Abstract
Plants’ reliance on sunlight for energy makes their light-driven circadian clock a critical regulator in balancing the energy needs for vital activities such as growth and defense. Recent studies show that the circadian clock acts as a strategic planner to prime active defense responses towards the morning or daytime when conditions, such as the opening of stomata required for photosynthesis, are favorable for attackers. Execution of the defense response, on the other hand, is determined according to the cellular redox state and is regulated in part by the production of reactive oxygen and nitrogen species upon pathogen challenge. The interplay between redox and the circadian clock further gates the onset of defense response to a specific time of the day to avoid conflict with growth-related activities. In this review, we focus on discussing the roles of the circadian clock as a robust overseer and the cellular redox as a dynamic executor of plant defense.
Keywords: Circadian clock, Redox rhythm, Plant immunity, Salicylic acid, Jasmonic acid, Cellular redox, Reactive oxygen species, Reactive nitrogen species, S-nitrosylation
1. Introduction
Plants lead challenging lives as sessile photosynthetic organisms: they cannot actively diversify their energy sources or escape from environmental challenges. This essentially turns their existence into an endeavor for better resource management. A well-established example is the tradeoff between growth and defense [1]: How are resources allocated towards defense without the risk of severely delayed growth and development? Since plants do not have specific immune cells against particular pathogens as do animals, they must also balance distinct defense mechanisms against attackers with contrasting infection strategies. When challenged by biotrophic pathogens, which thrive in living tissues, plants can kill infected cells to contain the invaders. However, this strategy may leave them vulnerable to necrotrophs that feed on dead cells. Therefore, immune responses need to be managed and balanced against each other, so that the most optimal defense strategy is deployed [2]. In this review, we present how the plant circadian clock and redox system respectively serve as a strategic planner and an executor of these immune strategies. For more comprehensive discussion on the interplay between the circadian clock and redox within the context of plant immunity, please see excellent reviews by Spoel and van Ooijen [3] and by Lu and colleagues [4].
The Earth’s rotation around its axis and the sun results in robust rhythmic changes in its environment. Living organisms have evolved circadian clocks to exploit this predictable environmental information and synchronize internal processes with the daily cycles [5,6]. Disharmony with the environment can have dire consequences, with the disruption of the circadian rhythm being a risk factor for multitude of diseases in humans [7]. Considering that plants rely on photosynthesis for the generation of energy and reducing power, it is hard to overestimate the importance of the circadian clock in managing numerous aspects of plant physiology and improving plant’s fitness [8–10], with at least 30% of the Arabidopsis thaliana transcripts showing the circadian rhythmicity [11]. Therefore, plants can use the circadian clock for the efficient planning of resource expenditure. For example, as a function of the clock, photosynthetic products (i.e., starch) accumulated during the day are exhausted in the service of growth-related activities at night right before dawn, demonstrating the “frugality” of plants in energy management [12]. As a high-cost and high-benefit trait, activation of plant immunity has also been found to be tightly regulated by the clock [4]. By using the circadian clock to schedule and gate defense to the time of high likelihood of an attack, plants can avoid energetically expensive and potentially deleterious effects of untimely immune induction [13].
In contrast to the regularity and robustness of the circadian clock, the cellular redox state is dynamic, determined by the current production of oxidized versus reduced metabolites. Plant immunity has long been associated with the production of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), and reactive nitrogen species (RNS), such as nitric oxide (NO) [14–16]. The balance between ROS and RNS production is crucial for a successful immune response [15], while their interplay is often a determinant of the fate of the infected cells [17]. As a major antioxidant in plants, glutathione plays a central role in regulating immunity [18–20]. In addition, the signaling of the two main plant defense hormones, salicylic acid (SA) and jasmonic acid (JA), responsible for defense against biotrophic and necrotrophic pathogens, respectively, are associated with opposing effects on cellular redox [21]. These redox-depended mechanisms ultimately ensure that plant defense combats the appropriate attacker [16] while potentially minimizing the costs.
2. When to defend and when to attack: the role of the circadian clock
Upon pathogen challenge, plants must first determine how much energy can be diverted towards defense. Plant energy generation in nature is diurnal, with photosynthesis occurring only during the day. Predictably, the opening of the microscopic epidermal pores (i.e., stomata) involved in the gas exchange for photosynthesis, is regulated by the circadian clock [22,23]. The aperture of the stomata is bigger during the day and smaller at night. Because stomata are also entry points for some pathogens, plants show greater resistance against surface-inoculated bacteria at night, when stomata have a smaller aperture [24,25] (Fig. 1). Moreover, stomatal closure in response to pathogens is among the earliest plant immune responses. After detecting pathogen-associated molecular patterns (PAMPs), such as flagellin, as a part of pattern-triggered immunity (PTI) [26], plants rapidly close the stomata [27]. Core morning clock genes CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) have been found to regulate stomatal immunity through their target GLYCINE-RICH RNA-BINDING PROTEIN 7 (GRP7), also known as COLD AND CIRCADIAN REGULATED 2 (CCR2) [24]. GRP7 is part of a peripheral loop of the circadian clock [28], which, besides being involved in stomatal defense, binds to some PAMP receptor transcripts and can upregulate the translation of at least one of them during an infection [29]. Additionally, night-expressed clock gene TIME FOR COFFEE (TIC) is required for both circadian oscillation of stomatal aperture and efficient stomatal defense [25]. Interestingly, darkness treatment by itself induces stronger stomatal closure than an infection under light [30], suggesting that other form of defense may be required in the presence of light to compensate for the weaker stomatal protection.
Fig. 1.
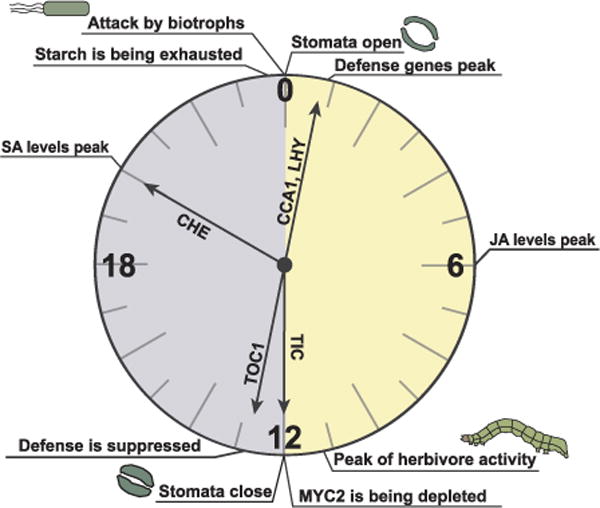
The many hands of the circadian clock. The circadian clock (genes represented by arrows in the figure) primes several active plant defenses against biotrophs towards the morning and daytime, when stomata are open, the energy is available due to photosynthesis and the conditions are favorable for pathogen invasion. The clock suppresses the active defense against biotrophs at night, relying on closed stomata to prevent pathogen invasion. JA signaling component MYC2 is depleted through the night and accumulates during the day. The nighttime peak of SA and the daytime peak of JA could anticipate the morning attack by biotrophs and peak herbivore activity just before dusk, respectively.
While stomatal closure after the infection during the daytime might be limited to prevent conflict with photosynthetic gas exchange, the light-dependent energy generation may allow plants to invest towards more proactive immune responses. Several A. thaliana genes involved in PTI and basal defense peak and/or are induced more strongly at dawn [13,25,31], when conditions such as high humidity and opened stomata are favorable for pathogen invasion [25,32]. Consequently, when a plant is infiltrated directly with bacteria, bypassing the stomata, it shows the highest resistance at dawn [25,31].
Certain pathogens have evolved specific proteins, called effectors, which are delivered into plant cells to overcome PTI and enhance virulence. As a counter act, plants express intracellular nucleotide-binding and leucine-rich repeat (NB-LRR) immune receptors to detect specific pathogen effectors or their activities to induce effector-triggered immunity (ETI) [33]. ETI is a more drastic defense response than PTI, normally involving programmed cell death (PCD) of the infected tissue. The immune receptor gene, RECOGNITION OF PERONOSPORA PARASITICA 4 (RPP4), which recognizes an effector in the oomycete pathogen Hyaloperonospora arabidopsidis (Hpa) Emwa1, is a target of the core clock component CCA1, and peaks in the morning, when the likelihood of Hpa infection is the highest [32]. This provides a direct genetic link between the circadian clock and defense.
Another direct clock target is the ISOCHORISMATE SYNTHASE 1 (ICS1) gene, which encodes a key enzyme in the defense hormone SA biosynthesis, and whose expression is driven by the evening-phased clock transcription factor CCA1 HIKING EXPEDITION (CHE) [34]. Both ICS1 gene expression and the concentration of SA peak in the middle of the night as a result of this regulation, possibly to anticipate infection in the morning. SA is not only required for local defense against biotrophs, but also serves as a necessary and sufficient signal for inducing the broad-spectrum systemic acquired resistance (SAR) in the distal tissue [33]. Notably, the che mutant has compromised SAR, but intact local defense, suggesting that oscillation of SA might be involved in gating SAR because “immunizing” the entire plants against future infection is an extra energy expenditure that should only be “budgeted” when plants have excess to spare [34]. Altogether, the circadian clock primes costly defense responses (e.g., expression of immune genes) towards the morning, when stomata must be opened to facilitate photosynthesis, coinciding with the infection window for some biotrophic pathogens (Fig. 1).
In contrast to SA, JA, the defense hormone against necrotrophs and herbivores, peaks in the middle of the day [35]. It is proposed that JA oscillation anticipates the activities of herbivores, such as Trichoplusia ni caterpillars, which peak just before dusk [35]. When such anticipation is disrupted by entraining plants’ circadian clocks out of phase with caterpillar activities, more tissue loss to herbivory is observed. Similarly, clock-deficient plants have been shown to be preferred by the caterpillars over wild type plants [35,36]. In addition to JA levels, the JA signaling pathway is also influenced by the clock. The clock component TIC facilitates proteasome-mediated degradation of MYC2, a key component in the JA-mediated defense against herbivores [37], through the night, while additionally priming the expression of the JA receptor gene CORONATINE INSENSITIVE 1 (COI1) towards dawn [38]. Altogether, the strongest accumulation of MYC2 happens during the daytime [38], which might help combat the higher herbivore activity during the day [35]. The day time JA peak does not seem to coincide with resistance against the necrotrophic fungus Botrytis cinerea, which is the strongest when the pathogen is inoculated at dawn [39,40]. This discrepancy was explained by the approximate 12-h delay required for the germination of B. cinerea conidiospores and the formation of infectious hyphae [40]. Based on these observations, it is reasonable to speculate that the temporal separation of SA and JA oscillations by the circadian clock allows plants to prepare defense for a specific type of the attacker while avoiding potential antagonism between SA and JA [41].
3. How to defend: the role of redox
While the plant circadian clock anticipates imminent invasion by various attackers [3,4], redox signaling is used to choose and execute the appropriate defense for the pathogen at hand [3,16] (Fig. 2). Immune responses are efficiently triggered only when both ROS and RNS signaling are simultaneously activated, and the sources of ROS/RNS often determine the nature of the defense response. Upon pathogen challenge, superoxide radical produced from the NADPH oxidase RESPIRATORY BURST OXIDASE HOMOLOGUE D (RBOHD) facilitates A. thaliana stomatal closure during PTI [42,43], while another NADPH oxidase, RBOHF, is required for full accumulation of SA [44]. Additionally, ROS production by the apoplastic peroxidases PRX33 and PRX34 are needed specifically for the induction of SA-dependent defense genes [45–48]. Meanwhile, production of nitric oxide through NO synthase-like activity is required for both stomatal closure [27] and the induction of defense genes [49,50].
Fig. 2.
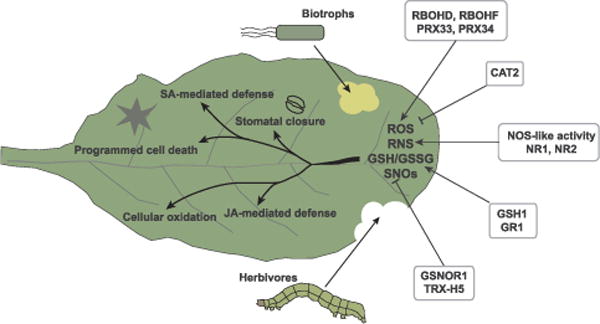
Cellular redox and ROS/RNS regulate plant immunity and balance potentially conflicting immune strategies against biotrophs versus herbivores/necrotrophs. Plants produce through NADPH oxidases RBOHD and RBOHF and apoplastic peroxidases PRX33 and PRX34 in response to pathogen challenge. Cellular ROS is scavenged by catalases, among CAT2 plays a significant role in regulating defense. RNS are produced through NOS-like activity or by nitrate reductases NR1 and NR2. The balance between oxidized (GSH) and (GSSG) forms of glutathione depends on glutathione reductase GR1 and γ-glutamylcysteine synthetase GSH1 activity. Cellular S-nitrosothiols are recycled partly by thioredoxin and are also affected by S-nitrosoglutathione (GSNO), which is recycled by the GSNO reductase, GSNOR1.
The interplay between ROS and RNS is more intricate during ETI when plants are forced to take the drastic measure of killing off the infected cells to contain the pathogen. Cell death is triggered when the NO/H2O2 ratio is between 0.25 and 2 [15], with both NO synthase-like activity [51] and nitrate reductases NR1 and NR2 [52] contributing to the generation of NO during ETI. The role of ROS signaling during ETI is complex. On one hand, RBOHD and RBOHF are required for the ROS production and cell death during ETI, respectively [53]. On the other hand, when rbohD and rbohF mutants were crossed with the lesion simulation disease 1 (lsd1) mutant, which exhibits superoxide-dependent runaway cell death (RCD) upon SA treatment [54], they exacerbated the RCD phenotype [55]. This implies that in addition to initiating PCD during ETI, the ROS production by RBOHs can also prevent uncontrollable cell death during ETI.
S-nitrosylation, the covalent attachment of the NO moiety to the cysteine thiol group, is an important mediator of NO signaling and forms yet another layer of redox regulation on PCD. S-nitrosoglutathione (GSNO) acts as a mobile donor of SNOs and affects the balance of S-nitrosylation in the cell [56]. Both the GSNO reductase 1 mutant of A. thaliana, gsnor1-3, and the nitrous oxide overexpressor 1 (nox1) mutant, have increased levels of S-nitrosothiols (SNOs) and stronger PCD during ETI [17]. Remarkably, in gsnor1-3 and nox1 mutants, both SA accumulation and NADPH oxidase activity are reduced, the latter of which can be explained by the inhibitory S-nitrosylation of RBOHD. This results in increased susceptibility of the gsnor1-3 and nox1 mutants to infection [57]. Surprisingly, while THIOREDOXIN H-TYPE 5 (TRX-H5) can partially restore immunity in nox1 plants through its denitrosylation activity, it is unable to do so in gsnor1-3 mutants [58]. This indicates that TRX-H5 selectively targets the proteins which were S-nitrosylated due to excessive free NO but not GSNO. The restoration of the PCD in RBOH-deficient mutants by the gsnor1-3 mutation suggests that S-nitrosylation may act as a backup mechanism for ETI even though it negatively affects basal resistance. Supporting this hypothesis, the gsnor1-3 mutation could reestablish the PCD-dependent resistance to Hpa Emwa1 in the salicylic acid induction deficient 2 (sid2) mutant [17].
In addition to their role in defense against biotrophic pathogens, both ROS and RNS are also necessary for defense against necrotrophs and herbivores. The lack of ROS production by apoplastic peroxidases in A. thaliana renders the plants more susceptible to B. cinerea [45], while addition of NO donors induces JA-mediated defense against root knot nematodes in tomato [59]. NO is also produced in response to wounding [60]. However, full induction of JA-signaling by gaseous NO in A. thaliana happens only in the absence of SA. Considering that gaseous NO can also induce SA production, this exclusive induction likely serves as a mechanism to avoid unnecessary simultaneous activation of the opposing pathways [60].
Apart from the ROS/RNS bursts described above, plant immunity is often accompanied by alteration of the cellular redox state, reflected by changes in the ratio of reduced (GSH) and oxidized (GSSG) forms of glutathione, as well as in total glutathione levels. These changes are necessary for execution of the long-term immune response, SAR, which is accompanied by global transcriptional reprogramming mediated by the redox-sensitive transcription regulator NONEXPRESSER OF PATHOGENESIS-RELATED GENES 1 (NPR1) [61]. NPR1 is normally sequestered in the cytoplasm in an oligomer form through disulfide bridges, which are also affected by S-nitrosylation [62]. SA induces glutathione production and increases the GSH/GSSG ratio, reducing NPR1 oligomer primarily via thioredoxin TRX-H5 [62,63]. NPR1 monomer is then translocated into the nucleus [63,64], where it acts as a transcriptional coactivator for the TGA family of transcription factors and induces defense genes [65,66].
In contrast to SA, JA depletes glutathione pools and lowers the GSH/GSSG ratio, shifting the cellular redox towards oxidizing conditions [21]. Unsurprisingly, glutathione plays a vital role in the regulation of the crosstalk between SA and JA. The suppression of JA signaling by SA depends on the changes in glutathione pools and is weakened in the presence of a glutathione synthesis inhibitor [67]. Additionally, the mutation in GLUTATHIONE-DISULFIDE REDUCTASE 1 (GR1) leads to oxidized cellular environment and attenuates SA-mediated defense while affecting JA signaling [68]. The redox-dependent SA-JA antagonism can also be manipulated by pathogens and herbivores to their advantage. Necrotrophic fungus B. cinerea increases SA levels to take advantage of the SA-mediated induction of glutaredoxin (a reducing enzyme which uses glutathione as a cofactor) GRXS13 to repress JA-mediated defense and increase host susceptibility [69]. Spodoptera exigua caterpillars also secrete effectors from salivary glands to induce the SA pathway and prevent oxidation of the cellular redox state necessary for the activation of JA-mediated defense [70]. Remarkably, while glutathione acts as a centerpiece in redox-mediated SA-JA antagonism, a mutation in γ-glutamylcysteine synthetase (GSH1), which catalyzes the first step of glutathione synthesis, results in susceptibility to not only biotrophic pathogens [18,20], but also necrotrophs [18] and herbivores [19]. This underscores the importance of proper redox balance during immune responses.
4. Balancing growth and immunity: combined influence of the clock and redox
Despite being necessary for plant survival, defense induction often carries a great cost. Plant growth and defense are locked in an eternal struggle for resource allocation [1]. Constitutive induction of plant immunity results in dwarfism [71,72], whereas the plant growth hormone auxin suppresses defense [73]. Interestingly, the crosstalk between defense and growth is, at least partially, redox-mediated. The catalase mutant cat2, which is under constitutive oxidative stress, exhibits dwarfism but has increased resistance against biotrophic pathogens [74–76]. It was found that SA directly binds to plant catalases to inhibit their activity [77,78]. In A. thaliana, the inhibition of CAT2 activity by SA leads to an increase in H2O2 and subsequent sulfenylation (oxidation of a thiol -SH into sulfenic acid -SOH) of tryptophan synthetase β subunit 1 (TSB1) [76]. Reduced levels of tryptophan, the auxin precursor, cause reduced growth, but stronger immunity. These findings demonstrate how plant defense hormones could manipulate cellular redox to suppress growth.
The plant circadian clock also plays a central role in mediating growth-defense tradeoff. The clock-mediated stomatal closure at night and the gating of active defense towards the morning are good demonstrations of how the circadian clock prevents unsustainable redirection of energy towards immunity. Additionally, the morning core clock component CCA1 regulates the expression of catalases, including CAT2, to manage ROS homeostasis and possibly also to affect defense through redox changes [79]. Moreover, circadian clock gates the induction of immunity towards morning to prevent conflicts with growth at night through interactions with the metabolic circadian redox rhythm [13]. The circadian redox rhythm was initially discovered as oscillations in NADH and NADPH levels as well as in the oxidation state of ROS scavenger peroxiredoxins in anucleate red blood cells (RBCs), in which transcription and translation activities are considered absent [80]. Unlike the genetic circadian clocks consisting of distinct transcription and translation feedback loops, the active site of peroxiredoxins, responsible for circadian redox rhythms, is conserved in all domains of life, hinting at a possible redox-based origin of circadian clocks [81]. Furthermore, the oscillations of NADH and NADPH in RBCs [80], in particular, hint at a more generic redox rhythm than simple oxidation/reduction cycles of peroxiredoxins. In plants, the circadian redox rhythm (i.e. NADP/H oscillation) is coupled to the genetic clock through the redox-sensitive immune regulator NPR1 [13]. This link is mediated through SA-induced cellular redox changes, which cause nuclear translocation of NPR1 [63]. However, acute perturbation of the redox rhythm by SA does not change the phase or the period of the genetic clock, but rather reinforces it through NPR1-mediated upregulation of both morning (LHY and PSEUDO-RESPONSE REGULATOR 7 (PRR7)) and evening (TIMING OF CAB EXPRESSION 1 (TOC1)) clock genes [13]. Since LHY is a positive regulator of defense genes while TOC1 is a repressor, increases in their expression lead to the gating of defense with higher sensitivity to SA induction in the morning than in the evening [13]. The biological significance of this clock-mediated gating is demonstrated by the severe loss of fresh weight in plants whose immunity is untimely induced by SA in darkness [13]. It has been recently demonstrated that an aqueous environment is essential for pathogen virulence [82]. It is also known that SA treatment results in repression of several aquaporin genes, possibly as a defense mechanism [13,83]. If this repression on water transport activities occurs at night, it interferes with growth-related activities. Therefore, it can be argued that one of the benefits of clock-mediated gating of the SA immune response towards the morning is to avoid conflict with water transport activities required for growth that occurs at night.
5. Future directions
In this review, we present how plants utilize the robustness of the circadian clock to plan and the sensitivity of redox signaling to execute an appropriate defense response while minimizing the cost of this response. It should be noted, however, that there is a daily rhythmic accumulation of ROS in plants, accompanied by the circadian expression of ROS-dependent genes [79]. Because many of these genes, such as catalases and NADPH oxidases, are also involved in plant defense, redox might add an extra layer of regulation to the clock control of plant defense[3,4]. However, ROS-dependent genes which regulate immunity are also often directly induced in the presence of the pathogen, as demonstrated by the specific spatiotemporal regulation of RBOHD and RBOHF expression during plant defense [84]. Thus, it is likely that circadian expression of these redox genes does not serve to anticipate pathogens, but rather is tied to the rhythmic metabolic ROS production as shown in [79]. It is also unknown which circadian-clock-dependent redox genes are responsible for setting the circadian redox rhythm, especially considering that peroxiredoxin oscillations persist in clock-impaired TOC1-overexpressing (TOC1-OX) plants [81]. Thus, the source of the circadian redox rhythm (NADP/H oscillation) remains unclear at least in plants.
While we understand that acute perturbation of the redox rhythm during SA-mediated immune induction reinforces the genetic clock to gate the immune response, not much is known about the role of the redox rhythm itself. Levels of monomeric NPR1 protein peak at the same time as NADPH [13], implying that the redox rhythm might periodically reduce NPR1. Nevertheless, the cause of the NPR1 monomer oscillations has not been unambiguously identified yet. Notably, SA levels also peak in phase with the NPR1 monomer, as a result of regulation by the clock component CHE [34]. Thus, it is possible that SA might influence oscillations of NPR1 monomer by affecting cellular redox [13,63]. Another possibility could be the stabilization of nuclear NPR1 at night by higher levels of SA [85]. Furthermore, the activity of CHE protein itself might be regulated by the cellular redox [86], closing the feedback loop between SA, circadian redox rhythm, NPR1 and the genetic circadian clock. A thorough investigation of the role of the immune hormone SA as a connecting signal between redox and the circadian clock might reveal the significance of having the ancient redox rhythm along with the comparatively modern genetic clock.
Similar to the interplay between the genetic circadian clock and the redox rhythm discussed above, integration of multiple ROS/RNS signals is required for efficient plant defense. This complexity allows the plant to select the appropriate defense strategy based on the source of ROS/RNS, preventing conflicting immune responses such as concurrent defense against herbivores and biotrophic pathogens. Furthermore, because ROS/RNS are required for defense against both biotrophs [27,42,48,50] and necrotrophs/herbivores [45,59], they could also play a significant role in limiting the SA-JA antagonism, which is often exploited by attackers [69,70]. For example, the cat2 mutation increases defense against biotrophs but decreases resistance to necrotrophs by suppressing JA biosynthesis [76]. In contrast, plants without apoplastic peroxidase activity are unable to mount efficient defense against both types of pathogens [45], implying that the source of ROS/RNS also affects how the plants balance SA- and JA-dependent defenses. A possible strategy to prevent excessive antagonism between these two essential pathways could be a preemptive partial activation of the opposing pathway, recently demonstrated by the noncanonical activation of JA signaling through SA receptors [87]. Interestingly, gaseous NO does induce elements of both JA and SA signaling pathways [60]. Further dissection of these clues may give us better understanding of a plant’s struggle to equipoise contrasting defense strategies.
Acknowledgments
This work was supported by the National Institutes of Health [grant R35-GM118036-02] and by the Howard Hughes Medical Institute. We thank Dr. Paul J. Zwack and Sophia Zebell for commenting on the manuscript.
Abbreviations
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- RNS
reactive nitrogen species
- NO
nitric oxide
- GSH1
γ-glutamylcysteine synthetase
- SA
salicylic acid
- JA
jasmonic acid
- PAMP
pathogen-associated molecular pattern
- PTI
pattern-triggered immunity
- CCA1
CIRCADIAN CLOCK ASSOCIATED 1
- LHY
LATE ELONGATED HYPOCOTYL
- GRP7
GLYCINE-RICH RNA-BINDING PROTEIN 7
- CCR2
COLD AND CIRCADIAN REGULATED 2
- TIC
TIME FOR COFFEE
- NB-LRR
nucleotide-binding and leucine-rich repeat
- ETI
effector-triggered immunity
- PCD
programmed cell death
- Hpa
Hyaloperonospora arabidopsidis
- RPP4
RECOGNITION OF PERONOSPORA PARASITICA 4
- ICS1
ISOCHORISMATE SYNTHASE 1
- CHE
CCA1 HIKING EXPEDITION
- SAR
systemic acquired resistance
- COI1
CORONATINE INSENSITIVE 1
- NAD
nicotinamide dinucleotide
- NADP
nicotinamide dinucleotide phosphate
- RBOHD
RESPIRATORY BURST OXIDASE HOMOLOGUE D
- RBOHF
RESPIRATORY BURST OXIDASE HOMOLOGUE F
- PRX33
PEROXIDASE 33
- PRX34
PEROXIDASE 34
- NR1
NITRATE REDUCTASE 1
- NR2
NITRATE REDUCTASE 2
- nox1
nitrous oxide overexpressor 1
- lsd1
lesion simulation disease 1
- RCD
runaway cell death
- GSNO
S-ni-trosoglutathione
- GSNOR1
GSNO reductase 1
- GSH
glutathione
- GSSG
glutathione disulfide
- SNO
S-nitrosothiol
- TRX-H5
THIOREDOXIN H-TYPE 5
- sid2
salicylic acid induction deficient 2
- NPR1
NONEXPRESSER OF PATHOGENESIS-RELATED GENES 1
- GRXS13
GLUTAREDOXIN 13
- GR1
GLUTATHIONE-DISULFIDE REDUCTASE 1
- CAT2
CATALASE 2
- TSB1
tryptophan synthetase β subunit 1
- PRR7
PSEUDO-RESPONSE REGULATOR 7
- TOC1
TIMING OF CAB EXPRESSION 1
- TOC1-OX
TOC1-overexpressing
References
- 1.Huot B, Yao J, Montgomery BL, He SY. Growth–defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant. 2014;7(8):1267–1287. doi: 10.1093/mp/ssu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spoel SH, Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe. 2008;3(6):348–351. doi: 10.1016/j.chom.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Spoel SH, van Ooijen G. Circadian redox signaling in plant immunity and abiotic stress. Antioxid Redox Signal. 2014;20(18):3024–3039. doi: 10.1089/ars.2013.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu H, McClung CR, Zhang C. Tick tock: circadian regulation of plant innate immunity. Annu Rev Phytopathol. 2017;55:287–311. doi: 10.1146/annurev-phyto-080516-035451. [DOI] [PubMed] [Google Scholar]
- 5.Reddy AB, Rey G. Metabolic and nontranscriptional circadian clocks: eukaryotes. Annu Rev Biochem. 2014;83(1):165–189. doi: 10.1146/annurev-biochem-060713-035623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West AC, Bechtold DA. The cost of circadian desynchrony: evidence, insights and open questions. BioEssays. 2015;37(7):777–788. doi: 10.1002/bies.201400173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355 doi: 10.1136/bmj.i5210. [DOI] [PubMed] [Google Scholar]
- 8.Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410(6832):1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- 9.Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309(5734):630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- 10.Greenham K, McClung CR. Integrating circadian dynamics with physiological processes in plants. Nat Rev Genet. 2015;16:598. doi: 10.1038/nrg3976. [DOI] [PubMed] [Google Scholar]
- 11.Michael TP, Mockler TC, Breton G, McEntee C, Byer A, Trout JD, Hazen SP, Shen R, Priest HD, Sullivan CM, Givan SA, Yanovsky M, Hong F, Kay SA, Chory J. Network discovery pipeline elucidates conserved time-of-day–specific cis-regulatory modules. PLoS Genet. 2008;4(2):e14. doi: 10.1371/journal.pgen.0040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graf A, Schlereth A, Stitt M, Smith AM. Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci. 2010;107(20):9458–9463. doi: 10.1073/pnas.0914299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou M, Wang W, Karapetyan S, Mwimba M, Marques J, Buchler NE, Dong X. Redox rhythm reinforces the circadian clock to gate immune response. Nature. 2015;523(7561):472–476. doi: 10.1038/nature14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance respons0. Cell. 1994;79(4):583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- 15.Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci. 2001;98(23):13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frederickson Matika DE, Loake GJ. Redox regulation in plant immune function. Antioxid Redox Signal. 2014;21(9):1373–1388. doi: 10.1089/ars.2013.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun B-W, Feechan A, Yin M, Saidi NBB, LeBihan T, Yu M, Moore JW, Kang J-G, Kwon E, Spoel SH, Pallas JA, Loake GJ. S-nitrosylation of NADPH oxidase regulates cell death in plant immunity. Nature. 2011:264–268. doi: 10.1038/nature10427. [DOI] [PubMed] [Google Scholar]
- 18.Parisy V, Poinssot B, Owsianowski L, Buchala A, Glazebrook J, Mauch F. Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 2007;49(1):159–172. doi: 10.1111/j.1365-313X.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- 19.Schlaeppi K, Bodenhausen N, Buchala A, Mauch F, Reymond P. The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis. Plant J. 2008;55(5):774–786. doi: 10.1111/j.1365-313X.2008.03545.x. [DOI] [PubMed] [Google Scholar]
- 20.Dubreuil-Maurizi C, Vitecek J, Marty L, Branciard L, Frettinger P, Wendehenne D, Meyer AJ, Mauch F, Poinssot B. Glutathione deficiency of the Arabidopsis mutant pad2-1 affects oxidative stress-related events, defense gene expression, and the hypersensitive response. Plant Physiol. 2011;157(4):2000–2012. doi: 10.1104/pp.111.182667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spoel SH, Loake GJ. Redox-based protein modifications: the missing link in plant immune signalling. Curr Opin Plant Biol. 2011;14(4):358–364. doi: 10.1016/j.pbi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Legnaioli T, Cuevas J, Mas P. TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. EMBO J. 2009;28(23):3745–3757. doi: 10.1038/emboj.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb AAR. The physiology of circadian rhythms in plants. New Phytol. 2003;160(2):281–303. doi: 10.1046/j.1469-8137.2003.00895.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Xie QG, Anderson RG, Ng GN, Seitz NC, Peterson T, McClung CR, McDowell JM, Kong DD, Kwak JM, Lu H. Crosstalk between the circadian clock and innate immunity in arabidopsis. PLoS Pathog. 2013;9(6) doi: 10.1371/journal.ppat.1003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korneli C, Danisman S, Staiger D. Differential control of pre-invasive and post-invasive antibacterial defense by the Arabidopsis circadian clock. Plant Cell Physiol. 2014;55(9):1613–1622. doi: 10.1093/pcp/pcu092. [DOI] [PubMed] [Google Scholar]
- 26.Bigeard J, Colcombet J, Hirt H. Signaling mechanisms in Pattern-Triggered Immunity (PTI) Mol Plant. 2015;8(4):521–539. doi: 10.1016/j.molp.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126(5):969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 28.Heintzen C, Nater M, Apel K, Staiger D. AtGRP7, a nuclear RNA-binding protein as a component of a circadian-regulated negative feedback loop in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94(16):8515–8520. doi: 10.1073/pnas.94.16.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicaise V, Joe A, Jeong Br, Korneli C, Boutrot F, Westedt I, Staiger D, Alfano JR, Zipfel C. Pseudomonas HopU1 modulates plant immune receptor levels by blocking the interaction of their mRNAs with GRP7. EMBO J. 2013;32(5):701–712. doi: 10.1038/emboj.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panchal S, Roy D, Chitrakar R, Price L, Breitbach ZS, Armstrong DW, Melotto M. Coronatine facilitates pseudomonas syringae infection of Arabidopsis leaves at night. Front Plant Sci. 2016;7:880. doi: 10.3389/fpls.2016.00880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhardwaj V, Meier S, Petersen LN, Ingle RA, Roden LC. Defence responses of Arabidopsis thaliana to infection by pseudomonas syringae are regulated by the circadian clock. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Barnaby JY, Tada Y, Li H, Tor M, Caldelari D, Lee DU, Fu XD, Dong XN. Timing of plant immune responses by a central circadian regulator. Nature. 2011;470(7332):110–U126. doi: 10.1038/nature09766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spoel SH, Dong X. How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol. 2012;12(2):89–100. doi: 10.1038/nri3141. [DOI] [PubMed] [Google Scholar]
- 34.Zheng XY, Zhou M, Yoo H, Pruneda-Paz JL, Spivey NW, Kay SA, Dong XNA. Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. Proc Natl Acad Sci USA. 2015;112(30):9166–9173. doi: 10.1073/pnas.1511182112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodspeed D, Chehab EW, Min-Venditti A, Braam J, Covington MF. Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc Natl Acad Sci. 2012;109(12):4674–4677. doi: 10.1073/pnas.1116368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodspeed D, Chehab EW, Covington MF, Braam J. Circadian control of jasmonates and salicylates. Plant Signal Behav. 2013;8(2):e23123. doi: 10.4161/psb.23123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kazan K, Manners JM. MYC2: the master in action. Mol Plant. 2013;6(3):686–703. doi: 10.1093/mp/sss128. [DOI] [PubMed] [Google Scholar]
- 38.Shin J, Heidrich K, Sanchez-Villarreal A, Parker JE, Davis SJ. Time for coffee represses accumulation of the MYC2 transcription factor to provide time-of-day regulation of jasmonate signaling in Arabidopsis. Plant Cell. 2012;24(6):2470–2482. doi: 10.1105/tpc.111.095430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hevia MA, Canessa P, Müller-Esparza H, Larrondo LF. A circadian oscillator in the fungus Botrytis cinerea regulates virulence when infecting Arabidopsis thaliana. Proc Natl Acad Sci. 2015;112(28):8744–8749. doi: 10.1073/pnas.1508432112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingle RA, Stoker C, Stone W, Adams N, Smith R, Grant M, Carre I, Roden LC, Denby KJ. Jasmonate signalling drives time-of-day differences in susceptibility of Arabidopsis to the fungal pathogen Botrytis cinerea. Plant J. 2015;84(5):937–948. doi: 10.1111/tpj.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- 42.Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones Jonathan D, Shirasu K, Menke F, Jones A, Zipfel C. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol Cell. 2014;54(1):43–55. doi: 10.1016/j.molcel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, Chen S, Zhou JM. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe. 2014;15(3):329–338. doi: 10.1016/j.chom.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Chaouch S, Queval G, Noctor G. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J. 2012;69(4):613–627. doi: 10.1111/j.1365-313X.2011.04816.x. [DOI] [PubMed] [Google Scholar]
- 45.Bindschedler LV, Dewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davies DR, Ausubel FM, Paul Bolwell G. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J. 2006;47(6):851–863. doi: 10.1111/j.1365-313X.2006.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Brien JA, Daudi A, Finch P, Butt VS, Whitelegge JP, Souda P, Ausubel FM, Bolwell GP. A peroxidase-dependent apoplastic oxidative burst in cultured Arabidopsis cells functions in MAMP-elicited defense. Plant Physiol. 2012;158(4):2013–2027. doi: 10.1104/pp.111.190140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daudi A, Cheng Z, O’Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP. The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell. 2012;24(1):275–287. doi: 10.1105/tpc.111.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mammarella ND, Cheng Z, Fu ZQ, Daudi A, Bolwell GP, Dong X, Ausubel FM. Apoplastic peroxidases are required for salicylic acid-mediated defense against Pseudomonas syringae. Phytochemistry. 2015;112:110–121. doi: 10.1016/j.phytochem.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95(17):10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeidler D, Zähringer U, Gerber I, Dubery I, Hartung T, Bors W, Hutzler P, Durner J. Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA. 2004;101(44):15811–15816. doi: 10.1073/pnas.0404536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394(6693):585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- 52.Modolo LV, Augusto O, Almeida IMG, Pinto-Maglio CAF, Oliveira HC, Seligman K, Salgado I. Decreased arginine and nitrite levels in nitrate reductase-deficient Arabidopsis thaliana plants impair nitric oxide synthesis and the hypersensitive response to Pseudomonas syringae. Plant Sci. 2006;171(1):34–40. [Google Scholar]
- 53.Torres MA, Dangl JL, Jones JDG. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci. 2002;99(1):517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science. 1996;273(5283):1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- 55.Torres MA, Jones JDG, Dangl JL. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet. 2005;37(10):1130–1134. doi: 10.1038/ng1639. [DOI] [PubMed] [Google Scholar]
- 56.Feechan A, Kwon E, Yun BW, Wang Y, Pallas JA, Loake GJ. A central role for S-nitrosothiols in plant disease resistance. Proc Natl Acad Sci. 2005;102(22):8054–8059. doi: 10.1073/pnas.0501456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yun B-W, Skelly MJ, Yin M, Yu M, Mun B-G, Lee S-U, Hussain A, Spoel SH, Loake GJ. Nitric oxide and S-nitrosoglutathione function additively during plant immunity. New Phytol. 2016;211(2):516–526. doi: 10.1111/nph.13903. [DOI] [PubMed] [Google Scholar]
- 58.Kneeshaw S, Gelineau S, Tada Y, Loake Gary J, Spoel Steven H. Selective protein denitrosylation activity of thioredoxin-h5 modulates plant immunity. Mol Cell. 2014;56(1):153–162. doi: 10.1016/j.molcel.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Zhou J, Jia F, Shao S, Zhang H, Li G, Xia X, Zhou Y, Yu J, Shi K. Involvement of nitric oxide in the jasmonate-dependent basal defense against root-knot nematode in tomato plants. Front Plant Sci. 2015;6:193. doi: 10.3389/fpls.2015.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang X, Stettmaier K, Michel C, Hutzler P, Mueller MJ, Durner J. Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta. 2004;218(6):938–946. doi: 10.1007/s00425-003-1178-1. [DOI] [PubMed] [Google Scholar]
- 61.Wang D, Amornsiripanitch N, Dong X. A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2006;2(11):e123. doi: 10.1371/journal.ppat.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science. 2008;321(5891):952–956. doi: 10.1126/science.1156970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mou Z, Fan W, Dong X. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 2003;113(7):935–944. doi: 10.1016/s0092-8674(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 64.Mateo A, Funck D, Mühlenbock P, Kular B, Mullineaux PM, Karpinski S. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J Exp Bot. 2006;57(8):1795–1807. doi: 10.1093/jxb/erj196. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Fan W, Kinkema M, Li X, Dong X. Interaction of NPR1 with basic leucine zipper protein transcription factors that bind sequences required for salicylic acid induction of the PR-1 gene. Proc Natl Acad Sci. 1999;96(11):6523–6528. doi: 10.1073/pnas.96.11.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou JM, Trifa Y, Silva H, Pontier D, Lam E, Shah J, Klessig DF. NPR1 differentially interacts with members of the TGA/OBF family of transcription factors that bind an element of the PR-1 Gene required for induction by salicylic acid. Mol Plant-Microbe Interact. 2000;13(2):191–202. doi: 10.1094/MPMI.2000.13.2.191. [DOI] [PubMed] [Google Scholar]
- 67.Koornneef A, Leon-Reyes A, Ritsema T, Verhage A, Den Otter FC, Van Loon LC, Pieterse CMJ. Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiol. 2008;147(3):1358–1368. doi: 10.1104/pp.108.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, Saindrenan P, Gouia H, Issakidis-Bourguet E, Renou JP, Noctor G. Arabidopsis GLUTATHIONE REDUCTASE1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol. 2010;153(3):1144–1160. doi: 10.1104/pp.110.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Camera S La, L’Haridon F, Astier J, Zander M, Abou-Mansour E, Page G, Thurow C, Wendehenne D, Gatz C, Métraux J-P, Lamotte O. The glutaredoxin ATGRXS13 is required to facilitate Botrytis cinerea infection of Arabidopsis thaliana plants. Plant J. 2011;68(3):507–519. doi: 10.1111/j.1365-313X.2011.04706.x. [DOI] [PubMed] [Google Scholar]
- 70.Paudel J, Copley T, Amirizian A, Prado A, Bede JC. Arabidopsis redox status in response to caterpillar herbivory. Front Plant Sci. 2013;4:113. doi: 10.3389/fpls.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell. 1997;9(9):1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y, Goritschnig S, Dong X, Li X. A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell. 2003;15(11):2636–2646. doi: 10.1105/tpc.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Z, Agnew JL, Cohen JD, He P, Shan L, Sheen J, Kunkel BN. Pseudomonas syringae type III effector AvrRpt2 alters Arabidopsis thaliana auxin physiology. Proc Natl Acad Sci. 2007;104(50):20131–20136. doi: 10.1073/pnas.0704901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 2007;52(4):640–657. doi: 10.1111/j.1365-313X.2007.03263.x. [DOI] [PubMed] [Google Scholar]
- 75.Chaouch S, Queval G, Vanderauwera S, Mhamdi A, Vandorpe M, Langlois-Meurinne M, Van Breusegem F, Saindrenan P, Noctor G. Peroxisomal Hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE1 in a daylength-related manner. Plant Physiol. 2010;153(4):1692–1705. doi: 10.1104/pp.110.153957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yuan HM, Liu WC, Lu YT. CATALASE2 coordinates SA-mediated repression of both auxin accumulation and ja biosynthesis in plant defenses. Cell Host Microbe. 2017;21(2):143–155. doi: 10.1016/j.chom.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 77.Conrath U, Chen Z, Ricigliano JR, Klessig DF. Two inducers of plant defense responses, 2,6-dichloroisonicotinec acid and salicylic acid, inhibit catalase activity in tobacco. Proc Natl Acad Sci USA. 1995;92(16):7143–7147. doi: 10.1073/pnas.92.16.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Z, Silva H, Klessig DF. Active oxygen species in the induction of plant systemic acquired resistance by salicylic acid. Science. 1993;262(5141):1883–1886. doi: 10.1126/science.8266079. [DOI] [PubMed] [Google Scholar]
- 79.Lai AG, Doherty CJ, Mueller-Roeber B, Kay SA, Schippers JHM, Dijkwel PP. CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc Natl Acad Sci USA. 2012;109(42):17129–17134. doi: 10.1073/pnas.1209148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469(7331):498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edgar RS, Green EW, Zhao Y, Ooijen G van, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, Maywood ES, Hastings MH, Baliga NS, Merrow M, Millar AJ, Johnson CH, Kyriacou CP, O’Neill JS, Reddy AB. Peroxiredoxins are conserved markers of circadian rhythms. Nature. 2012;485(7399):459–464. doi: 10.1038/nature11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xin XF, Nomura K, Aung K, Velásquez AC, Yao J, Boutrot F, Chang JH, Zipfel C, He SY. Bacteria establish an aqueous living space in plants crucial for virulence. Nature. 2016;539(7630):524–529. doi: 10.1038/nature20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pajerowska-Mukhtar Karolina M, Wang W, Tada Y, Oka N, Tucker Chandra L, Fonseca Jose P, Dong X. The HSF-like transcription Factor TBF1 is a major molecular switch for plant growth-to-defense transition. Curr Biol. 2012;22(2):103–112. doi: 10.1016/j.cub.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morales J, Kadota Y, Zipfel C, Molina A, Torres MA. The Arabidopsis NADPH oxidases RbohD and RbohF display differential expression patterns and contributions during plant immunity. J Exp Bot. 2016;67(6):1663–1676. doi: 10.1093/jxb/erv558. [DOI] [PubMed] [Google Scholar]
- 85.Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, Dong X. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature. 2012;486(7402):228–232. doi: 10.1038/nature11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Viola IL, Güttlein LN, Gonzalez DH. Redox modulation of plant developmental regulators from the class I TCP transcription factor family. Plant Physiol. 2013;162(3):1434–1447. doi: 10.1104/pp.113.216416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu L, Sonbol FM, Huot B, Gu Y, Withers J, Mwimba M, Yao J, He SY, Dong X. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat Commun. 2016;7:13099. doi: 10.1038/ncomms13099. [DOI] [PMC free article] [PubMed] [Google Scholar]


