Abstract
Glioblastoma (GBM) is the most commonly diagnosed type of brain cancer and the leading cause of brain cancer-related death. GBM contains a subpopulation of tumor-propagating glioblastoma stem-like cells that are thought to drive cancer progression and recurrence. Although several clinical trials are ongoing to explore new chemotherapeutic agents to treat GBM, the use of metformin (Met), a first-line drug for type 2 diabetes mellitus, in cancer remains controversial. Here, we show that combining Met with 9-cis retinoic acid (9-cis RA) reduced the proliferation rate of C6-GSCs (glioblastoma stem-like cells) in vitro. The results of flow cytometric analysis showed that treatment with 9-cis RA for 24 h induced 4.5% early and 38.0% late apoptosis in C6-GSCs. Twenty-four hours of Met treatment induced 23.6% early and 33.5% late apoptosis in C6-GSCs. Combination of Met and 9-cis RA treatment significantly increased both early and late apoptosis to 30.4% and 55.4%, respectively. The present findings suggest that not only 9-cis RA but also Met has the potential to induce early and late apoptotic GSCs death by affecting the functional cytoplasmic and nuclear organelles. At the protein level, there was increased cleaved caspase-3 but decreased procaspase-3 expression in Met-, 9-cis RA- and Met+9-cis RA-treated C6 GSCs, as detected by western blotting. The ratio of cleaved caspase-3/procaspase-3 was 1.6 times higher in Met+9-cis RA-treated groups compared to control. Ultimately, a combination of Met and 9-cis RA might be a possible therapeutic target for the treatment of GBM.
Keywords: Biochemistry, Cancer research, Stem cell research, Neuroscience, Cell biology, Molecular biology, Pathology
1. Introduction
Glioblastoma is a primary brain tumor in adult patients. The treatment of GBM remains difficult due to its recurrence after initial treatment with either surgical resection, chemotherapy, or radiotherapy. GBM contains a subpopulation of tumor-propagating glioblastoma stem-like cells (GSCs) that are thought to drive cancer progression and recurrence. These stem-like properties include the capacity to support unlimited growth, self-renewal, and multilineage differentiation into multiple cell types such as neurons, astrocytes and oligodendrocytes. GSCs are more invasive than non-GSC tumor cell and are intrinsically resistant to chemo- and radiotherapy, likely due to the upregulation of anti-apoptotic proteins and DNA repair enzymes [1, 2, 3, 4, 5]. Currently, there are many regimens to treat malignant gliomas.
Natural and synthetic derivatives of vitamin A, such as retinoic acid (RA), are responsible for most of the activity of vitamin A. By binding to the retinoic acid receptor (RAR) and retinoid X receptor (RXR), RA can mediate transcription of different sets of genes by controlling differentiation of a variety of cell types [6]. The cytotoxic effect of all-trans-RA on cell growth and induction of apoptosis is mediated through binding and activating RARs, such as RARα, RARβ, RARγ, and the 9-cis-RA receptor [7, 8]. 9-cis RA and all-trans RA are agonists of RARs [9]. 9-cis RA is a potently activator of all isoforms of RARs and RXRs. 9-cis RA reduces tumor proliferation and inhibits tumor growth [9]. In preclinical studies, 9-cis RA has been used in the prevention of mammary and prostate cancer [10, 11]. In addition, 9-cis RA and all-trans-RA can individually induce apoptosis of human liver cancer cells [12, 13]. 9-cis RA and 13-cis RA have been shown to inhibit proliferation but induce the differentiation of GSCs into neurons, astrocytes, and oligodendrocytes [14, 15, 16].
Metformin (Met) is a biguanide (1,1-dimethylbiguanide) molecule used for treatment of diabetes mellitus. It is the most commonly used oral normoglycemic agent for type 2 diabetes and exhibits anti-tumor effects [17]. Met has been considered as a potential anti-cancer agent since studies reported that diabetic patients given Met had a lower incidence of cancer when compared with diabetic patients treated with other drugs. In 2005, Evans et al [18] first reported the unexpected finding of a lower prevalence of cancer in diabetic patients treated with Met compared with other anti-diabetic treatments. Diabetic patients receiving Met had a lower cancer-specific mortality [18]. The in vitro antiproliferative effects of Met have been reported in cells from breast, glioma, prostate, colon, endometrium, lung, ovarian, and pancreatic cancers [19, 20, 21, 22, 23, 24]. Furthermore, Met has been shown to reduce cancer incidence and mortality in certain cancers [25]. Recently, Met has been shown to selectively inhibit GSCs with minor side effects [26, 27, 28]. The mechanism of Met antitumor effects is considered to be the activation of AMP-activated protein and the down-regulation of the Akt pathway [25]. Treatment of glioblastoma with Met has been shown to reduce the proliferation of tumor-initiating cells [29]. Met was found to synergize with other chemotherapy drugs to increase apoptosis in glioma stem-like cells [26]. Since differentiation and hyperglycemia are associated with increased risks of cancer progression, the aim of the present study was to evaluate the effect of combining Met and 9-cis RA on both early and late apoptotic cell death of C6-GSCs.
2. Materials and methods
2.1. Cell culture
In this study, C6-gliospheres were induced to represent the GSCs. Briefly, C6 cells from CLS (Cell Lines Service, Denmark) were cultured in DMEM and Ham's F-12, supplemented with 10% fetal bovine serum in a humidified atmosphere of 5% CO2 at 37 °C until confluent. Confluent C6 glioma cells were dissociated using a 0.25% trypsin (Gibco) and 0.25% EDTA solution and cultured in neurobasal medium (NBM) supplemented with B27 (1X; Gibco), heparin (2 μg/ml), 20 ng/ml recombinant human basic fibroblast growth factor (bFGF, Sigma, USA), and 20 ng/ml recombinant human epidermal growth factor (EGF, Sigma, USA). The gliospheres were cultured in 24-well plates in a 5% CO2 incubator at 37 °C with a medium change every 3–4 days. When C6 gliospheres reached more than 100 μm in diameter, they were treated with either 20 mM Met [26], 0.6 mM 9-cis RA [10] or 20 mM Met plus 0.6 mM 9-cis RA for 24 h. The cells in gliospheres were collected and analyzed by flow cytometry, immunofluorescence staining, western blot, and transmission electron microscopy.
2.2. Flow cytometric analysis
The immunoreactivity of the gliospheres to CD133 (a marker of GBM stem cells) was assessed using flow cytometry. Briefly, untreated gliospheres were harvested and incubated with a FITC-conjugated anti-CD133 antibody (E-bioscience, United Kingdom) for 1 h, and a secondary antibody was used as a control. Cells were analyzed on a Becton Dickinson FACScalibur. To detect early and late apoptosis using flow cytometry, cells were stained with FITC-conjugated Annexin V and propidium iodide (PI) from Sigma, USA. Briefly, approximately 1 × 106 cells treatment with Met and 9-cis RA, either alone or in combination, were fixed with −20 °C absolute alcohol for 20 min and treated with 5% bovine serum albumin for 20 min. Eight microliters of Annexin V-FITC and 7 μl PI were added and incubated for 30 min at room temperature in the dark. A further 500 μl (0.01 M) of phosphate buffered saline (PBS) was added to terminate the reaction. Routinely, at least 10,000 cells were assessed on a FACScalibur flow cytometer (Becton Dickinson). The percentage of intact (annexin V-/PI-), early apoptotic (annexin V+/PI-), late apoptotic (annexin V+/PI+), and necrotic (annexin V-/PI+) cells was calculated with the FACSDiva software, version 6.1.1 (BD,Biosciences).
2.3. Immunofluorescence staining
C6 gliosphere cells were fixed in with −20 °C absolute alcohol for 20 min, washed three times in 0.01 M PBS and blocked by 5% bovine serum albumin for 30 min at room temperature. Cells were incubated with FITC-conjugated CD 133 and Nestin antibodies (E-bioscience, United Kingdom) for 1 h. Cells were examined under a fluorescence microscope (Olympus, IX71/IX51, Japan).
2.4. Transmission electron microscopy (TEM) ultrastructural analysis
Cells were fixed in 2.5% glutaraldehyde at pH 7.2 (at room temperature) for 24 h and postfixed in 1% OsO4 in a 0.1 M cacodylate buffer for 1 h and stained with 0.2% uranyl acetate for half an hour. The cells were then dehydrated in a series of graded ethanol steps and finally embedded in Araldite. Ultrathin sections were double-stained with lead citrate and uranyl acetate and viewed under a JEOL 1200 EX electron microscope.
2.5. Western blot analysis
The expression of cleaved caspase-3 and procaspase-3 proteins was analyzed by western blotting using 12% SDS-PAGE. Cells were plated into 6-well plates (Corning). After treatments, the cells were washed with 0.01 M PBS. The exposed cells were then collected by adding RIPA lysis buffer containing a protease inhibitor cocktail. Next, the cells were scraped off the wells and collected. The lysates were centrifuged at 14,000 rpm at 4 °C for 15 min. The supernatant was collected and protein concentration was measured by Bradford reagent at 595 nm. An equal amount of protein from each sample was separated by SDS-PAGE and then transferred onto a nitrocellulose membrane (Bio-Rad Laboratories, CA, USA). Non-specific binding was blocked by 5% BSA in TBS-T for 1 h at room temperature. After that, the membranes were incubated in appropriate dilutions of antibodies, 1:1000 for mouse monoclonal primary against cleaved caspase-3 and procaspase-3 (Santa Cruz), and 1:5000 for mouse monoclonal primary against β-actin (Sigma), at 4 °C overnight. Then, membranes were incubated with anti-mouse IgG secondary antibody (Zymed Laboratories, CA, USA). The membranes were developed by ECL kit (Pierce Biotechnology). Quantitative analysis was performed by densitometry scanning with ScnImage software and expressed as relative bars normalized to β-actin.
2.6. Statistical analysis
The data were expressed as the means with standard deviations (SD) for at least three independent experiments performed in triplicate. The significant differences between treated cells and controls were determined by an independent t-test with significance levels of p < 0.05.
3. Results
3.1. Characteristics of C6-GSCs gliospheres
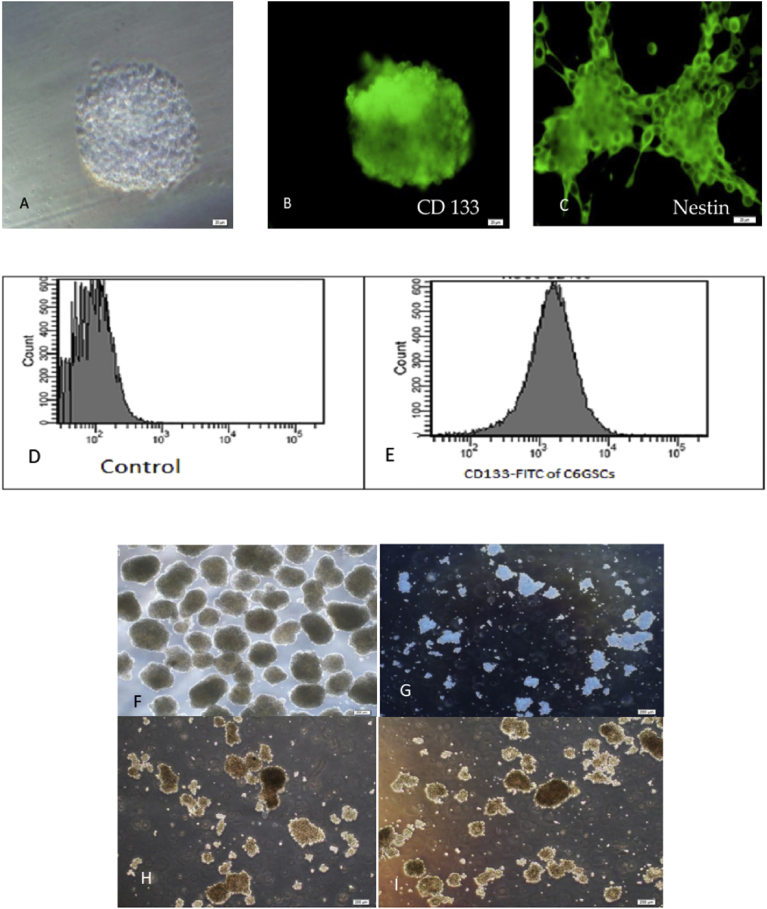
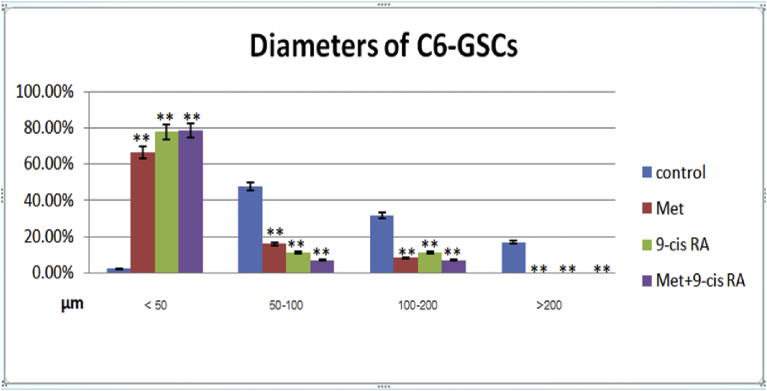
To evaluate the characteristics of C6-GSCs in gliospheres, the expression of CD133, a neural stem cell marker, was analyzed by immunofluorescence staining and flow cytometry. The majority of cells exhibited high expression of CD133. The whole gliosphere was stained for CD133 and Nestin (Fig. 1B and C). CD133 staining was analyzed by flow cytometry and subsequent analysis showed that a CD133-positive population was detected in 90.6% of C6-GSCs gliospheres as shown in Fig. 1E and D (control). Treating the gliospheres with either Met or 9-cis RA decreased both the size and number of gliospheres (Figs. 1F–I and 2). Combination of the two agents (Fig. 1I) significantly inhibited the self-renewal capability of C6-GSCs compared to single treatment with each agent.
Fig. 1.
Identification of C6-GSCs gliospheres. (A) phase contrast, (B) immunocytochemical staining of CD133 positive cells (scale bar = 20 μm). (C) immunocytochemical staining of Nestin positive cells (scale bar = 20 μm). CD133 staining analyzed by flow cytometry analysis showed that a CD133-positive population was detected in 90.6% of C6-GSCs in gliospheres (D and E). Flow cytometry analysis of C6-GSCs gliospheres labeled with FITC-conjugated antibodies against CD133 revealed 90.6% positive (E) compared with control (D). Representative micrographs (F–I) of C6-GSCs gliospheres after 24 h Met and 9-cis RA treatment, (F) control, (G) Met, (H) 9-cis RA, and (I) Met+9-cis RA (scale bar = 200 μm).
Fig. 2.
Diameters of C6 –GSCs shown as 4 categories, <50, 50–100, 100–200, and >200 μm. Quantitative analysis shows a difference in percentage of C6-GSCs with the indicated diameters among treatment and control groups. ** P < 0.01 vs single agent.
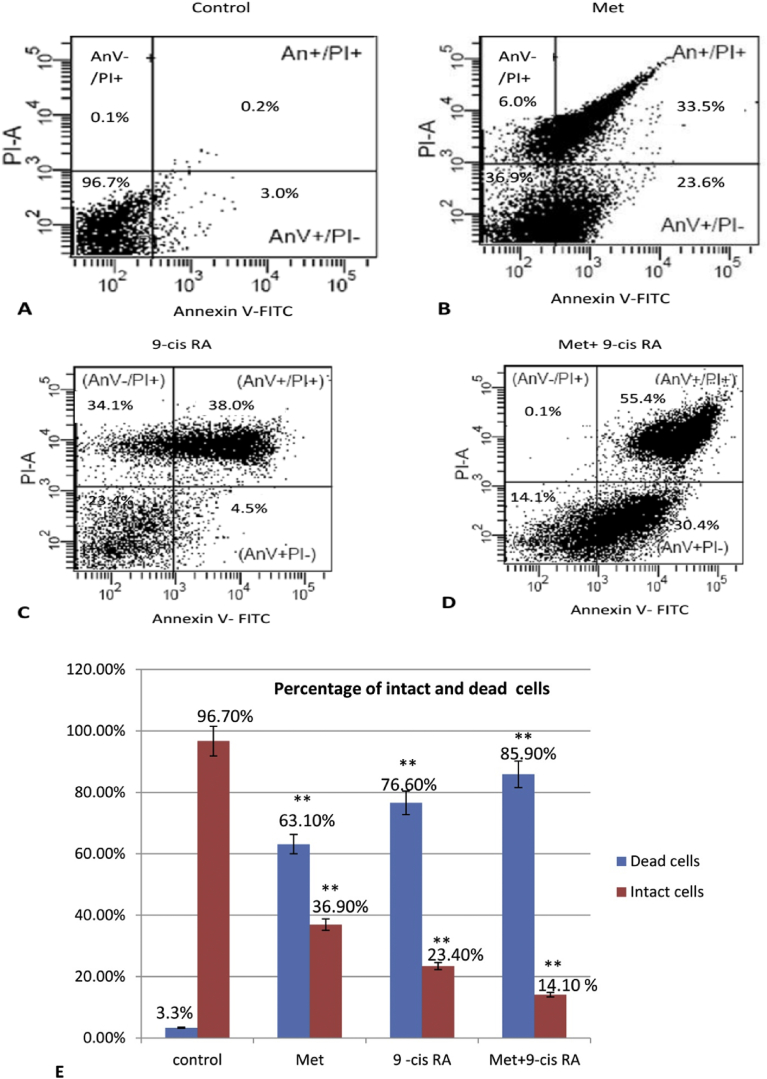
3.2. Effects of Met and 9-cis RA on apoptotic cell death in C6-GSCs
To determine the effect of Met and 9-cis RA on apoptotic cell death in C6-GSCs, gliospheres were treated with Met and 9-cis RA, alone or in combination for 24 h. Then, the percentage of apoptotic cells was analyzed by Annexin V/PI staining with flow cytometry. Following incubation with Met for 24 h, the percentage of apoptotic cells were 23.6% early and 33.5% late apoptosis (Fig. 3B). Treatment with 9-cis RA for 24 h induced 4.5% early and 38.0% late apoptosis (Fig. 3C). Combination of Met with 9-cis RA significantly increased the percentage of apoptotic cells to 30.4% early and 55.4% late apoptosis (Fig. 3D). When comparing control and each treated group about dead cells in C6-GSCs, the results indicated there was difference between intact and dead cells at statistically significant (p < .01) shown in Fig. 3E.
Fig. 3.
Combination treatment with Met and 9-cis RA significantly increased apoptosis in C6-GSCs gliospheres. Cells were treated with Met, 9-cis RA or Met+9-cis RA for 24 h, stained with Annexin V-FITC and propidium iodide, and analyzed by flow cytometry, (A) control (B) 20 mM Met, (C) 0.6 mM 9-cis RA, and (D) Met+9-cis RA. Quantitative analysis of intact and dead cells in C6-GSCs ** P < 0.01 vs each treated group are shown in E.
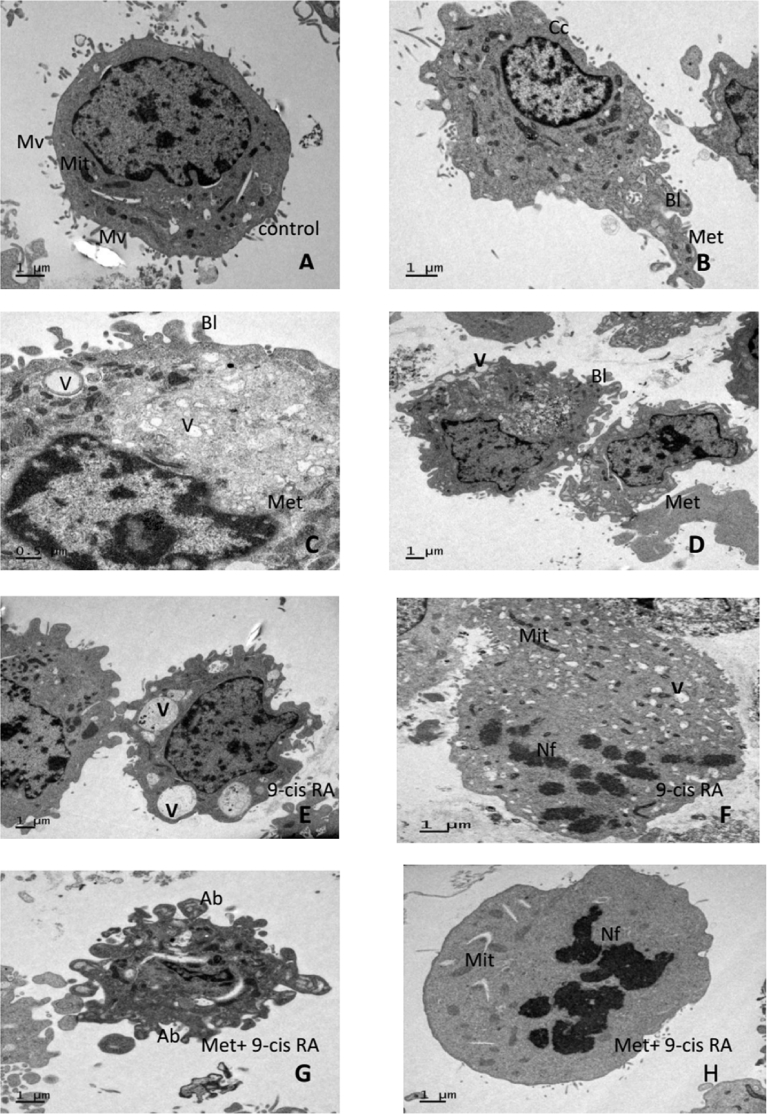
3.3. Effects of Met and 9-cis RA on C6-GSCs morphology
Transmission electron microscopy (TEM) was used to observe the morphology C6-GSCs. Cells treated with either Met or 9-cis RA (Fig. 4B–D) were smaller than control-treated cells (Fig. 4A). Karyopyknosis was observed as a crescent cap structure distributed throughout the karyoplasms in cells treated with Met or 9-cis RA. Both early and late apoptotic morphological changes were found in all of C6-GSCs after treatment with either Met or 9-cis RA. Cell shrinkage was also observed due to blebbing of the membrane. Additionally, nuclear and chromatin condensation were evident and the cytoplasm was reduced in volume, had many vacuoles but few lipid droplets, margination, and nuclear fragmentation (Fig. 4E and F). Some cells became apoptotic bodies with nuclear fragments during late apoptosis (Fig. 4G and H). The vacuoles were surrounded by membranes and some of these membranes were decorated by ribosomes, indicating that the intracellular vacuoles were derived from the rough endoplasmic reticulum (ER) and possibly smooth ER. The vacuoles were mostly empty although some contained membrane structures indicating cytoplasmic contents. However, the mitochondrial morphology remained normal in some cells even after 24 h of treatment. In summary, treatment with both Met and 9-cis RA for 24 h could affect nuclear and cytoplasmic organelles, but not mitochondrial morphology (Fig. 4B and H).
Fig. 4.
Representative TEM images of C6-GSCs. A: Control cell showing the typical chromatin pattern of nucleus (N) with numerous microvilli (Mv) arising from the cell membrane and mitochondria (Mit). B, C and D: Early apoptosis in cells treated with Met for 24 h showing shrinkage, membrane blebs (Bl), vacuolar cytoplasm(V), and nuclear chromatin condensation(Cc). E and F: Late apoptosis in cells treated with 9-cis RA for 24 h showing abundant cytoplasmic vacuoles (V) and nuclear fragments (Nf) with few lipid droplets. G and H: Apoptotic bodies (Ab) and nuclear fragments (Nf) in C6-GSCs treated with Met+9-cis RA for 24 h.
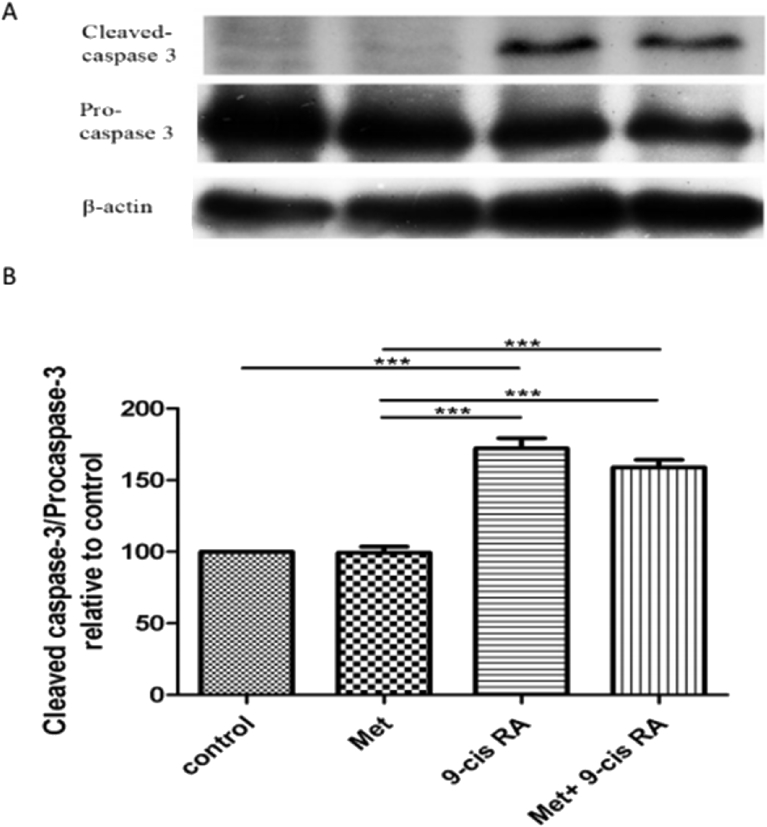
3.4. Effects of Met and 9-cis RA on the expression of cleaved caspase-3 and procaspase-3
The expression of cleaved caspase-3 protein increased but the expression of procaspase-3 protein in Met, 9-cis RA, and Met+9-cis RA-treated C6 GSCs compared to control (Fig. 5A). The band intensities were measured using an image analyzer and presented as the relative ratio. The results showed the relative ratio of cleaved caspase-3/procaspase-3 in Met +9-cis RA and 9-cis RA were 1.6, 1.7 times, respectively. They were higher than the relative ratio of the control (Fig. 5B).
Fig. 5.
A. Representative western blot for cleaved caspase-3 and procaspase-3 detection in the C6 GSCs. B. Densitometric analysis of cleaved caspase-3 and procaspase-3 levels. The ratio of cleaved caspase-3/procaspase-3 in 9-cis RA was the highest all groups. Data are expressed as means ± SD (n = 3) in each group.
4. Discussion
In the present study, we used an experimental model system of C6 gliospheres to represent GSCs. Treatment with very low concentrations of Met and 9-cis RA, either alone or in combination, was associated with a significant decrease in the surviving fraction of C6-GSCs. Combination of Met and 9-cis RA increased both early and late apoptosis in C6-GSCs. Met-treated cells showed higher number of early apoptotic cells death compared to 9-cis RA (23.6% vs 4.5%).
C6-GSCs showed significant changes in morphology when treated with Met, including shrinking, membrane blebbing, and vacuolization in the cytoplasm, all of which are characteristics of early apoptosis. Cells treated with 9-cis RA showed higher rates of apoptotic bodies and nuclear fragmentation, indicative of late apoptosis.
There are two pathways involved in drug-induced apoptosis: the death receptor pathway and the mitochondria pathway [29, 30]. In this study, we speculate that Met and 9-cis RA first induced functional changes of mitochondria, which ultimately led to apoptosis. It has been reported that Met inhibits mitochondrial oxidative phosphorylation and causes the disruption of respiration complex I, ATP production in the mitochondria, and cell polarity in low-energy conditions [1]. Met has been used previously in combination with temozolomide to induce early and late apoptosis in C6 glioma stem cells that resulted in 62.73% and 61.73% apoptotic cell death in U87- GSCs [26], which is concordant with this study. Met and 9-cis RA induced 85.80% apoptotic cell death in C6 glioma stem cells. Some studies have reported that treatment with Met decreased proliferation, blocked Go/G1 cell cycle progression, and induced cell death in GSCs through AMPK (adenosine monophosphate-activated protein kinase)-dependent inhibition of FOXO3 and AKT [30]. AMPK was active in gliomas and the anti-proliferative effects of Met on GSCs were AMPK-independent [31].
All trans-retinoic acid (ATRA) is an endogenous ligand for the RARs and binds to RAR subtypes. 9-cis RA is an endogenous ligand binds RARs and RXRs. 9–cis RA induces tissue transglutaminase type II (tTG2). tTG2 has been shown to be induced by retinoids in multiple cell types [32]. tTG2 is involved in protein cross-linking activity and apoptosis [33]. tTG2 activity is also by changes in morphology and by nuclear or DNA fragmentation consistent with features of cells undergoing early and late apoptosis [10, 32, 33]. In some reports, 9-cis RA may influence intrinsic and extrinsic pathways of apoptotic cells. In the case of intrinsic apoptosis, 9-cis RA acts on RARs and RXRs, which are specific response elements within the nucleus that inhibit cancer cell proliferation [2].
Moreover, 9-cis RA affects mitochondrial organelles. It releases cytochrome c, which activates caspase-9 then cleaves caspases-3, -6, and -7, thereby affecting morphological and biochemical features of apoptosis with the intrinsic pathway of late apoptosis, including chromatin condensation, chromatin margination, and nuclear fragmentation. These characteristics of late apoptosis were found in C6-GSCs treated with 9-cis RA in this study [6, 14, 34].
For the extrinsic pathway, 9-cis RA may stimulate the binding of specific ligands of the TNF family, including Fas ligand, Fas-L or Bax, to their death receptors such as Fas, Trail-R1, R2, R3 on the cell membrane. Bax/Bad translocation to the mitochondria then promotes cytochrome c release and activation of the apoptosome [6, 11, 13].
There are many reports showing that 9-cis RA is more potent when it is used in combination with anti-estrogen treatment of breast cancer [9, 10]. Moreover, all-trans RA was used in combination with Met to inhibit neuroblastoma growth in children by decreasing cell proliferation [9]. Some studies show that 13-cis RA has activity against glioblastoma stem cells (GB) [15, 16]. The synergistic mechanism between Met and 9-cis RA remains unknown.
The present study showed that combination of 9-cis RA with Met induced a higher percentage of late apoptosis in C6-GSCs than single treatment with 9-cis RA or Met. Met affected the morphology of early apoptosis whereas 9-cis RA bound to RARs, RXRs, and caused DNA fragmentation [10, 21, 34]. Moreover, the mechanism of apoptosis, confirmed by western blot, shows cleaved caspase-3 in Met-, 9-cis +RA- and Met+ 9-cis RA-induced apoptosis. Met and 9-cis RA can promote Bax or Fas-L to move to the membrane.
Release of cytochrome c from the mitochondria activates caspase-3 and inhibits procaspse-3 leading to formation of the apoptosome and apoptosis. C6-GSCs gliospheres used in the present study represent a version of cancer-inducing stem cells [3, 9, 26]. Met and 9-cis RA were synergistic in reducing the number of C6-GSCs. Therefore, they can reduce cancer incidence and eliminate cancer stem cells with minimal side effects [5, 19]. Ultimately, Met and 9-cis RA should be considered as drug of choices for treatment GBM.
9-cis RA binds to both RARs and RXRs. After activation by their specific ligands become chromatin fragments through apoptosis as an intrinsic pathway. At the same time, 9-cis RA effects the mitochondria organelles. It can move to the mitochondrial membrane and release cytochrome c, which activates caspase-3, inhibits procaspse-3, and leads to apoptosome formation and apoptosis.
In conclusion, combining Met with 9-cis retinoic acid (9-cis RA) reduced the proliferation rate of C6-GSCs in vitro. 9-cis RA and Met act synergistically and induce 85.8% of early and late apoptotic GSCs death. Met and 9-cis RA could affect C6-GSCs. Met had an early influence on cellular organelles and apoptosis as seen by shrinkage, membrane blebs, vacuolar cytoplasm, and nuclear chromatin condensation. 9-cis RA affected late apoptosis of C6-GSCs as evidenced by apoptotic bodies, chromatin condensation, chromatin margination, and nuclear fragmentation.
Declarations
Author contribution statement
Chanchai Songthaveesin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Wanna Sa-nongdej: Analyzed and interpreted the data; Wrote the paper.
Tanapol Limboonreung: Performed the experiments; Analyzed and interpreted the data.
Sukumal Chongthammakun: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by research grants from Mahidol University (122591). The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary Figure 1.
References
- 1.Chen J., Li Y., Yu T.S., McKay R.M., Burns D.K., Kernie S.G., Parada L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J., McKay R.M., Parada L.F. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 2012;149:36–47. doi: 10.1016/j.cell.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao S., Wu Q., McLendon R.E., Hao Y., Shi Q., Hjelmeland A.B., Dewhirst M.W., Bigner D.D., Rich J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Montana V., Sontheimer H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J. Neurosci. 2011;31:4858–4867. doi: 10.1523/JNEUROSCI.3825-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadahiro H., Yoshikawa K., Ideguchi M., Kajiwara K., Ishii A., Ikeda E., Owada Y., Yasumoto Y., Suzuki Y.M. Pathological features of highly invasive glioma stem cells in a mouse xenograft model. Brain Tumor Pathol. 2014;31:77–84. doi: 10.1007/s10014-013-0149-x. [DOI] [PubMed] [Google Scholar]
- 6.Bissonnette R.P., Brunner T., Lazarchik S.B., Yoo N.J., Boehm M.F., Green D.R., Heyman R.A. 9-cis retinoic acid inhibition of activation-induced apoptosis is mediated via regulation of fas ligand and requires retinoic acid receptor and retinoid X receptor activation. Mol. Cell Biol. 1995;15:5576–5585. doi: 10.1128/mcb.15.10.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seewaldt V.L., Johnson B.S., Parker M.B., Collins S.J., Swisshelm K. Expression of retinoic acid receptor beta mediates retinoic acid-induced growth arrest and apoptosis in breast cancer cells. Cell Growth Differ. 1995;6:1077–1088. [PubMed] [Google Scholar]
- 8.Kim M.J., Ciletti N., Michel S., Reichert U., Rosenfield R.L. The role of specific retinoid receptors in sebocyte growth and differentiation in culture. J. Invest. Dermatol. 2000;114:349–353. doi: 10.1046/j.1523-1747.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 9.Karsy M., Albert L., Tobias M.E., Murali R., Jhanwar-Uniyal M. All-trans retinoic acid modulates cancer stem cells of glioblastoma multiforme in an MAPK-dependent manner. Anticancer Res. 2010;30:4915–4920. [PubMed] [Google Scholar]
- 10.Gottardis M.M., Lamph W.W., Shalinsky D.R., Wellstein A., Heyman R.A. The efficacy of 9-cis retinoic acid in experimental models of cancer. Breast Cancer Res. Treat. 1996;38:85–96. doi: 10.1007/BF01803787. [DOI] [PubMed] [Google Scholar]
- 11.Christov K.T., Moon R.C., Lantvit D.D., Boone C.W., Steele V.E., Lubet R.A., Kelloff G.J., Pezzuto J.M. 9-cis-retinoic acid but not 4-(hydroxyphenyl)retinamide inhibits prostate intraepithelial neoplasia in Noble rats. Cancer Res. 2002;62:5178–5182. [PubMed] [Google Scholar]
- 12.Wu K., Kim H.T., Rodriquez J.L., Munoz-Medellin D., Mohsin S.K., Hilsenbeck S.G., Lamph W.W., Gottardis M.M., Shirley M.A., Kuhn J.G., Green J.E., Brown P.H. 9-cis-Retinoic acid suppresses mammary tumorigenesis in C3(1)-simian virus 40 T antigen-transgenic mice. Clin. Cancer Res. 2000;6:3696–3704. [PubMed] [Google Scholar]
- 13.Wan Y.J., Cai Y., Cowan C., Magee T.R. Fatty acyl-CoAs inhibit retinoic acid-induced apoptosis in Hep3B cells. Cancer Lett. 2000;154:19–27. doi: 10.1016/s0304-3835(00)00341-4. [DOI] [PubMed] [Google Scholar]
- 14.Tang K., Cao L., Fan S.Q., Wu M.H., Huang H., Zhou Y.H., Zhou M., Tang Y.L., Wang R., Zeng F., Liao P., Li X.L., Li G.Y. Effect of all-trans-retinoic acid on C6 glioma cell proliferation and differentiation. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:892–897. [PubMed] [Google Scholar]
- 15.See S.J., Levin V.A., Yung W.K., Hess K.R., Groves M.D. 13-cis-retinoic acid in the treatment of recurrent glioblastoma multiforme. Neuro Oncol. 2004;6:253–258. doi: 10.1215/S1152851703000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ying M., Wang S., Sang Y., Sun P., Lal B., Goodwin C.R., Guerrero-Cazares H., Quinones-Hinojosa A., Laterra J., Xia S. Regulation of glioblastoma stem cells by retinoic acid: role for Notch pathway inhibition. Oncogene. 2011;30:3454–3467. doi: 10.1038/onc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirpichnikov D., McFarlane S.I., Sowers J.R. Metformin: an update. Ann. Intern. Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 18.Evans J.M., Donnelly L.A., Emslie-Smith A.M., Alessi D.R., Morris A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowker S.L., Majumdar S.R., Veugelers P., Johnson J.A. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 20.Aljada A., Mousa S.A. Metformin and neoplasia: implications and indications. Pharmacol. Ther. 2012;133:108–115. doi: 10.1016/j.pharmthera.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Ben Sahra I., Laurent K., Loubat A., Giorgetti-Peraldi S., Colosetti P., Auberger P., Tanti T.F., Le Marchand-Brustel Y., Bost F. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 22.Buzzai M., Jones R.G., Amaravadi R.K., Lum J.J., DeBerardinis R.J., Zhao F., Viollet B., Thompson C.B. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 23.Rattan R., Giri S., Hartmann L.C., Shridhar V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J. Cell Mol. Med. 2011;15:166–178. doi: 10.1111/j.1582-4934.2009.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L.W., Li L.S., Zou D.W., Jin Z.D., Gao J., Xu G.M. Metformin induces apoptosis of pancreatic cancer cells. World J. Gastroenterol. 2008;14:7192–7198. doi: 10.3748/wjg.14.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakikhani M., Dowling R., Fantus I.G., Sonenberg N., Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 26.Yu Z., Zhao G., Li P., Li Y., Zhou G., Chen Y., Xie G. Temozolomide in combination with metformin act synergistically to inhibit proliferation and expansion of glioma stem-like cells. Oncol. Lett. 2016;11:2792–2800. doi: 10.3892/ol.2016.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolen S., Feldman L., Vassy J., Wilson L., Yeh H.C., Marinopoulos S., Wiley C., Selvin E., Wilson R., Bass E.B., Brancati F.L. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann. Intern. Med. 2007;147:386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 28.Rattan R., Ali Fehmi R., Munkarah A. Metformin: an emerging new therapeutic option for targeting cancer stem cells and metastasis. J. Oncol. 2012 doi: 10.1155/2012/928127. 12 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Würth R., Pattarozzi A., Gatti M., Bajetto A., Corsaro A., Parodi A., Sirito R., Massollo M., Marini C., Zona G., Fenoglio D., Sambuceti G., Filaci G., Daga A., Barbieri F., Florio T. Metformin selectively affects human glioblastoma tumor-initiating cell viability: a role for metformin-induced inhibition of Akt. Cell Cycle. 2013;12:145–156. doi: 10.4161/cc.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fulda S., Meyer E., Friesen C., Susin S.A., Kroemer G., Debatin K.M. Cell type specific involvement of death receptor and mitochondrial pathways in drug-induced apoptosis. Oncogene. 2001;20:1063–1075. doi: 10.1038/sj.onc.1204141. [DOI] [PubMed] [Google Scholar]
- 31.Liu X., Chhipa R.R., Pooya S., Wortman M., Yachyshin S., Chow L.M., Kumar A., Zhou X., Sun Y., Quinn B., McPherson C., Warnick R.E., Kendler A., Giri S., Poels J., Norga K., Viollet B., Grabowski G.A., Dasgupta B. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E435–E444. doi: 10.1073/pnas.1311121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Autuori F., Farrace, S. Oliverio, L. Piredda, M. Piacentini M.G. Tissue transglutaminase and apoptosis. Adv. Biochem. Eng. Biotechnol. 1998;62:129–136. [PubMed] [Google Scholar]
- 33.Gentile V., Thomazy V., Piacentini M., Fesus L., Davies P.J. Expression of tissue transglutaminase in Balb-C 3T3 fibroblasts: effects on cellular morphology and adhesion. J. Cell Biol. 1992;119:463–474. doi: 10.1083/jcb.119.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakai H., Shimizu M., Moriwaki H. A role for acyclic retinoid in the chemoprevention of hepatocellular carcinoma: therapeutic strategy targeting phosphorylated retinoid X receptor-α. Diseases. 2014;2:226–242. [Google Scholar]