Abstract
This study investigated the potential of stevioside to prevent oxidative DNA damage in the liver and kidney of type 2 diabetes mellitus (T2DM) using high fat-low streptozocin rat model. Rats were treated daily with 12.5, 25 and 50 mg/kg stevioside orally for 21 days. Levels of biomarkers of T2DM, lipid profile and oxidative stress were assayed spectrophotometrically. The DNA ladder assay method was used to assess DNA fragmentation in the liver and kidney while computational analysis was used to predict the mechanisms of antidiabetic properties of stevioside. Stevioside significantly (p < 0.05) decreased the levels of plasma glucose, insulin, dipeptidyl peptidase IV and activities of kidney angiotensin converting enzyme. Stevioside significantly reduced oxidative stress by decreasing the levels of lipid peroxidation and nitric oxide in the liver and kidney; thereby, reducing the extent of DNA fragmentation in the liver and kidney of the diabetic rats. The in silico analysis showed that the ability of stevioside to exert these effects is linked to its inhibition of beta-adrenergic receptor kinase and G-protein-coupled receptor kinase. The results of this study suggest that the prevention of DNA fragmentation may be an additional benefit of the use of stevioside in the management of T2DM.
Keywords: Nutrition, Metabolism
1. Introduction
Type 2 diabetes mellitus (T2DM) is a life-threatening metabolic disease that is reaching epidemic proportions [1]. T2DM is responsible for about 5 million deaths annually due largely to major complications like atherosclerotic coronary heart disease, cardiomyopathy, stroke and nephropathy. Globally, undiagnosed diabetes continues to predispose about 200 million people to the development of several long-term micro and macrovascular complications of unmanaged chronic hyperglycemia [1].
These complications are consequences of glucotoxicity-induced oxidative stress with concomitant damage to biomolecules [2]. In particular, the damage to DNA has been linked to enhanced cancer risk, and patients with diabetes mellitus have been shown to have an increased cancer incidence [3]. Hence, oxidative damage to DNA does not often lead to severe vascular complications with a phenotypic outcome synonymous with diabetes [3].
The rise in diabetes prevalence mirrors the rise in its main risk factors-overweight and obesity, as well as increase in sugar consumption [4, 5]. Hence, the urgent need to control this trend requires a scrupulous control of diet and sugar intake. However, the cognitive desire of human for sugar or sweet diet seems insatiable [6]. Therefore, it is imperative to investigate the role of non-caloric sweeteners in the management of diabetes.
Stevioside (PubChem CID:442089) is one of natural non-caloric sweeteners refined from the leaves of Stevia rebaudiana Bertoni. It is recognized to be 250 times sweeter than sucrose (4 g/L) and is used as non-caloric alternative to table sugar [7]. The Joint FAO/WHO Expert Committee on Food Additives recommended stevioside's admissible daily intake to be less than or equal to 4 mg/kg body weight, based on no-observed-adverse-effect level of 970 mg/kg body weight per day (383 mg/kg bw per day expressed as steviol equivalent) [8]. Stevioside is a glycoside and it is hydrolyzed to steviol (the aglycone) in the caecum by intestinal micro-flora under anaerobic conditions [9]. The plasma concentration of steviol has been reported to reach the peak after 15 min of oral administration, demonstrating rapid absorption [9]. Long-term oral intake of stevioside has been shown to be highly tolerated in human with no apparent side effect at dietary dosage [10]. Besides sweetness, many studies have reported the therapeutic benefits of glycosides derived from Stevia rebaudiana, which include anti-oxidant, anti-hyperglycemic, anti-hypertensive, anti-inflammatory, anti-tumor, anti-diarrheal, diuretic, and immunomodulatory actions [11, 12]. These benefits have contributed to its increased use and commercialization of its production [13].
Although, previous studies have reported the hypoglycemic property of stevioside [14], more detailed studies are still needed to understand the mechanism by which it improves T2DM and prevents its associated complications. This present study therefore seeks to investigate the effects of stevioside on oxidative damage in a high fat fed/streptozotocin-induced T2DM rat model. This model is associated with initial insulin resistance and/or glucose intolerance following streptozocin-induced destruction of functional β-cell mass; hence, mimicking the pathology of human T2DM [15, 16]. Using this model, we therefore, hypothesize that the antiglycemic and antioxidant properties of stevioside will be beneficial in protecting DNA from oxidative damage in the diabetic rats.
2. Materials and methods
2.1. Chemicals
Streptozotocin was a product of Sigma-Aldrich (St. Loius, MO, USA) while stevioside (95%) was a product of Anhui Minmetals Development Co., Hefei, Anhui, China. DNA extraction spin column kit was a product of Aidlab Biotechnologies Co. Ltd (Beijing, China) while kits for Enzyme-linked immunosorbent assays (ELISA) were products of Hangzhou Eastbiopharm Co., Hangzhou, China. All other chemicals were products of Sigma-Aldrich (St. Loius, MO, USA).
2.2. Animals
Thirty male inbred albino rats weighing between 150 and 200 g were used for this study. The rats were housed under normal temperature (22 ± 2 °C) with 12-h light and dark cycle. The animals were allowed to acclimatize for three weeks before commencement of the experiment. The experiment was approved by the Covenant University Ethical Committee (CU/BIOSCRECU/BIO/2015/004) and carried out according to the guidelines of the committee.
2.3. Experimental design
The rats were randomly divided into five groups of six rats each. Four of the groups were maintained on a high fat diet (HFD), which contained 50% fat (5% from vegetable oil and 45% from tallow) (Table 1), throughout the period of the experiment (9 weeks) while the last group was maintained on normal pellet diet (5% fat from vegetable oil) and served as the normal control. After 4 weeks, the HFD fed groups were given a low dose (35 mg/kg body weight) of streptozocin intraperitoneally while the normal control group was administered the vehicle (0.1 mL/kg body weight of 0.1 M citrate buffer), as described by Zhang et al. [17]. Another dose of streptozocin was administered at week 6 of the experiment, after which fasting blood glucose was checked and stevioside was then administered orally for 21 days. The rats were grouped as shown below;
Group 1: Normal Control: Rats fed normal diet (n = 6)
Group 2: Diabetic Control: Rats fed HFD (n = 6)
Group 3: Diabetic rats treated with 12.5 mg/kg stevioside: Rats fed HFD (n = 6)
Group 4: Diabetic rats treated with 25 mg/kg stevioside: Rats fed HFD (n = 6)
Group 5: Diabetic rats treated with 50 mg/kg stevioside: Rats fed HFD (n = 6)
Table 1.
Composition of diet.
| Component | Level (g/100 g) in diet |
|
|---|---|---|
| Normal diet | High fat diet | |
| Fish meal | 25 | 25 |
| Sucrose | 10 | 10 |
| Corn starch | 49.5 | 4.5 |
| Vegetable oil | 5 | 5 |
| Salt/mineral mixa | 5.5 | 5.5 |
| Cellulose | 5 | 5 |
| Tallow | 0 | 45 |
Salt/mineral mix contains the following (in g/100 g): calcium phosphate, 49.50; sodium powder, 11.80; potassium sulfate, 5.20; sodium chloride, 7.40; magnesium oxide, 2.40; potassium citrate, 22.40; ferric citrate, 0.60; manganous carbonate, 0.35; cupric carbonate, 0.03; zinc carbonate, 0.16; chromium potassium sulfate, 0.055; potassium iodate, 0.001; sodium selenate, 0.001; choline chloride, 0.50; thiamine HCl, 0.06; riboflavin, 0.06; niacin, 0.30; calcium pantothenate, 0.16; biotin, 0.01; vitamin B12, 0.10; vitamin D3, 0.025; vitamin E acetate, 1.00; pyridoxine, 0.07; folic acid, 0.02; vitamin A acetate, 0.08.
Twenty-four hours after the last dose of stevioside, blood was collected from the animals by cardiac puncture under light ether anesthesia after an overnight fast; liver and kidney were also excised for analysis. Tissue homogenates were prepared as described by Rotimi et al. [18, 19].
2.4. Biochemical analysis
2.4.1. Plasma chemistry
Glucose, bicarbonate, hydroxyl butyrate dehydrogenase, alkaline phosphatase (ALP), urea, creatinine kinase (CK), α-amylase, gamma-glutamyl transferase (GGT) and lactate dehydrogenase (LDH) were determined spectrophotometrically in the plasma using commercially available kits according to manufacturer's instructions (Jinan Biobase Biotech Co.,Ltd, Jinan, China) while insulin and dipeptidyl peptidase-4 (DPP-IV) were determined using enzyme linked immunosorbent assay (ELISA) kits (Hangzhou Eastbiopharm Co., Ltd. Hangzhou, China).
2.4.2. Determination of angiotensin converting enzyme (ACE) activity
ACE was determined in the kidney spectrophotometrically, using the conversion of N-Hippuryl–His-Leu hydrate, hydrochloric acid (HHL) to hippuric acid according to the method of Cushman and Cheung [20]. Briefly, assay solution consisted of 5 mM HHL in 100 mM sodium borate buffer (pH 8.3). The reaction was initiated by addition of 10 μL of kidney homogenate and incubated at 37 °C for 30 min. Thereafter, the reaction was terminated by the addition of 1 M HCl and the amount of hippuric acid released was monitored at 228 nm.
2.4.3. Plasma lipid profiles
Plasma cholesterol and triacylglycerols were determined spectrophotometrically using commercially available kits according to manufacturer's instructions (Jinan Biobase Biotech Co.,Ltd, Jinan, China). High Density Lipoprotein (HDL) and HDL3 were obtained from the plasma using the Dextran sulfate–MgCl2 precipitation method as described by Rifai et al. [21]. The supernatant obtained after centrifugation contained the HDL and HDL3 while the precipitate contained VLDL and VLDL3 respectively. Free fatty acid was determined spectrophotometrically as described by Rotimi et al. [18, 19].
2.4.4. Oxidant/antioxidant status assays
Lipid Peroxidation was determined by measuring the concentration of thiobarbituric acid reactive substances (TBARS) according to the method of Buege and Aust [22]. Nitric oxide (NO) concentration was determined by the Griess reaction using a method described by Yucel et al [17]. Glutathione (GSH) concentration in the tissues were determined according to the method described by Ellman [23] while glutathione-S-transferase (GST) activity was determined by measuring the formation of 1-chloro-2,4-dinitrobenzene and GSH conjugate at 340 nm according to the method described by Habig et al. [24]. Glutathione peroxidase (GPx) activity was determined according to the method of Rotruck et al. [25] while glutathione reductase (GR) activity was assayed as described by Mavis and Stellwagen [26]. Superoxide dismutase (SOD) activity was determined according to the pyrogallol autoxidation method of Marklund and Marklund [27] while peroxidase (PER) activity was determined according to the method of Fergusson and Chance [28]. The activities of the enzymes in each organ were expressed per mg protein as previously described [29], following the determination of protein concentration as described by Lowry et al. [30].
2.4.5. DNA extraction and analysis of intra-nucleosomal DNA fragmentation
Genomic DNA was extracted from the liver and kidney using the spin column technique according to manufacturer's instructions (Aidlab Biotechnologies Co. Ltd., China). Quality and concentration of DNA was determined spectrophotometrically at 260 nm using Nanodrop® spectrophotometer (Thermo Scientific, Fisher Scientific Company, Suwanee, GA, USA). After normalization of the DNA concentration, the DNA ladder assay method was used as described by Suman et al. [31], using 0.8% agarose. The agarose gel was stained with ethidium bromide and visualized using the UVP GelDoc-It® imaging system. The intensity of the DNA on agarose gel was analyzed using Image J software.
2.4.6. Computational analysis
The biological activities spectra that relates to T2DM, of stevioside was predicted using a prediction of activity spectra for substances (PASS)-based approach as described by Filimonov et al. [32]. PASS is an online algorithm that estimates predicted activity spectrum of a compound as probable activity (Pa) and probable inactivity (Pi). It utilizes a structure-activity relationship analysis to predict activities and it has been trained with a set containing more than 250,000 of biologically active compounds. The values of Pa and Pi vary between 0.000 and 1.000. Only activities with Pa > Pi are considered as possible for a particular compound. If Pa > 0.7, the probability of experimental pharmacological action is high, and if 0.5 < Pa < 0.7, the probability of experimental pharmacological action is less.
2.4.7. Statistical analysis
Data were expressed as mean ± SEM of six replicates in each group. Analysis of variance (ANOVA) was carried out to test for the level of homogeneity at p < 0.05 among the groups. Duncan's multiple range test was used to separate the heterogeneous groups.
3. Results
3.1. Effects of stevioside administration on biomarkers T2DM
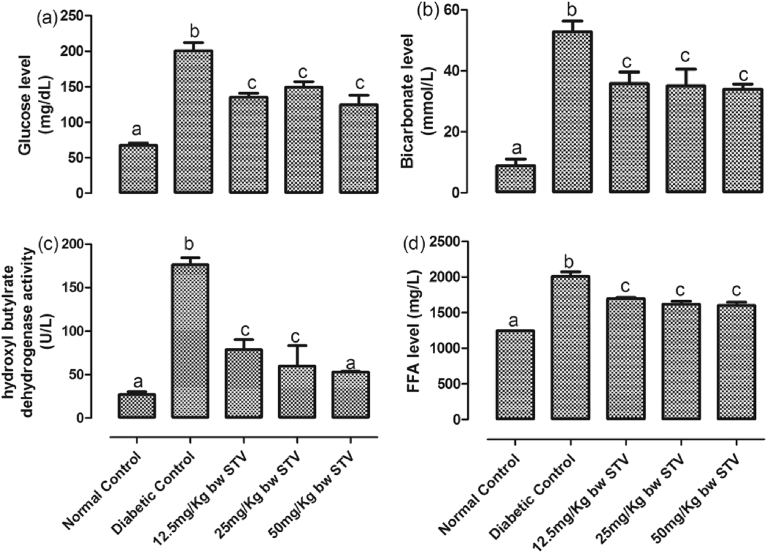
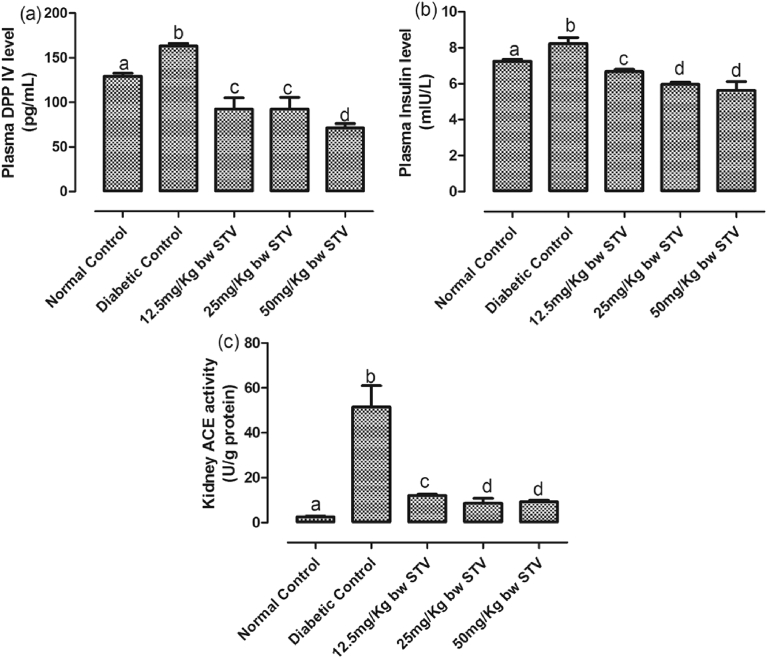
The levels of glucose, bicarbonate, free fatty acids (FFA) and activity of hydroxybutyrate dehydrogenase were assessed in the plasma of the rats [Fig. 1(a–d)]. Furthermore, the levels of plasma DPP IV and plasma insulin, as well as the activity of kidney ACE were assayed [Fig. 2(a–c)] as biomarkers of T2DM. T2DM, significantly (p < 0.05) increased the plasma concentrations of glucose, insulin, DPP IV and bicarbonate while all the doses of stevioside resulted in a significant (p < 0.05) decrease when compared with the diabetic control. Treatment with stevioside significantly (p < 0.05) decreased the concentrations of plasma DPP IV to levels significantly (p < 0.05) lower than the normal control. 50 mg/kg dose and, 25 and 50 mg/kg doses had the lowest concentrations of DPP IV and insulin respectively. T2DM also significantly (p < 0.05) increased the activity of ACE in the kidney and concentration of plasma FFA. Stevioside treatment resulted in a dose-dependent significant (p < 0.05) decreases in both biomarkers, with 25 mg/kg treatment giving the optimum reductions.
Fig. 1.
(a–d): Effects of stevioside on biomarkers of diabetes in the experimental rats. (a) level of plasma glucose, (b) level of plasma bicarbonate, (c) activity of plasma hydroxyl butylrate dehygrogenase, and (d) level of plasma free fatty acids. Bars represent mean ± SEM (n = 6). Bars with different statistical markers are significantly different at p < 0.05.
Fig. 2.
(a–c): Effects of stevioside on specific biomarkers of T2DM in the experimental rats. (a) level of plasma DPP IV, (b) level of plasma insulin, and (c) activity of kidney ACE. Bars represent mean ± SEM (n = 6). Bars with different statistical markers are significantly different at p < 0.05.
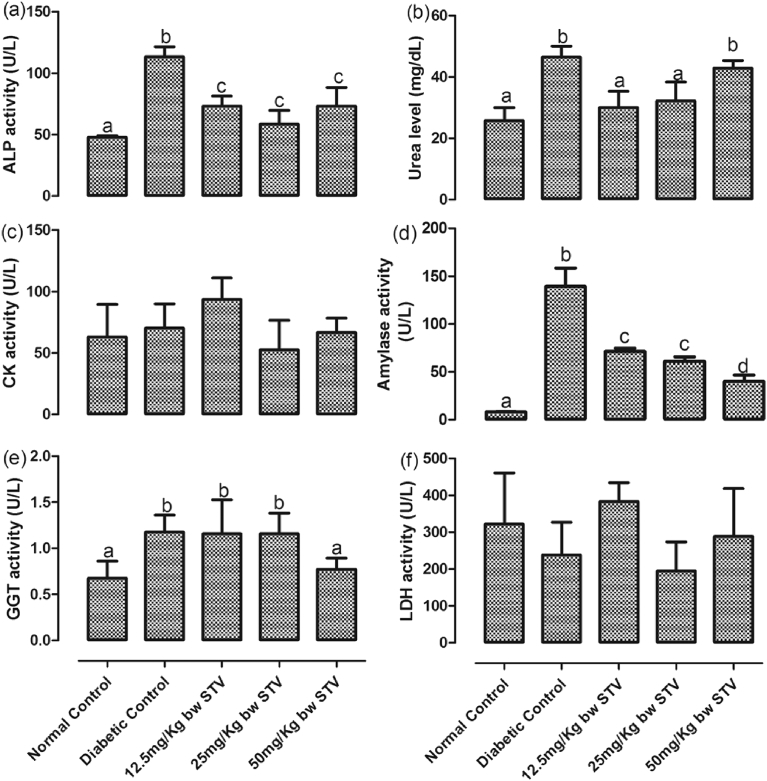
The activities of plasma ALP, CK, amylase, GGT and LDH, as well as the level of plasma urea are depicted in Fig. 3(a–f). T2DM significantly (p < 0.05) increased the activities of ALP, amylase and GGT, as well as the level of urea. The activities of ALP and amylase were significantly (p < 0.05) reduced by stevioside. The reduction of amylase activity was dose-dependent with 50 mg/kg having the highest reduction. Although, level of plasma urea was significantly (p < 0.05) reduced by stevioside treatment, this reduction was significantly (p < 0.05) reversed at 50 mg/kg. However, this dosage significantly (p < 0.05) reduced the activity of GGT.
Fig. 3.
(a–f): Effects of stevioside on biomarkers of organ function the experimental rats. (a) activity of plasma alkaline phosphatase, (b) level of plasma urea, (c) activity of plasma creatinine kinase, (d) activity of plasma amylase, (e) activity of plasma gamma-glutamyl transferase, and (f) activity of plasma lactate dehydrogenase. Bars represent mean ± SEM (n = 6). Bars with different statistical markers are significantly different at p < 0.05.
3.2. Stevioside administration reduced dyslipidemia
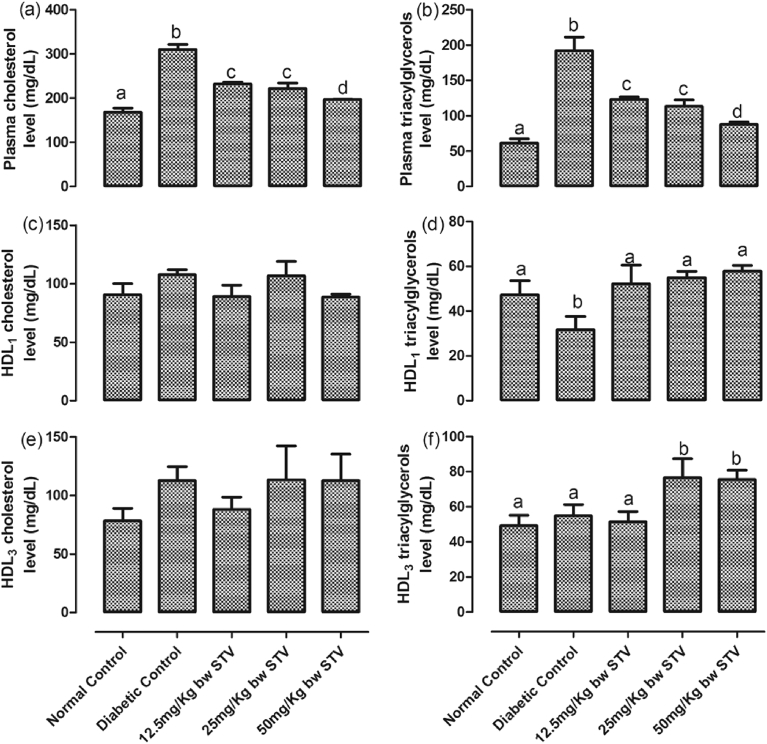
Fig. 4(a–f) shows the levels of cholesterol and triacylglycerols in the rats. T2DM significantly (p < 0.05) increased the concentrations of plasma cholesterol and triacylglycerols while it decreased the concentration of HDL1 triacylglycerols. There was no significant difference in the concentrations of HDL1 cholesterol, HDL3 cholesterol and HDL3 triacylglycerols compared with normal control. All the doses of stevioside significantly (p < 0.05) decreased plasma cholesterol and triacylglycerols compared with diabetic control, with the highest dose having the most significant (p < 0.05) decrease. All the doses of stevioside significantly reversed the concentrations of HDL1 triacylglycerols to levels not significantly different from the normal control while the 25 and 50 mg/kg doses significantly (p < 0.05) increased HDL3 triacylglycerols when compared with normal and diabetic controls.
Fig. 4.
(a–f): Effects of steviosdie on plasma lipid profile in the experimental rats. (a) level of plasma total cholesterol, (b) level of plasma total triacylglycerols, (c) level of HDL1 cholesterol, (d) level of HDL1 triacylglycerols, (e) level of HDL3 cholesterol, and (f) level of HDL3 triacylglycerols. Bars represent mean ± SEM (n = 6). Bars with different statistical markers are significantly different at p < 0.05.
3.3. Stevioside reversed oxidative stress and damage
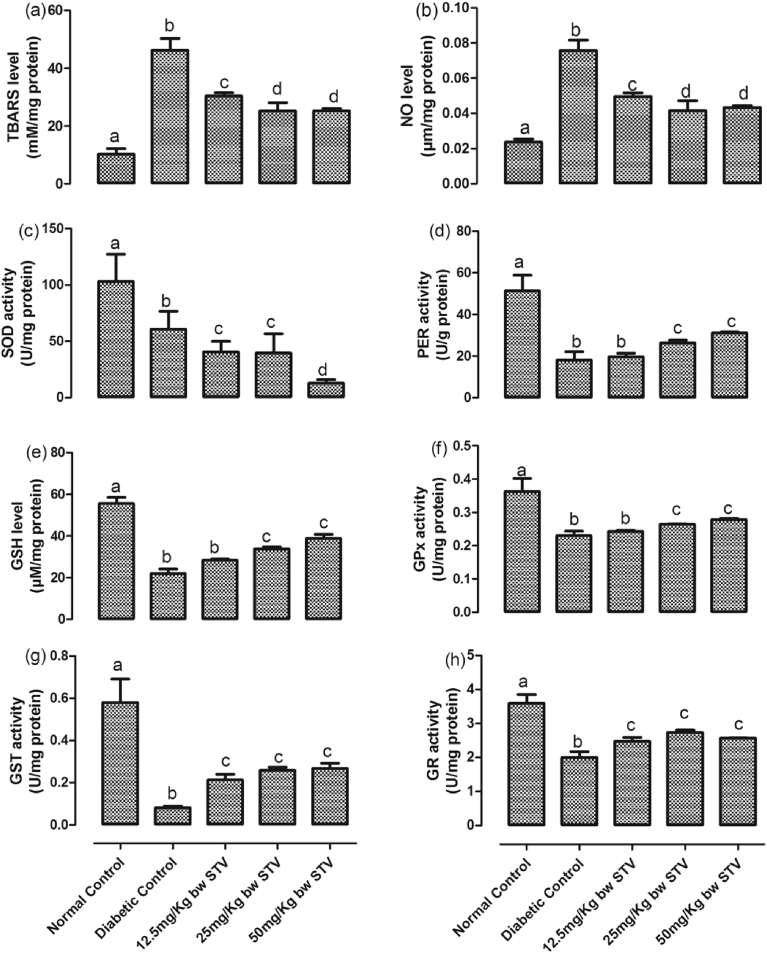
The activities of hepatic SOD, GST, GSH, GPx, PER, and GR were significantly decreased while the concentrations of TBARS and NO were significantly (p < 0.05) increased by T2DM (Fig. 5(a–h)). All the doses of stevioside significantly (p < 0.05) increased the activities of GST and GR when compared with diabetic control while it decreased SOD with the 50 mg/kg having the most decrease. Only 25 and 50 mg/kg dose increased the levels of GSH, GPx and PER. All the doses of stevioside significantly decreased the concentrations of TBARS and NO when compared with diabetic control. The decrease resulting from 25 and 50 mg/kg doses were not significantly (p > 0.05) different from each other.
Fig. 5.
(a–h): Effects of stevioside on biomarkers of oxidative stress in the liver of the experimental rats. (a) level of thiobarbituric reactive substances, (b) level of nitric oxide, (c) activity of superoxide dismutase, (d) activity of peroxidase, (e) level of reduced glutathione, (f) activity of glutathione peroxidase, (g) activity of glutathione-S-transferase, and (h) activity of glutathione reductase. Bars represent mean ± SEM (n = 6). Bars with different statistical markers are significantly different at p < 0.05.
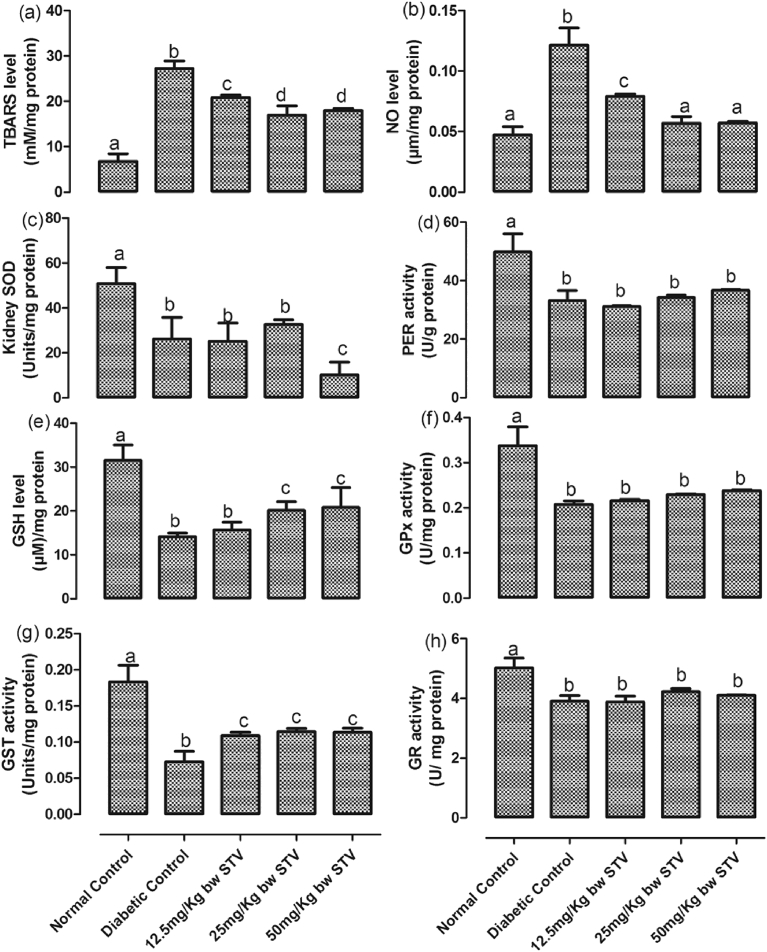
T2DM significantly (p < 0.05) decreased the levels of SOD, GST, GSH, GPx, PER and GR while it increased that of TBARS and NO in the kidney (Fig. 6(a–h)). All doses of stevioside significantly (p < 0.05) increased the activities of GST while only the 25 and 50 mg/kg doses of stevioside significantly (p < 0.05) increased the concentrations of GSH when compared with the diabetic control. All doses of stevioside were unable to increase the diabetic-induced decrease in the activities of GPx, PER and GR. This trend was also observed in SOD, however, the 50 mg/kg dose led to a further decrease in SOD activity when compared with the control. All doses of stevioside significantly (p < 0.05) decreased the diabetic-induced increase in the concentrations of TBARS and NO. While 25 and 50 mg/kg doses resulted in decreases in TBARS and NO significantly (p < 0.05) different from the 12.5 mg/kg group, these doses reduced only NO to levels not significantly (p < 0.05) different from the control group.
Fig. 6.
(a–h): Effects of stevioside on biomarkers of oxidative stress in the kidney of the experimental rats. (a) level of thiobarbituric reactive substances, (b) level of nitric oxide, (c) activity of superoxide dismutase, (d) activity of peroxidase, (e) level of reduced glutathione, (f) activity of glutathione peroxidase, (g) activity of glutathione-S-transferase, and (h) activity of glutathione reductase. Bars represent mean ± SEM (n = 6). Bars with different statistical markers are significantly different at p < 0.05.
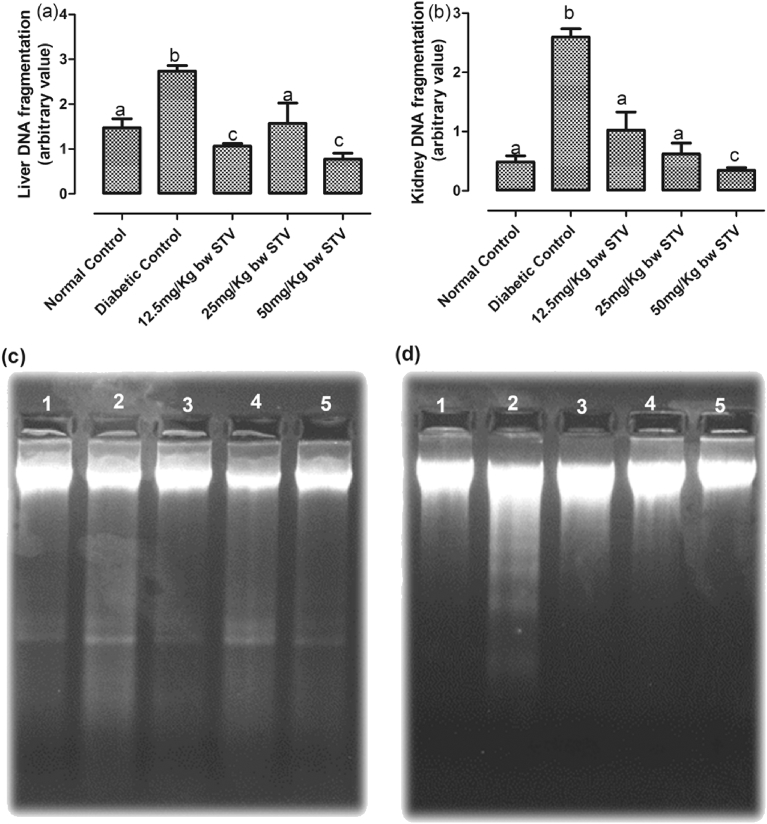
The level of liver and kidney DNA fragmentation was significantly (p < 0.05) increased in the diabetic control group compared with other groups. All doses of stevioside significantly (p < 0.05) decreased the fragmentation in the liver and kidney when compared with the diabetic control (Fig. 7(a–b)). The 12.5 and 25 mg/kg doses significantly (p < 0.05) decreased the kidney DNA fragmentation to levels not significantly (p > 0.05) different from the normal control while the 50 mg/kg dose was significantly (p < 0.05) lower than the normal control.
Fig. 7.
(a–d) Effect of stevioside on the intra-nucleosomal DNA fragmentation. (a) the pattern of DNA ladder of the liver, (b) the pattern of DNA ladder of the kidney, (c) agarose gel image of liver DNA fragmentation and (d) agarose gel image of kidney DNA fragmentation. Group 1: Normal Control, Group 2: Diabetic Control, Group 3: Diabetic rats treated with 12.5 mg/kg stevioside, Group 4: Diabetic rats treated with 25 mg/kg stevioside and Group 5: Diabetic rats treated with 50 mg/kg stevioside.
3.4. In silico predictions of biological activities of stevioside
Table 2 showed the PASS-based anti-T2DM activities of stevioside. Apart from its antidiabetic property, other biological activities include very high inhibition of beta-adrenergic receptor kinase, G-protein-coupled receptor kinase, alpha glucosidase inhibitor, beta glucuronidase inhibitor and glucan endo-1.3-beta-D-glucosidase inhibitor. PASS also indicated the antioxidant as well as the cholesterol and nitric oxide antagonistic properties of stevioside.
Table 2.
PASS-predicted antidiabetic activities of stevioside.
| Pa | Pi | Activity | |
|---|---|---|---|
| 1 | 0.799 | 0.013 | Beta-adrenergic receptor kinase inhibitor |
| 2 | 0.799 | 0.013 | G-protein-coupled receptor kinase inhibitor |
| 3 | 0.786 | 0.005 | Antidiabetic |
| 4 | 0.764 | 0.001 | Alpha glucosidase inhibitor |
| 5 | 0.721 | 0.005 | Antidiabetic (type 2) |
| 6 | 0.718 | 0.007 | Cholesterol antagonist |
| 7 | 0.637 | 0.009 | Beta glucuronidase inhibitor |
| 8 | 0.630 | 0.021 | Glucan endo-1.3-beta-D-glucosidase inhibitor |
| 9 | 0.575 | 0.004 | Nitric oxide antagonist |
| 10 | 0.498 | 0.007 | Antioxidant |
4. Discussion
Stevioside improved oxidative stress and damage associated with T2DM and also reduced the associated increase in biomarkers of T2DM. Although the admissible daily intake for steviol glycosides is 0–4 mg/kg body weight (expressed as steviol), the concentration used in this study were of pharmacological significance in rats and below the reported toxicity level [33, 34].
The results of this study showed that the rise in blood glucose was associated with a concomitant increase in biomarkers of T2DM, which include DPP IV, bicarbonate, hydroxybutyrate dehydrogenase dehydrogenase and FFA [35, 36]. This is consistent with the pattern observed in T2DM in humans and in rat model [37]. It is noteworthy that stevioside administration resulted in mild but significant reduction in plasma glucose and other biomarkers of T2DM measured. Of particular interest is the reduction in the level of DPP IV – a biomarker of T2DM that has been reported to be beneficial in the management of T2DM by previous authors [38, 39]. This further validates the in silico results indicating the potential of stevioside as an anti-T2DM agent. This could be due to the beta glucuronidase and glucan endo-1.3-beta-D-glucosidase inhibitory properties of stevioside. The inhibition of these enzymes regulate carbohydrate digestion and release of glucose in the gastrointestinal tract and has been recognized as potential mechanism of hypoglycemic agents [40]. Previous studies have reported the hypoglycemic property of stevioside but none has indicated the inhibition of these enzymes as part of the mechanisms.
A number of hypoglycemic agents that inhibit the release of glucose in the GIT often results in the buildup of bicarbonate. In contrast, the hypoglycemic property of stevioside was accompanied with decrease in bicarbonate level together with insulin. This is an indication that the glucose uptake by the cells was enhanced thereby reducing lipolysis. In T2DM, cells rely on lipolysis as an alternative source of energy by upregulating β-oxidation of FFA to form ketone bodies [36]. This condition, known as diabetic ketoacidosis, is a known life-threatening acute complication of diabetes. Diabetic acidosis is characterized by the build of bicarbonate in the blood and increase in the activity of β-hydroxybutyrate dehydrogenase. β-hydroxybutyrate dehydrogenase converts β-hydroxybutyrate to acetoacetate. The acetoacetate is further converted to acetoacetyl-CoA, which is then split into two acetyl-CoA that are utilized in the TCA cycle to generate ATP in peripheral tissues [41].
Increase in β-hydroxybutyrate dehydrogenase activity could serve as a surrogate biomarker for both insulin resistance and impaired glucose regulation [42]. This further support the increase in insulin level along with glucose and β-hydroxybutyrate dehydrogenase activity observed in the diabetic untreated animals. The shift in energy metabolism from glucose to fatty acid results in alterations in several signal transduction pathways, which is now known to involve the G protein-coupled receptor kinase 2 (GRK2)-integrated signal transduction pathways [43]. Emerging evidences now suggest that increase in GRK2 activity contribute to the development of T2DM, and inhibiting GRK2 could reverse an established insulin-resistance [44]. It of interest that stevioside has GRK2 inhibitory property, as shown by PASS analysis in silico results. Hence, the ability of stevioside to reverse insulin resistance and enhance uptake of glucose could be due to its ability to inhibit GRK2.
The hypoglycemic effect of stevioside was also associated with decrease in plasma amylase activity, as well as decrease in some biomarkers of organ function. However, it is of concern that stevioside at 50 mg/kg resulted in increase in plasma urea. This shows that at this concentration of stevioside could be toxic to the kidney. This finding is corroborated by previous studies that showed that showed that stevioside at high concentration could be nephrotoxic [44, 45]. Also, we had earlier expressed the need to consider nephrotoxic implication of hypoglycemic regime [46].
A major macrovascular complication associated with T2DM is hypertension, and this comorbidity is responsible for high mortality due to this disease [1, 46]. The pathogenesis of hypertension involves elevation of renal angiotensin converting enzyme [47] and previous studies have identified its elevation in T2DM [48]. Hence, inhibiting this enzyme is a major target of antihypertensive therapies [49]. The findings of this study showed that stevioside reduced the elevation of renal angiotensin converting enzyme in the diabetic rats. This could therefore, explain the antihypertensive property of stevioside earlier reported [50, 51], and validated in human hypertension [52].
The shift in cellular energy source from glucose to fatty acids and the associated insulinemia result in dyslipidemia and cardiovascular complications in T2DM [53]. The in silico analysis showed that stevioside possesses a strong cholesterol antagonistic property, and this could account for the reduction in cholesterol level. Although previous studies have reported the cholesterol lowering properties of stevioside, none has associated it to its cholesterol antagonistic property. Another possible mechanism of improving dyslipidemia is by inhibiting G-protein-coupled receptor kinase. Altered lipid metabolism following high-fat diet-induced dyslipidemia has been shown to upregulate kinase and it is a therapeutic target [42, 54].
A major biochemical consequence of dyslipidemia is the FFAs activation of NADPH oxidase, which induces reactive oxygen species (ROS) production and ROS induced oxidative stress [55]. The role of ROS in the pathogenesis of diabetes, and its complications, has been reported by many researchers [40, 46, 55]. In this study, oxidative stress in liver and kidney was characterized with increased lipid peroxidation and NO with a decrease in the level of GSH as well as the activities of PER, SOD, GST and GPx. The depletion of these antioxidants has been reputed to expose major macromolecules to the peroxidative damaging effects of ROS [55]. This leads to lipid peroxidation and oxidative fragmentation of DNA. The free radicals generated due to gluco-toxicity can be well scavenged by antioxidant phytochemicals. Interestingly, the findings from in silico investigation showed that stevioside has antioxidant and anti NO property; and these could have accounted for the in vivo effects in the liver and kidney of the diabetic rats. Although NO is a potent antioxidant, it is often converted to peroxynitrite in the presence of ROS and can damage biomolecules in this state [46].
An important finding of this study is the ability of stevioside to reverse intra-nucleosomal DNA fragmentation in the liver and kidney rats. This DNA fragmentation was more prominent in the kidney than the liver. This could be due to the activation of angiostensin-converting enzyme. Kawahito et al [56] stated that activation of ACE enhances glucotoxicity-induced apoptosis.
5. Conclusion
Our findings suggest that the use of stevioside could be beneficial in preventing oxidative DNA damage observed in T2DM. This is in addition to its antioxidant and antihyperglycemic properties, particularly in potentially inhibiting glucose absorption. This also further supports its nutraceutical role as a component of the S. rebaudiana-derived sugar substitute, especially in T2DM patients.
Declarations
Author contribution statement
Solomon Oladapo Rotimi, Oluwakemi Anuoluwapo Rotimi: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Isaacson Bababode Adelani, Chinonye Onuzulu, Patience Obi, Rotimi Okungbaye: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Carrera-Lanestosa A., Moguel-Ordonez Y., Segura-Campos M. Stevia rebaudiana Bertoni: a natural alternative for treating diseases associated with metabolic syndrome. J. Med. Food. 2017;20(10):933–943. doi: 10.1089/jmf.2016.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorentino T.V. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013;19(32):5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 3.Grindel A. Oxidative stress, DNA damage and DNA repair in female patients with diabetes mellitus type 2. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rippe J.M., Angelopoulos T.J. Relationship between added sugars consumption and chronic disease risk factors: current understanding. Nutrients. 2016;8(11):697. doi: 10.3390/nu8110697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rippe J.M., Angelopoulos T.J. Added sugars and risk factors for obesity, diabetes and heart disease. Int. J. Obes. (Lond) 2016;40(Suppl 1):S22–S27. doi: 10.1038/ijo.2016.10. [DOI] [PubMed] [Google Scholar]
- 6.Reed D.R., McDaniel A.H. The human sweet tooth. BMC Oral Health. 2006;6(Suppl 1):S17. doi: 10.1186/1472-6831-6-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crammer B. Sweet glycosides from the stevia plant. Chem. Br. 1986;22:915–918. [Google Scholar]
- 8.J.F.W.E.C.o.F.A. Meeting, W.H. Organization . World Health Organization; 2006. Safety Evaluation of Certain Food Additives. [Google Scholar]
- 9.Koyama E. Absorption and metabolism of glycosidic sweeteners of stevia mixture and their aglycone, steviol, in rats and humans. Food Chem. Toxicol. 2003;41(6):875–883. doi: 10.1016/s0278-6915(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh M.H. Efficacy and tolerability of oral stevioside in patients with mild essential hypertension: a two-year, randomized, placebo-controlled study. Clin. Ther. 2003;25(11):2797–2808. doi: 10.1016/s0149-2918(03)80334-x. [DOI] [PubMed] [Google Scholar]
- 11.Prata C. Glycosides from Stevia rebaudiana Bertoni possess insulin-mimetic and antioxidant activities in rat cardiac fibroblasts. Oxidative Med. Cell. Longev. 2017;2017:3724545. doi: 10.1155/2017/3724545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bender C., Graziano S., Zimmermann B.F. Study of Stevia rebaudiana Bertoni antioxidant activities and cellular properties. Int. J. Food Sci. Nutr. 2015;66(5):553–558. doi: 10.3109/09637486.2015.1038223. [DOI] [PubMed] [Google Scholar]
- 13.A. Roberts, I. Munro, Stevioside and related compounds: therapeutic benefits beyond sweetness, Pharmacol. Ther. 122 (3) (2009) e1–e2. [DOI] [PubMed]
- 14.Ilic V. Insight into anti-diabetic effect of low dose of stevioside. Biomed. Pharmacother. 2017;90:216–221. doi: 10.1016/j.biopha.2017.03.045. [DOI] [PubMed] [Google Scholar]
- 15.Skovso S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J. Diabetes Investig. 2014;5(4):349–358. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotimi S.O. Naringin enhances reverse cholesterol transport in high fat/low streptozocin induced diabetic rats. Biomed. Pharmacother. 2018;101:430–437. doi: 10.1016/j.biopha.2018.02.116. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp. Diabetes Res. 2008;2008:704045. doi: 10.1155/2008/704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotimi S.O. Tissue dyslipidemia in Salmonella-infected rats treated with amoxillin and pefloxacin. Lipids Health Dis. 2012;11:152. doi: 10.1186/1476-511X-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rotimi S.O. Amoxillin- and pefloxacin-induced cholesterogenesis and phospholipidosis in rat tissues. Lipids Health Dis. 2015;14:13. doi: 10.1186/s12944-015-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cushman D.W., Cheung H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971;20(7):1637–1648. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 21.Rifai N., Warnick G.R., Dominiczak M.H. Amer. Assoc. for Clinical Chemistry; 2000. Handbook of Lipoprotein Testing. [Google Scholar]
- 22.Buege J.A., Aust S.D. Elsevier; 1978. [30] Microsomal Lipid Peroxidation, Methods in Enzymology; pp. 302–310. [DOI] [PubMed] [Google Scholar]
- 23.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 24.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 25.Rotruck J.T. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 26.Mavis R.D., Stellwagen E. Purification and subunit structure of glutathione reductase from bakers' yeast. J. Biol. Chem. 1968;243(4):809–814. [PubMed] [Google Scholar]
- 27.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 28.Fergusson R.R., Chance B. Substrate specificity of peroxidase. Science. 1955;122(3167):466–467. doi: 10.1126/science.122.3167.466-a. [DOI] [PubMed] [Google Scholar]
- 29.Rotimi S.O. Hesperidin prevents lipopolysaccharide-induced endotoxicity in rats. Immunopharmacol. Immunotoxicol. 2016;38(5):364–371. doi: 10.1080/08923973.2016.1214142. [DOI] [PubMed] [Google Scholar]
- 30.Lowry O.H. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 31.Suman S., Pandey A., Chandna S. An improved non-enzymatic “DNA ladder assay” for more sensitive and early detection of apoptosis. Cytotechnology. 2012;64(1):9–14. doi: 10.1007/s10616-011-9395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filimonov D. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Comp. 2014;50(3):444–457. [Google Scholar]
- 33.Barriocanal L.A. Apparent lack of pharmacological effect of steviol glycosides used as sweeteners in humans. A pilot study of repeated exposures in some normotensive and hypotensive individuals and in Type 1 and Type 2 diabetics. Regul. Toxicol. Pharmacol. 2008;51(1):37–41. doi: 10.1016/j.yrtph.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Latha S., Chaudhary S., Ray R.S. Hydroalcoholic extract of Stevia rebaudiana bert. leaves and stevioside ameliorates lipopolysaccharide induced acute liver injury in rats. Biomed. Pharmacother. 2017;95:1040–1050. doi: 10.1016/j.biopha.2017.08.082. [DOI] [PubMed] [Google Scholar]
- 35.Gall W.E. α-Hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5(5) doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilding J.P. The importance of free fatty acids in the development of Type 2 diabetes. Diabet. Med. 2007;24(9):934–945. doi: 10.1111/j.1464-5491.2007.02186.x. [DOI] [PubMed] [Google Scholar]
- 37.Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp. Clin. Endocrinol. Diabetes. 2003;111(3):121–124. doi: 10.1055/s-2003-39781. [DOI] [PubMed] [Google Scholar]
- 38.Green B.D., Flatt P.R., Bailey C.J. Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diabetes Vasc. Dis. Res. 2006;3(3):159–165. doi: 10.3132/dvdr.2006.024. [DOI] [PubMed] [Google Scholar]
- 39.Augustyns K. The therapeutic potential of inhibitors of dipeptidyl peptidase IV (DPP IV) and related proline-specific dipeptidyl aminopeptidases. Curr. Med. Chem. 2005;12(8):971–998. doi: 10.2174/0929867053507298. [DOI] [PubMed] [Google Scholar]
- 40.Rotimi S.O., Omotosho O.E., Rotimi O.A. Persistence of acidosis in alloxan-induced diabetic rats treated with the juice of Asystasia gangetica leaves. Pharmacogn. Mag. 2011;7(25):25–30. doi: 10.4103/0973-1296.75887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newman J.C., Verdin E. β-hydroxybutyrate: much more than a metabolite. Diabetes Res. Clin. Pract. 2014;106(2):173–181. doi: 10.1016/j.diabres.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Guerra L. G protein–coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010;59(10):2407–2417. doi: 10.2337/db10-0771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Vila-Bedmar R. Reversal of diet-induced obesity and insulin resistance by inducible genetic ablation of GRK2. Sci. Signal. 2015;8(386):ra73. doi: 10.1126/scisignal.aaa4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melis M.S. Stevioside effect on renal function of normal and hypertensive rats. J. Ethnopharmacol. 1992;36(3):213–217. doi: 10.1016/0378-8741(92)90046-t. [DOI] [PubMed] [Google Scholar]
- 45.Toskulkac C. Acute toxicity of stevioside, a natural sweetener, and its metabolite, steviol, in several animal species. Drug Chem. Toxicol. 1997;20(1-2):31–44. doi: 10.3109/01480549709011077. [DOI] [PubMed] [Google Scholar]
- 46.Rotimi S.O. Inability of legumes to reverse diabetic-induced nephropathy in rats despite improvement in blood glucose and antioxidant status. J. Med. Food. 2010;13(1):163–169. doi: 10.1089/jmf.2008.0293. [DOI] [PubMed] [Google Scholar]
- 47.Bernstein K.E. Renal angiotensin-converting enzyme and blood pressure control. Curr. Opin. Nephrol. Hypertens. 2014;23(2):106. doi: 10.1097/01.mnh.0000441047.13912.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall S. Blood pressure control, microalbuminuria and cardiovascular risk in Type 2 diabetes mellitus. Diabet. Med. 1999;16(5):358–372. doi: 10.1046/j.1464-5491.1999.00045.x. [DOI] [PubMed] [Google Scholar]
- 49.Komers R., Komersova K. Therapeutic potential of ACE inhibitors for the treatment of hypertension in Type 2 diabetes. Expert Opin. Investig. Drugs. 2000;9(11):2601–2617. doi: 10.1517/13543784.9.11.2601. [DOI] [PubMed] [Google Scholar]
- 50.Hsu Y.H. Antihypertensive effect of stevioside in different strains of hypertensive rats. Zhonghua Yi Xue Za Zhi (Taipei) 2002;65(1):1–6. [PubMed] [Google Scholar]
- 51.Ferri L.A. Investigation of the antihypertensive effect of oral crude stevioside in patients with mild essential hypertension. Phytother. Res. 2006;20(9):732–736. doi: 10.1002/ptr.1944. [DOI] [PubMed] [Google Scholar]
- 52.Chan P. A double-blind placebo-controlled study of the effectiveness and tolerability of oral stevioside in human hypertension. Br. J. Clin. Pharmacol. 2000;50(3):215–220. doi: 10.1046/j.1365-2125.2000.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor R. Insulin resistance and type 2 diabetes. Diabetes. 2012;61(4):778–779. doi: 10.2337/db12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Belmonte S.L., Blaxall B.C. G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circ. Res. 2011;109(3):309–319. doi: 10.1161/CIRCRESAHA.110.231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawahito S., Kitahata H., Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J. Gastroenterol. 2009;15(33):4137. doi: 10.3748/wjg.15.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]