Abstract
Background
There is a peculiar phenomenon: two separate individuals (mother and foetus) have a mutually interactive dependency concerning their respective weight. Very thin mothers have a higher risk of small for gestational age (SGA) infants, and rarely give birth to a large for gestational age (LGA) infant. While morbidly obese women often give birth to LGA infants, and rarely to SGA. Normal birthweight (AGA) infants (>10th and <90th centile of a neonatal population) typically have the lowest perinatal and long-term morbidity. The aim of the current study is (1) to determine the maternal body mass index (BMI) range associated with a balanced risk (10% SGA, 10% LGA), and (2) to investigate the interaction between maternal booking BMI, gestational weight gain (GWG) and neonatal birthweight centiles.
Methods
16.5 year-observational cohort study (2001–2017). The study population consisted of all consecutive singleton term (37 weeks onward) live births delivered at University's maternity in Reunion island, French Overseas Department.
Findings
Of the 59,717 singleton term live births, we could define the booking BMI and the GWG in 52,092 parturients (87.2%). We had 2 major findings (1) Only women with a normal BMI achieve an equilibrium in the SGA/LGA risk (both 10%). We propose to call this crossing point the Maternal Fetal Corpulence Symbiosis (MFCS). (2) This MFCS shifts with increasing GWG. We tested the MFCS by 5 kg/m2 incremental BMI categories. The result is a linear law:
opGWG (kg) = −1.2 ppBMI (Kg/m²) + 42 ± 2 kg
Interpretation
IOM-2009 recommendations are adequate for normal and over-weighted women but not for thin and obese women: a thin woman (17 kg/m2) should gain 21.6 ± 2 kg (instead of 12.5–18). An obese 32 kg/m2 should gain 3.6 kg (instead of 5–9). Very obese 40 kg/m2 should lose 6 kg.
Keywords: Nutrition, Public health, Reproductive medicine
1. Introduction
Knowing the optimal gestational weight gain (GWG, from conception to birth) among the annual 135 million of human pregnancies worldwide is considered to be one of the “Holy Grail” for maternity health care providers, neonatologists and epidemiologists. Extensive literature exists on the subject with, in background, the current international cornerstone which is the 2009-IOM recommendations [1] based on the WHO-BMI classification [2] underweight women (before pregnancy) <18.5 kg/m2 should have a GWG between 12.5 and 18 kg, normal weight, 18.5–24.9 kg/m2, a GWG of 11.5–16 kg, overweight, 25–29.9 kg/m2, a GWG of 7–11.5 kg, and obese >30 kg/m2 a GWG of 5–9 kg. Evolution of ideas on the subject are well reported in a recent paper [3]: besides the eternal well accepted social dogma of “eating for two”, medicine tended to recommend a GWG of 7 kg per pregnancy before 1945 [3]. The experience of the 1944 Dutch famine lead to the liberalization of this view to evolve toward the real first international guidelines for GWG in 1990 [4]. Finally, with the increasing worldwide prevalence of overweight and obesity over the last 4-5 decades, revised IOM guidelines were proposed in 2009 [1], more tailored to maternal booking BMI in line with the WHO BMI.
Since then, multiple papers debated two main controversies: 1) are the IOM guidelines, made mainly on Caucasian population, adequate for other population such as Asian women for example? For Chinese, Japanese, and Korean scholars, the answer is clearly no [5, 6, 7, 8], and they even propose an Asian classification where overweight would begin at 23 kg/m2, and not at 25 [6, 7]). These authors also concluded that, the IOM recommendations are too low for underweight women [5, 6, 7, 8]. 2) the obesity problem: are the 5–9 kg recommendations also adequate for obese pregnant women? This is in fact the research core of different meta-analysis [9, 10, 11, 12]: for obese women shouldn't we accept a GWG below 5 kg, or even a gestational weight loss [9, 13, 14, 15]? The puzzle is further complicated by the fact that the American College of Obstetrics and Gynecology committee has in 2013 stated that if an obese woman is gaining weight below the recommendation, but has an appropriately growing fetus, the potential benefits might be more than those gaining weight within the guidelines [15].
Further, there is a growing consensus to differentiate within obese women 3 class of obesity [9, 16, 17]: class 1 (30–34.9 kg/m2), class 2 (35–39.9 kg/m2) and class 3 (40 kg/m2 and over). The debate is “should super obese women lose weight during pregnancy?”. According to Kiel et al [13], class 1 women should gain between 4.5 and 11 kg (vs IOM 5–9 kg, all obese), class 2 women should gain between 0 and 4 kg, and class 3 women should lose between 1 and 4 kg, while for Marguerison Zilko et al [18] or Oken et al [19] these class 3 women should lose 7 kg. Swank et al on their side studied specifically the “super obese”, i.e. >50 kg/m2 [20], and reported that in these women gaining weight below the recommendations (therefore less than 5 kg), was not associated with an increase of pre-term births or low birthweight, while there was a significant reduction of birthweight more than 4,000 g. While Kapadia et al [11], in a recent meta analysis, concluded that gestational weight loss should not be advocated in general for obese women.
Whatever, the central tenet of all these analyses is that all authors had chosen as primary outcomes of their studies: small for gestational age (SGA), large for gestational age (LGA) newborns. Taking SGA-LGA (international consensus to study this subject) as somewhere a final end-point seems rational considering that the finality of a pregnancy being to have a normal birthweight (AGA) infant, the gestational weight gain of the mother is completely linked to this goal. Both SGA and LGA are well known to have some immediate morbidities, but moreover long-term effect on the future life of the individual [21, 22, 23].
The 2 aims of this study are: First, to establish in term deliveries the “natural tendency” of SGA-LGA association per maternal BMI and, second to establish if there is an association between GWG and the 10% crossing point SGA-LGA for each maternal BMI category.
2. Material and methods
From January 1st, 2001, to 30 June 2017, the hospital records of all women delivered at the maternity of the University South Reunion Island (ap. 4,300 births per year) were abstracted in a standardized fashion. All data were entered into an epidemiological perinatal data base which contained information on obstetrical risk factors, description of deliveries and neonatal outcomes. As participants in the French national health care system, all pregnant women in Reunion Island have their prenatal visits, biological and ultrasound examinations, and anthropological characteristics recorded in their maternity booklet. In our term pregnancies the average of prenatal visits was 9.2 ± 2, and 4.2 ± 1.7 ultrasounds per pregnancy.
In the general analysis, there were three criteria of exclusion: Preterm births (<37 weeks), multiple births and fetal deaths (in utero fatal deaths, stillbirths and medical termination of pregnancy).
Small for gestational age (SGA, ≤10th percentile) and Large for gestational age babies (LGA, ≥90th percentile) have been defined by a local curve established from our perinatal data base [24].
Reunion Island is a French department in the Southern Indian Ocean. The peculiarity of this tropical region lays in the multiethnic origin of inhabitants [Africa and intermixed population (50%), Europe (27%), India (20%) and China (3%)]. Compared to Europe and mainland France, there is a younger reproductive population (the mean age at primiparity is 23 years). Finally, accessibility to maternity services is easy, and high-quality care is provided free of charge by the French healthcare system.
Epidemiological data have been recorded and analysed with the software EPI-INFO 7.1.5 (2008, CDC Atlanta, OMS), EPIDATA 3.0 and EPIDATA Analysis V2.2.2.183.
Ethics approval: This study analyzing data anonymously was exempt from approval of institutional review board (Comité de Protection des Personnes Sud-Ouest et Outre Mer III) and according to French legislation written consent.
3. Results
There were 60,870 term births (37 weeks onward) at the South Reunion maternity during the 16.5 year period (January 1, 2001–June 30, 2017). Multiple pregnancies (N = 508 pregnancies and 1,029 newborns), fetal deaths (in utero N = 108, 13 still births at term and 3 medical termination of pregnancy) were excluded from further analysis. The live-birth term population consisted of 59,717 singleton pregnancies. The final study population consisted of 52,092 patients (87.2%), where we could define the maternal pre-pregnancy BMI and the Gestational Weight Gain.
-
1.
What is the “natural tendency” of SGA-LGA association per maternal BMI?
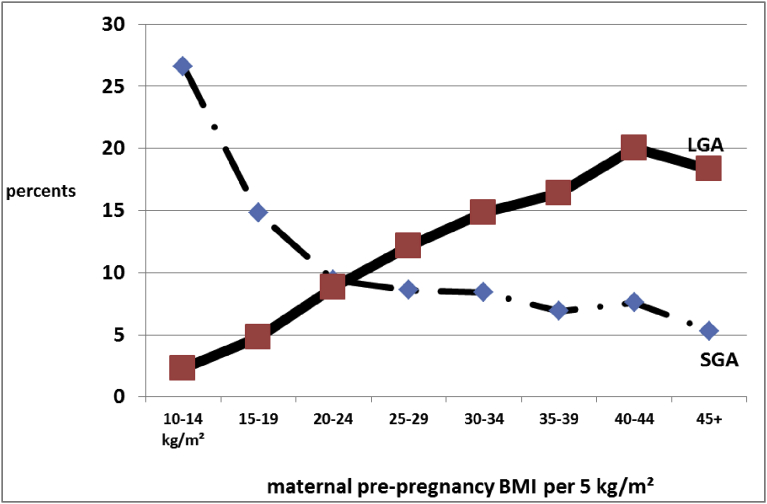
All is summarized in Fig. 1: we found that the natural tendency for thin women (10–14 kg/m2) was to spontaneously have some 25% of SGA babies and some 2% of LGA babies, while on the other side of the spectrum, very obese women (40–44 kg/m2) had spontaneously some 20% of LGA and 5% of SGA newborns, Fig. 1.
Fig. 1.
“Natural Tendency” of SGA and LGA incidences (percentages) per maternal pre-pregnancy BMI in our singleton term (≥37 weeks gestation) pregnancies. N = 52,092.
In Fig. 1, we see that 1) Only women with a normal BMI achieve an equilibrium in the SGA/LGA risk (both 10%). We propose to call this crossing point the Maternal Fetal Corpulence Symbiosis (MFCS).
-
2)
What is the association between GWG and the 10% crossing point SGA-LGA (MFCS) for each maternal BMI category?
Calculations for the Figures were based on the WHO BMI classification, and the (<18.5, 18.5–24.9, 25–29.9, 30 and plus) IOM recommendations. However, data in the tables will be presented using, 5 kg/m2 increments in order to have regular intervals. Figs. 2, 3, 4, and 5 show the incidences (%) of SGA-LGA newborns For all categories of maternal pre-pregnancy BMI, and GWG. The data clearly showed that there is a regular shift from the right to the left of the MFCS crossing point beginning in very thin mothers (10–14.9 kg/m2) to the obese (30–39.9 kg/m2). This shift appears to be quite regular, and even linear.
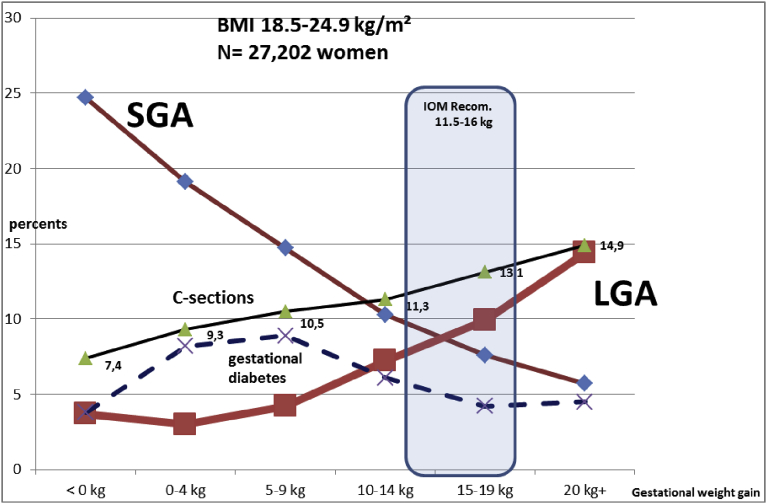
Fig. 2.
Evolution of the small for gestational age (SGA) and large for gestational age (LGA) term babies by maternal gestational weight gain (GWG). Underweight women (<18.5 kg/m2). N = 5252 women.
Fig. 3.
Evolution of the small for gestational age (SGA) and large for gestational age (LGA) term babies by maternal gestational weight gain (GWG). Normal Weight women (18.5–24.9 kg/m2). N = 27,202 women.
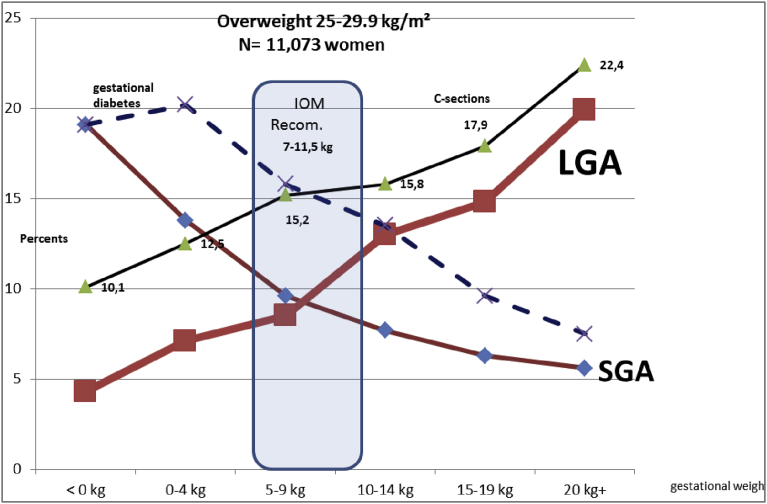
Fig. 4.
Evolution of the small for gestational age (SGA) and large for gestational age (LGA) term babies by maternal gestational weight gain (GWG). Over Weight women (25–29.9 kg/m2). N = 11,073 women.
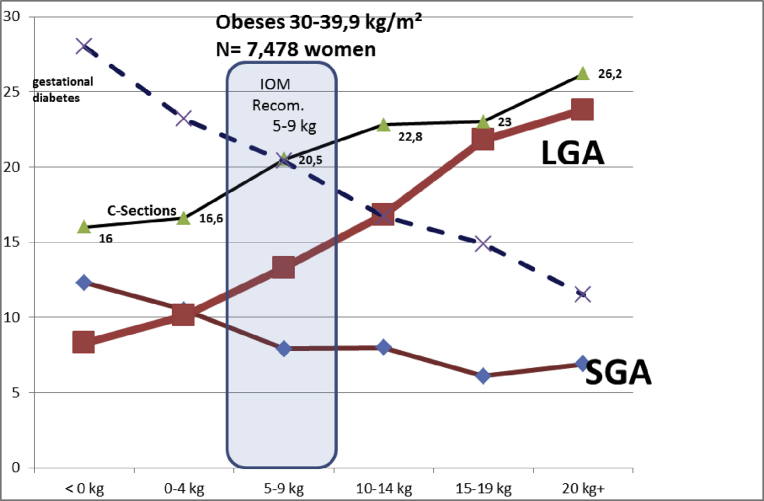
Fig. 5.
Evolution of the small for gestational age (SGA) and large for gestational age (LGA) term babies by maternal gestational weight gain (GWG). Obese women (30–39.9 kg/m2). N = 7,478 women.
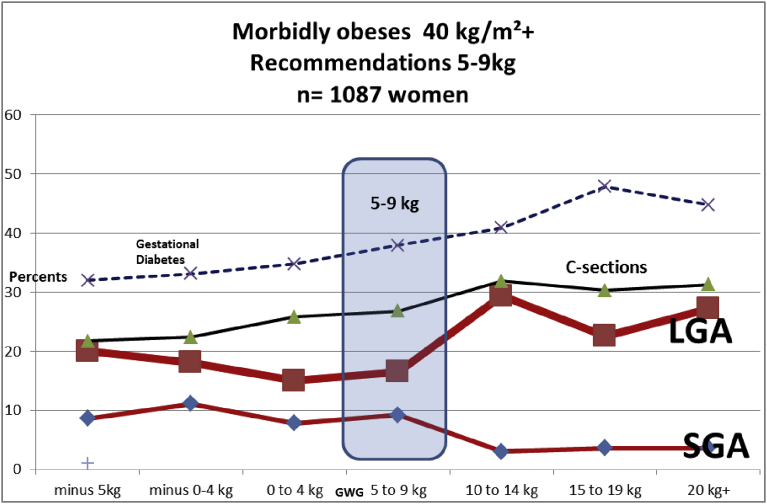
In Fig. 6, and Table 3, we tested all these items in very obese women: we could not achieve to have a SGA-LGA MFCS crossing point in our 1,087 very obese women.
Fig. 6.
Evolution of the small for gestational age (SGA) and large for gestational age (LGA) term babies by maternal gestational weight gain (GWG). Obese women (over 40 kg/m2). N = 1,087 women.
Table 3.
Incidence of SGA-LGA and different secondary variables, Obesity class II and III women (MFCS point #).
| OBESITY CLASS II: 35–39.9 kg/m2. N = 2,208 (MFCS point #) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gestational weight gain | Weight loss Minus 5 kg and below N = 16 |
Weight loss Minus 0–4.9 kg N = 57 |
Weight gain 0–4.9 kg N = 634 |
Weight gain 5–9.9 kg N = 648 |
Weight gain 10–14.9 kg N = 490 |
Weight gain 15–19.9 kg N = 227 |
Weight gain 20kg and over N = 136 |
GLOBAL INCIDENCE (%) N = 2208 |
| SGA Small for gestational age (%) | 2 (12.5) |
5 (8.8) |
58 (9.1) # |
48 (7.4) |
29 (5.9) |
5 (2.2) |
5 (3.6) |
(6.9) |
| LGA Large for gestational age (%) | 1 (6.2) |
7 (12.3) |
67 (10.6) # |
88 (13.6) |
103 (21.0) |
54 (23.8) |
43 (31.6) |
(16.4) |
|
Cesarean section N = 21,235 (%) |
2 (12.5) |
9 (15.8) |
122 (19.2) |
145 (22.4) |
132 (26.9) |
58 (25.6) |
39 (28.6) |
(23.0) |
|
Gestational Diabetes N = (%) |
7 (43.7) |
21 (36.8) |
167 (26.3) |
149 (22.9) |
91 (18.6) |
45 (19.8) |
22 (16.2) |
(23.5) |
| Chronic high blood pressure (%) | 1 (6.2) |
2 (3.5) |
29 (4.6) |
28 (4.3) |
17 (3.5) |
11 (4.9) |
7 (5.1) |
(4.3) |
|
Preeclampsia (%) |
0 (0) |
1 (1.8) |
8 (1.3) |
16 (2.5) |
12 (2.4) |
6 (2.7) |
9 (6.6) |
(2.4) |
| OBESITY CLASS III: 40 kg/m2 and over. N = 1,087 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gestational weight gain | Weight loss Minus 5 kg and below N = 35 |
Weight loss Minus 0–4.9 kg N = 72 |
Weight gain 0–4.9 kg N = 360 |
Weight gain 5–9.9 kg N = 284 |
Weight gain 10–14.9 kg N = 197 |
Weight gain 15–19.9 kg N = 84 |
Weight gain 20kg and over N = 55 |
GLOBAL INCIDENCE (%) N = 1,087 |
| SGA Small for gestational age (%) | 3 (8.6) |
8 (11.1) |
28 (7.8) |
26 (9.2) |
6 (3.0) |
3 (3.6) |
2 (3.6) |
(7.0) |
| LGA Large for gestational age (%) | 7 (20) |
13 (18.1) |
54 (15.0) |
47 (16.5) |
58 (29.4) |
19 (22.6) |
15 (27.2) |
(19.6) |
|
Cesarean section N = 21,235 (%) |
8 (22.8) |
17 (23.6) |
91 (25.3) |
78 (27.5) |
68 (34.5) |
26 (31.0) |
17 (30.9) |
(28.1) |
|
Gestational Diabetes N = (%) |
8 (22.8) |
22 (31.4) |
107 (29.7) |
65 (22.8) |
52 (27.2) |
19 (22.6) |
16 (29.0) |
(27.6) |
| Chronic high blood pressure (%) | 1 (2.8) |
6 (8.3) |
26 (7.2) |
23 (8.1) |
18 (9.1) |
12 (14.3) |
6 (10.9) |
(8.5) |
|
Preeclampsia (%) |
0 (0) |
1 (1.4) |
5 (1.4) |
10 (3.5) |
4 (2.0) |
4 (4.8) |
3 (5.4) |
(2.5) |
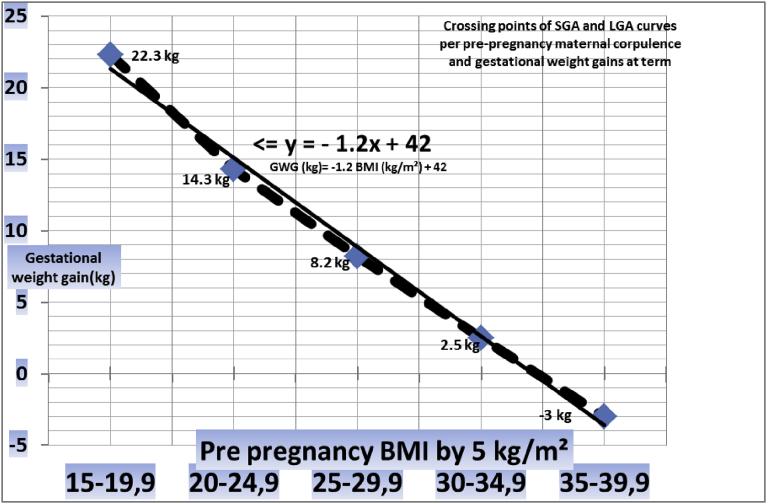
To test the possible linearity of the MFCS point drift per maternal pre-pregnancy categories, we recalculated all these variables with regular BMI intervals of 5 kg/m2, Tables 1, 2, and 3. The results are shown in the Tables and are summarized in Fig. 7: weight gains corresponding to MFCS crossing points describe a linear curve (R2 0.99), which can be calculated as:
| opGWG (kg) = −1.2 ppBMI (Kg/m²) + 42 ± 2 kg |
(opGWG: optimal gestational weight gain, ppBMI: maternal pre-pregnancy body mass index)
Table 1.
Incidence of SGA-LGA and different secondary variables, underweight and normal weighted women (MFCS point #).
| UNDERWEIGHT: 15–19.9 kg/m. N = 11,071 | |||||||
|---|---|---|---|---|---|---|---|
| Gestational weight gain | Weight loss Minus 1 kg and below N = 1 |
Weight gain 0–4.9 kg N = 225 |
Weight gain 5–9.9 kg N = 1799 |
Weight gain 10–14.9 kg N = 4694 |
Weight gain 15–19.9 kg N = 3029 |
Weight gain 20kg and over N = 1323 |
GLOBAL INCIDENCE (%) N = 11,071 |
| SGA Small for gestational age (%) | – | 71 (31.6) |
431 (24.0) |
714 (15.2) |
317 (10.5) |
108 (8.1) # |
(14.8) |
| LGA Large for gestational age (%) | – | 3 (1.3) |
30 (1.7) |
193 (4.1) |
189 (6.2) |
113 (8.5)# |
(4.8) |
|
Cesarean section N = 21,235 (%) |
– | 16 (7.1) |
143 (7.9) |
450 (9.6) |
297 (9.8) |
143 (10.8) |
(9.5) |
|
Gestational Diabetes N = (%) |
– | 11 (4.9) |
99 (5.5) |
159 (3.4) |
84 (2.8) |
41 (3.1) |
(3.6) |
| Chronic high blood pressure (%) | – | 0 (0) |
3 (0.2) |
13 (0.3) |
8 (0.3) |
5 (0.4) |
(0.3) |
|
Preeclampsia (%) |
– | 1 (0.4) |
10 (0.6) |
17 (0.4) |
17 (0.6) |
18 (1.4) |
(0.6) |
| NORMAL WEIGHT: 20–24.9 kg/m2. N = 21,206 | |||||||
|---|---|---|---|---|---|---|---|
| Gestational weight gain | Weight loss Minus 1 kg and below N = 18 |
Weight gain 0–4.9 kg N = 887 |
Weight gain 5–9.9 kg N = 4100 |
Weight gain 10–14.9 kg N = 8365 |
Weight gain 15–19.9 kg N = 5243 |
Weight gain 20kg and over N = 2593 |
GLOBAL INCIDENCE (%) N = 21,206 |
| SGA Small for gestational age (%) | 4 (22.2) |
161 (18.2) |
539 (13.1) |
779 (9.3) # |
369 (7.0) |
139 (5.3) |
(9.4) |
| LGA Large for gestational age (%) | 3 (16.6) |
28 (3.2) |
192 (4.7) |
664 (7.9) # |
567 (10.8) |
407 (15.6) |
(8.8) |
|
Cesarean section N = 21,235 (%) |
1 (5.5) |
82 (9.2) |
484 (11.1) |
985 (11.8) |
743 (14.2) |
420 (16.2) |
(12.7) |
|
Gestational Diabetes N = (%) |
1 (5.5) |
72 (8.2) |
390 (9.5) |
565 (6.8) |
240 (4.6) |
124 (4.7) |
(6.6) |
| Chronic high blood pressure (%) | 0 (0) |
4 (0.5) |
30 (0.7) |
40 (0.5) |
30 (0.6) |
13 (0.5) |
(0.6) |
|
Preeclampsia (%) |
0 (0) |
3 (0.3) |
19 (0.5) |
51 (0.6) |
48 (0.9) |
50 (1.9) |
(0.8) |
Table 2.
Incidence of SGA-LGA and different secondary variables, overweight and obesity class I women (MFCS point #).
| OVERWEIGHT: 25–29.9 kg/m. N = 11,073 | |||||||
|---|---|---|---|---|---|---|---|
| Gestational weight gain | Weight loss Minus 1 kg and below N = 44 |
Weight gain 0–4.9 kg N = 1280 |
Weight gain 5–9.9 kg N = 2955 |
Weight gain 10–14.9 kg N = 3686 |
Weight gain 15–19.9 kg N = 1990 |
Weight gain 20kg and over N = 1113 |
GLOBAL INCIDENCE (%) N = 11,073 |
| SGA Small for gestational age (%) | 10 (22.7) |
183 (14.3) |
285 (9.6) # |
284 (7.7) |
125 (6.3) |
63 (5.6) |
(8.6) |
| LGA Large for gestational age (%) | 1 (2.2) |
88 (6.9) |
251 (8.5) # |
481 (13.0) |
295 (14.8) |
223 (20.0) |
(12.1) |
|
Cesarean section N = 21,235 (%) |
2 (4.5) |
159 (12.4) |
448 (15.2) |
583 (15.8) |
357 (17.9) |
250 (22.5) |
(16.2) |
|
Gestational Diabetes N = (%) |
6 (13.6) |
258 (20.1) |
459 (15.5) |
493 (13.4) |
189 (9.5) |
83 (7.4) |
(13.6) |
| Chronic high blood pressure (%) | 0 (0) |
30 (2.3) |
62 (2.1) |
65 (1.8) |
25 (1.3) |
14 (1.3) |
(1.8) |
|
Preeclampsia (%) |
0 (0) |
9 (0.7) |
17 (0.6) |
37 (1.0) |
19 (1.0) |
29 (2.6) |
(1.0) |
| OBESITY CLASS I: 30–34.9 kg/m2. N = 5,270 | |||||||
|---|---|---|---|---|---|---|---|
| Gestational weight gain | Weight loss Minus 1 kg and below N = 80 |
Weight gain 0–4.9 kg N = 1107 |
Weight gain 5–9.9 kg N = 1615 |
Weight gain 10–14.9 kg N = 1426 |
Weight gain 15–19.9 kg N = 649 |
Weight gain 20kg and over N = 393 |
GLOBAL INCIDENCE (%) N = 5,270 |
| SGA Small for gestational age (%) | 10 (12.5) |
109 (9.8) # |
123 (7.6) |
125 (8.8) |
47 (7.2) |
31 (7.9) |
(8.4) |
| LGA Large for gestational age (%) | 3 (3.7) |
115 (10.4) # |
220 (13.6) |
222 (15.6) |
137 (21.1) |
83 (21.1) |
(14.8) |
|
Cesarean section N = 21,235 (%) |
10 (12.5) |
180 (16.3) |
328 (20.3) |
320 (22.4) |
154 (23.7) |
103 (26.2) |
(20.8) |
|
Gestational Diabetes N = (%) |
16 (20.0) |
254 (22.9) |
318 (19.7) |
229 (16.1) |
95 (14.6) |
43 (10.9) |
(18.5) |
| Chronic high blood pressure (%) | 1 (1.2) |
29 (2.6) |
66 (4.1) |
54 (3.8) |
19 (2.9) |
12 (3.1) |
(3.4) |
|
Preeclampsia (%) |
0 (0) |
10 (0.9) |
17 (1.1) |
23 (1.6) |
13 (2.0) |
15 (3.8) |
(1.5) |
Fig. 7.
Crossing points (MFCS) of SGA and LGA curves per pre-pregnancy maternal corpulence and gestational weight gains at term.
Post testing of the equation, Table 4: we tested the MFCS points in pregnant women with a BMI at the extreme of the spectrum (MFCS points #)
-
1)
Those who “theoretically” by the equation should gain 20 kg and plus: BMI < 19 kg/m2, N = 6830
-
2)
Those who “theoretically” by the equation should lose weight: BMI 36 kg/m2 and plus, N = 2494
Table 4.
Post testing of the equation in thin women (BMI < 19 kg/m2).
| UNTIL WEIGHT GAIN 25 kg and plus. N = 6830 women (MFCS point #) | |||||||
|---|---|---|---|---|---|---|---|
| Gestational weight gain |
Weight loss N = 1 |
Weight gain 0–4.9 kg N = 123 |
Weight gain 5–9.9 kg N = 1111 |
Weight gain 10–14.9 kg N = 2904 |
Weight gain 15–19.9 kg N = 1854 |
Weight gain 20–24.9kg N = 646 |
Weight gain 25kg and over N = 191 |
| SGA Small for gestational age (%) | -- | 39 (31.7) |
305 (27.5) |
465 (16.0) |
215 (11.6) |
64 (9.9) # |
7 (3.6) |
| LGA Large for gestational age (%) | -- | 2 (1.6) |
15 (1.4) |
103 (3.5) |
101 (5.4) |
48 (7.4) # |
17 (8.9) |
| Post testing of the equation in very obese women (BMI 36 kg/m2 and over) | ||||||||
|---|---|---|---|---|---|---|---|---|
| UNTIL WEIGHT LOSS 10 kg and below. N = 2687 women (MFCS points #) | ||||||||
| Gestational weight gain | Weight loss Minus 10 kg and below N = 17 |
Weight loss Minus 5–9.9 kg N = 32 |
Weight loss Minus 0–4.9 kg N = 120 |
Weight gain 0–4.9 kg N = 832 |
Weight gain 5–9.9 kg N = 731 |
Weight gain 10–14.9 kg N = 554 |
Weight gain 15–19.9 kg N = 248 |
Weight gain 20kg and over N = 154 |
| SGA Small for gestational age (%) | 2 (11.7) |
3 (9.4) # |
12 (10.0) |
71 (8.5) |
63 (8.6) |
30 (5.4) |
6 (2.4) |
6 (3.9) |
| LGA Large for gestational age (%) | 3 (17.6) |
4 (12.5) # |
20 (16.7) |
16 (12.7) |
107 (14.6) |
140 (25.3) |
56 (22.6) |
46 (29.9) |
4. Discussion
Notthstanding hundreds of studies and meta-analysis made on the subject [5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 25, 26], no consensus has been reached regarding the optimal GWG for different maternal BMI categories [9, 10, 11, 12].
Our data show that the so-called SGA-LGA crossing point (10% SGA and 10% LGA) happens “naturally” for normal weighted women (20–24 kg/m2), Fig. 1. This is a surprising findings, as the very definition of SGA (10th percentile of a given neonatal population) and LGA (90Th percentile) have never been designed to correspond in any matter with maternal BMI. The fact that this 10% SGA/LGA point corresponds to a given maternal BMI category suggest that there is a kind of biological maternal-foetal connection. This was proposed by Kapadia et al [9, 11] who wrote that it should have “a graded relationship between maternal weight gain (or loss) and infant size” [11].
The 2nd main findings was that for all categories of maternal pre-pregnancy BMI and GWG, we did notice that there is a linear shift from the left of the MFCS crossing point beginning in very thin mothers (10–14.9 kg/m2) to the obese (30–39.9 kg/m2), Tables 1, 2, and 3. The fact that this linear association between maternal pre-pregnancy BMI and GWG has now finally been deciphered will greatly facilitate an individualized approach when advising women about their optimal GWG without needing to put them in fixed categories (underweight, normal, overweight, obese, very obese, super obese etc…). This will enable maternity care providers and the pregnant women to agree on the optimal GWG, e.,g. “you have a BMI of 17.5 kg/m2, our common goal is that you should try to gain 21 kg during this pregnancy (±2 kg), versus “your BMI is 33 kg/m2, you need to try to restrict your weight gain to 2.4 kg”. While very obese women e.g. 38 kg/m2, should try to lose 3–4 kg. These findings should resolve the ongoing debate among researchers facing populations with a high incidence of obesity, many of these were already claiming that these women should lose weight during pregnancy [13, 18].
We would like to encourage other populations (particularly Asian population representing 25–30% of mankind), to establish their own maternal BMI-GWG association. Our formula is valid for all pre-pregnancy maternal BMI including obesity class II (<40 kg/m2). For the “super obese” BMI > 40 kg/m2 we could not establish the MFCS crossing point (Fig. 6, Table 3). This could partially be due to the fact that BMI > 40 is rare our population.: we had in our population “only” 1,087 women over 40 kg/m2 with 243 women who had lost weight in that category (and only 21 women having lost more than 10 kg). If we were to extend extend the formula to these women, for example a woman with a 47 kg/m2 BMI should lose 14 kg. We tested in Table 4 specifically those women who should have lost weight according to our equation (i.e. 36 kg/m2 and over), and results seem to be in that line. Further studies in populations with a high rate of super obesity are required to check if there is a MFCS crossing point in these pregnant women [16, 20, 27], and hopefully to arrive at individualized gestational weight loss guidelines. Maternal obesity is associated with increased rates of many complications during pregnancy for the mother, the fetus and the neonate, including preeclampsia, gestational diabetes, fetal malformations, the risk of stillbirth and fetal overgrowth with as a result increased birthweights. Different factors of maternal metabolism may contribute to higher fat mass in neonates born to women with obesity. In a recent study by Mitanchez et al, [28] the authors compared non-diabetic and diabetic obese women and showed that they had the same level of insulin resistance as measured by HOMA-IR at 37 weeks and the same level of HbA1c at delivery. They furthermore demonstrated that pregnant women with obesity who have normal glucose tolerance still had a higher glucose profile (glycemia and HbA1c) than pregnant women of normal weight, thereby exposing the fetus to relative hyperglycemia. The authors concluded that regardless of gestational diabetes, deregulation of glucose metabolism is present in obese women and may contribute to fat mass in the neonates. A fascinating finding of this study by Mitanchez et al was that these effects were largely limited to girls.
5. Conclusion
Future perspectives: Without too much efforts different populations can establish their own local linear curve, moreover if they have also their specific centile curves for their SGA and LGA infants. Establishing such curves would allow easy development of smart-phone applications entering weight and the height of a woman, will inform the pregnant woman and her care provider about her individual optimal GWG, and probably also the GWG for the last trimester of pregnancy (we are testing, but the GWG is also a linear curve from 22 weeks gestation to 40 weeks).
Declarations
Author contribution statement
Pierre-Yves Robillard: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Gus Dekker: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Malik Boukerrou, Nathalie Le Moullec: Conceived and designed the experiments.
Thomas Hulsey: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.IOM . Institute of Medicine (US), National Research Council (US); 2009. Weight Gain during Pregnancy: Reexamining the Guidelines. Committee to Reexamine IOM Pregnancy Weight Guidelines. [Google Scholar]
- 2.WHO . 2000. Obseity: Preventing and Managing the Global Epidemic Report of a WHO Consultation. 0512–3054 (Print) 0512-3054 (Linking) [PubMed] [Google Scholar]
- 3.Gilmore L.A., Klempel-Donchenko M., Redman L.M. Pregnancy as a window to future health: excessive gestational weight gain and obesity. Semin. Perinatol. 2015;39(4):296–303. doi: 10.1053/j.semperi.2015.05.009. Epub 2015 Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IOM, Sciences NAO . Institute of Medicine (US), National Research Council (US), National Academy of Engineering (US), and National Academy of Sciences (US); 1990. Nutrition during Pregnancy: Part I: Weight Gain, Part II: Nutrients Supplements. Committee on Nutrition Status During Pregnancy and Lactation. [Google Scholar]
- 5.Li C., Liu Y., Zhang W. Joint and independent associations of gestational weight gain and pre-pregnancy body mass index with outcomes of pregnancy in Chinese women: a retrospective cohort study. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136850. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morisaki N., Nagata C., Jwa S.C., Sago H., Saito S., Oken E., Fujiwara T. Pre-pregnancy BMI-specific optimal gestational weight gain for women in Japan. J. Epidemiol. 2017;27(10):492–498. doi: 10.1016/j.je.2016.09.013. Epub 2017 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi S.K., Lee G., Kim Y.H., Park I.Y., Ko H.S., Shin J.C. Determining optimal gestational weight gain in the Korean population: a retrospective cohort study. Reprod. Biol. Endocrinol. 2017;15(1):67. doi: 10.1186/s12958-017-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nomura K., Kido M., Tanabe A., Nagashima K., Takenoshita S., Ando K. Investigation of optimal weight gain during pregnancy for Japanese Women. Sci. Rep. 2017;7(1):2569. doi: 10.1038/s41598-017-02863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapadia M.Z., Park C.K., Beyene J., Giglia L., Maxwell C., McDonald S.D. Can we safely recommend gestational weight gain below the 2009 guidelines in obese women? A systematic review and meta-analysis. Obes. Rev. 2015;16(3):189–206. doi: 10.1111/obr.12238. Epub 2015 Jan 18. [DOI] [PubMed] [Google Scholar]
- 10.Schumann N.L., Brinsden H., Lobstein T. A review of national health policies and professional guidelines on maternal obesity and weight gain in pregnancy. Clin. Obes. 2014;4(4):197–208. doi: 10.1111/cob.12062. Epub 2014 Jun 23. [DOI] [PubMed] [Google Scholar]
- 11.Kapadia M.Z., Park C.K., Beyene J., Giglia L., Maxwell C., McDonald S.D. Weight loss instead of weight gain within the guidelines in obese women during pregnancy: a systematic review and meta-analyses of maternal and infant outcomes. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0132650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein R.F., Abell S.K., Ranasinha S., Misso M., Boyle J.A., Black M.H., Li N. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. J. Am. Med. Assoc. 2017;317(21):2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiel D.W., Dodson E.A., Artal R., Boehmer T.K., Leet T.L. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet. Gynecol. 2007;110(4):752–758. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- 14.Beyerlein A., Schiessl B., Lack N., von Kries R. Optimal gestational weight gain ranges for the avoidance of adverse birth weight outcomes: a novel approach. Am. J. Clin. Nutr. 2009;90:1552–1558. doi: 10.3945/ajcn.2009.28026. Epub 2009 Oct 7. [DOI] [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet. Gynecol. 2013;121:210–212. doi: 10.1097/01.aog.0000425668.87506.4c. [DOI] [PubMed] [Google Scholar]
- 16.Faucher M.A., Barger M.K. Gestational weight gain in obese women by class of obesity and select maternal/newborn outcomes: a systematic review. Women Birth. 2015;28(3):e70–e79. doi: 10.1016/j.wombi.2015.03.006. Epub 2015 Apr 9. [DOI] [PubMed] [Google Scholar]
- 17.Oza-Frank R., Keim S.A. Should obese women gain less weight in pregnancy than recommended? Birth. 2013;40(2):107–114. doi: 10.1111/birt.12037. Epub 2013 Apr 26. [DOI] [PubMed] [Google Scholar]
- 18.Margerison Zilko C.E., Rehkopf D., Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am. J. Obstet. Gynecol. 2010;202(6) doi: 10.1016/j.ajog.2009.12.007. 574.e1–8. Epub 2010 Feb 4. [DOI] [PubMed] [Google Scholar]
- 19.Oken E., Kleinman K.P., Belfort M.B., Hammitt J.K., Gillman M.W. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am. J. Epidemiol. 2009;170(2):173–180. doi: 10.1093/aje/kwp101. Epub 2009 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swank M.L., Marshall N.E., Caughey A.B., Main E.K., Gilbert W.M., Melsop K.A., Chung J.H. Pregnancy outcomes in the super obese, stratified by weight gain above and below institute of medicine guidelines. Obstet. Gynecol. 2014;124(6):1105–1110. doi: 10.1097/AOG.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 21.Barker D.J., Winter P.D., Osmond C., Margetts B., Simmonds S.J. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 22.Boney C.M., Verma A., Tucker R., Vohr B.R. Metabolic syndrome in childhood: association with birth weight, maternal obesity,and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds R.M., Allan K.M., Raja E.A., Bhattacharya S., McNeill G., Hannaford P.C., Sarwar N., Lee A.J., Bhattacharya S., Norman J.E. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347 doi: 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.F. Bonsante, P.Y. Robillard, S. Iacobelli. Courbes périnatales Sud-Réunion. Rapport 2001-2014 du Relevé épidémiologique périnatal Sud-Réunion. http://www.repere.re/fileadmin/user_upload/RAPPORT_Epidemio_2014_Sud-Reunion.pdf.
- 25.Nohr E.A., Vaeth M., Baker J.L., Sørensen TIa, Olsen J., Rasmussen K.M. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am. J. Clin. Nutr. 2008 Jun;87(6):1750–1759. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- 26.Riskin-Mashiah S., Damti A., Younes G., Auslander R. Pregestational body mass index, weight gain during pregnancy and maternal hyperglycemia. Gynecol. Endocrinol. 2011;27(7):464–467. doi: 10.3109/09513590.2010.495436. Epub 2010 Jul 20. [DOI] [PubMed] [Google Scholar]
- 27.Andraweera P.H., Dekker G.A., Leemaqz S., McCowan L., Roberts C.T., SCOPE consortium The obesity associated FTO gene variant and the risk of adverse pregnancy outcomes: evidence from the SCOPE study. Obesity (Silver Spring) 2016;24(12):2600–2607. doi: 10.1002/oby.21662. Epub 2016 Oct 21. [DOI] [PubMed] [Google Scholar]
- 28.Mitanchez D., Jacqueminet S., Nizard J., Tanguy M.L., Ciangura C., Lacorte J.M., De Carne C., Foix L'Hélias L., Chavatte-Palmer P., Charles M.A., Dommergues M. Effect of maternal obesity on birthweight and neonatal fat mass: a prospective clinical trial. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181307. [DOI] [PMC free article] [PubMed] [Google Scholar]