.71 nm, it is still not comparable to the 20.7% reduction in RMSD from 0.29 nm to 0.23 nm observed for the protofilament in the PHF34-HP system (Table S2). The RMSFs reflect an increased flexibility of other systems, compared to that in the PHF34-HP system (Table S3). Heparin reduces the flexibility of most regions in the protofilaments. In the PHF34-HP system, in addition to the slightly decreased rigidity of β3, the rigidity of the overall structure is enhanced. However, for other protofilaments, there is only localized enhancement of rigidity. PHF34 has the highest probability of β-sheet, followed by PHF23, PHF13 and PHF12. These results show that the repeats R3-R4 are most suited to interact with heparin and accelerate tau fibrillization.
Why is heparin able to selectively stabilize R3-R4 to adopt a C-shaped structure? We examine the contact probability between heparin and each protofilament by excluding the edge strands (Fig. S9 ESI†). In the PHF34-HP system, there are three regions involving seven residues (R349, Q351, K353, I360, H362 K369, and I371) that have strong contacts with heparin. Positively charged K353 and K369 attractively interact with polyanion heparins. Q351 also displays high contact probability with heparin. Residues I371 and Q351 are likely to serve as a gate to keep heparin inside the groove. The attraction between R249 and heparin also helps stabilizing the C-shaped structure. Other three systems only have two or three residues strongly interacting with heparin, which can maintain the local but not global structures. In the PHF12-HP system, K290 and H299 have high contact probabilities with heparin. However, RMSF values revealed that although the strong interaction between K290 and heparin can stabilize the β4~β6 regions, the interaction between H299 and heparin destabilizes the β3 and β7 (Table S3). Similarly, fewer basic residues in other repeat systems (K321 in PHF13 and PHF23) do not provide sufficient interactions to stabilize the C-shaped structure.
Previous experiments22, 23 reported that the core of tau filaments is formed by three or four repeats. Only bi-repeat constructs were considered in above-mentioned simulations. When all four repeats are considered (such as in K18) two questions arise: 1) Can R1-R2 also be stabilized in C-shape? 2) Is the C-shaped R3-R4 motif compatible with a different shape of the R1-R2 motif? To answer these questions, we simulate K18 with the C-shaped R3-R4 in combination with different shapes of R1-R2 motif. We termed them K18-1, -2, -3, -4, -5, -6, and -7. All the initial structures are shown in Fig. 3A.
Figure 3.
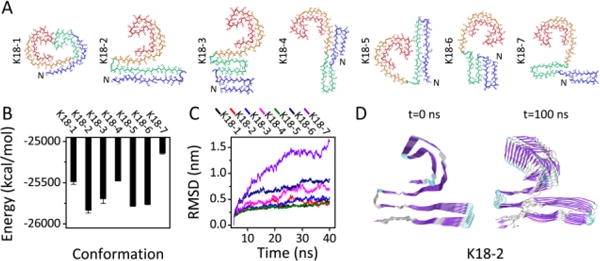
Seven constructed octameric K18 protofilaments and their Stabilities. (A) The initial structures. For clarity, only one chain in each octamer is presented with R1 in blue, R2 in green, R3 in orange and R4 in red (B) The GBMV energy of each protofilament. (C) The RMSD trajectories of each protofilament. (D) Cartoon representations of initial and final snapshots of the most stable structure of K18: K18-2.
Figure 3 shows that, K18-2, where R1-R2 is in linear and R3-R4 is in C-shape, has the lowest energy and the smallest RMSD, followed by K18-5 (with a linear shaped R1-R2) and K18-3 (with a compact U-shaped R1-R2). K18-1, the structure with two C-shaped motifs, has small RMSD, but high energy. (Fig. 3C) By comparing the GBMV energies with those in our previous work,12 we found that the energies of all K18 structures containing the C-shaped R3-R4 motif are respectively lower than those of conformers with the same R1-R2 conformations. Interestingly, we found that in K18-2, 16 residues (i.e. K290 to S305) in R2 are well packed with R3. The cryo-EM indicated that residues from R1 or R2 may form an additional, less-ordered β-sheet.14 The relative position between these 16 residues and the C-shaped R3-R4 resembles that in the cryo-EM electron density map of tau filament cores.14 While residues from the N-terminal region may also contribute to the observed cryo-EM, our simulation indicated that the stability of C-shaped of R3-R4 does not depend on the structurally unsolved region. Overall, our data indicate that the K18 protofilament is most likely to adopt the K18-2 conformation (Fig. 3D).
In summary, our data indicate that only the R3-R4 motif can maintain the C-shaped structure, while R1-R2 tends to be linear. The high β-sheet probability and stable interface of R2-R3 suggest that R2-R3 might also be a good candidate. However, whether it can exist in C-shape remains to be seen. Heparin may stabilize the C-shaped R3-R4 motif. Notably, the interactions in other repeat combinations are not strong enough to stabilize them in the C-shaped β-sheet motif. Simulations of K18 with the C-shaped R3-R4 in combination with different shapes of R1-R2 demonstrate that the C-shaped R3-R4 motif is compatible with either a linear or compact U-shaped R1-R2 motif. The constructed K18 with a linear R1-R2 and C-shaped R3-R4 is the most stable one among all K18 conformations, indicating it’s most possible to be the tau filament core. In the future, it will be interesting to see how the N- and C-terminal flanking regions in tau might affect structural stability and if the C-shaped motif is sensitive to mutations and posttranslational modifications.
Supplementary Material
Acknowledgments
This work was supported by NIH contract HHSN261200800001E and by the Intramural Research Program of the CCR, NCI, NIH. G. W thanks the NSF of China (Grant No. 11674065) and the National Key R&D Program of China (Grant No. 2016YFA0501702). MD simulations were performed at the Biowulf cluster at the NIH and National High-Performance Computing Center of Fudan University. We thank Dr. Scheres for providing the cryo-EM structures.
Footnotes
Electronic Supplementary Information (ESI) available: 3 Tables and 10 figures.
Notes and References
- 1.Polanco JC, Li C, Bodea LG, Martinez-Marmol R, Meunier FA, Gotz J. Nat Rev Neurol. 2018;14:22–39. doi: 10.1038/nrneurol.2017.162. [DOI] [PubMed] [Google Scholar]
- 2.He Z, Guo JL, McBride JD, Narasimhan S, Kim H, Changolkar L, Zhang B, Gathagan RJ, Yue C, Dengler C, Stieber A, Nitla M, Coulter DA, Abel T, Brunden KR, Trojanowski JQ, Lee VM. Nat Med. 2018;24:29–38. doi: 10.1038/nm.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qi R, Luo Y, Wei G, Nussinov R, Ma B. The Journal of Physical Chemistry Letters. 2015;6:3276–3282. [Google Scholar]
- 4.Bolos M, Perea JR, Avila J. Biomol Concepts. 2017;8:37–43. doi: 10.1515/bmc-2016-0029. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Gotz J. Nat Rev Drug Discov. 2017;16:863–883. doi: 10.1038/nrd.2017.155. [DOI] [PubMed] [Google Scholar]
- 6.Crowther RA, Goedert M. J Struct Biol. 2000;130:271–279. doi: 10.1006/jsbi.2000.4270. [DOI] [PubMed] [Google Scholar]
- 7.Jangholi A, Ashrafi-Kooshk MR, Arab SS, Riazi G, Mokhtari F, Poorebrahim M, Mahdiuni H, Kurganov BI, Moosavi-Movahedi AA, Khodarahmi R. Arch Biochem Biophys. 2016;609:1–19. doi: 10.1016/j.abb.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Luo Y, Yu X, Dinkel P, Zheng J, Margittai M, Nussinov R, Wei G, Ma B. Chemical Communications. 2013;49:3582–3584. doi: 10.1039/c3cc00241a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamcik J, Sanchez-Ferrer A, Ait-Bouziad N, Reynolds NP, Lashuel HA, Mezzenga R. Angew Chem Int Ed Engl. 2016;55:618–622. doi: 10.1002/anie.201508968. [DOI] [PubMed] [Google Scholar]
- 10.Miller Y, Ma B, Nussinov R. Biochemistry. 2011;50:5172–5181. doi: 10.1021/bi200400u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqua A, Luo Y, Meyer V, Swanson MA, Yu X, Wei G, Zheng J, Eaton GR, Ma B, Nussinov R, Eaton SS, Margittai M. Journal of the American Chemical Society. 2012;134:10271–10278. doi: 10.1021/ja303498q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Luo Y, Dinkel P, Zheng J, Wei G, Margittai M, Nussinov R, Ma B. The Journal of biological chemistry. 2012;287:14950–14959. doi: 10.1074/jbc.M112.340794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowther RA. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:2288–2292. doi: 10.1073/pnas.88.6.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, Crowther RA, Ghetti B, Goedert M, Scheres SHW. Nature. 2017;547:185–190. doi: 10.1038/nature23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Brain Research Brain Research Reviews. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 16.Pérez M, Valpuesta JM, Medina M, Montejo DGE, Avila J. Journal of Neurochemistry. 1996;67:1183–1190. doi: 10.1046/j.1471-4159.1996.67031183.x. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi S, Suzuki N, Masuda M, Hisanaga S, Iwatsubo T, Goedert M, Hasegawa M. Journal of Biological Chemistry. 2005;280:7614. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Huvent I, Lippens G, Eliezer D, Zhang A, Li Q, Tessier P, Linhardt RJ, Zhang F, Wang C. Biophysical Journal. 2017;112:921. doi: 10.1016/j.bpj.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perry G, Siedlak SL, Richey P, Kawai M, Cras P, Kalaria RN, Galloway PG, Scardina JM, Cordell B, Greenberg BD. Journal of Neuroscience. 1991;11:3679–3683. doi: 10.1523/JNEUROSCI.11-11-03679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathalie Sibille, Alain Sillen, Arnaud Leroy§, Jeanmichel Wieruszeski, Barbara Mulloy A. Isabelle Landrieu and Guy Lippens. Biochemistry. 2006;45:12560. doi: 10.1021/bi060964o. [DOI] [PubMed] [Google Scholar]
- 21.Carlson SW, Branden M, Voss K, Sun Q, Rankin CA, Gamblin TC. Biochemistry. 2007;46:8838–8849. doi: 10.1021/bi700403a. [DOI] [PubMed] [Google Scholar]
- 22.von Bergen M, Barghorn S, Muller SA, Pickhardt M, Biernat J, Mandelkow EM, Davies P, Aebi U, Mandelkow E. Biochemistry. 2006;45:6446–6457. doi: 10.1021/bi052530j. [DOI] [PubMed] [Google Scholar]
- 23.Wischik CM, Novak M, Edwards PC, Klug A, Tichelaar W, Crowther RA. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


