Abstract
We investigated whether preschoolers with poor phonological awareness (PA) skills had impaired cortical basis for detecting speech feature, and whether speech perception influences future literacy outcomes in preschoolers. We recorded ERP responses to speech in 52 Chinese preschoolers. The results showed that the poor PA group processed speech changes differentially compared to control group in mismatch negativity (MMN) and late discriminative negativity (LDN). Furthermore, speech perception in kindergarten could predict literacy outcomes after literacy acquisition. These suggest that impairment in detecting speech features occurs before formal reading instruction, and that speech perception plays an important role in reading development.
Introduction
A large body of research has shown that the ability to recognize, manipulate, and decode basic phonological units is critical for successful reading acquisition (Cao, Bitan, Chou, Burman, & Booth, 2006; Castles & Friedmann, 2014; Hämäläinen et al., 2017; Hulme, Bowyer-Crane, Carroll, Duff, & Snowling, 2012; Ramus & Szenkovits, 2008; Shankweiler & Lundquist, 1992; Ziegler & Goswami, 2005). Although recent research has highlighted the multifactorial nature of developmental dyslexia (DD), with deficits in a range of perceptual and cognitive processes (Pennington, 2006; Pennington, Schlitt, Jackson, Schulz, & Schust, 2012), the core feature that characterizes dyslexia across languages is a deficit in phonological processing (Lyon, Shaywitz, & Shaywitz, 2003; Ramus & Szenkovits, 2008; Snowling, 2001). On this view, phonological deficits that result from suboptimal phonological representations lead to poor decoding (Elbro, 1996; Fowler, Brady, & Shankweiler, 1991; Snowling & Hulme, 1994).
Although the underlying cause of the phonological deficit remains unclear, some research indicates that suboptimal phonological representations in DD arise from deficits in processing speech sound information, including impairments in representation, recognition, and retrieval of speech (Fowler et al., 1991; Muter, Hulme, Snowling, & Taylor, 1998). Indeed, several studies provide support for the phonological deficit in DD arising from lower level, perceptual impairments in speech perception or lower level auditory sensory processing (Boets, Ghesquière, Van Wieringen, & Wouters, 2007; Goswami, 2011, 2015; Steinbrink, Zimmer, Lachmann, Dirichs, & Kammer, 2014; Tallal, 2004). These studies have reported dysfunctions in speech perception and categorization in children with DD (Kraus et al., 1996; Tallal, Miller, & Fitch, 1993), and estimates suggest that at least one-third of DD children have deficits in perceiving speech sounds and even basic acoustic contrasts (Bishop, 2007; Goswami, 2011; Hämäläinen, Salminen, & Leppänen, 2013; Mcbride-Chang, 1995; Ramus et al., 2003; Schulte-Körne & Bruder, 2010; Tallal & Gaab, 2006). However, support for deficits in speech perception in DD is mixed, with many studies failing to show differences between dyslexics and controls (Adlard & Hazan, 1998; Démonet, Taylor, & Chaix, 2004; Manis et al., 1997; Ramus et al., 2003; Sperling, Lu, Manis, & Seidenberg, 2003). Indeed, other researchers suggest that rather than deficits in speech perception or auditory processing per se, children with DD may have difficulty in accessing phonemes (Boets et al., 2013) or utilizing phonemic information (Frost et al., 2009; Preston et al., 2016).
In general, children are diagnosed with DD after Grade 2 in elementary school. Research using neuroimaging has shown that children with DD in this age have atypical structure and function in dorsal and ventral components of the reading system (McCandliss & Noble, 2003; Pugh et al., 2001; Sandak, Mencl, Frost, & Pugh, 2004; Schlaggar & McCandliss, 2007). While these studies have been important for identifying the brain bases of DD, research done with school-age children provides a post-diagnostic snapshot rather than a biomarker of risk for DD. Critically, research conducted with younger children, before formal literacy instruction, has revealed neuroanatomical differences in children can be observed in regions known to support phonological processing (Raschle, Zuk, & Gaab, 2012). Further, auditory event-related potentials (ERPs) to speech sounds measured before school-age were also found to be associated with later reading outcomes (Espy, Molfese, Molfese, & Modglin, 2004; Hämäläinen et al., 2017, 2013; Maurer et al., 2009). Following up on this work, we examine relations among neural response to speech (ERPs) and later reading in a cohort of Chinese preschoolers followed longitudinally. Importantly, we include children who are at risk for DD, as indicated by poor phonological awareness (PA) at study entry. The use of PA to define risk is supported by a large literature that has shown that PA predicts successful literacy acquisition and separates DD from typical readers in both alphabetic and nonalphabetic languages (Bryant, MacLean, Bradley, & Crossland, 1990; Ziegler & Goswami, 2005). Moreover, PA can be measured in our sample of pre-readers and will be uncontaminated by reciprocal influences from reading instruction. Although PA has been linked to DD, using preschool PA to define risk, which we term “phonological deficit risk,” rather than family history, is a novel approach, particularly for Chinese.
Much of the work linking early phonological processing ability to reading acquisition or failure has entailed research in alphabetic orthographies. Studying early phonological processing in preliterate children with a nonalphabetic orthography such as Chinese allows associating additional novel properties with both pre-reading phonological skills and later reading skills. One particular property of interest for Chinese is lexical tone. Different from the linguistic usage of pitch variation in nontonal languages, tonal languages like Chinese differentiate lexical meanings by pitch changes. Although lexical tone is a suprasegmental feature, it behaves like segmental features similar to consonants and vowels (Schirmer, Tang, Penney, Gunter, & Chen, 2005). Thus, perception of lexical tone or “lexical tone awareness” may be associated with reading and poorer in Chinese children with dyslexia (e.g., Cheung et al., 2009; Li & Ho, 2011; Shu, Peng, & McBride-Chang, 2008; Zhang et al., 2012). Indeed, Chan and Siegel (2001) reported that Cantonese subjects who had poor reading scores also performed poorly in tone perception. Similarly, Siok and Fletcher (2001) discovered a significant correlation between tone awareness and character recognition. Zhang et al. (2012) found that Chinese children with DD had deficits on categorical perception of lexical tone. These findings suggest that lexical tone perception plays an important role in reading development in Chinese language.
Much of the previous work investigating speech perception in DD has used tasks that require categorizing speech sounds (Blomert, Mitterer, & Paffen, 2004; Cheung et al., 2009; Mody, Studdert-Kennedy, & Brady, 1997; Serniclaes, Sprenger-Charolles, Carré, & Demonet, 2001; Werker & Tees, 1987; Zhang et al., 2012); however, because explicit categorization tasks require attention and motivation, the use of passive electrophysiological designs to measure speech perception in young children is gaining popularity. A number of studies have examined auditory ERPs to speech in longitudinal studies of reading outcome (Espy et al., 2004; Hämäläinen et al., 2013; Maurer et al., 2009).
In the current study, we focus on two ERPs that have been used to index speech processing and discrimination, the mismatch negativity (MMN) and late discriminative negativity (LDN). The MMN component is an ERP that can reflect discrimination among repeated stimuli and detection of novel stimuli at the pre-attentive stage (Näätänen, 1995; Näätänen, Paavilainen, Rinne, & Alho, 2007) when the processing is automatized even for high-level language processing like syntax and semantics (Pulvermüller & Shtyrov, 2003). In typically developed school-age children, MMN responses to auditory stimuli are similar to adults in latency and topography (e.g., Corbera, Escera, & Artigas, 2006; Hämäläinen, Leppänen, Guttorm, & Lyytinen, 2008; Huttunen, Halonen, Kaartinen, & Lyytinen, 2007; Lachmann, Berti, Kujala, & Schröger, 2005; Sharma et al., 2006). However, the MMN component in younger children has longer latency, compared to adults, despite similar topography (Cheour, Leppänen, & Kraus, 2000). In addition to the MMN, some researchers have observed a second negativity, occurring between 300 and 600 msec after stimulus onset in oddball paradigms, which is named LDN (sometimes also referred to as “late MMN;” Korpilahti, Krause, Holopainen, & Lang, 2001; Kushnerenko et al., 2001). The LDN component is also thought to reflect processing of novel stimuli (Čeponienė et al., 2004; Cheour, Korpilahti, Martynova, & Lang, 2001; Schulte-Körne, Deimel, Bartling, & Remschmidt, 2001). Several studies have shown that both MMN and LDN are attenuated in DD (e.g., Corbera et al., 2006; Neuhoff et al., 2012; Schulte-Körne et al., 2001).
In the present study, we measure auditory MMN and LDN in an oddball paradigm as an index of Chinese preschoolers’ speech perception before they began formal schooling (and therefore formal literacy instruction). We divided preschoolers based on PA skills and followed them longitudinally for 1 year to examine both early speech perception and later literacy outcome for those with phonological deficits relative to typically developing children.
We were interested in the following key questions: (1) how does speech perception in preschoolers with phonological deficits compare to those with typical phonological skills? (2) Do phonological deficits affect perception of consonants and lexical tone differently in young children? (3) Can we identify early neural correlates of poor PA that predict later literacy outcome?
Method
Participants
Data were collected as part of a longitudinal study with a cohort of preschoolers, prior to formal literacy instruction, at a mean age of 6.39 years old upon entry to the study (SD 0.31; range 5.60–6.99). A total of 106 Chinese preschoolers were invited to participate in this project. Inclusion criteria were native Chinese speaker, no neurological disease or psychiatric disorders, no ADHD, no impaired sight (uncorrected) or hearing. All children were invited to participate in this longitudinal study before receiving formal instructions (Time 1) and 1 year later (Time 2). Fifty-two of them attended both the ERP experiments and behavioral measurements in Time 1. From the 52 children, we identified a low PA group of 20 children (13 girls, 7 boys)—those with behavioral performance on the PA tasks below the 20th percentile of the performance of the whole longitudinal group (N = 106) on at least two of the four measures of the PA tasks or below the 10th percentile of that on at least one of the four measures. The remaining 32 children (15 girls, 17 boys) we included in a comparison group with the two groups matched on age and nonverbal IQ (based on Raven’s Matrices scores). After the children received formal instruction 1 year later, 43 children (21 girls, 22 boys) received the reading skills tests again. Table 1 presents statistics (mean scores and standard deviations) for the low PA and control group both at entry into the study (Time 1) and after the year of formal literacy instruction (Time 2). No outliers were found in the children who attended the ERP experiments. The distributions for performances in some PA tasks (lexical tone detection, syllable deletion, phoneme deletion) at Time 1 were not normal, so we report the group contrasts (low PA group vs. controls) using the Mann–Whitney U test for those three tasks in Table 1; the distribution for performances for rime detection, age, and nonverbal IQ were normal, so the group contrasts employed independent samples t-tests. The participants and their parents gave written consent before taking part in the experiments, and they received gift books as compensation. The ethical committee of the Beijing Normal University approved the research protocol.
Table 1.
Descriptive statistics for behavioral measures and group differences tested at Time 1 and Time 2.
| Low PA group (N = 20)
|
Control group (N = 32)
|
Z/ta | |||
|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | ||
| T1 Age (year) | 6.36 (0.32) | 5.88–6.99 | 6.41 (0.32) | 5.6–6.93 | −0.56 |
| T1 Nonverbal IQ | 23.30 (11.90) | 5–43 | 24.72 (9.73) | 12–44 | −0.45 |
| T1 Phonological awareness tests | |||||
| T1 Rime detection | 10.25 (3.19) | 3–16 | 12.75 (2.02) | 9–16 | −3.47** |
| T1 Lexical tone detection | 7.80 (5.16) | 0–20 | 12.41 (4.66) | 6–23 | −3.62** |
| T1 Syllable deletion | 11.50 (3.69) | 4–16 | 14.91 (1.69) | 10–16 | −3.63** |
| T1 Phoneme deletion | 1.35 (2.78) | 0–9 | 4.88 (5.04) | 0–21 | −3.07** |
| T2 Character recognition | 48.72 (24.03) | 12–93 | 66.72 (31.88) | 10–117 | −2.1* |
Note:
Group contrasts for age, nonverbal IQ, rime detection at Time 1, and character recognition at Time 2 used t-test (t); group contrasts for lexical tone detection, syllable deletion, and phoneme deletion at Time 1 used Mann–Whitney test (Z).
p < .05;
p < .01.
Behavioral measures
Group identification measures: PA
Four PA tasks were included to measure Chinese PA. Those tasks were both tested at kindergarten (Time 1) and Grade 1 (Time 2). Performance on those four tasks was the criterion for subgroups (low PA and control group). Mean Z scores of the four tasks (rime detection, lexical tone detection, phoneme deletion, syllable deletion) were adopted as the composite score for PA. Cronbach’s alphas for the mean scores of reading accuracy were α = 0.66.
Rime detection. The rime detection subtest consisted of 16 trials (Li, Shu, McBride-Chang, Liu, & Peng, 2012). Children listened to one monosyllabic target (e.g. /mao1/ [meaning cat]) and two options (e.g., bao1 [meaning bag] and san1 [meaning mountain]) in one trial. And they were required to choose one from the two options, which sounded more similar to the target. Score was total number correct.
Lexical tone detection. The lexical tone detection subtest consisted of 24 trials. Each trial had three monosyllabic words, two of which shared the same lexical tone. Children were asked to pick the one that had a different lexical tone compared to the other two. For example, /chu1/, /shao1/, /wei4/ in one trial, the answer is /wei4/, because the other two share the same lexical tone. Score was total number correct.
Syllable deletion. The syllable deletion subtest consisted of 16 trials (Lei et al., 2011). Children were required to produce a new word by deleting the given monosyllable from a disyllabic or trisyllabic phrase. For example, we asked children say /qi4 che1 zhan4/ [meaning bus station] without /zhan4/ [meaning station], and the correct answer was /qi4 che1/ [meaning bus]. Score was total number correct.
Phoneme deletion. The phoneme deletion subtest consisted of 26 trials (Pan et al., 2015). Children were asked to say a syllable by deleting the given phoneme from a monosyllabic word. For example, we asked children say /mei4/ without the /m/, the correct answer was /ei4/. Score was total number correct.
Nonverbal IQ
Raven’s Standard Progressive Matrices (Raven, Court, & Raven, 1996) was used as measure of nonverbal IQ at kindergarten (Time 1). Children were required to choose one fragment that best fits the original picture out of six to eight options. This task contains 5 sets, and each set consisted of 12 items. This task had to be terminated when children’s answers for five consecutive trials were wrong. One point was awarded for each correct answer.
Literacy skills
Character recognition
This task consisted of 150 Chinese characters, all of which were supposed to have been learned by Grade 6 (Shu, Chen, Anderson, Wu, & Xuan, 2003). Children were required to name all these characters. One point was given for each correct naming.
ERP stimuli
Two pairs of Chinese monosyllables were used as stimuli, which were read by a male native Chinese speaker: lexical tone pairs /ji1/ and /ji4/ (Tone 1, the high level tone; and Tone 4, the high-falling tone) and consonant pairs /ba1/ and /ta1/. The lexical tone pairs differed in pitch contour (in fundamental frequency, F0). The acoustic features of F0 in /i1/ were 191.7 Hz and those in /i4/ were onset = 203.6 Hz, end point = 135.6 Hz. The consonant pairs differed in the initial voice onset time (VOT) of consonants. The acoustic features of VOT in /ba1/ were 12.4 msec and those in /ta/ were 42.4 msec. And the first formant (F1) and the second formant (F2) for stimuli /i/ were 340.2/1,907.4 and 808.9 Hz/1,122.3 Hz for /a/. These monosyllables were digitally edited using Sound-Forge (SoundForge9; Sony Corporation, Tokyo, Japan) to have 140 ms duration and 70 dB.
ERP procedure
Participants were seated comfortably in an acoustically and electrically shielded room and instructed to ignore the presented sounds while watching a self-selected silent movie. Lexical tone pairs and consonant pairs were presented in four separate experiment sessions. Each session started with 30 standard stimuli, followed by standard stimuli (86.5%, 513 trials) and deviant stimuli (13.5%, 80 trials). Those standards and deviants were pseudo-randomly presented with at least three successive standards between deviants. The stimuli were presented to the participants with a randomly distributed interstimulus interval of 450–500 msec via a loudspeaker located approximately 1 m in front of the participant at 70 dB root mean square intensity level. Lexical tone pairs (/ji1/ and /ji4/) contained two sessions, the first one presented /ji1/ as a standard and /ji4/ as a deviant; the second presented /ji4/ as a standard and /ji1/ as deviant. Consonant pairs (/ba1/ and /ta1/) also contained two sessions in which /ba1/ or /ta1/ was presented as a standard stimulus. The sequence of these four sessions was counterbalanced among participants. The whole experiment lasted for approximately 30 min, and children could have a rest (3–5 min) between each block.
Electroencephalogram recording
Continuous EEG was recorded using a Geodesic HydroCel Sensor Net (GSN) consisting of 128 electrodes evenly distributed across the scalp and referenced against the vertex electrode (Electrical Geodesics Inc.) at a sampling rate of 1,000 Hz. EEG signals were amplified by the EGI Net Amp 200 Amplifier with NetStation 4.2 software. The GSN also included the electrodes next to, and below, the eyes for recording horizontal and vertical eye movements. Signals were filtered online with a band pass filter of 0.05–100 Hz. All the electrodes were physically referenced to Cz (fixed by the EGI system). The impedance of each electrode was kept below 50 kΩ throughout the recording.
ERP data analysis
ERP waveform analysis was completed offline using BESA 5.3 (Brain Electrical Source Analysis, Gräfelfing, Germany) and EEGLAB (v13.4.4b) software package (Delorme & Makeig, 2004) running in Matlab R2014b. The raw EEG data were first band-passed at 0.3–30 Hz and then re-referenced to an average of all electrodes across the scalp. Channels with a flat-line duration higher than 5 sec and those poorly correlated with their interpolated reconstruction based on neighboring channels (correlation thresholds = 0.85) were considered abnormal and rejected. No more than 5.4% (≤7) of the channels were discarded (mean number of rejected channels = 3). The signal was segmented between −100 and 700 msec relative to the stimulus onset and baseline corrected (−100–0 ms relative to stimulus onset). All deviant stimuli (consonant and lexical tone) and only the standard stimuli (consonant and lexical tone) before the deviant stimuli were extracted. Independent component analysis (ICA) was used to identify and remove eye blinks or other movement artifacts. In this study, we adopted Infomax ICA algorithms (Makeig, Bell, Jung, & Sejnowski, 1995) to process EEG data, and we used default parameters as implemented in the runica function of the EEGLAB (v13.4.4b) toolbox (Delorme & Makeig, 2004). Artifact rejection for the EEG was performed by a maximum voltage criterion of ±75 V on all scalp electrodes. Analyses were performed on the remaining trials (average non-rejected trials: 115/160 for consonant deviant stimuli, 117/160 for lexical tone deviant stimuli, 116/160 for consonant standard stimuli, 117/160 for lexical tone standard stimuli).
Difference waves (deviant minus standard) were calculated for the MMN and LDN for each deviant. Time windows and electrode pools were selected based on previous literature (Zhang et al., 2012) and visual inspection of the topographies. The mean amplitudes of MMN (250–350 msec after the stimulus) and LDN (500–600 msec after the stimulus) were calculated from the symmetric window of 40 msec around the grand average peak latency. The frontal–central electrodes (F3, F4, FCz, FC3, and FC4) were adopted for statistical analyses. Linear regression analysis was executed with character recognition as a dependent variable, gender and age as control variables, preschool-age PA scores (mean Z scores of PA tasks) and ERPs to lexical tone, and consonant stimuli as independent variables.
Results
ERP experiments at Time 1
The ERP waveforms from the average signals of frontal–central electrodes (F3, F4, FCz, FC3, and FC4) for the standards, deviants, and the difference waveforms are shown in Figure 1. Mixed measures analyses of variances were run per time window (MMN, LDN) with three factors: group (low PA group, control), phoneme category (consonant, lexical tone), and stimulus type (standard, deviant).
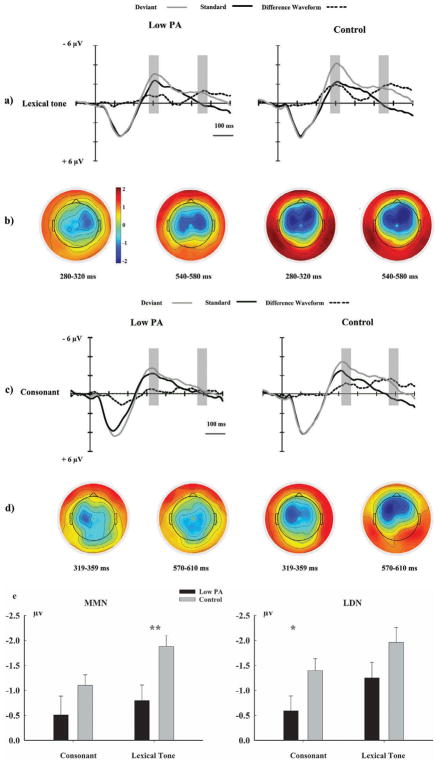
Figure 1.
(a) ERP waveforms from the average signals of frontal–central electrodes (F3, F4, FCz, FC3, and FC4) elicited by the lexical tone deviants, the standards, and difference waveforms (deviant–standard) in the control and the low PA groups, respectively; (b) maps display the topographic distribution of the mean amplitudes for the lexical tone contrast in the MMN and LDN analysis windows of the control and the low PA groups, respectively. (c) ERP waveforms from the average signals of frontal–central electrodes (F3, F4, FCz, FC3, and FC4) elicited by the consonant deviants, the standards, and difference waveforms (deviant–standard) in the control and the low PA groups, respectively; (d) maps display the topographic distribution of the mean amplitudes for the consonant contrasts in the MMN and LDN analysis windows of the control and the low PA groups, respectively. (e) MMN and LDN mean amplitude values (vertical bars represent one standard error) from the average signals of frontal–central electrodes (F3, F4, FCz, FC3, and FC4) in each group. Note: *p<0.05; **p<0.01.
MMN
For the MMN, a significant main effect of stimulus type (F(1,50) = 48.74, p < .001, ) was found. The phoneme category × stimulus type interaction was significant (F(1,50) = 5.15, p = .028, ). Importantly, the group × stimulus type interaction was significant (F(1,50) = 7.46, p = .009, ). The main effects of stimulus type were significant both in the low PA group (F(1,50) = 7.34, p = .009, ) and in the control group (F(1,50) = 61.31, p < .001, ) in the subsequent simple effect test. In the follow-up pairwise analysis, we found that the difference between the standard and deviant stimulus in the control group was much larger than that in the low PA group (t(50) = 2.73, p = .009). In addition, we conducted planned contrasts on the difference waveforms to directly compare groups on the size of the MMN effect for the lexical tone and consonant contrast conditions (see Figure 1(e)). This analysis revealed a smaller MMN effect for lexical tone in the low PA group compared to that in control group (t(50) = 2.92, p = .005), but no group difference for the consonant condition. These results suggest that although MMNs to speech stimuli existed in both groups, the MMN effect was weak in the low PA group compared to that in the control group.
LDN
For the LDN, the main effects of phoneme category (F(1,50) = 14.11, p < .001, ), stimulus type (F(1,50) = 62.78, p < .001, ), and group (F(1,50) = 4.33, p = .043, ) were all significant. The phoneme category × stimulus type interaction was significant (F(1,50) = 5.60, p = .022, ). The group × phoneme category interaction (F(1,50) = 5.01, p = .030, ) was significant. And in the subsequent simple effect test, in the low PA group, the main effect of phoneme category was not significant (F(1,50) = 0.94, p = .338, ), while in the control group, the main effects of phoneme category were significant (F(1,50) = 23.35, p < .001, ). It suggested that lexical tone and consonant contrasts induced different auditory potentials in control group, yet stimulus type did not interact with these two factors. Hence, we do not discuss phoneme category differences in the control group further. More importantly, the group × stimulus type interaction (F(1,50) = 5.35, p = .025, ) was significant. In the subsequent simple effect test, both in the low PA group (F(1,50) = 12.79, p = .001, ) and the control group (F(1,50) = 68.10, p < .001, ), the main effects of stimulus type were significant. In the follow-up pairwise analysis, we found that the difference between the standard and deviant stimulus in the control group was much larger than that in the low PA group (t(50) = 2.31, p = .025). In addition, we also conducted planned contrasts on the difference waveforms to directly compare groups on the size of the LDN effect for the lexical tone and consonant contrast conditions (see Figure 1(e)). This analysis revealed a smaller LDN effect for consonant stimuli in the low PA group compared to that in the control group (t(50) = 2.10, p = .040), but no group differences for the lexical tone condition.
Prediction to literacy skills at Time 2
To assess contributions of PA and auditory processing to literacy skill measured at Time 2, hierarchical regression analyses were executed with character recognition at Time 2 as a dependent variable, all independent variables were forced (entered) into the model. In this model, gender and age as control variables were entered in step 1, preschool-age PA scores (mean Z scores of PA tasks) were entered in step 2, and ERPs to lexical tone and consonant stimuli were entered in step 3 (see Table 2). The control variables had a 14.8% contribution, and PA could explain a larger percentage (ΔR2 = 16.3%). Moreover, the auditory processing at Time 1 also significantly predicted to literacy skill at Time 2 (ΔR2 = 15.9%). In other words, PA and speech perception at kindergarten predicted future literacy outcomes.
Table 2.
Hierarchical multiple regression predicting character recognition at Time 2 (Significant variables are marked in bold).
| Variable | B | SE | Beta | Significant | Adjusted R2 | ΔR2 |
|---|---|---|---|---|---|---|
| Dependent variable: character recognition. | ||||||
| Step 1 | 0.085 | 0.148 | ||||
| Gender | 9.97 | 8.67 | 0.17 | 0.257 | ||
| Age 1 | 4.16 | 69.85 | 0.04 | 0.953 | ||
| Age 2 | 31.86 | 67.92 | 0.32 | 0.642 | ||
| Step 2 | 0.241 | 0.163 | ||||
| T1_PA | 18.49 | 6.08 | 0.41 | 0.004 | ||
| Step3 | 0.350 | 0.159 | ||||
| T1_Consonant MMN | 5.75 | 3.20 | 0.28 | 0.081 | ||
| T1_Consonant LDN | 1.42 | 3.23 | 0.07 | 0.662 | ||
| T1_Tone MMN | −0.69 | 3.83 | −0.03 | 0.859 | ||
| T1_Tone LDN | −5.97 | 2.71 | −0.33 | 0.034 | ||
Discussion
In this study, we examined speech perception of the low PA preschoolers and controls, and its influence on their future reading skills. We found reduced difference waveforms for the MMN (induced by lexical tone) and the LDN (induced by consonant) for both consonant and lexical tone stimuli in the low PA group compared to the control group, suggesting a low-level deficit in speech perception of preschoolers. Specifically, this study found that the neural-level indices of risk in preschool, defined here by poor PA, could predict literacy outcomes in later grade. Overall, our results are consistent with what has been observed in previous studies conducted in alphabetic languages and support the hypothesis that speech perception plays a critical role in reading development (Preston et al., 2016; Pugh et al., 2013). Given that phonological processing both predicts and is influenced by learning to read, it is especially noteworthy that we see this pattern linking phonological abilities and speech in pre-readers.
We found significant differences between the low PA and control groups in different stimuli (lexical tone and consonant). Given that reduced MMN and LDN to speech reflect poor discrimination and detection of speech stimuli (e.g., Cheour et al., 2001; Maurer, Bucher, Brem, & Brandeis, 2003), the current results suggest that children who have a deficit in PA also have impairments in speech perception and that these deficits are present before formal reading instruction. It suggests that the interaction between PA and speech perception happens at a very early stage. Preschoolers (Boets et al., 2010; Boets, Wouters, VanWieringen, & Ghesquiere, 2007; Espy et al., 2004; Gerrits & De Bree, 2009; Hämäläinen et al., 2017, 2013; Law, Vandermosten, Ghesquière, & Wouters, 2017; Lovio, Näätänen, & Kujala, 2010; Maurer et al., 2009; Plakas, Van Zuijen, Van Leeuwen, Thomson, & Van Der Leij, 2013) and even infants (Guttorm, Leppänen, Hämäläinen, Eklund, & Lyytinen, 2010; Guttorm et al., 2005; Leppänen, Pihko, Eklund, & Lyytinen, 1999; Pihko et al., 1999; Richardson, Leppänen, Leiwo, & Lyytinen, 2003) who have risk for DD are found to have impairments in speech perception. This result is in line with another recent longitudinal investigation of pre-reading English-speaking children with temporal auditory processing measurements (Law et al., 2017). Law and colleagues found significant differences between DD and non-impaired readers on measures of PA across 3 years and that auditory processing in kindergarten predicted later literacy skills.
Previous studies have suggested that speech perception modulates development of reading skills (Goswami, 2011; Goswami et al., 2002; Guttorm et al., 2010; Law et al., 2017; Molfese, 2000; Thomson, Fryer, Maltby, & Goswami, 2006). For example, Molfese (2000) found that the auditory ERP responses to speech and nonspeech stimuli in newborns can predict their reading problems after 8 years. Guttorm et al. (2010) also found that newborn responses to syllables are related to phonological abilities, verbal short memory, and reading abilities in preliterate children. The present study replicates and extends these previous studies, which are mostly carried out in alphabetic languages, by examining both suprasegmental and segmental features and revealing the nature of brain–behavior relations. That is, the amplitude of the difference waveforms for MMN and LDN, which were induced by lexical tone and consonant stimuli, was significantly correlated with later measures of literacy skills (character recognition, see in Appendix A). Thus, individuals with better neural responses to speech stimuli before formal instruction have higher reading proficiency 1 year later. These findings support the explanations for the associations between speech sound processing in young children and future’s reading skill (e.g., Hulme et al., 2012; Ziegler & Goswami, 2005).
The regression analyses of preliterate ERP responses including MMN and LDN components of lexical tone and consonant demonstrated that speech perception in kindergarten speech perception ability also predicts growth in measures of literacy skill (Chinese character recognition). The lexical tone LDN predicted literacy skill when kids were in Grade 1, even after controlling for the relevant behavioral performance (PA) in kindergarten. This result demonstrates a link between early perception in lexical tone and development of Chinese character recognition. Moreover, the current study compared the lexical tone contrasts with consonant contrasts to address a question surrounding the specific role of lexical tone. However, we did not observe a main effect of phoneme category and group differences were similar for both in the lexical tone and consonant conditions, revealing that lexical tone perception and consonant perception have a similar relationship to PA. This result is consistent with other studies investigating the influence of both suprasegmental and segmental features on semantic processing (Schirmer et al., 2005).
Conclusion
The current longitudinal study goes beyond previous studies by demonstrating that ERP indices of speech perception in Chinese preschool children can predict reading skill at school age. We find that Chinese preschoolers with low PA have atypical speech perception at both the suprasegmental and segmental levels compared to controls. Moreover, the neural-level indices of risk factors before formal instruction were associate and predictive of later linguistic skills. This work provides empirical evidence for the associations between low-level speech sound processing and reading development.
Acknowledgments
This research was supported by National Key Basic Research Program of China (2014CB846103), by Natural Science Foundation of China (31671126, 31611130107), by Beijing Municipal Science & Technology Commission (Z151100003915122), by the Fundamental Research Funds for the Central Universities (2017XTCX04), by the Interdiscipline Research Funds of Beijing Normal University, and by the National Institute of Child Health and Human Development (grant number P01 HD001994) to Haskins Laboratories.
Appendix A. Supplementary material
As can be seen in Table A.1, the consonant LDN showed a significant correlation with PA (r = −0.332, p = .030). The lexical tone MMN also showed a correlation with PA (r = −0.412, p = .006). The tone LDN showed significant correlations with literacy skills, character recognition (r = −0.329, p = .031).
Table A.1.
Correlations between ERP amplitudes and behavior in Time 2 (Significant variables are marked in bold).
| Consonant MMN | Consonant LDN | Tone MMN | Tone LDN | |
|---|---|---|---|---|
| T2_Phonological awareness | −0.136 | −.332* | −.412** | −0.157 |
| T2_Character recognition | 0.176 | −0.077 | −0.228 | −.329* |
Note:
p < .05;
p < .01.
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/hdvn.
References
- Adlard A, Hazan V. Speech perception abilities in children with developmental dyslexia. Quarterly Journal of Experimental Psychology Section A: Human Experimental Psychology. 1998;51:153–177. doi: 10.1080/713755750. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Using mismatch negativity to study central auditory processing in developmental language and literacy impairments: Where are we, and where should we be going? Psychological Bulletin. 2007;133:651–672. doi: 10.1037/0033-2909.133.4.651. [DOI] [PubMed] [Google Scholar]
- Blomert L, Mitterer H, Paffen C. In search of the auditory, phonetic, and/or phonological problems in dyslexia context effects in speech perception. Journal of Speech, Language, and Hearing Research. 2004;47(5):1030–1047. doi: 10.1044/1092-4388(2004/077). [DOI] [PubMed] [Google Scholar]
- Boets B, De Beeck HPO, Vandermosten M, Scott SK, Gillebert CR, Mantini D, … Ghesquière P. Intact but less accessible phonetic representations in adults with dyslexia. Science. 2013;342(6163):1251–1254. doi: 10.1126/science.1244333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B, Ghesquière P, Van Wieringen A, Wouters J. Speech perception in preschoolers at family risk for dyslexia: Relations with low-level auditory processing and phonological ability. Brain and Language. 2007;101(1):19–30. doi: 10.1016/j.bandl.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Boets B, Smedt B, Cleuren L, Vandewalle E, Wouters J, Ghesquiere P. Towards a further characterization of phonological and literacy problems in Dutch-speaking children with dyslexia. British Journal of Developmental Psychology. 2010;28(1):5–31. doi: 10.1348/026151010X485223. [DOI] [PubMed] [Google Scholar]
- Boets B, Wouters J, Van Wieringen A, Ghesquiere P. Auditory processing, speech perception and phonological ability in pre-school children at high-risk for dyslexia: A longitudinal study of the auditory temporal processing theory. Neuropsychologia. 2007;45(8):1608–1620. doi: 10.1016/j.neuropsychologia.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Bryant PE, MacLean M, Bradley LL, Crossland J. Rhyme and alliteration, phoneme detection, and learning to read. Developmental Psychology. 1990;26(3):429–438. doi: 10.1037/0012-1649.26.3.429. [DOI] [Google Scholar]
- Cao F, Bitan T, Chou TL, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. Journal of Child Psychology and Psychiatry. 2006;47(10):1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castles A, Friedmann N. Developmental dyslexia and the phonological deficit hypothesis. Mind & Language. 2014;29(3):270–285. doi: 10.1111/mila.12050. [DOI] [Google Scholar]
- Čeponienė R, Lepistö T, Soininen M, Aronen E, Alku P, Näätänen R. Event-related potentials associated with sound discrimination versus novelty detection in children. Psychophysiology. 2004;41(1):130–141. doi: 10.1111/psyp.2004.41.issue-1. [DOI] [PubMed] [Google Scholar]
- Chan CK, Siegel LS. Phonological processing in reading Chinese among normally achieving and poor readers. Journal of experimental Child Psychology. 2001;80(1):23–43. doi: 10.1006/jecp.2000.2622. [DOI] [PubMed] [Google Scholar]
- Cheour M, Korpilahti P, Martynova O, Lang AH. Mismatch negativity and late discriminative negativity in investigating speech perception and learning in children and infants. Audiology and Neurotology. 2001;6(1):2–11. doi: 10.1159/000046804. [DOI] [PubMed] [Google Scholar]
- Cheour M, Leppänen PH, Kraus N. Mismatch negativity (mmn) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clinical Neurophysiology Official Journal of the International Federation of Clinical Neurophysiology. 2000;111(1):4–16. doi: 10.1016/S1388-2457(99)00191-1. [DOI] [PubMed] [Google Scholar]
- Cheung H, Chung KK, Wong SW, McBride-Chang C, Penney TB, Ho CS. Perception of tone and aspiration contrasts in Chinese children with dyslexia. Journal of Child Psychology and Psychiatry. 2009;50(6):726–733. doi: 10.1111/j.1469-7610.2008.02001.x. [DOI] [PubMed] [Google Scholar]
- Corbera S, Escera C, Artigas J. Impaired duration mismatch negativity in developmental dyslexia. Neuroreport. 2006;17(10):1051–1055. doi: 10.1097/01.wnr.0000221846.43126.a6. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single trial EEG dynamics. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Taylor MJ, Chaix Y. Developmental dyslexia. The Lancet. 2004;363(9419):1451–1460. doi: 10.1016/S0140-6736(04)16106-0. [DOI] [PubMed] [Google Scholar]
- Elbro C. Early linguistic abilities and reading development: A review and a hypothesis. Reading and Writing. 1996;8(6):453–485. doi: 10.1007/BF00577023. [DOI] [Google Scholar]
- Espy KA, Molfese DL, Molfese VJ, Modglin A. Development of auditory event-related potentials in young children and relations to word-level reading abilities at age 8 years. Annals of Dyslexia. 2004;54(1):9–38. doi: 10.1007/s11881-004-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler AE, Brady SA, Shankweiler DP. How early phonological development might set the stage for phoneme awareness. Phonological Processes in Literacy: A Tribute to Isabelle Y. Liberman. 1991;106:97–117. [Google Scholar]
- Frost SJ, Landi N, Mencl WE, Sandak R, Fulbright RK, Tejada ET, … Pugh KR. Phonological awareness predicts activation patterns for print and speech. Annals of Dyslexia. 2009;59(1):78–97. doi: 10.1007/s11881-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits E, De Bree E. Early language development of children at familial risk of dyslexia: Speech perception and production. Journal of Communication Disorders. 2009;42(3):180–194. doi: 10.1016/j.jcomdis.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Goswami U. A temporal sampling framework for developmental dyslexia. Trends in Cognitive Sciences. 2011;15(1):3–10. doi: 10.1016/j.tics.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Goswami U. Sensory theories of developmental dyslexia: Three challenges for research. Nature Reviews Neuroscience. 2015;16(1):43–54. doi: 10.1038/nrn3836. [DOI] [PubMed] [Google Scholar]
- Goswami U, Thomson J, Richardson U, Stainthorp R, Hughes D, Rosen S, Scott SK. Amplitude envelope onsets and developmental dyslexia: A new hypothesis. Proceedings of the National Academy of Sciences. 2002;99(16):10911–10916. doi: 10.1073/pnas.122368599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PH, Hämäläinen JA, Eklund KM, Lyytinen HJ. Newborn event-related potentials predict poorer pre-reading skills in children at risk for dyslexia. Journal of Learning Disabilities. 2010;43(5):391–401. doi: 10.1177/0022219409345005. [DOI] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PH, Poikkeus AM, Eklund KM, Lyytinen P, Lyytinen H. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2005;41(3):291–303. doi: 10.1016/S0010-9452(08)70267-3. [DOI] [PubMed] [Google Scholar]
- Hämäläinen J, Landi N, Loberg O, Lohvansuu K, Pugh K, Leppänen PHT. Brain event-related potentials to phoneme contrasts and their correlation to reading skills in school-age children. International Journal of Behavioral Development. doi: 10.1177/0165025417728582. 0(0), 0165025417728582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen JA, Leppänen PHT, Guttorm TK, Lyytinen H. Event-related potentials to pitch and rise time change in children with reading disabilities and typically reading children. Clinical Neurophysiology. 2008;119(1):100–115. doi: 10.1016/j.clinph.2007.09.064. [DOI] [PubMed] [Google Scholar]
- Hämäläinen JA, Salminen H, Leppänen PHT. Basic auditory processing deficits in dyslexia—Systematic review of the behavioral, event-related potential/field evidence. Journal of Learning Disabilities. 2013;46:413–427. doi: 10.1177/0022219411436213. [DOI] [PubMed] [Google Scholar]
- Hulme C, Bowyer-Crane C, Carroll JM, Duff FJ, Snowling MJ. The causal role of phoneme awareness and letter-sound knowledge in learning to read: Combining intervention studies with mediation analyses. Psychological Science. 2012;23(6):572–577. doi: 10.1177/0956797611435921. [DOI] [PubMed] [Google Scholar]
- Huttunen T, Halonen A, Kaartinen J, Lyytinen H. Does mismatch negativity show differences in reading-disabled children compared to normal children and children with attention deficit? Developmental neuropsychology. 2007;31(3):453–470. doi: 10.1080/87565640701229656. [DOI] [PubMed] [Google Scholar]
- Korpilahti P, Krause CM, Holopainen I, Lang AH. Early and late mismatch negativity elicited by words and speech-like stimuli in children. Brain and Language. 2001;76(3):332–339. doi: 10.1006/brln.2000.2426. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB. Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science. 1996;273(5277):971–973. doi: 10.1126/science.273.5277.971. [DOI] [PubMed] [Google Scholar]
- Kushnerenko E, Cheour M, Ceponiene R, Fellman V, Renlund M, Soininen K, … Naatanen R. Central auditory processing of durational changes in complex speech patterns by newborns: An event-related brain potential study. Developmental Neuropsychology. 2001;19(1):83–97. doi: 10.1207/S15326942DN1901_6. [DOI] [PubMed] [Google Scholar]
- Lachmann T, Berti S, Kujala T, Schröger E. Diagnostic subgroups of developmental dyslexia have different deficits in neural processing of tones and phonemes. International Journal of Psychophysiology. 2005;56(2):105–120. doi: 10.1016/j.ijpsycho.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Law JM, Vandermosten M, Ghesquière P, Wouters J. Predicting future reading problems based on pre-reading auditory measures: A longitudinal study of children with a familial risk of dyslexia. Frontiers in psychology. 2017;8:124. doi: 10.3389/fpsyg.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Pan J, Liu H, McBride-Chang C, Li H, Zhang Y, … Shu H. Developmental trajectories of reading development and impairment from ages 3 to 8 years in Chinese children. Journal of Child Psychology and Psychiatry. 2011;52(2):212–220. doi: 10.1111/j.1469-7610.2010.02311.x. [DOI] [PubMed] [Google Scholar]
- Leppänen PH, Pihko E, Eklund KM, Lyytinen H. Cortical responses of infants with and without a genetic risk for dyslexia: I. age effects. Neuroreport. 1999;10(5):969–973. doi: 10.1097/00001756-199904060-00014. [DOI] [PubMed] [Google Scholar]
- Li H, Shu H, McBride-Chang C, Liu H, Peng H. Chinese children’s character recognition: Visuoorthographic, phonological processing and morphological skills. Journal of Research in Reading. 2012;35(3):287–307. doi: 10.1111/jrir.2012.35.issue-3. [DOI] [Google Scholar]
- Li WS, Ho CSH. Lexical tone awareness among Chinese children with developmental dyslexia. Journal of Child Language. 2011;38(4):793–808. doi: 10.1017/S0305000910000346. [DOI] [PubMed] [Google Scholar]
- Lovio R, Näätänen R, Kujala T. Abnormal pattern of cortical speech feature discrimination in 6-year-old children at risk for dyslexia. Brain Research. 2010;1335:53–62. doi: 10.1016/j.brainres.2010.03.097. [DOI] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz SE, Shaywitz BA. A definition of dyslexia. Annals of Dyslexia. 2003;53(1):1–14. doi: 10.1007/s11881-003-0001-9. [DOI] [Google Scholar]
- Makeig S, Bell AJ, Jung TP, Sejnowski TJ. Independent component analysis of electroencephalographic data. International Conference on Signal Processing; IEEE; 1995. pp. 1548–1551. [Google Scholar]
- Manis FR, McBride-Chang C, Seidenberg MS, Keating P, Doi LM, Munson B, Petersen A. Are speech perception deficits associated with developmental dyslexia? Journal of Experimental Child Psychology. 1997;66(2):211–235. doi: 10.1006/jecp.1997.2383. [DOI] [PubMed] [Google Scholar]
- Maurer U, Bucher K, Brem S, Benz R, Kranz F, Schulz E, … Brandeis D. Neurophysiology in preschool improves behavioral prediction of reading ability throughout primary school. Biological Psychiatry. 2009;66:341–348. doi: 10.1016/j.biopsych.2009.02.031. [DOI] [PubMed] [Google Scholar]
- Maurer U, Bucher K, Brem S, Brandeis D. Altered responses to tone and phoneme mismatch in kindergartners at familial dyslexia risk. Neuroreport. 2003;14(17):2245–2250. doi: 10.1097/00001756-200312020-00022. [DOI] [PubMed] [Google Scholar]
- Mcbride-Chang C. Phonological processing, speech perception, and reading disability: An integrative review. Educational Psychologist. 1995;30:109–121. doi: 10.1207/s15326985ep3003_2. [DOI] [Google Scholar]
- McCandliss BD, Noble KG. The development of reading impairment: A cognitive neuroscience model. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9(3):196–205. doi: 10.1002/(ISSN)1098-2779. [DOI] [PubMed] [Google Scholar]
- Mody M, Studdert-Kennedy M, Brady S. Speech perception deficits in poor readers: Auditory processing or phonological coding? Journal of Experimental Child Psychology. 1997;64(2):199–231. doi: 10.1006/jecp.1996.2343. [DOI] [PubMed] [Google Scholar]
- Molfese DL. Predicting dyslexia at 8 years of age using neonatal brain responses. Brain and Language. 2000;72(3):238–245. doi: 10.1006/brln.2000.2287. [DOI] [PubMed] [Google Scholar]
- Muter V, Hulme C, Snowling M, Taylor S. Segmentation, not rhyming, predicts early progress in learning to read. Journal of Experimental Child Psychology. 1998;71(1):3–27. doi: 10.1006/jecp.1998.2453. [DOI] [PubMed] [Google Scholar]
- Näätänen R. The mismatch negativity: A powerful tool for cognitive neuroscience. Ear and Hearing. 1995;16(1):6–18. doi: 10.1097/00003446-199502000-00002. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clinical Neurophysiology. 2007;118(12):2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Neuhoff N, Bruder J, Bartling J, Warnke A, Remschmidt H, Müller-Myhsok B, Schulte-Körne G. Evidence for the late MMN as a neurophysiological endophenotype for dyslexia. PloS One. 2012;7(5):e34909. doi: 10.1371/journal.pone.0034909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Song S, Su M, McBride C, Liu H, Zhang Y, … Shu H. On the relationship between phonological awareness, morphological awareness and Chinese literacy skills: Evidence from an 8-year longitudinal study. Developmental Science. 2015;19(6):982–991. doi: 10.1111/desc.12356. [DOI] [PubMed] [Google Scholar]
- Pennington BF. From single to multiple deficit models of developmental disorders. Cognition. 2006;101(2):385–413. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: Multiple approaches for a multifactorial disease. Disease Models & Mechanisms. 2012;5(1):9–18. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihko E, Leppänen PH, Eklund KM, Cheour M, Guttorm TK, Lyytinen H. Cortical responses of infants with and without a genetic risk for dyslexia: I. Age effects. Neuroreport. 1999;10(5):901–905. doi: 10.1097/00001756-199904060-00002. [DOI] [PubMed] [Google Scholar]
- Plakas A, Van Zuijen T, Van Leeuwen T, Thomson JM, Van Der Leij A. Impaired non-speech auditory processing at a pre-reading age is a risk-factor for dyslexia but not a predictor: An ERP study. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2013;49(4):1034–1045. doi: 10.1016/j.cortex.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Preston JL, Molfese PJ, Frost SJ, Mencl WE, Fulbright RK, Hoeft F, … Pugh KR. Print-speech convergence predicts future reading outcomes in early readers. Psychological Science. 2016;27(1):75–84. doi: 10.1177/0956797615611921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Landi N, Preston JL, Mencl WE, Austin AC, Sibley D, … Molfese P. The relationship between phonological and auditory processing and brain organization in beginning readers. Brain and Language. 2013;125(2):173–183. doi: 10.1016/j.bandl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, … Shaywitz BA. Neurobiological studies of reading and reading disability. Journal of Communication Disorders. 2001;34(6):479–492. doi: 10.1016/S0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Shtyrov Y. Automatic processing of grammar in the human brain as revealed by the mismatch negativity. Neuroimage. 2003;20(1):159–172. doi: 10.1016/S1053-8119(03)00261-1. [DOI] [PubMed] [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, Frith U. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126(4):841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Ramus F, Szenkovits G. What phonological deficit? The Quarterly Journal of Experimental Psychology. 2008;61(1):129–141. doi: 10.1080/17470210701508822. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. Proceedings of the National Academy of Sciences. 2012;109(6):2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Standard progressive matrices. Oxford, England: Oxford Psychologists Press; 1996. [Google Scholar]
- Richardson U, Leppänen PH, Leiwo M, Lyytinen H. Speech perception of infants with high familial risk for dyslexia differ at the age of 6 months. Developmental Neuropsychology. 2003;23(3):385–397. doi: 10.1207/S15326942DN2303_5. [DOI] [PubMed] [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Pugh KR. The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Scientific Studies of Reading. 2004;8(3):273–292. doi: 10.1207/s1532799xssr0803_6. [DOI] [Google Scholar]
- Schirmer A, Tang SL, Penney TB, Gunter TC, Chen HC. Brain responses to segmentally and tonally induced semantic violations in Cantonese. Journal of Cognitive Neuroscience. 2005;17(1):1–12. doi: 10.1162/0898929052880057. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G, Bruder J. Clinical neurophysiology of visual and auditory processing in dyslexia: A review. Clinical Neurophysiology. 2010;121(11):1794–1809. doi: 10.1016/j.clinph.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G, Deimel W, Bartling J, Remschmidt H. Speech perception deficit in dyslexic adults as measured by mismatch negativity (MMN) International Journal of Psychophysiology. 2001;40(1):77–87. doi: 10.1016/S0167-8760(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Serniclaes W, Sprenger-Charolles L, Carré R, Demonet JF. Perceptual discrimination of speech sounds in developmental dyslexia. Journal of Speech, Language, and Hearing Research. 2001;44(2):384–399. doi: 10.1044/1092-4388(2001/032). [DOI] [PubMed] [Google Scholar]
- Shankweiler D, Lundquist E. On the relations between learning to spell and learning to read. Advances in Psychology. 1992;94:179–192. [Google Scholar]
- Sharma M, Purdy SC, Newall P, Wheldall K, Beaman R, Dillon H. Electrophysiological and behavioral evidence of auditory processing deficits in children with reading disorder. Clinical Neurophysiology. 2006;117:1130–1144. doi: 10.1016/j.clinph.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Shu H, Chen X, Anderson RC, Wu N, Xuan Y. Properties of school Chinese: Implications for learning to read. Child Development. 2003;74(1):27–47. doi: 10.1111/cdev.2003.74.issue-1. [DOI] [PubMed] [Google Scholar]
- Shu H, Peng H, McBride-Chang C. Phonological awareness in young Chinese children. Developmental Science. 2008;11(1):171–181. doi: 10.1111/desc.2008.11.issue-1. [DOI] [PubMed] [Google Scholar]
- Siok WT, Fletcher P. The role of phonological awareness and visual-orthographic skills in Chinese reading acquisition. Developmental Psychology. 2001;37(6):886–899. doi: 10.1037/0012-1649.37.6.886. [DOI] [PubMed] [Google Scholar]
- Snowling M, Hulme C. The development of phonological skills. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1994;346(1315):21–27. doi: 10.1098/rstb.1994.0124. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. From language to reading and dyslexia. Dyslexia. 2001;7(1):37–46. doi: 10.1002/(ISSN)1099-0909. [DOI] [PubMed] [Google Scholar]
- Sperling AJ, Lu ZL, Manis FR, Seidenberg MS. Selective magnocellular deficits in dyslexia: A “phantom contour” study. Neuropsychologia. 2003;41(10):1422–1429. doi: 10.1016/S0028-3932(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Zimmer K, Lachmann T, Dirichs M, Kammer T. Development of rapid temporal processing and its impact on literacy skills in primary school children. Child Development. 2014;85(4):1711–1726. doi: 10.1111/cdev.2014.85.issue-4. [DOI] [PubMed] [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nature Reviews Neuroscience. 2004;5(9):721–728. doi: 10.1038/nrn1499. [DOI] [PubMed] [Google Scholar]
- Tallal P, Gaab N. Dynamic auditory processing, musical experience and language development. Trends in Neurosciences. 2006;29:382–390. doi: 10.1016/j.tins.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller S, Fitch RH. Neurobiological basis of speech: A case for the preeminence of temporal processing. Annals of the New York Academy of Sciences. 1993;682(1):27–47. doi: 10.1111/j.1749-6632.1993.tb22957.x. [DOI] [PubMed] [Google Scholar]
- Thomson JM, Fryer B, Maltby J, Goswami U. Auditory and motor rhythm awareness in adults with dyslexia. Journal of Research in Reading. 2006;29(3):334–348. doi: 10.1111/jrir.2006.29.issue-3. [DOI] [Google Scholar]
- Werker JF, Tees RC. Speech perception in severely disabled and average reading children. Canadian Journal of Psychology/Revue Canadienne De Psychologie. 1987;41(1):48–61. doi: 10.1037/h0084150. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang L, Shu H, Xi J, Wu H, Zhang Y, Li P. Universality of categorical perception deficit in developmental dyslexia: An investigation of Mandarin Chinese tones. Journal of Child Psychology and Psychiatry. 2012;53(8):874–882. doi: 10.1111/j.1469-7610.2012.02528.x. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: A psycholinguistic grain size theory. Psychological Bulletin. 2005;131(1):3. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]