Abstract
Introduction
The mammography screening programme has been the subject of criticism for some time. Invitation to take part is currently based only on the risk factors of age and female sex, whereby women with an above-average risk are screened too seldom and women with a low risk are possibly screened too often. In future, an individualised risk assessment could make a risk-adapted procedure possible in breast cancer screening. In the RISIKOLOTSE.DE project, schemes are devised to calculate the individual breast cancer risk and evaluate the results. The aim is to assist doctors and screening participants in participatory decision-making. To gauge the baseline situation in the target groups, qualitative and quantitative surveys were conducted.
Method
At the start of the project, a guideline-based focus group discussion was held with 15 doctors and representatives of the public health service. The transcript of this discussion was evaluated by means of a qualitative content analysis.
Results
The participants assessed the concept of risk-adapted screening positively overall. At the same time, the majority of them were of the opinion that the results of individualised risk calculation can be understood and evaluated adequately only by doctors. The great communication requirement and lack of remuneration were given as practical obstacles to implementation.
Discussion
The suggestions and new ideas from the focus group ranged from administrative and regulatory changes to new forms of counselling and adaptable practice aids. An important indicator for the RISIKOLOTSE.DE conception and for planning future surveys was that risk calculation for mammography screening 2.0 was regarded as a purely medical function and that the concept of participatory decision-making played hardly any part in the discussion.
Key words: focus group, mammography screening, breast cancer risk, risk-adapted screening, participatory decision-making
Zusammenfassung
Einleitung Das Mammografie-Screening-Programm steht seit einiger Zeit in der Kritik. Die Einladung zur Teilnahme beruht derzeit nur auf den Risikofaktoren Alter und weibliches Geschlecht, wodurch Frauen mit überdurchschnittlichem Risiko zu selten, Frauen mit niedrigem Risiko möglicherweise zu häufig untersucht werden. Künftig könnte eine individualisierte Risikobewertung ein risikoadaptiertes Vorgehen bei der Brustkrebs-Früherkennung ermöglichen. Im Projekt RISIKOLOTSE.DE werden Angebote erarbeitet, um das individuelle Brustkrebsrisiko zu berechnen und die Ergebnisse zu bewerten. Ziel ist es, Ärzte und Screening-Teilnehmerinnen bei der partizipativen Entscheidungsfindung zu unterstützen. Um die Ausgangssituation bei den Zielgruppen zu erfassen, wurden qualitative und quantitative Erhebungen durchgeführt.
Methode Zu Projektbeginn wurde eine leitfadenbasierte Fokusgruppendiskussion mit 15 Ärzten und Vertretern des öffentlichen Gesundheitsdienstes durchgeführt. Das Transkript dieser Diskussion wurde mittels einer qualitativen Inhaltsanalyse ausgewertet.
Ergebnisse Die Teilnehmer bewerteten das Konzept der risikoadaptierten Früherkennung insgesamt positiv. Gleichzeitig waren sie mehrheitlich der Meinung, dass die Ergebnisse der individualisierten Risikokalkulation nur von Ärzten adäquat verstanden und bewertet werden können. Als praktische Hürden bei der Umsetzung wurden besonders der hohe Kommunikationsaufwand und die fehlende Vergütung angeführt.
Diskussion Die Vorschläge und Impulse aus der Fokusgruppe reichten von administrativen bzw. regulatorischen Änderungen über neue Beratungsformen bis hin zu adaptierbaren Praxishilfen. Für die Konzeption von RISIKOLOTSE.DE und die Planung weiterer Erhebungen war ein wichtiger Hinweis, dass die Risikoberechnung für das Mammografie-Screening 2.0 als rein ärztliche Aufgabe gesehen wurde und dass das Konzept der partizipativen Entscheidungsfindung bei der Diskussion kaum eine Rolle spielte.
Schlüsselwörter: Fokusgruppe, Mammografie-Screening, Brustkrebsrisiko, risikoadaptiertes Screening, partizipative Entscheidungsfindung
Introduction
Breast cancer is the most common cancer in women 1 . A national breast cancer screening programme was introduced in Germany from 2005 2 . Women from 50 – 69 years of age are currently invited for screening. This “Mammography screening 1.0” is controversial, however 3 , 4 , 5 . Through the programme, breast cancer should be detected and treated early to increase the chances of cure. This should reduce the disease burden and lower mortality. A particular problem, however, is the risk of overdiagnosis and false positive results. For affected women, this means not only substantial psychological stress, but the wrong cancer diagnosis also results in unnecessary operations. Every woman who takes part in the screening must therefore be informed about the procedure, especially as it is associated with radiation exposure 3 – 5 .
Only the womanʼs age is taken into account for participation, whereas other risk factors influence the disease risk 6 . The programme therefore does not meet the individually different need for screening. In many women, mammography is performed without clear benefit. Other women, especially younger women, are not included in the programme despite the presence of risk factors. Apart from a familial predisposition, the time of menarche and menopause plays a part, for example, and also hormone replacement therapy and life style. More recent risk models for breast cancer take some of these factors into account. They enable individualised breast cancer screening – “Mammography screening 2.0”. The aim is to make mammography screening more efficient. Thus, women with an increased breast cancer risk could have additional investigations if appropriate, such as ultrasonography or magnetic resonance imaging. Conversely, women with a low risk could possibly forego screening examinations. Studies show in addition that some women outside the 50 to 69-year age group might benefit from screening 7 , 8 . Adjustment of the screening interval to the individual breast cancer risk is advocated in some quarters 9 , 10 .
Risk-adapted screening is already used in Germany for a certain group, namely, high-risk women. For familial reasons, they have a much higher risk of developing breast cancer than the general population. These women require more comprehensive screening methods as regular mammography screening does not suffice because of the early age at which the disease occurs. This programme is undisputed in carriers of mutations; in this case, the finding of a pathogenic mutation in the high-risk genes, BRCA2, BRCA2, CDH1 or TP53 or in the moderately penetrant genes CHEK2, PALB2, RAD51C/D, NBN or ATM is crucial 11 . The decision is more difficult in women with only a calculated high-risk situation (more than 20% mutation probability or 30% lifelong breast cancer risk) 12 . Their risks are calculated with a standardised risk calculation method – currently Cyrillic (based on the Claus model 13 ). However, the model has now been superseded scientifically and technically.
Besides these method-related challenges, studies have also shown profound problems of understanding mammography screening per se. The benefits and risks of mammography screening 1.0 are not assessed correctly, and some of the knowledge deficits are substantial 14 . The benefit is markedly overestimated while the risks are largely ignored 15 . Substantial knowledge deficits about screening were also found in doctors 16 . In mammography 2.0, dealing with the risk models represents an additional challenge.
This is where the RISIKOLOTSE.DE research project comes in. Information and tools will be provided in an online platform that will allow the breast cancer risk to be calculated, understood and evaluated. The target groups are doctors and laypersons: doctors will be supported in risk communication and counselling, laypersons in weighing the benefits and risks of taking part. Participatory decision-making will be enabled thereby.
The target groups were included in the conception right at the start of the project. One of the measures was a focus group discussion with experts about the question of adequate counselling of potential screening participants.
Methods
Focus group composition and running
The focus group is an exploratory investigation to enable a comprehensive and reality-based insight into the experiences and opinions of practice experts 17 , 18 . The discussion took place in the gynaecology department in the Klinikum rechts der Isar of Munich Technical University and lasted 2.5 hours. Doctors and representatives of the public health service were selected (“experts”); they were informed by post and e-mail about the RISIKOLOTSE.DE project and invited to the discussion. Out of 31 experts, 15 in total took part in the discussion ( Table 1 ). The only reasons cited for declining the invitation were time-related and organisational difficulties. Eleven persons did not react to the invitation. The focus group discussion was moderated using an open guideline. To establish current counselling practice, two case examples were presented at the start. The focus group members were to vote spontaneously on whether participation in screening can be recommended to the women in either case.
Table 1 Focus group participants.
| Women | Men | Total | |
|---|---|---|---|
| Gynaecologists | 6 | 1 | 7 |
|
2 | 0 | 2 |
|
4 | 1 | 5 |
| General physicians | 1 | 1 | 2 |
| Radiologists | 1 | 2 | 3 |
|
1 | 1 | 2 |
|
0 | 1 | 1 |
| Human geneticists | 0 | 1 | 1 |
| Public health service | 1 | 1 | 2 |
| Total | 9 | 6 | 15 |
Content analysis
With the agreement of the participants, the focus group discussion was recorded on video and transcribed verbally. A standardised transcription guideline was developed based on the methodical literature 19 , 20 . The transcript was evaluated systematically by qualitative content analysis based on Mayring 21 . The summarising content analysis used here, one of the three basic techniques of qualitative content analysis, follows inductive logic 21 , 22 . All text passages with content were first entered chronologically in a table, together with speaker pseudonym and time marker. According to the methodological specifications 21 , 23 , relevant statements were marked, paraphrased and generalised. Based on this, categories were formed with which the results could be summarised.
Results
Part 1: Discussion of mammography screening 1.0
The discussion concentrated initially on the current counselling situation for mammography screening.
Two brief case examples by way of introduction
Two brief case examples were presented by way of introduction to the discussion. The participants were to decide whether they would advise the women for or against mammography.
For the 42-year-old woman in case 1, whose cousin had breast cancer, there was no clear advice. By contrast, nearly all of them advised the 51-year-old woman in case 2 to take part in the mammography screening.
The two case examples show where the weaknesses of the existing mammography screening lie. The invitation to screening is currently based only on the age of women between 50 and 69 years. This screening recommendation appears to represent a binding guideline for doctors. On the other hand, there is no uniform recommendation on mammography for women under 50 years.
However, individual risk calculation with the IBIS risk calculation procedure, which includes other risk factors 24 , results in an increased breast cancer risk for the 42-year-old woman and an average risk for the 51-year old ( Fig. 1 ) compared with the general population. Nevertheless, only the 51-year-old woman is included in the screening programme according to the current standards 25 , 26 .
Fig. 1.
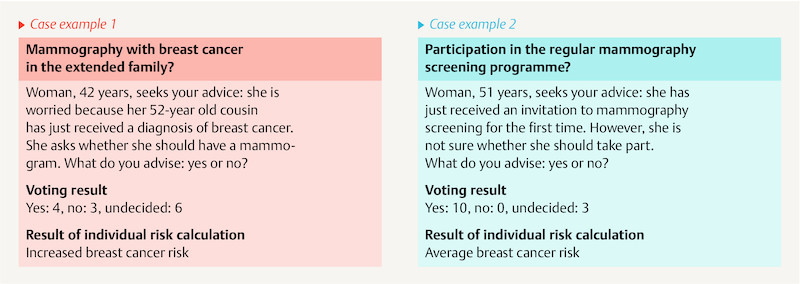
Voting result of the case examples.
Assessments of counselling practice
Nearly all participants conceded self-critically that they would usually talk only about positive aspects of screening during a consultation. After critical press reports about mammography screening, however, there would be corresponding enquiries from the women seeking advice, which the majority assessed as problematic.
Assessments of womenʼs need for counselling
Doctors working in the ambulant area in particular reported that they are confronted “really often” (general physician) with questions about the existing mammography screening. It was remarked critically that no advisory and information discussion is envisaged in the regular screening programme [authorsʼ comment: this was changed in the meantime and there is now an entitlement to this].
Assessments of the doctorsʼ counselling competence
Participants differed in their assessment of their own counselling competence and the assessment became more self-critical in the course of the discussion, as two examples show: “It is often the case that the women but also the doctors donʼt understand it” (gynaecologist), or “I am already a bit uncertain because I originally thought that screening can really only be good (…) But it is not quite so simple” (general physician). In routine clinical practice, screening recommendations were also made “instinctively” (general physician).
Part 2: Discussion of mammography screening 2.0
The second part of the focus group discussion concentrated on counselling for individualised mammography screening.
Assessments of individualised screening
The concept of individualised risk-adapted screening was assessed positively overall by the participants. The limitation in mammography 1.0 to the risk factors age and sex was criticised unanimously: “We do know that that doesnʼt suffice” (gynaecologist). Thus, “mammograms are done in women who derive no benefit from them” (gynaecologist). In this connection, the financial aspects for the healthcare system were also addressed: “Because of limited resources, it must be considered (…) whether it is actually necessary for us to screen all women” (public health service).
Assessments of womenʼs need for counselling in mammography screening 2.0
The majority of the participants were of the opinion that the results of the individualised risk calculation should be interpreted (only) by doctors: “The [medical] interpretation is always needed” (gynaecologist). Concern was expressed about enabling lay persons to use the risk calculator. Misunderstandings and incorrect interpretation by lay persons were referred to repeatedly: “You only need to imagine a woman with a family history (…), who keeps on clicking, forgets something and lands at a supposedly low risk and tells herself: everythingʼs OK” (gynaecologist). Possible positive aspects of independent use of risk calculators by lay persons (e.g., empowerment) were not mentioned by the participants.
Assessments of the doctorsʼ counselling competence in mammography screening 2.0
Several participants commented that risk interpretation and communication is itself a complex task, which would overtax many doctors. Counselling about the results of an individualised risk calculation is even more challenging: “I must classify the risk and we already see from genetics that that is not so simple” (gynaecologist). The objection was made that doctors could learn these competences: “I find that every doctor must be able to handle the subject of risk communication” (gynaecologist).
Assessments of implementation of individualised screening
“I think there are women who have such a low risk that they need less or no screening” (radiologist). On the other hand, women with an increased risk would benefit from earlier, more frequent or longer participation and from additional investigations such as ultrasonography or MR imaging. Mammography screening 2.0 could thus lead to “provision of better care for the overall population, (…) by simply redistributing them [= resources]” (radiologist). Implementation of risk-adapted screening was also classified as feasible. Several participants objected that implementation would be time-consuming: “That is too time-consuming, thatʼs our problem” (gynaecologist). The practice-based participants in particular stressed that the added effort would have to be remunerated. One suggestion was to introduce special risk consultation hours. The online platform RISIKOLOTSE.DE would have to offer practical aids for counselling, in the participantsʼ view.
Revision of the screening guidelines is needed fundamentally before the procedure can be implemented in practice. The participants expressed considerable concerns about advising a woman against screening mammography because of a low calculated disease risk or to deviate from the previous procedure.
Discussion
The counselling situation for mammography screening 1.0 was judged critically by the members of the focus group. They are aware of a high counselling need but do not feel entirely competent for this. It is apparent in the literature also that communication in the framework of the previous “normal” mammography screening represents a challenge. Women should be enabled to make an informed decision for or against taking part 27 . This informed consent assumes that the benefits and risks can be understood, correctly interpreted and applied to their own situation. The necessary health competence or health literacy also includes basic understanding of statistics. The actual decision should ideally be made jointly in the form of shared decision-making. The statistical statements about positive and negative effects of screening are often misinterpreted by both doctors and by laypersons seeking advice 28 . The benefit is sometimes massively overestimated while the risks are rather trivialised 29 .
Individualised risk-adapted mammography screening 2.0 was assessed positively by the majority in the discussion and also in the literature, though implementation was regarded critically. Besides the time required for consultation, concerns were expressed regarding oneʼs own competence and also regarding the current guidelines.
Overall, it was apparent that the concept of participatory decision-making did not play a major role in the discussion. Many doctors assume in general that they already practise joint decision-making. Studies suggest that the assessment is deceptive and that there is a perception–reality gap 30 . The participants emphasised that risk calculation in particular should rather be reserved to doctors, and lay persons were not trusted by the majority to be able to take responsibility for this. The deficits in their own understanding that they admitted at the same time were not perceived as inconsistent. The information that lay persons already have free access today to different breast cancer calculators on the internet 24 , 31 was largely ignored.
An important objection was the presumed time required for individualised risk consultation. It is well known that the available time per patient is limited especially in the ambulant area. The suggestion from among the participants to develop practical counselling aids and if necessary design new forms of counselling will be included in RISIKOLOTSE.DE planning.
Besides the usual limitations of the method 17 , 32 , 33 , a limitation that must be conceded is that the focus group members were recruited exclusively from the Munich region. In addition, the group size with n = 15 participants was greater than recommended in the specialist literature 33 , as an unexpectedly large number of experts wanted to take part in the discussion.
Conclusion
The focus group discussion proved to be an adequate method of obtaining new ideas from the target groups of doctors and the public health service for the conception of the online platform RISIKOLOTSE.DE.
The research project has hardly any influence on two obstacles to implementation of mammography screening 2.0: on the current screening guidelines and on remuneration of the medical counselling discussions. Only guidance can be given to political and funding institutions. On the other hand, there is evidence that the concept of participatory decision-making is not firmly established in mammography screening counselling practice. It can be assumed that this would not change in the context of mammography screening 2.0. It was found that there is a need to provide comprehension and implement participatory decision-making in routine medical practice, and also a need for fundamental schemes for risk communication. This knowledge will feed directly into the conception of the RISIKOLOTSE.DE online platform.
Acknowledgements
Our heartfelt thanks to all focus group participants for their commitment and valuable suggestions. We thank Prof. Kiechle and Dr. Pommer-Jung for their generous support in organising and carrying out the project.
Danksagung
Wir bedanken uns nachdrücklich bei allen Teilnehmern der Fokusgruppe für das Engagement und die wertvollen Anregungen. Vielen Dank an Frau Prof. Kiechle und Frau Dr. Pommer-Jung für die großzügige Unterstützung bei der Organisation und Durchführung des Projektes.
Conflict of Interest/Interessenkonflikt The authors declare that they have no conflict of interest./ Die Autoren geben an, dass kein Interessenkonflikt besteht.
These authors contributed equally to the study.
Diese Autoren haben zu gleichen Teilen zu der Arbeit beigetragen.
References/Literatur
- 1.Quante A S, Ming C, Rottmann M. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer Med. 2016;5:2649–2656. doi: 10.1002/cam4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert U, Altland H, Duda V. Kurzfassung der aktualisierten Stufe-3-Leitlinie Brustkrebs-Früherkennung in Deutschland 2008. Geburtsh Frauenheilk. 2008;68:251–261. doi: 10.1055/s-2008-1027320. [DOI] [PubMed] [Google Scholar]
- 3.Gøtzsche P, Jørgensen K J. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013;(06):CD001877. doi: 10.1002/14651858.CD001877.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Independent UK Panel on Breast Cancer Screening . The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–1786. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012Online:http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxlast access: 10.01.2016
- 6.Quante A S, Whittemore A S, Shriver T. Breast cancer risk assessment across the risk continuum: genetic and nongenetic risk factors contributing to differential model performance. Breast Cancer Res. 2012;14:R144. doi: 10.1186/bcr3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellquist B N, Duffy S W, Abdsaleh S. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years. Cancer. 2011;117:714–722. doi: 10.1002/cncr.25650. [DOI] [PubMed] [Google Scholar]
- 8.van Schoor G, Moss S M, Otten J D. Effective biennial mammographic screening in women aged 40-49. Eur J Cancer. 2010;46:3137–3140. doi: 10.1016/j.ejca.2010.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Venturini E, Losio C, Panizza P. Tailored breast cancer screening program with microdose mammography, US, and MR Imaging: short-term results of a pilot study in 40-49-year-old women. Radiology. 2013;268:347–355. doi: 10.1148/radiol.13122278. [DOI] [PubMed] [Google Scholar]
- 10.Evans D G, Warwick J, Astley S M. Assessing individual breast cancer risk within the U.K. National Health Service Breast Screening Program: a new paradigm for cancer prevention. Cancer Prev Res (Phila) 2012;5:943–951. doi: 10.1158/1940-6207.CAPR-11-0458. [DOI] [PubMed] [Google Scholar]
- 11.Schmutzler R. Konsensusempfehlung des Deutschen Konsortiums Familiärer Brust- und Eierstockkrebs zum Umgang mit Ergebnissen der Multigenanalyse. Geburtsh Frauenheilk. 2017;77:733–739. [Google Scholar]
- 12.Kiechle M. Brustkrebsfrüherkennung – Zielgruppen, Methoden, Nutzen und Nebenwirkungen. Onkologe. 2016;22:550–557. [Google Scholar]
- 13.Claus E B, Risch N, Thompson W D. Autosomal dominant inheritance of early-onset breast cancer. Implications for risk prediction. Cancer. 1994;73:643–651. doi: 10.1002/1097-0142(19940201)73:3<643::aid-cncr2820730323>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Albert U, Kalder M, Schulte H. Das populationsbezogene Mammografie-Screening-Programm in Deutschland: Inanspruchnahme und erste Erfahrungen von Frauen in 10 Bundesländern. Gesundheitswesen. 2012;74:61–70. doi: 10.1055/s-0030-1268441. [DOI] [PubMed] [Google Scholar]
- 15.Dierks M-L, Schmacke N. Mammografie-Screening und informierte Entscheidung – mehr Fragen als Antworten. Gesundheitsmonitor Newsletter. 2014;1:1–15. [Google Scholar]
- 16.Wegwarth O, Schwartz L M, Woloshin S. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med. 2012;156:340–349. doi: 10.7326/0003-4819-156-5-201203060-00005. [DOI] [PubMed] [Google Scholar]
- 17.Morgan D L. Focus groups. Annu Rev Sociol. 1996;22:129–152. [Google Scholar]
- 18.Przyborski A, Riegler J. Berlin, Heidelberg, New York: Springer Verlag; 2010. Gruppendiskussion und Fokusgruppe; pp. 436–448. [Google Scholar]
- 19.Dresing T, Pehl T. Marburg: Eigenverlag; 2011. Praxisbuch Transkription. Regelsysteme, Software und praktische Anleitungen für qualitative ForscherInnen. [Google Scholar]
- 20.Dresing T, Pehl T. Berlin, Heidelberg, New York: Springer Verlag; 2010. Transkription; pp. 436–448. [Google Scholar]
- 21.Mayring P. Berlin, Heidelberg, New York: Springer Verlag; 2010. Qualitative Inhaltsanalyse; pp. 601–613. [Google Scholar]
- 22.Mayring P, Fenzl T. Wiesbaden: Springer Verlag; 2014. Qualitative Inhaltsanalyse; pp. 543–556. [Google Scholar]
- 23.Gläser-Zikuda M. Wiesbaden: Verlag für Sozialwissenschaften | Springer Fachmedien; 2011. Qualitative Auswertungsverfahren; pp. 109–119. [Google Scholar]
- 24.Tyrer J, Duffy S W, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- 25.Krebsinformationsdienst Deutsches Krebsforschungszentrum Risikofaktoren für Brustkrebs. Heidelberg: Deutsches Krebsforschungszentrum [updated 2014 Aug 14; cited 2016 Jan 10]Online:https://www.krebsinformationsdienst.de/tumorarten/brustkrebs/brustkrebsrisiken-uebersicht.php%23inhalt3last access: 07.05.2018
- 26.Gemeinsamer Bundesausschuss . Richtlinie des Gemeinsamen Bundesausschusses über die Früherkennung von Krebserkrankungen (Krebsfrüherkennungs-Richtlinie/KFE-RL). In der Fassung vom 18 Jun 2009, veröffentlicht im Bundesanzeiger 2009, Nr. 148a, zuletzt geändert am 24 Jul 2014, veröffentlicht im Bundesanzeiger AT 31.12.2014 B4, in Kraft getreten am 01 Jan 2015
- 27.Stefanek M E. Uninformed compliance or informed choice? A needed shift in our approach to cancer screening. J Natl Cancer Inst. 2011;103:1821–1826. doi: 10.1093/jnci/djr474. [DOI] [PubMed] [Google Scholar]
- 28.Wegwarth O, Gigerenzer G. The barrier to informed choice in cancer screening: statistical illiteracy in physicians and patients. Recent Results Cancer Res. 2018;210:207–221. doi: 10.1007/978-3-319-64310-6_13. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann T C, Del Mar C. Patientsʼ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175:274–286. doi: 10.1001/jamainternmed.2014.6016. [DOI] [PubMed] [Google Scholar]
- 30.Stiggelbout A M, Van der Weijden T, De Wit M P. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256. doi: 10.1136/bmj.e256. [DOI] [PubMed] [Google Scholar]
- 31.Gail M H, Brinton L A, Byar D P. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- 32.Baur N, Blasius J. Wiesbaden: Springer Verlag; 2014. Methoden der empirischen Sozialforschung; pp. 543–556. [Google Scholar]
- 33.Vogel S. Wiesbaden: Springer Verlag; 2014. Gruppendiskussion; pp. 581–586. [Google Scholar]



