Abstract
Catheter-directed therapy (CDT) is now acknowledged as a treatment option for select patients with acute massive or submassive pulmonary embolism (PE), and more patients are being considered for CDT if there is available expertise. Therefore, interventionalists should be aware of the variety of catheter-based treatment options, specific pitfalls to avoid during therapy, and the appropriate treatment endpoints. This article reviews currently available techniques and protocols for treating acute massive and submassive PE, with tips to safely and successfully perform percutaneous PE interventions.
Keywords: Pulmonary Embolism, catheter, thrombolysis, thrombectomy, interventional radiology
Objectives: Upon completion of this article, the reader will be able to demonstrate to the interventionalist the technical tips for treating acute massive and submassive PE. The article focuses on intraprocedural protocols and provides an overview of devices currently available for percutaneous PE thrombectomy.
Accreditation: This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Acute pulmonary embolism (PE) is a significant public health problem. It is estimated that at least 600,000 symptomatic cases of PE and ∼300,000 PE-related deaths occur annually in the United States. 1 Thrombotic obstruction of the pulmonary arteries results in a hypoxemic state with local release of pulmonary artery (PA) vasoconstrictors. The overall increase in PA vascular resistance impacts right ventricular (RV) afterload resulting in RV hypokinesis, dilatation, and tricuspid regurgitation. If PA obstruction does not resolve, patients may develop severe RV strain, LV failure, and life-threatening hemodynamic shock.
Acute PE is classified into three categories: low-risk, submassive (intermediate-risk), and massive (high-risk). 2 Massive PE is defined by hemodynamic shock—systolic blood pressure less than 90 mm Hg for more than 15 minutes or requiring ionotropic support to maintain a systolic blood pressure higher than 90 mm Hg. Prior studies have demonstrated the benefits of catheter-directed therapy (CDT) in massive PE with high rates of clinical success (as measured by hemodynamic improvement and survival), and low complication rates compared with rates of major hemorrhage reported with systemic thrombolysis. 3
Submassive PE is defined by right heart strain without hemodynamic shock. While the optimal protocol for treating submassive PE is evolving, a prior meta-analysis demonstrated that systemic thrombolysis may improve mortality, 4 and increasing evidence supports CDT for submassive PE, 5 6 7 which may have a lower risk of bleeding versus systemic thrombolysis. Systemic administration of tissue plasminogen activator (tPA) carries a major hemorrhage rate of 9 to 20% including a 3 to 5% risk of hemorrhagic stroke. 3 8 CDT allows direct delivery of tPA into the thrombus, which appears to decrease the overall dose of tPA, and this allows many patients with contraindications to systemic thrombolysis to be considered for CDT. In some cases, when there are absolute contraindications to any pharmacologic thrombolysis, CDT with mechanical and/or aspiration technique has been performed without the use of any thrombolytics. 3 For massive PE, the goal of CDT is to remove obstructing PA thrombus to alleviate RV strain, improve cardiac output, and restore hemodynamic stability. For submassive PE, the goal of CDT is to alleviate RV strain and prevent development of hemodynamic shock. This article reviews technical tips and tricks to safely and successfully perform catheter-based PE interventions.
Relevant Procedural Anatomy
The bifurcation of the PA occurs superiorly, just to the left of the ascending aorta, dividing into the right and left pulmonary arteries below the aortic arch. On fluoroscopy, the bifurcation is usually inferior, anterior, and to the left of the carina near the fifth thoracic vertebra. The right PA is longer than the left and extends horizontally in a rightward direction before splitting into the ascending branch supplying the right upper lobe and the descending branch supplying the right middle and lower lobes. The left PA progresses in a more cranial and posterior direction prior to splitting into an ascending branch supplying the left upper lobe and a descending branch supplying the lingula and left lower lobe. Pulmonary arterial vasculature is highly elastic with a large capacitance for increasing blood flow. The average systolic pulmonary artery pressure (PAP) is 15 to 30 mm Hg and the mean is 9 to 18 mm Hg. 9 Patients with chronic PE and longstanding pulmonary hypertension will have elevated baseline PAPs and this should be correlated with other clinical findings.
Preprocedural Preparation
For patients with massive PE or severe RV strain, consideration should be made for obtaining an anesthesia consultation for the procedure, and the risks versus benefits of general anesthesia should be discussed. It should be noted that sedatives for intubation and positive pressure ventilation may decrease preload, worsen RV function and cause hypotension. A cardiac anesthetist should also be considered for critically ill patients with multiple comorbidities. Preprocedural ultrasound of the common femoral or internal jugular vein should be done to confirm patency. Prior to traversing the right heart en route to catheterizing the main PA, presence of left bundle branch block should be excluded. For patients with a left bundle branch block, transvenous pacing should be available prior to the procedure, as manipulation of wires and catheters in the right heart may cause right bundle branch block and consequently complete heart block. A variety of transvenous pacing options are now available and can be provided by cardiology.
Catheterizing the Main Pulmonary Artery
Percutaneous access may be obtained via the common femoral or jugular veins. Ultrasound-guided access is recommended to minimize bleeding risk and to avoid inadvertent puncture of arterial structures as the consequences of an arterial puncture or multiple venous punctures are augmented by thrombolytic infusion. The pulmonary arterial system can be accessed with a variety of methods depending on operator preference and available equipment. From the common femoral vein, the right heart outflow tract may be catheterized using a curved catheter, Grollman, or pigtail catheter along with a hydrophilic Glidewire (Terumo, Somerset, NJ) and torque control device. Some operators curve the back end of a Bentson wire and insert this as a stiffener remaining within the catheter to help advance the catheter into the PA. For instance, this allows a pigtail catheter to take on a slightly more angulated curve and facilitates its passage through the tricuspid valve. Once the tricuspid is traversed, another forward motion and 180-degree turn will pass it through the outflow tract into the PA. From the internal jugular vein, a C2 catheter (Cook, Bloomington, IN) and a Glidewire with torque control device may be used to select the PA. Careful manipulation under fluoroscopy should always be performed to reduce risk of vascular injury from wire perforation. Additionally, there should be constant monitoring by other staff for ventricular arrhythmias which would prompt changing the catheter or wire position. Sustained ventricular tachycardia despite wire/catheter repositioning can be managed with 150 mg IV amiodarone given over 10 minutes 10 and DC cardioversion if necessary.
Once the pulmonary outflow tract is accessed with the hydrophilic wire, it can be exchanged for a nonhydrophilic rail wire such as a Rosen wire which provides stability for vascular sheath placement in the PA. If subsequent thrombolytic infusion is planned, at least one sheath access should be at least 2 Fr larger than the infusion catheter to allow adequate PA pressure measurements through the sheath sideport. For example, a 7 or 8 Fr vascular sheath (e.g., Flexor sheath, Cook, Bloomington, IN) extending from the access point to the main pulmonary trunk would accommodate a 5-Fr Unifuse infusion catheter (Angiodynamics, Latham, NY) to permit simultaneous PAP measurements through the sheath. Additionally, if two sheaths are utilized for dual-catheter infusions, the sheaths may be placed via separate punctures using a combination of the internal jugular vein (IJV) and/or common femoral vein (CFV), and sometimes two separate punctures in the same vein. Squeezing two infusion catheters into a single large sheath is generally not recommended due to leaking around the catheters, unless a sheath is available with sufficient hemostatic valve or adjunct port apparatus that can accommodate both infusion catheters.
Pressure Measurements and Pulmonary Angiography
Prior to performing pulmonary angiography, the degree of pulmonary hypertension and underlying cardiopulmonary reserve are important considerations. These factors must be weighed to determine a safe rate and volume of contrast injection into the pulmonary circulation. When systolic PAP exceeds 55 mm Hg or RV end diastolic pressure is greater than 20 mm Hg, the mortality associated with pulmonary angiography using large-volume power injection is as high as 3%. 11 12 Therefore, in the massive PE patient with elevated PAP, power injection should be avoided. In the submassive PE patient, a selective contrast injection into the main left or right PA should not exceed 20 mL volume at a rate of 10 mL/second. Lower injection parameters may be considered depending on the degree of heart failure and pulmonary hypertension, to achieve adequate vessel opacification without endangering the patient. The typical digital subtraction angiography (DSA) frame rate for pulmonary angiography is 6 frames per second and this should ideally be performed during a breath hold for several seconds or as long as can be tolerated by the patient. Once the DSA is performed, the largest thrombosed arterial branch should be identified and correlated with prior cross-sectional imaging such as a chest CTA if available. The DSA reference should then serve as a roadmap for further selective catheterization.
Catheter-Directed Therapy for Massive Pulmonary Embolism
Modern CDT is defined by the use of low-profile catheters and devices (≤10 Fr), catheter-directed mechanical fragmentation and/or aspiration of emboli with existing low-profile catheters, and intraclot thrombolytic injection if a local drug is infused. 3 Therefore, unless specifically contraindicated, a variety of devices can be used to attempt PE thrombolysis. A simple, widely available technique is use of a rotating pigtail catheter. A pigtail catheter may be used to mechanically debulk central thrombus and also to infuse thrombolytic drug through the side holes ( Fig. 1 ). If needed, further clot fragmentation can be performed with an angioplasty balloon size smaller than the target arterial diameter. Another method is aspiration thrombectomy which can be performed manually with any 8- to 10-Fr end-hold catheter such as a Pronto catheter (Vascular Solutions, Minneapolis, MN) or 8-Fr JR 4 (Cook, Bloomington, IN). Although rare, one potential risk of mechanical fragmentation is distal embolization of clot into previously patent vessels which can increase PAP, and the operator should be prepared to treat distal branch obstruction if necessary. 13 A variety of other devices may facilitate mechanical and/or aspiration thrombectomy. Regardless of which device(s) is used earlier, simultaneous administration of low-dose local tPA can be synergistic in treating massive PE. An obstructing clot has been shown to cause a proximal vortex and formation of eddy currents which can wash away systemically administered thrombolytic agent from the target embolus. 14 Therefore, direct injection of local tPA into the clot during simultaneous fragmentation is likely advantageous compared with systemic administration as a greater surface area of fragmented clot is exposed to the thrombolytic agent. It should be remembered that the treatment endpoint for massive PE is not angiographic improvement but hemodynamic improvement with resolution of shock. Once this is achieved, the decision can be made whether or not to place infusion catheters and to continue low-dose thrombolytic infusion to resolve residual RV strain and pulmonary hypertension (see submassive PE protocol later). Regarding mechanical and aspiration thrombectomy for massive PE, although most devices below are U.S. Food and Drug Administration (FDA)-approved for peripheral thrombectomy, it should be noted that none are currently FDA-approved for use in the pulmonary circulation.
Fig. 1.
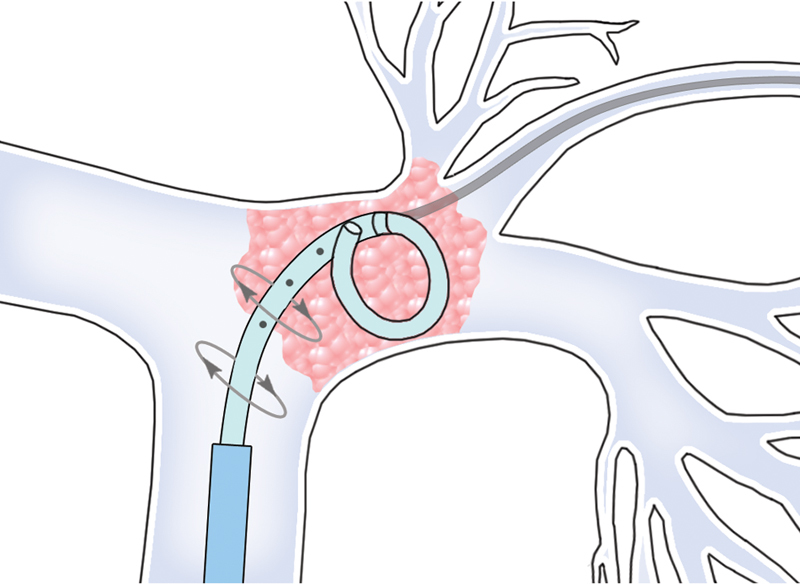
The rotating pigtail method may be used to treat proximal acute massive PE. A guidewire can be inserted through a hole just proximal to the pigtail curve, as this may facilitate rotation of the pigtail.
Aspirex System
The Aspirex system (Straub Medical, Wangs, Switzerland) is available in Europe in 6 to 10 Fr sizes and is currently undergoing the FDA 510k clearance process for peripheral thrombectomy in the United States. This device employs a rotating Archimedes screw (up to 40,000 rotations per minute) within a flexible catheter and it may be inserted and used over a guidewire. During activation, negative pressure is created within the catheter lumen to aspirate and macerate thrombus through an L-shaped aspiration port ( Fig. 2 ). The system uses the patient's own blood to transport the thrombus back through the catheter and to cool the rotating screw; however, the aspiration mechanism can sometimes create a vacuum in the target vessel resulting in low flow. If this occurs, infusion of additional fluids (i.e., saline solution) through the catheter can help facilitate device operation. Additional fluid can be supplied by pressure infusion of saline solution via the side-port of the introducer. Pressure infusion requires a sheath which is at least 1 Fr larger than the size of the selected Aspirex catheter, keeping in mind that the suggested aspiration volumes are 45 mL/min (6 Fr), 75 mL/min (8 Fr), and 180 mL/min (10 Fr). 15 Additionally, the pitch of the Archimedes screw, the number of rotations per minute, and the size of the aspiration port can be adjusted to minimize vessel collapse and injury. During aspiration, blood loss must be closely monitored in the collecting chamber.
Fig. 2.

Aspirex catheter.
Penumbra Indigo System
The Penumbra Indigo (Penumbra, Alameda, CA) device is an aspiration thrombectomy catheter ( Fig. 3 ) connected to a high-pressure suction pump. It is available in 6 or 8 Fr size known as the CAT6 or CAT8. During suction, rapid blood loss may occur and must be monitored in the collecting chamber as the current system does not allow recycling of aspirated blood. As the catheter may clog during operation, it is often used with a wire separator (SEP) to attempt declogging during the procedure. If the separator fails to declog the catheter, then the entire catheter must be removed and flushed. The Penumbra device may also be used in conjunction with the Cleaner device (Argon, Plano, TX; see below).
Fig. 3.
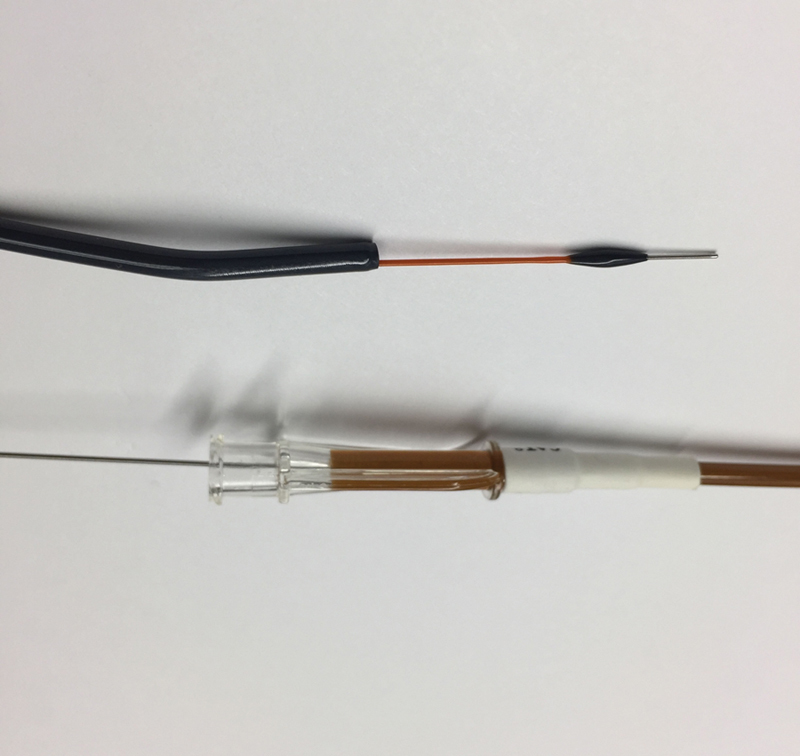
Penumbra CAT8 aspiration catheter with wire separator.
Argon Cleaner
The Argon Cleaner (Argon, Plano, TX) is a 6- to 7-Fr mechanical thrombectomy device that can fragment clot without causing hemolysis, but insertion requires prior sheath tip placement at the target vessel as the Cleaner in its current form cannot be inserted over a wire. It may be used in conjunction with the Indigo catheter as the 6-Fr Cleaner fits coaxially within the Penumbra CAT8 lumen ( Fig. 4 ). For larger vessels, partial retraction of the inner sinusoidal wire can create a larger diameter sweep of the device.
Fig. 4.
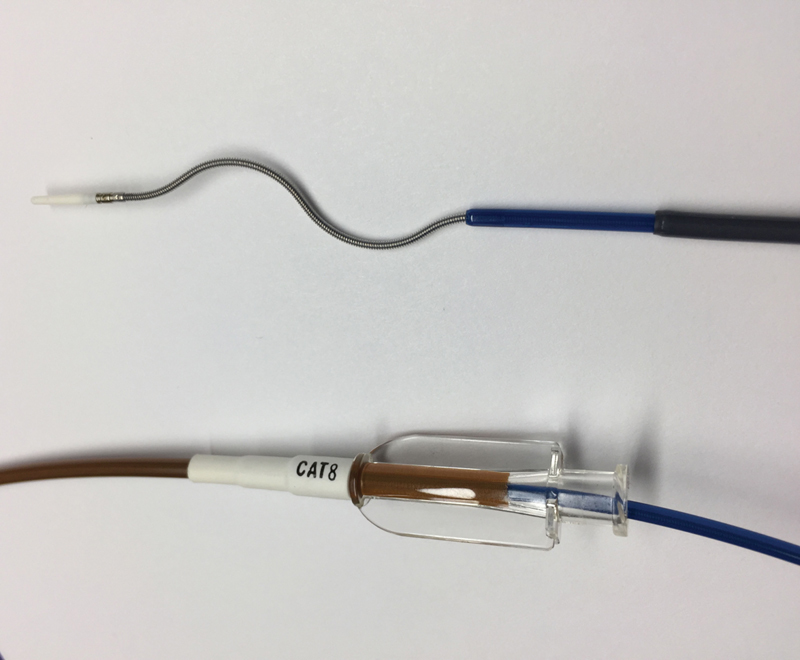
A 6-Fr (135 cm) Argon Cleaner device placed through an 8-Fr CAT8 (110 cm) catheter.
JETi Thrombectomy System
The 6-to 8-Fr JETi device (Walk Vascular, Irvine, CA) is an aspiration catheter system that employs a focused high-pulse saline jet along the inner catheter lumen tip that fragments and lubricates clot during aspiration ( Fig. 5 ). The device is connected to a suction generator which can be either a Penumbra pump or a less expensive option which is the Gomco 405 Aspirator (Allied Healthcare Products, Saint Louis, MO). During suction, a micro transducer within the saline drive unit activates the saline jet and audibly communicates aspirate flow status to help improve procedural efficiency and minimize blood loss. The saline jet may reduce the risk of catheter clogging while avoiding hemolysis, as the jet resides on the inner lumen of the catheter. The current generation device may be placed through an outer curved sheath which can be rotated to optimize contact with target thrombus. The aspiration mechanism can sometimes create a vacuum in the target vessel resulting in low flow, and similar to the Aspirex system, this can be managed with infusion of additional fluids (i.e., saline solution) through the outer sheath. As with all aspiration systems, blood loss must be closely monitored in the collecting chamber, as the current system does not allow recycling of blood aspirated through the pump.
Fig. 5.
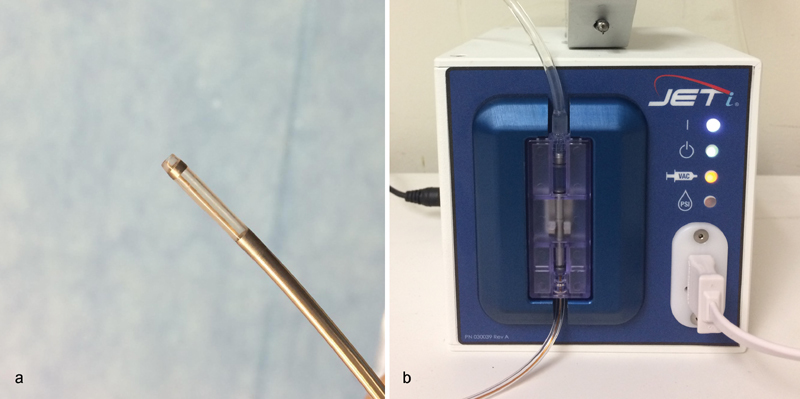
( a) JETi thrombectomy catheter. ( b) JETi Saline Drive Unit.
FlowTriever
The FlowTriever Retrieval/Aspiration System (Inari, Irvine, CA) is 510k cleared for peripheral thrombectomy and is currently undergoing an IDE trial called FLARE for the treatment of acute PE. The FlowTriever device has three components (inner wire form with nitinol disks, outer aspiration catheter, and proximal retraction/aspiration handle). The inner wire form is composed of three soft, braided nitinol disks ( Fig. 6 ) which are deployed over a guidewire by unsheathing within the thrombosed target vessel. The disks are intended to engage and disrupt clot without injuring the vessel walls. Once thrombus is engaged, the retraction aspirator handle is pulled once and combines the forces of aspiration and mechanical retraction to remove the nitinol disks containing clot through the large aspiration catheter. The device comes in three sizes which can be selected based on the size of the target vessel. The current generation device intended to treat PE is inserted through a 22-Fr aspiration sheath; therefore, it necessitates a larger incision at the venotomy site and placement of this large-diameter sheath into the PA. For those who wish to avoid large sheath placement and who do not need the aspiration component, the inner nitinol wire form may be inserted through a 12-Fr flexor sheath and used for clot fragmentation and disruption. As with all mechanical or aspiration devices, this may be used with or without local thrombolytic injection.
Fig. 6.
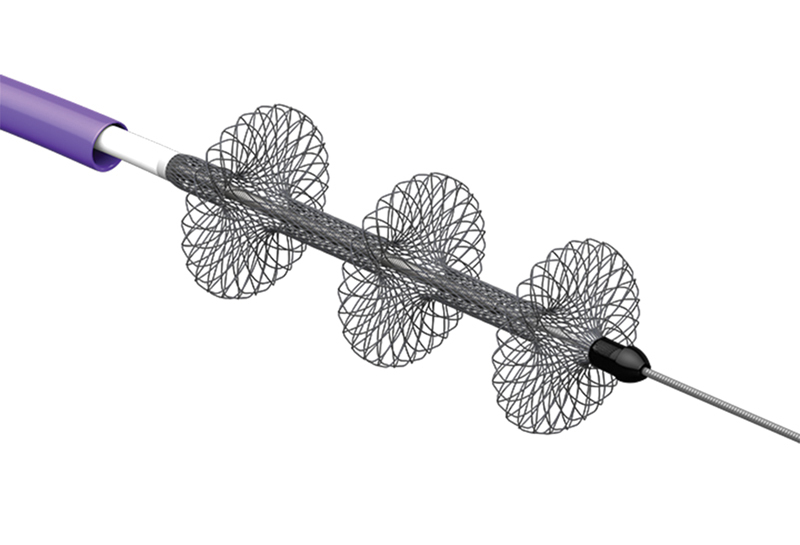
Inari FlowTriever disks and aspiration catheter. (Image courtesy of Inari Medical (Reprinted with permission from Inari Medical).)
AngioJet Rheolytic Thrombectomy
The AngioJet (Possis Medical, Minneapolis, MN) is the only device that carries a black-box warning by the FDA specifically for acute PE. 16 This is due to many reported minor and major complications including hemolysis, renal failure, bradyarrhythmia, apnea, bleeding, and procedure-related deaths when used to treat acute PE. 3 Therefore, use of AngioJet for acute PE is currently not recommended unless as part of an experimental protocol.
AngioVac
The AngioVac (Angiodynamics, Latham, NY) device is a large-bore aspiration system that can evacuate large clot burdens while recirculating filtered blood; however, the device is expensive, requires large-bore sheath placement (26 and 16 Fr), and requires general anesthesia along with a perfusionist as the patient is placed on extracorporeal veno-veno bypass. Another limitation is the stiff suction catheter which can be difficult to safely navigate into the pulmonary circulation. With AngioVac, major complications have been reported including RV free wall perforation 17 and distal clot embolization. 18 Other issues such as balloon rupture of the catheter tip as it passes through the struts of an IVC filter or through chronic clot have also been reported. 18 For those who wish to attempt AngioVac use for treating acute PE, initial PA catheterization using a flow-directed balloon catheter for accurate wire placement is recommended to avoid inadvertent wire passage through cardiac structures such as chordae tendineae. This helps avoid cardiac injury when advancing the large AngioVac cannula into the PA. 18 Another consideration is shaping the tip of the AngioVac device, which is stiff to maneuver, to assist passage into the main PA. 18
Catheter-Directed Therapy of Submassive Pulmonary Embolism
Since patients with submassive PE by definition are not in hemodynamic shock, aggressive mechanical maneuvers are usually unnecessary and may be associated with higher risk of complications such as distal embolization into patent vessels which could alter hemodynamics. 13 Therefore, the use of aggressive mechanical clot fragmentation for submassive PE is currently regarded as experimental. 19 For submassive PE, current data support gentle image-guided infusion catheter placement into thrombosed pulmonary arteries followed by local thrombolytic infusion. 5 6 7 Using a digital roadmap or overlay reference angiogram, a steerable hydrophilic wire and angled catheter can be used to select the thrombosed branches and a DSA hand injection (2–3 mL contrast) can be performed to confirm appropriate catheter position prior to infusion catheter placement. Examples of infusion catheters include the Unifuse catheter (Angiodynamics, Latham, NY), Fountain catheter (Merit Medical, South Jordan, UT), and EKOS (EKOS, Bothell, WA) catheter. Ideally, the infusion treatment length should extend from the most distal thrombosed PA segment (that can be safely catheterized) to the main PA. Additionally, the proximal side holes can extend into the pulmonary trunk and into the sheath itself, as this allows thrombolytic drug to distribute into other pulmonary branches. The outer vascular sheath can be locked with heparinized saline or run at TKO (to keep open) rate. Once the catheter(s) with multiple side holes are in appropriate position, thrombolysis can be performed with alteplase (tPA; Genentech, South San Francisco, CA). If a single catheter is used, tPA can be infused at 1.0 mg/hour and if bilateral catheters are used, a rate of 0.5 mg/hour can be used. Alternatively, a weight-based dose of 0.01 mg/kg/hour tPA can be infused, but the total rate should rarely exceed 1 mg/hour. The tPA can be reconstituted in normal saline solution until there is 0.1 mg tPA/mL of solution. This will stay biochemically stable and active at ambient temperatures for as long as 24 hours when diluted to a concentration as low as 0.01 mg/mL. 20 Once tPA infusion is initiated, full-dose IV heparin can be readjusted to subtherapeutic levels at a rate of 300 to 500 units/hour or less than two times normal PTT to reduce bleeding risk. The purpose of low-dose heparin is to minimize peri-sheath clot formation. Once the infusion is started, a final fluoroscopic image should be obtained to confirm appropriate positioning of the infusion catheters and to serve as a baseline reference. The patient should be transferred to an ICU or monitored bed and monitored closely for bleeding complications and/or changes in neurologic status. If a patient is at greater risk of bleeding or if the infusion is continued beyond 24 hours, monitoring of fibrinogen levels may be considered. If the fibrinogen level decreases below 150 to 200 mg/dL, the infusion should be reduced or discontinued. However, if additional thrombolysis is desired in these circumstances, concomitant FFP infusions may be administered along with close monitoring to permit ongoing tPA infusion. 21
The ultrasound-assisted EKOS infusion catheter is FDA-cleared for the treatment of acute PE. Although the EKOS system was intended to decrease the duration of thrombolytic infusion, no studies to date have shown any significant difference in treatment outcomes using EKOS versus standard infusion catheters such as the Unifuse or multi-side hole pigtail catheters. 7 22 23 In some patients following overnight infusion, if there is residual elevation of PAPs and persistent severe RV strain, consideration may be given to continuing the thrombolytic infusion if bleeding risk remains low. The treatment endpoints are improvement in pulmonary hypertension directly transduced through the pulmonary sheath (assuming no baseline pulmonary hypertension), alleviation of severe heart strain assessed by bedside echo (this should be the ultimate metric if there is baseline pulmonary hypertension), and/or development of bleeding complications. Once thrombolysis is completed, the tPA drip should be stopped and the infusion catheters and sheaths may be removed several minutes later at bedside followed by direct compression over the venotomy site(s). A D-Stat dressing may be applied in some patients to help achieve hemostasis. Fifteen minutes after hemostasis, full therapeutic anticoagulation can be resumed with continued monitoring of the venous access sites. Echocardiographic assessment should ideally be performed within 48 hours after CDT to confirm resolution of RV strain and to serve as a baseline for follow-up studies. After this, a follow-up echo and clinic visit are recommended within 1 to 3 months.
Acknowledgments
The authors acknowledge Rhonda Lee for her assistance in preparing Fig. 1 .
References
- 1.Heit J A, Cohen A T, Anderson F A. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the U.S. Blood. 2005;106:910. [Google Scholar]
- 2.Kabrhel C, Jaff M R, Channick R N, Baker J N, Rosenfield K. A multidisciplinary pulmonary embolism response team. Chest. 2013;144(05):1738–1739. doi: 10.1378/chest.13-1562. [DOI] [PubMed] [Google Scholar]
- 3.Kuo W T, Gould M K, Louie J D, Rosenberg J K, Sze D Y, Hofmann L V. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20(11):1431–1440. doi: 10.1016/j.jvir.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee S, Chakraborty A, Weinberg I et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311(23):2414–2421. doi: 10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]
- 5.Kucher N, Boekstegers P, Müller O J et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(04):479–486. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 6.Piazza G, Hohlfelder B, Jaff M R et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: The SEATTLE II Study. JACC Cardiovasc Interv. 2015;8(10):1382–1392. doi: 10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Kuo W T, Banerjee A, Kim P S et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest. 2015;148(03):667–673. doi: 10.1378/chest.15-0119. [DOI] [PubMed] [Google Scholar]
- 8.Fiumara K, Kucher N, Fanikos J, Goldhaber S Z. Predictors of major hemorrhage following fibrinolysis for acute pulmonary embolism. Am J Cardiol. 2006;97(01):127–129. doi: 10.1016/j.amjcard.2005.07.117. [DOI] [PubMed] [Google Scholar]
- 9.Moscucci M. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. Grossman & Baim's Cardiac Catheterization, Angiography, and Intervention. 8th ed; p. 991. [Google Scholar]
- 10.Valji K. Philadelphia, PA: Saunders; 2012. The Practice of Interventional Radiology. 3rd ed; p. 443. [Google Scholar]
- 11.Hofmann L V, Lee D S, Gupta A et al. Safety and hemodynamic effects of pulmonary angiography in patients with pulmonary hypertension: 10-year single-center experience. AJR Am J Roentgenol. 2004;183(03):779–786. doi: 10.2214/ajr.183.3.1830779. [DOI] [PubMed] [Google Scholar]
- 12.Mills S R, Jackson D C, Older R A, Heaston D K, Moore A V. The incidence, etiologies, and avoidance of complications of pulmonary angiography in a large series. Radiology. 1980;136(02):295–299. doi: 10.1148/radiology.136.2.7403500. [DOI] [PubMed] [Google Scholar]
- 13.Nakazawa K, Tajima H, Murata S, Kumita S I, Yamamoto T, Tanaka K. Catheter fragmentation of acute massive pulmonary thromboembolism: distal embolisation and pulmonary arterial pressure elevation. Br J Radiol. 2008;81(971):848–854. doi: 10.1259/bjr/93840362. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz-Rode T, Kilbinger M, Günther R W. Simulated flow pattern in massive pulmonary embolism: significance for selective intrapulmonary thrombolysis. Cardiovasc Intervent Radiol. 1998;21(03):199–204. doi: 10.1007/s002709900244. [DOI] [PubMed] [Google Scholar]
- 15.Aspirex Instructions for Use http://www.straubmedical.com/faq_aspirexs_en.html. Accessed July 22, 2017
- 16.Minneapolis, MN: Possis Medical; 2008. Angiojet Xpeedior [product insert] [Google Scholar]
- 17.Al-Hakim R, Park J, Bansal A, Genshaft S, Moriarty J M. Early experience with AngioVac aspiration in the pulmonary arteries. J Vasc Interv Radiol. 2016;27(05):730–734. doi: 10.1016/j.jvir.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Resnick S A, O'Brien D, Strain D et al. Single-center experience using AngioVac with extracorporeal bypass for mechanical thrombectomy of atrial and central vein thrombi. J Vasc Interv Radiol. 2016;27(05):723–7290. doi: 10.1016/j.jvir.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Kuo W T, Sista A K, Baerlocher M O et al. Society of Interventional Radiology position statement on catheter-directed therapy for acute pulmonary embolism. J Vasc Interv Radiol. 2018;29(03):293–297. doi: 10.1016/j.jvir.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Semba C P, Weck S, Patapoff T. Alteplase: stability and bioactivity after dilution in normal saline solution. J Vasc Interv Radiol. 2003;14(01):99–102. doi: 10.1097/01.rvi.0000052297.26939.05. [DOI] [PubMed] [Google Scholar]
- 21.Kuo W T.Endovascular therapy for acute pulmonary embolism J Vasc Interv Radiol 20122302167–790000., quiz 179 [DOI] [PubMed] [Google Scholar]
- 22.Baker R, Samuels S, Benenati J F, Powell A, Uthoff H. Ultrasound-accelerated vs standard catheter-directed thrombolysis--a comparative study in patients with iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2012;23(11):1460–1466. doi: 10.1016/j.jvir.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Engelberger R P, Spirk D, Willenberg T et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv. 2015;8(01):e002027. doi: 10.1161/CIRCINTERVENTIONS.114.002027. [DOI] [PubMed] [Google Scholar]


