Abstract
Background
Glucose-6-phosphate isomerase and collagen type II antibody induced arthritis models (K/BxN and CAIA, respectively) have an inflammatory and a post-inflammatory phase. Both phases display robust tactile allodynia. In previous work, inflammatory phase allodynia was reversed by gabapentin and ketorolac, whereas in late phase only gabapentin was effective. Here we sought to determine if the effects of these two drugs during the early and late phases of the two arthritis models were observed in the conditioned place preference (CPP) paradigm, indicating a differential drug effect on the aversive state.
Methods
Male C57BL/6 mice received K/BxN serum intraperitoneally, while male BALB/c mice received collagen type II antibody cocktail intravenously. After onset of inflammation and allodynia, we assessed effects of i.p. gabapentin (100 mg/kg) or ketorolac (15 mg/kg) using a CPP paradigm: 2 days adaptation, 2 days conditioning (vehicle in morning and drug in afternoon), preference testing on day 5.
Results
Consistent with the effects upon allodynia, both gabapentin and ketorolac produced a preference for the drug-paired compartment in the early phase of the K/BxN model, while gabapentin, but not ketorolac, resulted in a place preference during late phase. In the CAIA model, consistent with differential effects upon allodynia, gabapentin produced a preference in the early phase and a trend in the late phase, whereas ketorolac was ineffective at either time.
Conclusions
CPP validated the aversive state in the inflammatory and post-inflammatory phases of the K/BxN and CAIA arthritis models and correspondence between the anti-hyperpathic pharmacology as defined by thresholds and CPP.
Keywords: Conditioned place preference (CPP), gabapentin, ketorolac, arthritis, tactile allodynia, KBxN, CAIA
Introduction
Rheumatoid arthritis is a chronic autoimmune disease displaying synovial inflammation, matrix destruction, and joint pain (Sokka et al., 2007). The collagen antibody-induced arthritis (CAIA) model (Bas et al., 2012) and the K/BxN serum transfer model (Christianson et al., 2010) display joint inflammation that lasts for several weeks, followed by resolution of the inflammation. In both models, tactile allodynia develops with the onset of inflammation and persists long after inflammation has resolved. Of note, in the KBxN model, ketorolac and diclofenac (cyclo-oxygenase inhibitors), as well as gabapentin (binding to the α2∂ subunit of the voltage gated calcium channel), reversed allodynia during the inflammatory phase, but only gabapentin reversed allodynia during the post-inflammatory phase (Bas et al., 2012; Christianson et al., 2010). These findings indicate that pain signaling manifested by changes in mechanical thresholds in the early and late phases of both models are driven by different mechanisms.
The presumed aversive nature of the early and late (post-inflammatory) phases is predicated on the hypothesis that paw withdrawal reflects escape from an aversive state evoked by the low intensity tactile stimulus (Bas et al., 2012; Christianson et al., 2010; Inglis et al., 2007). Accordingly, simple relief of that ongoing state would be considered to possess a positive reinforcing component, which would support behaviors generating that relief. This positive reinforcing component may be characterized in rodents by using a conditioned place preference (CPP) paradigm. This assay is based on the assumption that if the animal is in a painful state and given an analgesic drug in a particular environment to alleviate the pain, it will associate the pain-relieving effect with that environment and later demonstrate a preference for the same particular environment without drug administration (King et al., 2011; Park et al., 2013; Qu et al., 2011; Sufka, 1994; Sufka and Roach, 1996; Wei et al., 2013). We sought to determine if, in accordance with the differential effects of gabapentin and ketorolac on the tactile allodynia observed in the early and late phases of the K/BxN persistent arthritis models, comparable distinctions would be observed supporting CPP in both phases of the K/BxN and CAIA models. Previous work shows that neither ketorolac nor gabapentin will support a CPP in a naïve animal (Park et al., 2013). Accordingly, we hypothesized that i) in the early phase both gabapentin and ketorolac will reverse tactile allodynia and support a CPP and ii) in the late phase only gabapentin would reverse the allodynia and support a CPP. In the present studies, in the K/BxN model gabapentin indeed blocked early and late phase allodynia and supported CPP in both phases. In contrast, ketorolac reversed the allodynia in the early but not late phase, and supported the CPP only in the early phase. Unexpectedly, early phase CAIA allodynia was unaltered by ketorolac and correspondingly failed to support a CPP, while gabapentin induced CPP only in the late phase. These observations support the aversive nature of the early and late phase CAIA and K/BxN arthritic state and emphasize their associated pharmacology.
Methods
1. Animals
All experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee at the University of California, San Diego. Male C57BL/6 and BALB/c mice (25-30 g) were used in these studies. The mice were housed in plastic cages with wood chip bedding in a temperature-controlled (~23°C) room and kept on a 12-h light/dark cycle with access to food and water ad libitum.
2. Induction of collagen antibody-induced arthritis and K/BxN serum arthritis
CAIA was induced in BALB/c mice by intravenous injection of a cocktail containing five monoclonal CII antibodies (1 mg in 100 μl PBS, Chondrex Inc., Redmond, WA, USA) on day 0 and followed by lipopolysaccharide (10 μg in 20 μl PBS i.p.) on day 5 (Bas et al., 2012; Su et al., 2015, in press). For induction of K/BxN serum arthritis, C57BL/6 mice were injected intraperitoneally (i.p.) with K/BxN serum (100 μl) on day 0 and day 2 (Christianson et al., 2010). The development of arthritis was evaluated by a semi-quantitative visual scoring using a scale of 0–4 per each paw (Choe et al., 2003). One point was given if swelling was observed for i) the ankle, ii) the footpad, iii) the knuckles, and/or iv) any or all of the digits, yielding a maximum score of 4 per paw and 16 per mouse.
3. Conditioned place preference (CPP)
Using a modification of the method (Okun et al., 2012) we tested CPP for gabapentin and ketorolac in mice subjected to collagen antibody-induced arthritis and K/BxN transfer.
Briefly, the system consisted of four separate locally constructed units. Each unit consisted of three adjoining clear Plexiglas © compartments. Each compartment measured 90x90x165 mm, with the middle compartment separated from the other two by walls with removable entries. The middle compartment was a neutral chamber, while the other two compartments were distinctly different in terms of wall pattern (diagonal black stripes vs. black squares with total light admitted into each chamber being identical) and removable flooring with one of two textures (granular vs. grid). The time the animal spent in each chamber was monitored by recording the disruption of three LED light paths crossing the entryway and space of each of the two non-neutral chambers. The output from these LED’s was collected by a PC running a Labview© based data collection program. The testing paradigm occurred over five days. During the first two “adaptation” days (days 1 and 2) mice were placed in the middle chamber and allowed to freely move between the three chambers for 30 minutes. The time spent in each chamber was recorded. On the mornings of the following two “conditioning” days (days 3 and 4), 10 minutes after vehicle injection, mice were placed in one of two outer chambers for 30 minutes. In the afternoons they received drug treatments and 10 minutes later they were restricted to the other outer chamber for 30 minutes. On the “test” day (day 5), the mice were placed in the middle chamber and had free access to all three compartments for 30 minutes and the time spent in each chamber was recorded. To define the drug effect, the average time spent in the drug-paired chamber during the two adaptation days was subtracted from the time spent in the same chamber on the test day. Assignment of the chambers to drug- or vehicle-paired compartments was counter-balanced. Mice were subjected to the CPP test twice, once during the early phase and once in the late phase of both models. In each case, they were randomly assigned to receive the α2δ binding molecule i.p. gabapentin (100 mg/kg), the NSAID ketorolac (15 mg/kg), or saline (vehicle), with the only condition being that the animal did not receive the same treatment twice. As will be noted, examination of the data revealed no carryover between preferences established in the first and second exposure (separated by 18 days).
4. Assessment of mechanical hypersensitivity
All behavioral tests were conducted at fixed times between 9:00 a.m. and 5:00 p.m. The thresholds for mechanical allodynia were measured with von Frey filaments (Semmes-Weinstein von Frey aesthesiometer, Stoelting Co., Wood Dale, IL, USA), ranging from 2.44 to 4.31 (0.03-2.00 g). The mice were positioned in Plexiglas © enclosures on top of a wire mesh surface and habituated to the environment for 2 hours prior to testing. Each filament was applied perpendicularly on the mid-hind paw and the testing was performed according to the Dixon up-down method (Dixon, 1980). A cut-off value of 2 g was determined. The 50% probability of withdrawal threshold was calculated as previously described (Chaplan et al., 1994) and expressed in grams. Withdrawal thresholds for both hind paws were determined and averaged.
To assess the analgesic effects of gabapentin and ketorolac, once during the early phase (CAIA: day 17, K/BxN: day 6) and in the late phase (CAIA: day 46, K/BxN: day 24), mice were randomly assigned to receive i.p. gabapentin (100 mg/kg), ketorolac (15 mg/kg) or saline (vehicle) and were tested for mechanical hypersensitivity prior to 30, 60 and 180 minutes after drug injection in the CAIA model, and prior to and 45 minutes after drug injection in the K/BxN model.
5. Statistical analysis
Results are expressed as mean ± standard error (SE). Statistical analysis was performed using GraphPad Prism (version 5.0, GraphPad Software, San Diego, CA, USA). All data were analyzed using one-way ANOVA followed by either Bonferroni or Newman Keuls post-hoc test. A P value of < 0.05 was considered significant.
Results
1. CII antibodies and K/BxN serum produce significant clinical signs of arthritis and mechanical hypersensitivity
Injection of CII antibodies and K/BxN serum led to the development of clinical signs of arthritis and pronounced mechanical hypersensitivity (Bas et al., 2012; Christianson et al., 2010). The duration of the joint inflammation was different in the two models. Intravenous CII antibodies induced joint inflammation with digital redness and swelling that was detectable on day 3, peaked around day 25, and was still present at the end of the study, day 47 (Fig. 1a). In contrast, in the K/BxN model joint inflammation was transient with increased arthritis scores from day 2 through day 24. The joint inflammation was completely resolved by day 28 (Fig. 1c). Significant mechanical hypersensitivity was observed from day 5 in the CAIA model (Fig. 1b) and from day 2 in the K/BxN model (Fig. 1d), and this state of hypersensitivity lasted throughout the study (day 47 and day 28, respectively) compared to control mice.
Figure 1.
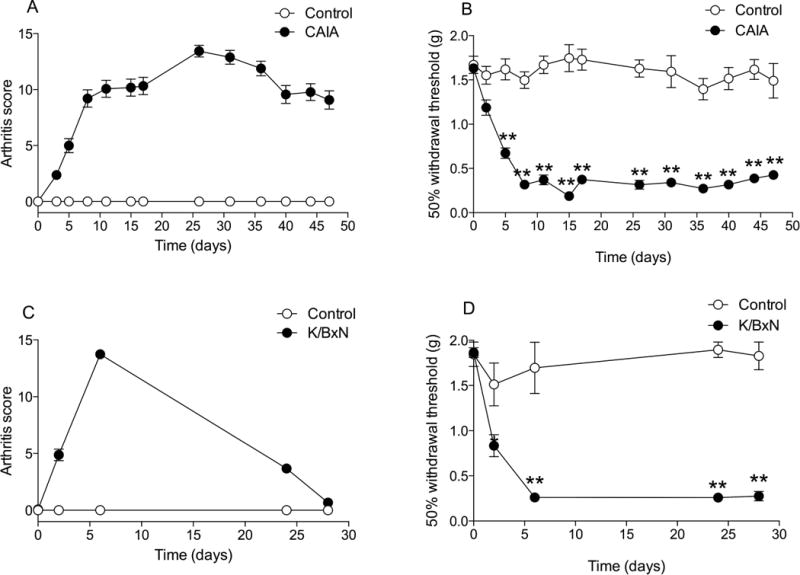
Change of clinical signs after the initiation of A) collagen II antibody cocktail (CAIA) or C) K/BxN serum treatment. Figure presents the tactile threshold plotted vs. time after the initiation of B) CAIA or D) K/BxN serum treatment. Results are presented as mean ± standard error (n = 43 for CAIA group, n = 16 for K/BxN group, n = 4 for both control groups) (*P < 0.001, **P < 0.0001).
2. Conditioned place preference in the CAIA model produced by gabapentin only in the early time point
During the two adaption days, when animals were allowed to move freely between the three chambers, the time spent in the vehicle- or the drug-paired chamber was similar in all three treatment groups (saline, 871 ± 48 sec, n=14; gabapentin, 768 ± 83 sec, n=10; ketorolac, 766 ± 57 sec, n=18), indicating there were no drug pre-treatment preferences for a particular chamber in either of the groups. On the test day of the early phase of the model (day 15), gabapentin (227 ± 80 sec, p<0.05), but not ketorolac (111 ± 94 sec, p>0.05), evoked significant place preference for the drug-paired chamber, compared to the vehicle group (−106 ± 49 sec) (Fig. 2a). In the late phase of CAIA arthritis (day 44), there was no preference during the adaptation days between the saline/drug groups (saline, 771 ± 75 sec, n=8; gabapentin, 757 ± 69 sec, n=9; ketorolac, 771 ± 28 sec, n=10). Although gabapentin animals spent a numerically greater amount of time in the drug treatment chamber (146 ± 78 sec, p>0.05) than the vehicle animals (−87 ± 40 sec), neither gabapentin nor ketorolac (22 ± 103 sec, p>0.05) reached statistical significance in place preference in the late phase of the test day as compared to the vehicle group (Fig. 2b).
Figure 2.
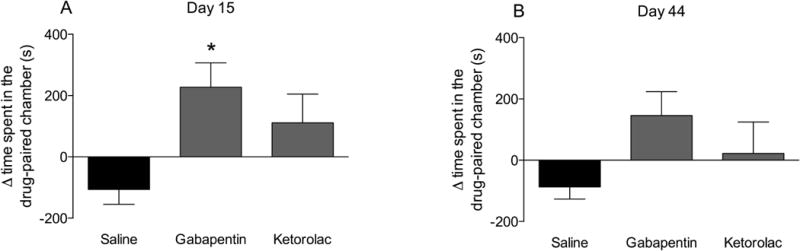
Conditioned place preference in the CAIA mouse carried out during the A) early (day 15) and B) late phase (day 44). Mean time spent in drug paired chamber (day 5) – mean time spent in drug paired chamber on days 1 and 2 in CAIA induced mice that were treated with gabapentin (100 mg/kg, i.p.), ketorolac (15 mg/kg, i.p.) or saline on the drug exposure days (days 3 and 4). Results are presented as mean ± standard error (n = 8-18 per group) (*P < 0.05).
3. Conditioned place preference in the K/BxN model induced by gabapentin in both phases
Times spent in the vehicle- or drug-paired chambers during the two adaptation days when animals were allowed to move freely between the three chambers did not differ across treatment groups (saline group, 776 ± 98 sec, n=4; gabapentin group, 726 ± 113 sec, n=6 and ketorolac group 781 ± 258 sec, n=6, p>0.05). On the test day during the early phase (day 6 of the K/BxN model) the mice previously injected with gabapentin (597 ± 98 sec, p<0.05) or ketorolac (322 ± 51 sec, p<0.05) showed a statistically significant place preference for the drug-paired chamber compared to vehicle (−137 ± 292 sec) (Fig. 3a). In the late phase of the model (day 28) no preference was detected on the adaptation days (saline group, 776 ± 98 sec, n=4; gabapentin group, 726 ± 113 sec, n=6; ketorolac group, 772 ± 153 sec, n=5). Mice previously injected with gabapentin (597 ± 98, p<0.05) but not ketorolac (187 ± 155 sec, p>0.05) or vehicle (137 ± 292 sec, p>0.05) displayed place preference (Fig. 3b).
Figure 3.
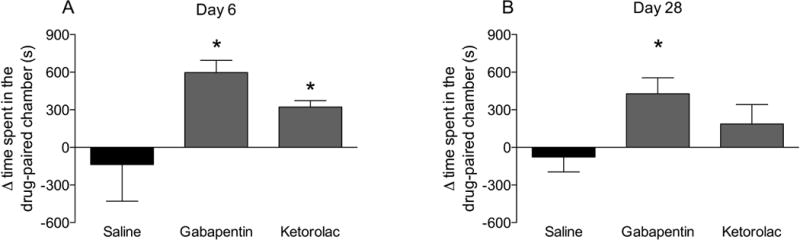
Conditioned place preference in the K/BxN mouse carried out during the A) early (day 6) and B) late phase (day 28). Mean time spent in drug paired chamber (day 5) – mean time spent in drug paired chamber on days 1 and 2 in K/BxN serum transferred mice that were treated with gabapentin (100 mg/kg, i.p.), ketorolac (15 mg/kg, i.p.) or saline on the drug exposure days (days 3 and 4). Results are presented as mean ± standard error (n = 5-7 per group) (*P < 0.05).
4. CAIA-induced mechanical hypersensitivity is reversed by gabapentin, but not ketorolac
To evaluate the effects of gabapentin and ketorolac on mechanical hypersensitivity in the CAIA model, drugs were injected i.p. on days 17 and 46 and changes in mechanical thresholds were recorded over the following 3 hours. On day 17 in the CAIA model when both inflammation and mechanical hypersensitivity was pronounced, systemic injection of gabapentin (n=10), but not ketorolac (n=18) increased the tactile thresholds 30 and 60 min after drug injections compared to the vehicle group (p<0.001; n=10) (Fig. 4a). On day 46, injection of gabapentin reduced the mechanical hypersensitivity 30 and 60 min after injection (p<0.001; n=10), while the injection of ketorolac did not alter mechanical hypersensitivity compared to the saline-injected group (Fig. 4b).
Figure 4.
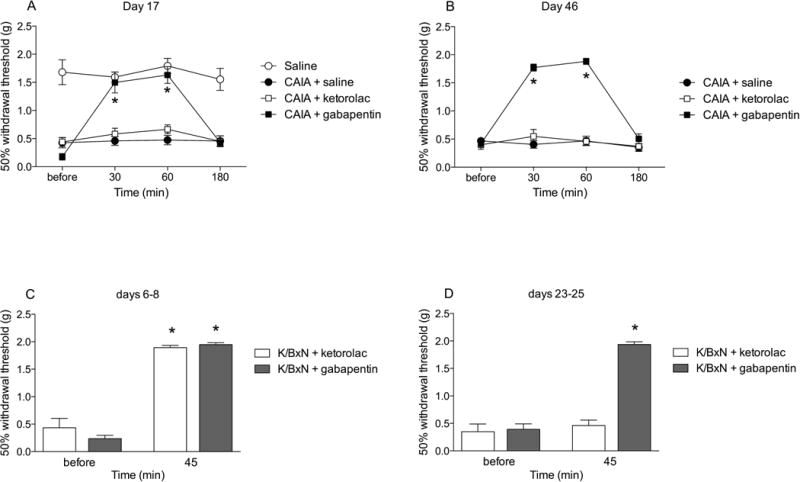
Figure plots the change in withdrawal thresholds assessed in CAIA mice carried out during A) early (day 17) and B) late phase (day 46), as well as C) early (days 6-8) and D) late (days 23-25) phases of the K/BxN model at intervals before and after the injection gabapentin (100 mg/kg, i.p.), ketorolac (15 mg/kg, i.p.) or saline. Results are presented as mean ± standard error (n = 4–18 per group) (*P < 0.0001).
5. Mechanical hypersensitivity induced in the K/BxN model is reversed in both phases by gabapentin, while only in the early phase by ketorolac
In previous work we had shown in the K/BxN model that tactile allodynia in the early phase (days 6-9) was reversed by both gabapentin and ketorolac, while in the late phase (days 22-25) the allodynia was reversed by gabapentin but not ketorolac (Christianson et al., 2010). After showing the reversal effect of gabapentin on mechanical hypersensitivity of the CAIA mice 30 and 60 minutes post-injection, we decided to investigate the effects of gabapentin and ketorolac in the K/BxN mice 45 minutes after injection in both phases (days 6-8 and 23-25). Systemic gabapentin injection reduced mechanical hypersensitivity in both phases (p<0.001; n=8) (Fig. 4c), while ketorolac only showed reversal effects during the early phase (p<0.001; n=8) (Fig. 4d) when compared to mechanical hypersensitivity prior to drug injections.
Discussion and Conclusions
The K/BxN and CAIA mouse models employ antibodies leading to a bilateral inflammatory phase and a post-inflammatory phase. In contrast to models of systemic inflammation, (Freund’s adjuvant), antibodies targeting joint epitopes result in changes in joint morphology and function with relatively little systemic pathology (Bas et al., 2012; Christianson et al., 2010). Not surprisingly, the inflammatory phase is associated with an evident tactile allodynia. Unexpectedly, after resolution of inflammation, allodynia persists. In the original work with the K/BxN and CAIA models our groups showed that the first phase was diminished by the anti-inflammatory agents ketorolac and diclofenac, respectively, and by the centrally acting anti-hyperpathic agent gabapentin, while the post-inflammatory phase was sensitive to gabapentin but not the anti-inflammatory agents (Bas et al., 2012; Christianson et al., 2010). An important caveat to the use of chronic inflammatory models in pain research is the relevance of the evoked threshold assessments to define the “pain” state. Thus, there are arguments about the meaning of such endpoints in the preclinical evaluation of drug efficacy. As an effort to address this question the conditioned place preference paradigm (CPP) has been implemented to define effects of analgesics in ongoing pain states (King et al., 2011; Qu et al., 2011). In the present version of the CPP, there is the assertion that i) the animal is in an aversive state of discomfort; ii) the drug targets that pain state; and iii) the drug has no intrinsic rewarding properties in the normal animal at doses believed to be antinociceptive.
Accordingly, we sought to extend the K/BxN-CAIA analgesic pharmacology to the CPP model to address the hypotheses that drug-induced reversal of the allodynia would be accompanied by development of a CPP, and that the pharmacology of the CPP in the early and late phases would be distinct. The present studies revealed that indeed there is a distinction between early and late phase pharmacology in the allodynia and that this covaries with the ability to establish a CPP. Unexpectedly, we found that the actions of anti-inflammatory agents ketorolac and diclofenac were different in the two models, an observation confirmed by covariance between the effects on thresholds and CPP. In the following sections we will consider issues raised by these findings.
K/BxN-CAIA models
Injection of antibodies against collagen type II or K/BxN serum results in robust joint inflammation that persists for two to three weeks in the K/BxN model and for more than six weeks in the CAIA model. We previously reported that mechanical hypersensitivity develops within two to five days after injection of antibodies/sera in these models and the hypersensitive state remains unchanged long after the inflammation had resolved (Bas et al., 2012; Christianson et al., 2010). Current work shows that BALB/c mice subjected to CAIA developed a more severe degree of disease and the arthritis scores remained relatively high even at day 47, while joint inflammation was completely resolved by day 40 in our previous studies (Agalave et al., 2014; Bas et al., 2012). These periods thus define the choice of the inflammatory (early) and post-inflammatory (late) time points examined here.
Anti-inflammatory drugs
Ketorolac, a mixed cyclo-oxygenase inhibitor, reduces inflammation-evoked hyperalgesia (Inglis et al., 2005; Lopez-Munoz et al., 2004; Rooks et al., 1985). In agreement with our earlier work, ketorolac possessed antiallodynic action in the early inflammatory but not the late phase of the K/BxN model (Christianson et al., 2010).
Unexpectedly, however, ketorolac failed to reverse the mechanical hypersensitivity in both the early and late phases of the CAIA model. The reason for this lack of ketorolac effect during ongoing inflammation in the CAIA model is not known. Previous work with the CAIA model demonstrated that diclofenac was highly efficacious in reversing the allodynia in the inflammatory phase of the CAIA model (Bas et al., 2012). While both diclofenac and ketorolac are potent cyclo-oxygenase inhibitors, both variously inhibit lipoxygenase enzymes and activate the nitric oxide-cGMP antinociceptive pathway. Novel mechanisms for diclofenac may include inhibition of peroxisome proliferator activated receptor gamma (PPARgamma), block of acid-sensing ion channels and reduced interleukin-6 production (Buczynski et al., 2010; Gan, 2010). Aside from mechanistic differences distinguishing the CAIA vs. K/BxN actions, the more pronounced joint inflammation in the CAIA model may lead to a state of hypersensitivity that at the time of testing was not predominantly driven by prostaglandins.
Centrally acting anti-hyperpathic actions
Gabapentin and its congeners interact with the α2δ subunit of the voltage sensitive calcium channel and is considered to be efficacious in models of facilitated processing initiated by both tissue and nerve injury (Hwang and Yaksh, 1997; Jun and Yaksh, 1998; Yoon and Yaksh, 1999), as well as by direct activation of dorsal horn systems activated by NMDA and NK1 receptors (Partridge et al., 1998). With one exception (see (Nagakura et al., 2003), systemic or intrathecal gabapentin reduced mechanical hypersensitivity in intra-articular kaolin-carrageenan and intraplantar carrageenan-induced acute inflammatory pain (Field et al., 1997; Lu and Westlund, 1999) and in the mono-iodoacetate model of osteoarthritis (Fernihough et al., 2004; Vonsy et al., 2009). Furthermore, our groups have demonstrated that gabapentin has antiallodynic effects in both the K/BxN serum transfer and the CAIA models (Bas et al., 2012; Christianson et al., 2010). Thus, although gabapentin is predominantly associated with reversal of neuropathic pain, it is also effective in models of inflammation-induced hypersensitivity. Our previous work showed that both the early and late phase mechanical hypersensitivity was transiently abolished by gabapentin. In the present work gabapentin similarly displayed an antiallodynic action in both the early and late phases of the K/BxN and CAIA models. This action of gabapentin in the late phase is considered to be consistent with the assertion that the late phase represents a phenotype that has neuropathic components including induction of ATF3 and GAP43 in the DRG (Christianson et al., 2010; Su et al., 2015, in press). Consistent with this hypothesis is an increased expression of GAP43 in persistent joint inflammation indicating that joint nerve fibers may undergo sprouting and local neuroma formation in the affected joint (Jimenez-Andrade and Mantyh, 2012).
Drug effects in the KBxN/CAIA CPP
The present studies revealed a close covariance between the ability of a treatment to alter the tactile allodynia in either the early or late phase of the K/BxN or the CAIA model and the ability of that treatment to support a CPP assessed during the respective early and late phases. Thus, in the K/BxN model, gabapentin reversed early and late phase allodynia and supported a CPP in both the early and late phases. In contrast, ketorolac reversed allodynia in the early but not the late phase and correspondingly supported a CPP in the early but not the late phase.
In the CAIA model, gabapentin was effective in reversing the allodynia in the early and the late phases, whereas ketorolac, for reasons that are not evident, failed to alter CAIA allodynia in either period. Examination of the place preference revealed that there was a statistically significant CPP for gabapentin in the early phase, and in the late phase it was associated with a numerical trend towards establishing a place preference, which did not achieve statistical significance. Ketorolac, while it failed to alter the CAIA allodynia for reasons that are not clear, consistent with its absence of effect on allodynia, failed to show any trend towards supporting a CPP in either the early or late phases in the CAIA model. Importantly, in previous work we have shown that these doses of either ketorolac or gabapentin failed to support a CPP in normal, nonarthritic mice (Park et al., 2006), suggesting that the drugs alone were not associated with any intrinsic positive reinforcing actions in the absence of an aversive state.
In short, these results provide support for the assertion that efficacy in altering the allodynia in both models was consistent with the association of a rewarding component to the drug action presumably through the reduction of the aversive nature of the arthritic states. Similar findings have recently been shown in a cisplatin-induced neuropathy model, where gabapentin, but not ketorolac, was effective in reversing tactile allodynia and evoking drug-paired preference in the CPP paradigm (Park et al., 2013). The correlations observed in these studies between tactile allodynia (evoked) and CPP pharmacology in the early (inflammatory) and late (post-inflammatory) phase does not indicate that both pain assessments reflect mechanistically identical processes. However, these results are in accord with the point that the concept of “spontaneous pain” may be of little value in describing systems in models of inflammation or polyneuropathy where ongoing afferent input is present (Bennett, 2012). In this regard, the mechanisms generating the “ongoing” pain state may be indistinguishable if not identical to those initiating the facilitated state in response to the injury.
In conclusion, we believe that, based on these studies, the CAIA and K/BxN models have a uniform sensitivity to the actions of gabapentin in the early and late phases of allodynia. Ketorolac is without antiallodynic actions in the CAIA model, while it has efficacy in the early but not late phase of the K/BxN model. Finally, these data support the assertion that relief of the allodynia in the early and late phases is associated with positive reinforcing properties that support the development of a place preference in the mouse.
What is already known about this topic
-
-
KBxN/CAIA inflammatory models yield allodynia persisting beyond reversal of inflammation
-
-
Anti-inflammatory and antihyperalgesic agents reverse early phase allodynia while late phase responds only to the latter.
What does this study add?
-
-
Anti-inflammatory and antihyperalgesic agents working in early phase versus allodynia, support conditioned place preferences in early phase.
-
-
Antihyperalgesic agents working in late phase versus allodynia, support conditioned place preferences in late phase.
-
-
Normalization of early and late phase allodynia is positively reinforcing.
Acknowledgments
We would like to thank Shelle A. Malkmus, Jennifer A. Stokes, James Skahen, and Yemisi Odina who helped with these experiments.
Funding Sources
This work was supported by grants NS16541 (TLY), DA02110 (TLY), the Swedish Research Council (CIS), the Swedish Association for Strategic Research (CIS), Ragnar Söderberg’s Foundation (CIS), the Erik och Edit Fernström stiftelse (KS) and T32 AR 64194-2 (SAW).
Footnotes
There are no conflicts of interest.
Author Contributions
HJ Park: Shared first authorship. Contributed to study design, performed experiments for KBxN CPP studies, analyzed data, completed rough draft of manuscript;
K Sandor: Shared first authorship. Performed experiments for CAIA CPP model studies, analyzed data, drafted her CAIA CPP section, prepared figures 1–3 for submission, contributed to manuscript preparation and revision.
J McQueen: Performed experiments for CAIA CPP model studies, analyzed data, drafted her CAIA CPP section.
SA Woller: Performed experiments for KBxN tactile allodynia studies, analyzed data, drafted her KBxN tactile allodynia section, prepared figure 4 for submission, contributed to revision of manuscript
CI Svensson: Provided overview/management of the CAIA studies and contributed to the manuscript preparation and revision
MP Corr: Provided overview of the KBxN studies, contributed to the manuscript preparation and revision
TL Yaksh: Contributed to the study design and supervision of the studies, manuscript preparation and revision
References
- Agalave NM, Larsson M, Abdelmoaty S, Su J, Baharpoor A, Lundback P, Palmblad K, Andersson U, Harris H, Svensson CI. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain. 2014;155:1802–1813. doi: 10.1016/j.pain.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Bas DB, Su J, Sandor K, Agalave NM, Lundberg J, Codeluppi S, Baharpoor A, Nandakumar KS, Holmdahl R, Svensson CI. Collagen antibody-induced arthritis evokes persistent pain with spinal glial involvement and transient prostaglandin dependency. Arthritis Rheum. 2012;64:3886–3896. doi: 10.1002/art.37686. [DOI] [PubMed] [Google Scholar]
- Bennett GJ. What is spontaneous pain and who has it? J Pain. 2012;13:921–929. doi: 10.1016/j.jpain.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Buczynski MW, Svensson CI, Dumlao DS, Fitzsimmons BL, Shim JH, Scherbart TJ, Jacobsen FE, Hua XY, Yaksh TL, Dennis EA. Inflammatory hyperalgesia induces essential bioactive lipid production in the spinal cord. J Neurochem. 2010;114:981–993. doi: 10.1111/j.1471-4159.2010.06815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Choe JY, Crain B, Wu SR, Corr M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J Exp Med. 2003;197:537–542. doi: 10.1084/jem.20021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson CA, Corr M, Firestein GS, Mobargha A, Yaksh TL, Svensson CI. Characterization of the acute and persistent pain state present in K/BxN serum transfer arthritis. Pain. 2010;151:394–403. doi: 10.1016/j.pain.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. Efficient Analysis of Experimental-Observations. Annu Rev Pharmacol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, Kidd B, Bevan S, Winter J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997;121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin. 2010;26:1715–1731. doi: 10.1185/03007995.2010.486301. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Yaksh TL. Effect of subarachnoid gabapentin on tactile-evoked allodynia in a surgically induced neuropathic pain model in the rat. Reg Anesth. 1997;22:249–256. doi: 10.1016/s1098-7339(06)80010-6. [DOI] [PubMed] [Google Scholar]
- Inglis JJ, Nissim A, Lees DM, Hunt SP, Chernajovsky Y, Kidd BL. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Res Ther. 2005;7:R807–816. doi: 10.1186/ar1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis JJ, Notley CA, Essex D, Wilson AW, Feldmann M, Anand P, Williams R. Collagen-induced arthritis as a model of hyperalgesia: functional and cellular analysis of the analgesic actions of tumor necrosis factor blockade. Arthritis & Rheumatism. 2007;56:4015–4023. doi: 10.1002/art.23063. [DOI] [PubMed] [Google Scholar]
- Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther. 2012;14:R101. doi: 10.1186/ar3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JH, Yaksh TL. The effect of intrathecal gabapentin and 3-isobutyl gamma-aminobutyric acid on the hyperalgesia observed after thermal injury in the rat. Anesth Analg. 1998;86:348–354. doi: 10.1097/00000539-199802000-00025. [DOI] [PubMed] [Google Scholar]
- King T, Qu C, Okun A, Mercado R, Ren J, Brion T, Lai J, Porreca F. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011;152:1997–2005. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Munoz FJ, Diaz-Reval MI, Terron JA, Campos MD. Analysis of the analgesic interactions between ketorolac and tramadol during arthritic nociception in rat. Eur J Pharmacol. 2004;484:157–165. doi: 10.1016/j.ejphar.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Lu Y, Westlund KN. Gabapentin attenuates nociceptive behaviors in an acute arthritis model in rats. J Pharmacol Exp Ther. 1999;290:214–219. [PubMed] [Google Scholar]
- Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, Wanibuchi F, Yamaguchi T. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther. 2003;306:490–497. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- Okun A, Liu P, Davis P, Ren J, Remeniuk B, Brion T, Ossipov MH, Xie J, Dussor GO, King T, Porreca F. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153:924–933. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Lee Y, Lee J, Park C, Moon DE. The effects of botulinum toxin A on mechanical and cold allodynia in a rat model of neuropathic pain. Can J Anaesth. 2006;53:470–477. doi: 10.1007/BF03022619. [DOI] [PubMed] [Google Scholar]
- Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent Hyperalgesia in the Cisplatin-Treated Mouse as Defined by Threshold Measures, the Conditioned Place Preference Paradigm, and Changes in Dorsal Root Ganglia Activated Transcription Factor 3: The Effects of Gabapentin, Ketorolac, and Etanercept. Anesth Analg. 2013;116:224–231. doi: 10.1213/ANE.0b013e31826e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge BJ, Chaplan SR, Sakamoto E, Yaksh TL. Characterization of the effects of gabapentin and 3-isobutyl-gamma-aminobutyric acid on substance P-induced thermal hyperalgesia. Anesthesiology. 1998;88:196–205. doi: 10.1097/00000542-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152:1641–1648. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks WH, 2nd, Maloney PJ, Shott LD, Schuler ME, Sevelius H, Strosberg AM, Tanenbaum L, Tomolonis AJ, Wallach MB, Waterbury D, et al. The analgesic and anti-inflammatory profile of ketorolac and its tromethamine salt. Drugs Exp Clin Res. 1985;11:479–492. [PubMed] [Google Scholar]
- Sokka T, Kautiainen H, Toloza S, Makinen H, Verstappen SMM, Hetland ML, Naranjo A, Baecklund E, Herborn G, Rau R, Cazzato M, Gossec L, Skakic V, Gogus F, Sierakowski S, Bresnihan B, Taylor P, McClinton C, Pincus T. QUEST-RA: quantitative clinical assessment of patients with rheumatoid arthritis seen in standard rheumatology care in 15 countries. Ann Rheum Dis. 2007;66:1491–1496. doi: 10.1136/ard.2006.069252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Gao T, Shi TJ, Qiong X, Xu X, Wiesenfeld-Hallin Z, Hokfelt T, Svensson CI. Phenotypic changes in dorsal root ganglion and spinal cord in the collagen antibody-induced arthritis mouse model. J Comp Neurol. 2015 doi: 10.1002/cne.23749. in press. [DOI] [PubMed] [Google Scholar]
- Sufka KJ. Conditioned Place Preference Paradigm - a Novel-Approach for Analgesic Drug Assessment against Chronic Pain. Pain. 1994;58:355–366. doi: 10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Sufka KJ, Roach JT. Stimulus properties and antinociceptive effects of selective bradykinin B1 and B2 receptor antagonists in rats. Pain. 1996;66:99–103. doi: 10.1016/0304-3959(96)02990-9. [DOI] [PubMed] [Google Scholar]
- Vonsy JL, Ghandehari J, Dickenson AH. Differential analgesic effects of morphine and gabapentin on behavioural measures of pain and disability in a model of osteoarthritis pain in rats. Eur J Pain. 2009;13:786–793. doi: 10.1016/j.ejpain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Wei H, Viisanen H, Amorim D, Koivisto A, Pertovaara A. Dissociated modulation of conditioned place-preference and mechanical hypersensitivity by a TRPA1 channel antagonist in peripheral neuropathy. Pharmacol Biochem Behav. 2013;104:90–96. doi: 10.1016/j.pbb.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Yoon MH, Yaksh TL. The effect of intrathecal gabapentin on pain behavior and hemodynamics on the formalin test in the rat. Anesth Analg. 1999;89:434–439. doi: 10.1097/00000539-199908000-00034. [DOI] [PubMed] [Google Scholar]


