Abstract
In contrast with other cells generated by the root apical meristem in Arabidopsis, pericycle cells adjacent to the protoxylem poles of the vascular cylinder continue to cycle without interruption during passage through the elongation and differentiation zones. However, only some of the dividing pericycle cells are committed to the asymmetric, formative divisions that give rise to lateral root primordia (LRPs). This was demonstrated by direct observation and mapping of mitotic figures, cell-length measurements, and the histochemical analysis of a cyclin-GUS fusion protein in pericycle cells. The estimated duration of a pericycle cell cycle in the root apical meristem was similar to the interval between cell displacement from the meristem and the initiation of LRP formation. Developmentally controlled LRP initiation occurs early, 3 to 8 mm from the root tip. Thus the first growth control point in lateral root formation is defined by the initiation of primordia in stochastic patterns by cells passing through the elongation and young differentiation zones, up to where lateral roots begin to emerge from the primary root. Therefore, the first growth control point is not restricted to a narrow developmental window. We propose that late LRP initiation is developmentally unrelated to the root apical meristem and is operated by a second growth control point that can be activated by environmental cues. The observation that pericycle cells divide and lateral root primordia form without intervening mitotic quiescence suggests that lateral organ formation in roots and shoots might not be as fundamentally different as previously thought.
The control of lateral root initiation is poorly understood. In most plant species, the positions of lateral root primordia (LRPs) along the main root axis are stochastic and do not follow a pattern. LRPs originate from founder cells in the pericycle, the outermost layer of the vascular cylinder (stele) of the root. Founder cells are those pericycle cells of the parent root that give rise to a primordium of a lateral root. It is not understood whether or when specific pericycle cells are determined to become founder cells. Moreover, lateral root density is influenced by the soil environment of the root and it is not understood how environmental signal pathways converge with developmental control to regulate the rate of lateral root initiation.
There is some evidence that lateral root formation is specified within the root apical meristem. It was shown that lateral root initiation in pteridophytes is closely related to the cell division pattern of parent-root apical cells and their derivatives (Mallory et al., 1970; Chiang and Gifford, 1971; Lin and Raghavan, 1991). In angiosperms, several studies showed that the determination of the site of future lateral roots occurs much in advance of visible initiation of the primordium (Foard et al., 1965; Charlton, 1977; Hinchee and Rost, 1992). In some members of the Araceae (Clowes, 1961), Pontederiaceae (Mallory et al., 1970; Charlton, 1975), and Cucurbitaceae (Mallory et al., 1970; Demchenko, 1999) families, primordia are initiated within the meristem of the parent root. In some Polygonaceae and Cucurbitaceae, primordia are initiated during late embryogenesis when the parent root cells are apparently in a meristematic state (O'Dell and Foard, 1969; Clowes, 1982; Dubrovsky, 1986, 1987). When pea (Pisum sativum) roots were grown at high temperature, primordia failed to form. When transferred to a permissive temperature, new primordia were initiated, but only in the new growth. This finding suggested that the competence for lateral root initiation might be restricted to a developmental window within the root apical meristem (Gladish and Rost, 1993).
The first divisions leading to LRP formation have often been assumed to occur after dedifferentiation of pericycle cells (Laskowski et al., 1995; Malamy and Benfey, 1997). In maize (Zea mays) seedlings, the time interval between meristem exit and LRP initiation by pericycle cells was found to be relatively short (8.3 h; Dubrovsky and Ivanov, 1984), leaving little if any time for dedifferentiation. It has been proposed that pericycle cells giving rise to primordia might continue to cycle after the cells leave the meristem (Blakely et al., 1982).
Auxins and other hormones are essential for LRP initiation (Torrey, 1986; Celenza et al., 1995). Early primordium development continues to depend significantly on the parent root (Laskowski et al., 1995). In Arabidopsis, the stimulation of founder cell division initiates a strict sequence of morphogenetic events, accompanied by differential gene expression, which leads to the formation of a functional lateral root (Malamy and Benfey, 1997). A few Arabidopsis genes are known to be required for lateral root development. The ALF4 locus is required for LRP initiation (Celenza et al., 1995), whereas the ROOTMERISTEMLESS (Cheng et al., 1995) and HOBBIT (Willemsen et al., 1998) genes are required during later steps of meristem formation.
To resolve some of the early steps of lateral root development in a genetically tractable system, we analyzed cell division activity in Arabidopsis pericycle cells, starting with their exit from the meristem. We show that pericycle cells in the zone of LRP initiation maintain cell division activity following their displacement from the meristem. However, only some of the pericycle cell divisions in the differentiation zone ultimately lead to LRP initiation. Most mitoses in the pericycle opposite the protoxylem poles contribute to the shorter length (on average) of cells at this position compared with pericycle cells opposite the protophloem.
RESULTS
Continued Cell Division Activity in Pericycle Cells after Displacement from the Root Apical Meristem
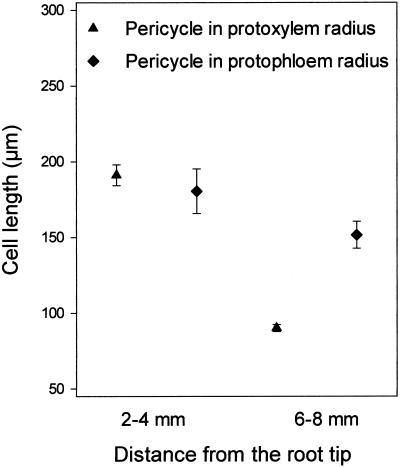
Under the growth conditions used in the experiments reported here, the root apical meristem and the elongation zone in Arabidopsis, which together comprise the growing part of the root, was 1.23 ± 0.03 mm (mean ± se, n = 21) long. In Arabidopsis roots 5 to 15 mm from the tip, pericycle cells in the protoxylem radius are about half as long as those in the protophloem radius (Laskowski et al., 1995). In our study, 4 to 6 mm from the root tip, the pericycle cells in the protoxylem radius were 90 ± 2 μm and in the protophloem radius the cells were 152 ± 9 μm (mean ± se; statistical details are given in Fig. 1). We examined whether these differences in cell size originated in the meristem, as has been reported for size differences between two types of epidermal cells (Berger et al., 1998) or if these differences were caused by mitoses following traverse of the elongation zone. We analyzed cell size in two root segments. Detailed examination of cell length of pericycle cells in the protoxylem radius revealed two populations: The majority of cells were long cells ranging from 73 to 312 μm, although relatively short cells ranging from 7 to 23 μm were also observed. These short cells were components (see “Discussion”) of LRPs and were excluded from the analysis of cell length differences in the protoxylem and protophloem radii. There were no differences in cell length between pericycle cells in the protoxylem radius and the protophloem radius as cells were displaced from the elongation zone, 2 to 4 mm from the root tip (P > 0.05, Student's t test). In contrast, pericycle cells opposite the protoxylem poles were substantially shorter than those opposite protophloem poles in the zone 6 to 8 mm from the tip, (P < 0.001, Student's t test; Fig. 1). Pericycle cells in the protophloem radius in this zone were slightly shorter than the cells 2 to 4 mm from the tip (P > 0.05, Student's t test), but the calculated t value for these samples was close to the threshold t value (t from data was 1.76, threshold t value was 1.67), indicating only a small difference. This finding suggested that pericycle cells in the protoxylem radius undertook further cell division subsequent to their displacement from the elongation zone leading to the production of either shorter cells or LRPs.
Figure 1.
Pericycle cell length in protoxylem and protophloem radii 2 to 4 mm and 6 to 8 mm from the tip. For cells in the protoxylem radius, means ± se are given for 48 cells in 11 roots (2–4 mm portion) and for 164 cells in 11 roots (6–8 mm portion). For cells in the protophloem radius, means ± se are given for 27 cells in nine roots (2–4 mm portion) and for 35 cells in seven roots (6–8 mm portion). When in the protoxylem radius (2–4 mm), a cell had recently divided or was in division, it was treated as one cell.
Pericycle Cells Rapidly Initiate LRP Formation after Leaving the Meristem of the Primary Root
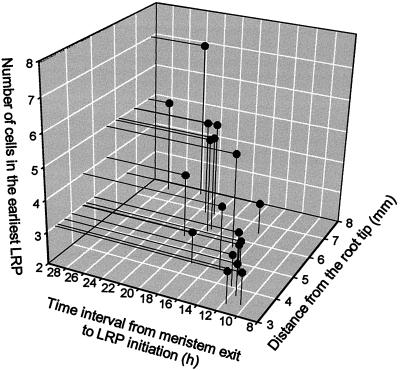
The first divisions leading to the formation of an LRP are anticlinal divisions of pericycle cells in the protoxylem radius. Short pericycle cells were frequently observed in clusters in three adjoining cell files next to the protoxylem; therefore, we hypothesized that such short cells were the earliest signs of LRP formation (Fig. 2). The hypothesis was strengthened when more advanced stages of LRP development were detected frequently in the more proximal root portion (away from the root tip). The most distal (closest to the root apex) primordia were found in the pericycle 3 to 8 mm from the root tip and contained three to seven cells (Fig. 3). This finding indicated that the time interval from when pericycle cells left the meristem (close to the time since the last division in the meristem) to the first visible events of LRP initiation was relatively short. This was confirmed by calculating the time interval between displacement from the meristem and the initiation of LRP formation (Ti). Ti was on average 13.6 h (n = 9) and 16 h (n = 5) for the most distal primordia containing three and four cells in a cell file (Table I). The average cycle time in the meristem (T) for pericycle and cortex cells was similar (Table II). The cell cycle duration of pericycle and cortex cells in the meristematic zone ranged in individual roots from 11.1 to 15.5 h, and 13.7 to 17.4 h, respectively, and was close to the duration defined for cortex cells with a different method (Beemster and Baskin, 1998). Thus the absence of a pronounced interval of mitotic quiescence in pericycle cells after their displacement from the meristem implied that the first stage of LRP initiation occurred earlier than previously demonstrated.
Figure 2.
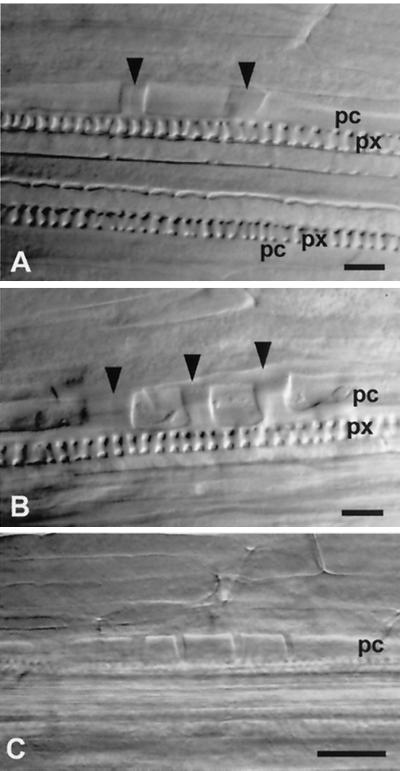
Earliest stages of LRP development detected on whole mounts of three different roots by using Nomarski optics. A, Three-cell stage 4.51 mm from the root tip. B, Four-cell stage 4.61 mm from the root tip. Note that LRPs on plates A and B also can be considered as two- and three-cell stages, respectively, because their right or left cell walls (arrowheads) can separate a founder cell from a neighbor pericycle cell. C, Five-cell stage at 6.71 mm from the root tip. pc, Pericycle; px, protoxylem. Bar = 25 μm.
Figure 3.
Characteristics of the earliest LRPs (detected on whole mounts) by their location in the primary root, by number of cells, and by the interval of time between displacement from the meristem and the initiation of LRP formation. The number of cells in the earliest LRP corresponds to the number of cells in a pericycle cell file, but not to the total number of LRP cells. The minimum number of LRP cells in a cell file was three cells, similar to LRPs shown in Figure 2A. This three-cell stage can be interpreted as a two-cell stage (see notes in Fig. 2, A and B).
Table I.
Estimation of time between displacement of pericycle cells from the meristem to the first detected LRPs of three- and four-cell stage (Ti)
| Stage of LRP Development | V | Lg | Le | Lp | Te | Tbf | Ti |
|---|---|---|---|---|---|---|---|
| μm h−1 | μm | h | |||||
| Three-cell (n = 9) | 362 ± 14 | 1,203 ± 57 | 826 ± 51 | 4,402 ± 324 | 4.6 ± 0.2 | 8.9 ± 1.0 | 13.6 ± 0.9 |
| Four-cell (n = 5) | 349 ± 17 | 1,289 ± 51 | 922 ± 44 | 5,509 ± 461 | 5.3 ± 0.2 | 12.3 ± 1.6 | 16.0 ± 2.0 |
Three- and four-cell stages of LRP development can be interpreted as two- and three-cell stages (see legend, Fig. 2). The number of cells in the earliest LRP corresponds to the number of cells in a pericycle cell file, but not to the total number of LRP cells. V is the rate of root growth; Lg is the length of the growing part of the root (the sum of the length of the meristem and the length of the elongation zone); Le is the length of the elongation zone; Lp is the distance from the root meristem-root cap junction to the earliest-detected primordium; Te is the duration of cell elongation; Tbf is a time after cells had completed elongation and before a primordium formation. Each variable was determined for an individual root. Data are means ± se.
Table II.
Rate of primary root growth (V), length of fully elongated cells (le), average cell number in a cell file (Nm) of pericycle and cortical cells in the meristem, and the duration of the cell cycle (T) in the meristem of Arabidopsis estimated by the rate-of-cell production method (Ivanov and Dubrovsky, 1997)
| Variables | Pericycle | Cortex |
|---|---|---|
| V, μm h−1 | 407 ± 14 | 407 ± 14 |
| le, μm | 203 ± 6 | 212 ± 6 |
| Nm | 39.0 ± 1.2 | 41.6 ± 1.2 |
| T, h | 13.6 ± 0.6 | 15.0 ± 0.6 |
Means ± se are given for nine roots, where Nm, V, and T were estimated for each individual root. For estimation of the average length of fully elongated cells (le), 73 pericycle cells in both the protoxylem and protophloem radii on sections of 10 roots (2–4 mm from the tip) and 50 cells of cortex on sections of nine roots were measured.
Pericycle Cells Maintain Proliferative Activity in the Differentiation Zone of the Root
To test whether LRP formation initiated close to the root apex, and whether all small cell clusters gave rise to LRPs, we analyzed proliferation in the pericycle using three different approaches: first, direct examination of the mitotic figures on sections and whole mounts of the roots; second, the analysis of cell packets; and third, the analysis of spatio-temporal patterns of cells accumulating a labile, cyclin-GUS fusion protein (Colón-Carmona et al., 1999).
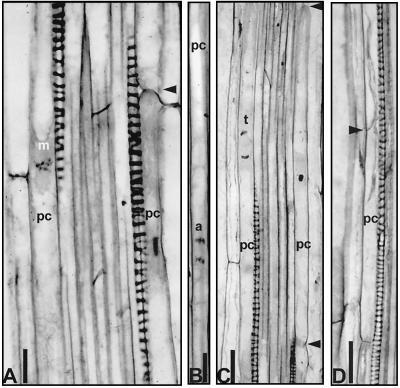
Longitudinal sections of the roots 2 to 7 mm from the tip were examined for mitotic activity. Mitoses were observed in the protoxylem-associated pericycle cells. Mitotic figures were commonly located at the center of elongated vacuolated pericycle cells that were unusually long for dividing cells, ranging from 175 to 230 μm (Fig. 4, A–C). To study distribution of mitotic figures and LRPs along the root, whole mounts of the Feulgen-stained roots were analyzed. These permanent preparations permitted the detection of mitotic figures in pericycle cells by using bright field microscopy (Fig. 5, C and E) or laser scanning confocal microscopy (Fig. 5, A, B, and D). The mitotic figures were classified either as single divisions in the pericycle or as divisions in LRP cells and mapped (Fig. 6).
Figure 4.
Mitotic figures in pericycle cells on root sections. A, Metaphase 2 to 3 mm from the tip. B, Anaphase 4 to 6 mm from the root tip. C, Telophase 2 to 4 mm from the tip. D, A cell packet 221 μm long with young cell wall (arrowhead) 2 to 4 mm from the root tip. Arrowheads on A and C indicate convex end cell walls. Bar on A, C, and D = 20 μm; bar on B = 10 μm.
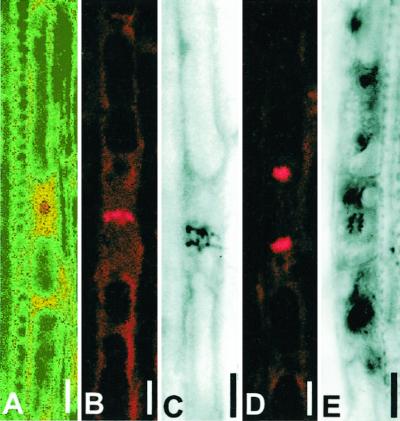
Figure 5.
Mitotic figures on whole mounts in pericycle cells and LRP 2 to 6 mm from the root tip stained by Feulgen. A, Prophase. B, Metaphase. C, Early anaphase. D, Telophase. E, Periclinal division in young LRP located 4.96 mm from the tip. Images A, B, and D were obtained using confocal microscopy and taken in different software modes. Images C and E were captured by bright-field microscopy. Bar = 5 μm.
Figure 6.
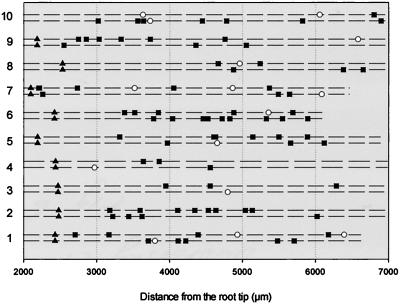
Distribution of mitoses and LRPs detected in whole mounts stained by Feulgen 2 to 7 mm from the root tip of 10 individual roots in 8-d-old seedlings. A dashed line represents a protoxylem strand. Triangles indicate the level at which differentiated protoxylem can be clearly recognized. Squares represent singular mitotic figures and circles represent LRPs. LRP was defined as a compact cluster of interphase or dividing nuclei.
Maturation of the protoxylem was essentially complete at a distance of about 2 mm from the tip (Fig. 6). The mitotic figures were found in cells of one to three adjacent files of the pericycle, all in contact with a protoxylem strand. The number of single mitotic figures varied between 0 and 6 per mm in the zone of the root 2 to 6 mm from the tip (Fig. 6). When single mitotic figures and LRPs were treated as different stages of LRP development, their distribution along the root was random. In different roots, along one protoxylem strand, either LRPs or single mitotic figures were most distally located. Within the same root, the presence of most distal LRPs and single mitotic figures along two protoxylem strands did not correlate (for example, in roots 3, 4, and 10 in Fig. 6, the most distally located single mitotic figures were found along one strand and the most distally located LRPs along another). In some roots, 2 to 6 mm from the tip along one protoxylem strand, only single mitotic figures, but not LRPs, were found (as in roots 2–6, 8, and 9 in Fig. 6). This finding suggested that some of the single mitotic figures might subsequently lead to LRP initiation.
To understand whether the spatial distribution of single mitotic figures and LRPs associated with different protoxylem strands along the root was regular, they were treated as independent events in pericycle development. One-millimeter root segments of the 3- to 6-mm root portion were analyzed. The single mitotic figures within a 1-mm segment of the root were associated with both protoxylem strands in 40% of the cases (n = 35). The respective percentage of cases for LRPs was only 7% (n = 35), indicating that LRPs had a higher tendency to be regularly distributed, then single, mitotic figures. This finding suggested that mitotic figures found in the pericycle were not exclusively related to LRP initiation.
After elongation, pericycle cells usually had convex end walls (Fig. 4, A and C). When such a cell divides, it becomes a parent of a cell packet. A cell packet represents a group of cells descendent from one mother cell and enclosed in the original cell wall of that cell. The new cell wall was commonly perpendicular or at some angle, and thinner (Fig. 4D) than the longitudinal wall. It was common that two-cell and sometimes three-cell packets were found. This independently indicated pericycle cell proliferation activity outside the meristem. Divisions frequently generated progeny of unequal size but there were no preferences for the proximal-distal orientation of the unequal daughters within a two-cell packet (data not shown).
A similar pattern of proliferation was found in the pericycle of transgenic plants carrying a CycB1;1::iudA construct. In histochemical assays, the activity of the cyclin-GUS chimeric protein is restricted to G2 and M1 phases (Colón-Carmona et al., 1999). CycB1;1 promoter-driven GUS staining in young LRPs has been described (Ferreira et al., 1994; Colón-Carmona et al., 1999). We focused on cyclin activity in singular pericycle cells. Within 2 to 6 mm from the root tip, GUS staining was detected in both pericycle cells (Fig. 7) and young LRPs (not shown). The staining was found in G2 cells (Fig. 7A) and in cells undergoing mitosis (Fig. 7B, metaphase). Single GUS-stained cells in the pericycle in the protoxylem radii were found up to the level where young lateral roots start to appear on the surface of the parent root.
Figure 7.
GUS staining of pericycle cells in the protoxylem radius in cyclin B1;1-GUS transgenic line 2 to 6 mm from the tip. A, Cells in G2 of cell cycle. B, Cell in metaphase. Nomarski optics. Bar = 10 μm.
Taken together, these data indicate high proliferative activity in the pericycle outside the meristem. However, not all mitotic activity observed directly precedes LRP initiation. This was verified in a separate experiment.
Only Some of the Dividing Pericycle Cells Are Committed for LRP Formation
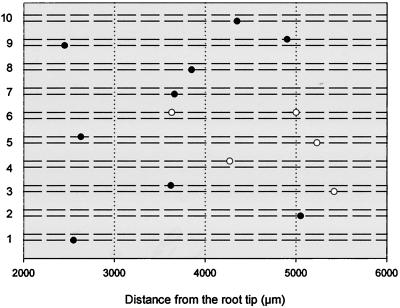
In 8-d-old seedlings, as many as two LRPs were found in addition to mitotic figures within 3 to 6 mm from the root tip (Fig. 6). An overall estimation showed there were 0.33 LRPs per millimeter of the root length in this root portion (n = 10). To examine the fate of cells in which we detected mitotic figures, a 2- to 6-mm root portion was marked (the marks were made on Petri dishes at 2 and 6 mm from the tip in the 8-d-old roots) and excised and analyzed 6 d later. At this time no further mitotic figures were observed. LRPs and lateral roots were mapped for each individual root and were found to be randomly located (Fig. 8). The density of LRPs and lateral roots per millimeter of root length at d 14 was 0.36 per mm (within the portion that corresponded to the zone 3–6 mm from the root tip in 8-d-old plants, n = 10), which was similar to that observed at d 8 (P > 0.05, Student's t test). This demonstrated that under our growth conditions, (a) LRP initiation is restricted to the differentiation zone because no new LRPs were initiated later, and (b) only some of the dividing pericycle cells (12.5%, n = 10) in the 3- to 6-mm zone were committed to LRP initiation and most of the singular mitoses observed did not lead to LRP initiation. We also grew wild-type plants in soil under the same conditions. The density of lateral roots in the corresponding zone was estimated to be 0.35 per mm of the root length (n = 8), not significantly different from agar-grown plants (P > 0.05, Student's t test).
Figure 8.
Distribution of LRPs and lateral roots detected in whole mounts stained by Feulgen in 10 individual roots 14 d after the start of seed germination. Root portions 2 to 6 mm from the root tip were marked at 8 d and seedlings continued growth for 6 more d. A dashed line represents a protoxylem strand. White circles represent LRPs, black circles represent lateral roots. Lateral roots were defined as those primordia that started emergence or emerged from the parent root.
DISCUSSION
Pericycle cells in intact wild-type Arabidopsis seedlings continue to proliferate after leaving the meristem. Using the length of the cells (Fig. 1) and the data on distribution of mitoses (Fig. 6), we estimated the mitotic index in three cell files opposite one protoxylem strand between 3 and 4 mm from the tip to be 11.3% (n = 20). This high mitotic activity is comparable with the mitotic index in the primary roots of various species, ranging from 6% to 12% (Burholt and Van't Hof, 1971; Rost and Sammut, 1982; Yee and Rost, 1982; Barlow and Rathfelder, 1985; Rost and Reynolds, 1985). Although continued cell proliferation behind the meristem was not demonstrated, D-type cyclin (Cyc4;1 At) transcription in single pericycle cells in the protoxylem radius (De Veylder et al., 1999) and continued expression of the cdc2a gene in pericycle cells (Martinez et al., 1992; Hemerly et al., 1993) further supports our conclusion. Continued proliferation of pericycle cells in xylem radii was also reported for other species; however, its relation to lateral root initiation remained unclear (Luxová and Murín, 1973; Rost et al., 1988).
In plants, generally cells that leave the meristem are in different phases of the cell cycle, except mitosis (Ivanov, 1981; Demchenko, 1987). If we assume that all pericycle cells in the protoxylem radii within approximately the first 10 mm from the root apex of Arabidopsis are proliferating, then they should also be in different phases of the cell cycle, including mitosis. The patchy pattern of GUS activity in pericycle cells in the construct studied here (Fig. 7) and random distributions of cells in mitosis of wild-type plants (Fig. 6) support this statement. Thus the pericycle cells in the protoxylem radii in the region analyzed represent columns of meristematic tissue behind the apical meristem. Unlike typical meristems, these cells are highly vacuolated albeit competent to proliferative (cell multiplication) and formative (asymmetric divisions resulting in the establishment of a new cell fate; Scheres and Benfey, 1999) divisions. Our study thus suggests that extensive dedifferentiation of pericycle cells apparently is not required for the formation of LRP in the root zones we analyzed. The maintenance of proliferation in pericycle cells in protoxylem radii can be considered a specific differentiation state for this cell type. The use of differentiation markers for these cells would clarify the relationship between lack of quiescence and differentiation.
The competence of pericycle cells for division and their high proliferative activity is a specific feature of these plant cells. The length of dividing cells was close to 200 μm. We are unaware of any other case where cells of these dimensions can maintain proliferation activity in intact plant tissues. In tissue culture conditions, elongated cells (for example, differentiated fiber cells longer than 660 μm) can maintain proliferation (Van't Hof and Saha, 1997).
Our data on cell size, cell packet analysis, and distribution of mitoses indicate that after pericycle cells leave the meristem three distinct trajectories can lead to LRP formation: (a) elongated cells can directly become founder cells (because early primordia can be detected close to the apex, as in root 4 in Fig. 6), (b) elongated cells can go through a formative division that defines the LRP founder cells (because few mitoses along the same protoxylem strand can precede LRP formation, as in roots 1, 6, and 9 in Fig. 6), and (c) elongated cells first go through a proliferative division and then through a formative division (few mitoses along one protoxylem strand and there is still an absence of primordia 2–7 mm from the tip; roots 2 or 5 in Fig. 6 indicate such a possibility). Our observations suggest that in Arabidopsis, specification of pericycle cells for LRP development is defined just after cells exit the meristem. However, scenario (a) implies that a prepattern determining the position of LRPs is laid down in the meristem. Thus we presently cannot exclude that early determination of future cell development had occurred already in the meristem.
Based on the number of pericycle cells in the meristem (Table II), we calculate that pericycle cells progress through five or six division cycles after proliferation of the pericycle initial cell closest to the root cap-root body junction and then leave the meristem. Thus, overall in pericycle development, a formative division for LRP initiation represents the sixth or seventh round of division for progeny of the pericycle cell initials. After that, a new cell lineage, LRP, is established. In contrast with animal systems where asymmetric divisions of stem cells restrict cell differentiation options (Holtzer et al., 1972; Dienstman and Holtzer, 1975; Holtzer and Holtzer, 1976), here the formative division broadens differentiation options by initiating a new meristem capable of generating all root organ-specific cell types.
Several growth control points operate during lateral root development (Malamy and Benfey, 1997). Here we show that the first growth control point is defined after pericycle cells exit the meristem by the establishment of LRP founder cells. The competence of pericycle cells for formative divisions resulting in founder cell formation is not restricted to a narrow developmental window. Early LRP initiation occurred in the root zone 3 to 8 mm from the root tip; no new primordia were initiated later (Figs. 3, 6, and 8). Single GUS-positive cells in the pericycle of CycB1;1::uidA transgenic plants were found, however, up to the level where young lateral roots start emergence (for example, 34 mm from the root tip in 40-mm-long roots). We do not know how many cell cycles these cells passed after their exit from the meristem, but we suggest that at this level, new founder cells can still be established. Thus the young differentiation zone can be considered as the zone of early lateral root initiation; however, the first growth control point can operate also at later stages. In 14-d-old seedlings, single mitotic figures in the pericycle were not found (Fig. 8), indicating the absence of late LRP initiation. Other studies would suggest that pericycle cells between already developed lateral roots do not lose their ability to form LRPs; for example, when strongly stimulated by auxin (Blakely et al., 1982; Laskowski et al., 1995; Doerner et al., 1996). We can hypothesize that induction of LRP formation at such late stages represents another growth control point, also related to root initiation. Whether founder cells for such an induction were established during the operation of the first growth control point and then maintained in a latent state, or formative divisions leading to founder cell formation can occur later in development in more mature root portions, is an open question. An alternative option is that for operation of the second growth control point no founder cell formation is needed. Experiments showing that practically all pericycle cells in the xylem radii participated in LRP formation (Goldacre, 1959; Blakely et al., 1982; Laskowski et al., 1995) indicate such a possibility. In contrast with other tissues in the root differentiation zone, pericycle cells do not endoreduplicate (Blakely and Evans, 1979). Maintenance of cell proliferation is perhaps an important mechanism to keep cell competence for LRP initiation at a later stage for operation of the second growth control point. The second growth control point might operate only as an emergency response to tissue damage or environmental changes (or be induced experimentally by hormonal treatments). Under examined growth conditions, no late LRP initiation was recorded in this study.
In general, it is considered that lateral roots initiate in an acropetal pattern (Charlton, 1996). Our data indicate that because not all dividing pericycle cells give rise to a founder cell, the first growth control point does not operate strictly acropetally. Sometimes in the zone of early initiation, we observed earlier stages of LRP formation basipetally to the later stages (data not shown). This observation reflects the stochastic pattern of operation of the first growth control point. A similar pattern was reported for roots of Pontederia cordata (Charlton, 1975) and maize (MacLeod, 1990).
After commitment to primordium formation, cells within the shoot apical meristem organize lateral organ formation without an intervening period of mitotic quiescence (Laufs et al., 1998; Bowman and Eshed, 2000). Our observation that lateral root initiation by founder cell division does not involve interruption in cell cycling suggests that the basic principles governing the timing of organ formation in shoots and roots might not be as fundamentally different as previously assumed. However, morphogenesis of lateral organs in roots is displaced from the apex because cell expansion moves the responsible pericycle cells away from the apical meristem.
MATERIALS AND METHODS
Plant Growth Conditions, Sectioning, and Whole Mounts
Seeds of Arabidopsis, Columbia 2, were placed in an uncovered glass Petri dish and sterilized under a UV-C lamp (model XX-15L, Black Ray, San Gabriel, CA) for 6 min, shaking the Petri dish after 3 min for complete exposure of the seeds to the irradiation. Seeds were transferred to square Petri dishes containing 10% (v/v) diluted Murashige and Skoog medium (Life Technologies, Inc., Gaithersburg, MD), pH 5.7, and supplemented with 0.01 mgL−1 thiamine, 0.05 mgL−1 pyridoxine, 0.05 mgL−1 nicotinic acid, 3% (w/v) Suc, and 1.5% (w/v) agar. Ten seeds per dish were arranged in a line and the dishes were maintained vertically at 20°C in 16 h of light (45 μmol s−1 m−2) and 8 h of dark. Unless otherwise indicated, all observations and measurements were done on roots 8 d after the start of germination.
Roots were fixed overnight in 1.5% (v/v) glutaraldehyde and 0.3% (v/v) paraformaldehyde in 25 mm of PIPES (1,4-piperazinediethanesulfonic acid). The fixed material was gradually dehydrated (10% increase per step, 15 min per step, starting with 10% [v/v] ethanol) and then embedded in historesin (Leica Instruments GmbH, Heidelberg) by incubation in an ethanol:historesin mixture in proportions of 3:1, 1:1, and 1:3 (v/v; 2 h in each), and then in pure historesin overnight. Plastic blocks were sectioned on a Reichert-Jung 2050 Supercut microtome (Cambridge Instruments GmbH, Nussloch, Germany). The thickness was 2.5 μm for longitudinal sections and 3.5 μm for transverse sections. Sections were mounted on gelatin-coated slides (Baum and Rost, 1996), and after 30 min of hydrolysis in 5 n of HCl at room temperature, were stained by Feulgen (De Tomasi, 1936) for 1 h. The same material subsequently was stained by periodic acid-Schiff reaction (Baum and Rost, 1996) and counterstained for 30 s in 0.05% (w/v) Toluidine Blue O.
To detect mitotic figures and LRPs on whole mounts, roots were fixed overnight either as done for sectioning or in a mixture of 70% (v/v) ethanol:glacial acetic acid (3:1, v/v). When fixed as done for sectioning, the material was gradually dehydrated to 70% (v/v) ethanol and then post-fixed in a mixture of 70% (v/v) ethanol:glacial acetic acid (3:1, v/v) for 30 min. The material was washed three times in 70% (v/v) ethanol for 5 min each, gradually hydrated, and stained by Feulgen as was done for the sections. To obtain permanent preparations, the stained roots were dehydrated, infiltrated, and embedded on a slide in 60 μL of the historesin premixed with a hardener, covered by a coverslip, and sealed with nail polish. Arabidopsis transgenic lines carrying the mitotic cyclin CycB1,1::uidA reporter construct (Colón-Carmona et al., 1999) were used to identify cells undergoing cell division. To detect GUS activity histochemically, the material was incubated in 90% (v/v) ice-cold acetone for 30 min, stained for 9 h as described by Hemerly et al. (1993), and cleared by using the method of Malamy and Benfey (1997). The material was analyzed and photographed with a Vanox AHBT photomicroscope (Olympus, Tokyo) using ASA 160T Ektachrome film (Eastman-Kodak, Rochester, NY) or an XC-75 CCD camera (Sony, Tokyo). Cell measurements were made with an ocular micrometer. The whole mounts of roots stained by Feulgen were also viewed under a TCS confocal microscope (Leica Instruments GmbH) using a Krypton laser at 568 nm and various software modes.
Unless otherwise indicated, all chemicals were from Sigma Chemical Company (St. Louis). The number of replicates of each experiment is indicated in the text. Data were analyzed by Student's independent t test.
Determination of Temporal Growth Parameters
We assumed steady-state growth conditions. The interval between displacement from the meristem and the initiation of LRP formation (Ti) was determined by Equation 1:
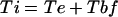 |
1 |
where Te is the duration of cell elongation (time interval from the exit from the meristem to the completion of elongation) and Tbf is an interval between the time of completion of cell elongation and the time of primordium initiation. Te was calculated by Equation 2:
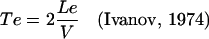 |
2 |
where Le is the length of the elongation zone in μm (where cells that left the meristem are in the process of rapid cell elongation), and V is the rate of root growth (μm h−1). A factor of 2 is introduced in the equation to indicate that when the most distal cells just entering the elongation zone have completed elongation, the root tip will have been displaced by a distance equal to two lengths of the elongation zone (Ivanov, 1974). Tbf was determined by Equation 3:
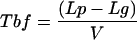 |
3 |
where Lp is the distance from the root meristem-root cap junction to the earliest detected primordium in a root, and Lg is the length of the growing part of the root (the sum of the length of the meristem and the length of the elongation zone). For the determination of Ti, the position of the root tip was marked on the surface of the Petri dish and the growth increments were measured in 24 h under a binocular microscope to the nearest 0.5 mm. The location of the earliest detected LRPs was determined on the cleared roots mounted in 50% (v/v) glycerol (Malamy and Benfey, 1997) using Nomarski optics. Only roots lying on a slide in the protoxylem plane (when both protoxylem strands were clearly visible in the same focal plane) were analyzed for determination of the location of most distal primordia. The lengths of the growing part of the root (Lg) were determined as the distance between the root body-root cap junction and the location of the first-detected root hair bulges. In Arabidopsis, the initiation of root hair formation corresponds to the termination of cell elongation (Dubrovsky et al., 1994). All data (Lg, Le, and Lp) were collected on individual roots. The average cycle time in the meristem was determined for cortical and pericycle using the rate-of-cell production method (Ivanov and Dubrovsky, 1997). Because Arabidopsis roots do not grow steadily during the first 10 d after germination (Beemster and Baskin, 1998), root growth increments were measured 6 h before root fixation. The number of cells in a cell file within the meristem (Nm) was determined on longitudinal sections. The proximal meristem border was defined based on the changes in cell length (Dubrovsky, 1997). Nm and V were determined for each individual root. The length of fully elongated cells was determined on sections of a sample of roots grown in the same Petri dishes and with same average rate of root growth as those fixed individually.
ACKNOWLEDGMENTS
We thank Natalia Doktor for her help with preparation of the illustrations and Dr. Ellis Glazier for editing the English language text.
Footnotes
This work was supported by a UCMEXUS grant from the University of California. Work in the P.W.D. lab was supported in part by the U.S. Department of Agriculture (grant no. 95–37304–2228). J.G.D. was supported by the Mexican Council for Science and Technology during his sabbatical leave.
LITERATURE CITED
- Barlow PW, Rathfelder EL. Cell division and regeneration in primary root meristems of Zea mays recovering from cold treatment. Environ Exp Bot. 1985;25:303–314. [Google Scholar]
- Baum SF, Rost TL. Root apical organization in Arabidopsis thaliana: 1. Root cap and protoderm. Protoplasma. 1996;192:178–188. [Google Scholar]
- Beemster GTS, Baskin TI. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 1998;116:1515–1526. doi: 10.1104/pp.116.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Chen-Yi H, Dolan L, Schiefelbein J. Control of cell division in the root epidermis of Arabidopsis thaliana. Dev Biol. 1998;194:235–245. doi: 10.1006/dbio.1997.8813. [DOI] [PubMed] [Google Scholar]
- Blakely LM, Durham M, Evans TA, Blakely RM. Experimental studies on lateral root formation in radish seedling roots: I. General methods, developmental stages, spontaneous formation of laterals. Bot Gaz. 1982;143:341–352. [Google Scholar]
- Blakely LM, Evans TA. Cell dynamics studies on the pericycle of radish seedling roots. Plant Sci Lett. 1979;14:79–83. [Google Scholar]
- Bowman JL, Eshed Y. Formation and maintenance of the shoot apical meristem. Trends Plant Sci. 2000;5:110–115. doi: 10.1016/s1360-1385(00)01569-7. [DOI] [PubMed] [Google Scholar]
- Burholt DR, Van't Hof J. Quantitative thermal-induced changes in growth and cell proliferation kinetics of Helianthus roots. Am J Bot. 1971;58:386–393. [Google Scholar]
- Celenza JL, Jr, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Charlton WA. Distribution of lateral roots and sequence of lateral initiation in Pontederia cordata L. Bot Gaz. 1975;136:225–235. [Google Scholar]
- Charlton WA. Evaluation of sequence and rate of lateral root initiation in Pontederia cordata L. by means of colchicine inhibition of cell division. Bot Gaz. 1977;138:71–79. [Google Scholar]
- Charlton WA. Lateral root initiation. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: The Hidden Half. Ed 2. New York: Marcel Dekker; 1996. pp. 149–173. [Google Scholar]
- Cheng JC, Seeley KA, Sung ZR. RML1 and RML2, Arabidopsis genes required for cell proliferation at the root tip. Plant Physiol. 1995;107:365–376. doi: 10.1104/pp.107.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S-H, Gifford EM. Development of the root of Ceratopteris thalictroides with special reference to apical segmentation. J Indian Bot Soc. 1971;50A:96–106. [Google Scholar]
- Clowes FAL. Apical Meristems. F.A. Philadelphia: Davis Company; 1961. [Google Scholar]
- Clowes FAL. Changes in cell population kinetics in an open meristem during root growth. New Phytol. 1982;91:741–748. [Google Scholar]
- Colón-Carmona A, You R, Haimovitch-Gal T, Doerner P. Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 1999;20:503–508. doi: 10.1046/j.1365-313x.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- Demchenko KN. Cell proliferation during lateral root initiation. PhD thesis. St. Petersburg, Russia: V.L. Komarov Botanical Institute of the Russian Academy of Sciences; 1999. [Google Scholar]
- Demchenko NP. Changes in population structure of epidermal, endodermal, and pericycle cells in the course of their development in the wheat root. Tsitologia. 1987;29:174–181. [Google Scholar]
- De Tomasi JA. Improving the technic of the Feulgen stain. Stain Technol. 1936;11:137–144. [Google Scholar]
- De Veylder L, de Almeida Engler J, Burssens S, Manevski A, Lescure B, Van Montagu M, Engler G, Inzé D. A new D-type cyclin of Arabidopsis thaliana expressed during lateral root primordia formation. Planta. 1999;208:453–462. doi: 10.1007/s004250050582. [DOI] [PubMed] [Google Scholar]
- Dienstman SR, Holtzer H. Myogenesis: a cell lineage interpretation. In: Reinert J, Holtzer H, editors. Cell Cycle and Cell Differentiation. Heidelberg: Springer-Verlag; 1975. pp. 1–25. [Google Scholar]
- Doerner P, Jørgensen J-E, You R, Steppuhn J, Lamb C. Control of root growth and development by cyclin expression. Nature. 1996;380:520–523. doi: 10.1038/380520a0. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG. Origin of tissues of embryonic lateral root in the cucumber, tissue interactions, and positional control in development. Ontogenez. 1986;17:176–189. (English translation from Russian appeared in Sov J Dev Biol, New York, N.Y., Consultant Bureau 17: 119–128) [Google Scholar]
- Dubrovsky JG. Latent embryonic root system in the cucumber. Bot Zh. 1987;72:171–176. [Google Scholar]
- Dubrovsky JG. Determinate primary-root growth in seedlings of Sonoran Desert Cactaceae: its organization, cellular basis, and ecological significance. Planta. 1997;203:85–92. [Google Scholar]
- Dubrovsky JG, Ivanov VB. Certain mechanisms of lateral root initiation in germinating maize roots. Fiziol Biokhim Kul't Rast. 1984;16:279–284. [Google Scholar]
- Dubrovsky JG, Puente ME, Bashan Y. Arabidopsis thaliana as a model system for the study of the effect of Azospirillum brasilense Sp-245 on root hair growth. Soil Biol Biochem. 1994;26:1657–1664. [Google Scholar]
- Ferreira PCG, Hemerly A, de Almeida Engler J, Van Montagu M, Engler G, Inzé D. Developmental expression of the Arabdopsis cyclin gene cyc1At. Plant Cell. 1994;6:1763–1774. doi: 10.1105/tpc.6.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foard DE, Haber AH, Fishman TN. Initiation of lateral root primordia without completion of mitosis and without cytokinesis in uniseriate pericycle. Am J Bot. 1965;52:580–590. [Google Scholar]
- Gladish DK, Rost TL. The effects of temperature on primary root growth dynamics and lateral root distribution in garden pea (Pisum sativum L. cv Alaska) Environ Exp Bot. 1993;33:243–258. [Google Scholar]
- Goldacre PL. Potentiation of lateral root induction by root initials in isolated flax roots. Aust J Biol Sci. 1959;12:388–394. [Google Scholar]
- Hemerly AS, Ferreira P, de Almeida Engler J, Van Montagu M, Engler G, Inze D. cdc2a expression in Arabidopsis is linked with competence for cell division. Plant Cell. 1993;5:1711–1723. doi: 10.1105/tpc.5.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchee MAW, Rost TL. The control of lateral root development in cultured pea seedlings: III. Spacing intervals. Bot Acta. 1992;105:127–131. [Google Scholar]
- Holtzer H, Holtzer S. Lineages, quantal cell cycles and cell diversification. In: Müller-Bérat N, Rosenfeld C, Tarin D, Viza D, editors. Progress in Differentiation Research. Amsterdam: North-Holland Publishing Company; 1976. pp. 3–10. [Google Scholar]
- Holtzer H, Weintraub H, Mayne R, Mochan B. The cell cycle, cell lineage and cell differentiation. In: Moscona A, Monroy A, editors. Current Topics in Developmental Biology. New York: Academic Press; 1972. pp. 229–256. [DOI] [PubMed] [Google Scholar]
- Ivanov VB. Kletochnye Osnovy Rosta Rastenii. Moscow: Nauka; 1974. [Google Scholar]
- Ivanov VB. Cellular basis of root growth. Sov Sci Rev. 1981;D2:365–392. [Google Scholar]
- Ivanov VB, Dubrovsky JG. Estimation of the cell-cycle duration in the root meristem: a model of linkage between cell-cycle duration, rate of cell production, and rate of root growth. Int J Plant Sci. 1997;158:757–763. [Google Scholar]
- Laskowski MJ, Williams ME, Nusbaum HC, Sussex IM. Formation of lateral root meristems is a two-stage process. Development. 1995;121:3303–3310. doi: 10.1242/dev.121.10.3303. [DOI] [PubMed] [Google Scholar]
- Laufs P, Grandjean O, Jonak C, Kiêu K, Traas J. Cellular parameters of the shoot apical meristem in Arabidopsis. Plant Cell. 1998;10:1375–1390. doi: 10.1105/tpc.10.8.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B-L, Raghavan V. Lateral root initiation in Marsilea quadrifolia: I. Origin and histogenesis of lateral roots. Can J Bot. 1991;69:123–135. doi: 10.1139/b91-018. [DOI] [PubMed] [Google Scholar]
- Luxová M, Murín A. The extent and differences in mitotic activity of the root tip of Vicia faba L. Biol Plant. 1973;15:37–43. [Google Scholar]
- MacLeod RD. Lateral root primordium inception in Zea mays L. Environ Exp Bot. 1990;30:225–234. [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Mallory TE, Chiang S-H, Cutter EG, Gifford EM. Sequence and pattern of lateral root formation in five selected species. Am J Bot. 1970;57:800–809. [Google Scholar]
- Martinez MC, Jørgensen J-E, Lawton MA, Lamb CJ, Doerner PW. Spatial pattern of cdc2 expression in relation to meristem activity and cell proliferation during plant development. Proc Natl Acad Sci USA. 1992;89:7360–7364. doi: 10.1073/pnas.89.16.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell DH, Foard DE. Presence of lateral root primordia in the radicle of buckwheat embryos. Bull Torrey Bot Club. 1969;96:1–3. [Google Scholar]
- Rost TL, Jones TJ, Falk RH. Distribution and relationship of cell division and maturation events in Pisum sativum (Fabaceae) seedling roots. Am J Bot. 1988;75:1571–1583. [Google Scholar]
- Rost TL, Reynolds T. Reversal of chlorsulfuron-induced inhibition of mitotic entry by isoleucine and valine. Plant Physiol. 1985;77:481–482. doi: 10.1104/pp.77.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost TL, Sammut M. Regulation of cell division in pea root tips after wounding: a possible role for ethylene. Protoplasma. 1982;111:1–9. [Google Scholar]
- Scheres B, Benfey PN. Asymmetric cell division in plants. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:505–537. doi: 10.1146/annurev.arplant.50.1.505. [DOI] [PubMed] [Google Scholar]
- Torrey JG. Endogenous and exogenous influences on the lateral root formation. In: Jackson MB, editor. New Root Formation in Plants and Cuttings. Dordrecht, Germany: Martinus Nijhoff Publishers; 1986. pp. 31–66. [Google Scholar]
- Van't Hof J, Saha S. Cotton fibers can undergo cell division. Am J Bot. 1997;84:1231–1235. [PubMed] [Google Scholar]
- Willemsen V, Wolkenfelt H, de Vrieze G, Weisbeek P, Scheres B. The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development. 1998;125:521–531. doi: 10.1242/dev.125.3.521. [DOI] [PubMed] [Google Scholar]
- Yee VF, Rost TL. Polyethylene glycol induced water stress in Vicia faba seedlings: cell division, DNA synthesis and a possible role for cotyledons. Cytologia. 1982;47:615–624. [Google Scholar]