Abstract
In the U.S., the FDA has initiated a public dialogue about reducing the nicotine content of cigarettes. A reduced-nicotine standard could increase withdrawal symptoms among current smokers. We examined the impact of switching smokers to cigarettes that varied in nicotine content on withdrawal symptoms over 6 weeks. A secondary analysis (N=839) of a 10-site, double-blind clinical trial of non-treatment seeking smokers was completed. Participants were instructed to smoke study cigarettes, containing 0.4 to 15.8 mg of nicotine/g of tobacco, for 6-weeks and were then abstinent overnight. Using latent growth curves, trajectories of individual withdrawal symptoms were compared between the reduced nicotine content (RNC) conditions and a normal nicotine content (NNC) condition. Path analyses compared symptoms after overnight abstinence. Relative to NNC cigarettes, participants smoking RNC cigarettes had increased anger/irritability/frustration and increased appetite/weight gain during the initial weeks, but the symptoms resolved by Week 6. Individuals who were biochemically-verified as adherent with using only the 0.4 mg/g cigarettes had higher sadness levels (Cohen’s d = .40) at Week 6 compared with the NNC condition, although symptoms were mild. After a post-Week 6 overnight abstinence challenge, some RNC conditions relative to NNC condition exhibited reduced withdrawal. Individuals who were biochemically-confirmed as adherent to the lowest nicotine condition experienced only mild and transient symptom elevations. Thus, a reduced-nicotine standard for cigarettes produced a relatively mild and temporary increase in withdrawal among non-treatment seeking smokers (ClinicalTrials.gov number, NCT01681875).
Keywords: reduced nicotine cigarettes, withdrawal symptoms, nicotine exposure, adherence
Introduction
In the United States (U.S.), cigarette smoking contributes to at least 480,000 deaths annually (US Department of Health and Human Services, 2014). The Food and Drug Administration (FDA) has the authority to reduce (to non-zero levels) the nicotine content of cigarettes (Congress, 2009). The FDA recently announced that they intend to “pursue lowering nicotine in cigarettes to non-addictive levels” and “seek input on the potential public health benefits and any possible adverse effects of lowering nicotine in cigarettes” (FDA, 2017). Because nicotine is the primary addictive substance in tobacco that sustains smoking (Corrigall, 1999; Harvey et al., 2004; USDHSS, 1988), reducing nicotine levels in cigarettes to below a level that results in dependence would be expected to substantially reduce national rates of smoking and related diseases (Benowitz & Henningfield, 1994; Donny, Walker, Hatsukami, & Bullen, 2016; Zeller & Hatsukami, 2009). Consistent with these predictions, smoking cigarettes containing substantially-reduced levels of nicotine in the tobacco decreased nicotine exposure, nicotine dependence, and smoking compared to smoking conventional cigarettes (Benowitz et al., 2012; Donny et al., 2015; Donny, Houtsmuller, & Stitzer, 2007; Hatsukami et al., 2013a; Hatsukami et al., 2010) and has promoted abstinence (Hatsukami, et al., 2010; Walker et al., 2012). An anticipated negative consequence of nicotine reduction is withdrawal. The present study evaluated the time course of individual withdrawal symptoms during a 6 week randomized clinical trial comparing reduced nicotine content (RNC) to normal nicotine content (NNC) cigarettes in non-treatment seeking smokers.
Relative to not smoking, smoking RNC cigarettes suppresses total withdrawal scores (Donny, et al., 2007; Tidey, Rohsenow, Kaplan, Swift, & AhnAllen, 2013). Furthermore, on average, total withdrawal scores do not increase when smokers switch from their usual brand to RNC cigarettes compared to NNC cigarettes (Benowitz, et al., 2012; Donny, et al., 2015; Donny, et al., 2007; Donny & Jones, 2009; Hatsukami, et al., 2010; Pickworth, Nelson, Rohrer, Fant, & Henningfield, 1999). However, investigations focusing on total withdrawal scores could overlook instances when not all of the symptoms are suppressed equally. Select symptoms, such as craving, are effectively suppressed by RNC relative to NNC cigarettes (Baldinger, Hasenfratz, & Bättig, 1995; Benowitz et al., 2007; Buchhalter, Acosta, Evans, Breland, & Eissenberg, 2005; Buchhalter, Schrinel, & Eissenberg, 2001; Donny, et al., 2015; Donny & Jones, 2009; Hatsukami, et al., 2010; Pickworth, et al., 1999; Westman, Behm, & Rose, 1996) and nonsmoking conditions (Donny, et al., 2007; Rose, Behm, Westman, & Johnson, 2000; Tidey, et al., 2013). Whereas, relative to smoking NNC cigarettes, RNC cigarettes may not completely suppress restlessness, impatience, difficulty concentrating (Buchhalter, et al., 2005), irritability, eating (Benowitz, et al., 2007; Buchhalter, et al., 2005; Donny & Jones, 2009), and weight gain (Benowitz, et al., 2012; Rupprecht et al., 2016). Thus, the latter symptoms may be more effectively suppressed by the pharmacological effects of nicotine combined with the sensory aspects of smoking (Buchhalter, et al., 2005) as opposed to by RNC cigarettes that retain many of the sensory aspects of smoking but have substantially reduced nicotine levels.
Importantly, the extent to which nicotine reduction would lead to withdrawal discomfort can change over time. Select symptoms appear to be completely suppressed within the first hours and day of RNC cigarette use, such as irritability (Baldinger, et al., 1995; Westman, et al., 1996), difficulty concentrating, sluggishness (Baldinger, et al., 1995), craving (Baldinger, et al., 1995; Buchhalter, et al., 2005; Buchhalter, et al., 2001; Faulkner et al., 2017), restlessness, anxiety/nervousness (Baldinger, et al., 1995; Buchhalter, et al., 2001), hunger, desire for sweets (Buchhalter, et al., 2005; Buchhalter, et al., 2001; Faulkner, et al., 2017), negative affect (Faulkner, et al., 2017), and depression (Buchhalter, et al., 2001). In contrast, other symptoms have been shown to emerge in subsequent days, including difficulty concentrating, restlessness, increased eating, and impatience, and remain elevated for five days (Buchhalter, et al., 2005). As the RNC cigarettes use in these studies has been limited to five days or less, it remains unknown how long the latter symptoms would remain elevated. Furthermore, it is unknown if dose-response effects of nicotine on these symptoms would emerge after extended use of RNC cigarettes.
Understanding the effects of extended nicotine reduction and subsequent abstinence on individual withdrawal symptoms is important for understanding how a nicotine reduction policy would impact smokers over time. To examine this, we conducted a secondary analysis of a double-blind, multi-site clinical trial of daily smokers not interested in quitting smoking, conducted by Donny et al. (2015), randomly assigned participants to smoke cigarettes of varying nicotine content for 6 weeks. The aim of the primary trial was to evaluate the effects of smoking cigarettes with varying nicotine contents. Effects on a total withdrawal score were reported; however, effects of the cigarette conditions on individual withdrawal symptoms and the time course of these effects were not examined. To this end, the present study sought to extend the findings of Donny et al. (2015) and prior studies by examining the dose-response relationship of nicotine on specific withdrawal symptoms over time, during and beyond the first week of switching, in order to better understand the discomfort from these cigarettes among non-treatment seeking smokers. A unique feature of the design was the inclusion of two control conditions – usual brand and NNC cigarettes – which allows for determining the extent to which switching cigarette brands affects withdrawal. We also considered potential moderators of RNC cigarette effects on withdrawal symptoms, including nicotine dependence level, sex, and non-adherence to RNC cigarettes. We hypothesized that difficulty concentrating, irritability, restlessness, and increased eating would be elevated early in the trial when smoking RNC relative to NNC cigarettes, but then would begin to resolve; however, the exact timing of resolution was exploratory. During abstinence, we hypothesized that individual withdrawal symptoms would be lower in the RNC conditions than the NNC cigarette group because RNC cigarette use over the 6-week period would reduce nicotine dependence. To test these hypotheses, analyses included assessments of individual withdrawal symptoms at daily (i.e., during first week of switching to RNC cigarettes) and weekly (i.e., for 6 weeks) units of measurement, and also evaluated withdrawal during a period of overnight smoking abstinence at the end of 6 weeks of study cigarette use.
Methods
Participants
From 2013–2014, adult daily smokers were recruited using flyers, direct mailings, television/radio, and other advertisements at 10 study sites. Inclusion criteria included: ≥age 18; smoking ≥five cigarettes per day (CPD); expired carbon monoxide (CO) >8 ppm or urine cotinine >100 ng/ml. Exclusion criteria included: intention to quit smoking in next 30 days; regular use of other tobacco products (>9 of past 30 days); frequent binge drinking (>9 of past 30 days); significant or unstable medical/psychiatric conditions; positive illicit drug toxicology screen other than cannabis; pregnancy/breastfeeding; exclusively using ‘roll your own’ cigarettes. A total of 839 eligible participants were randomized and were paid up to $835 for participation.
Study Design
The data comes from a previously-published 7-arm, double-blind, 10-site randomized trial included a 2-week baseline and 6-week experimental period (Donny et al., 2015). During baseline, participants smoked their usual brand of cigarettes. Participants were then randomly assigned to smoke cigarettes varying in nicotine content: 0.4 mg/g; 0.4 mg/g-high tar (HT; defined a priori as exploratory); 1.3 mg/g; 2.4 mg/g; 5.2 mg/g; 15.8 mg/g (defined a priori as the primary control); and usual brand cigarette. Study cigarettes were supplied by the National Institute on Drug Abuse (NIDA; NOT-DA-14-004). Following baseline, participants were provided a free 14-day supply of cigarettes (baseline cigarettes per day times 14) at each weekly visit. Participants were instructed to not use other cigarettes, received brief weekly counseling aimed at increasing compliance, and completed weekly laboratory assessments. At the end of the six-week period, smokers were paid $90 to abstain from all nicotine and tobacco products for ≥18 hours. Additional abstinence assessments (i.e., abstinence visit) were conducted only if CO was <50% of Week 6 visit or <6 ppm. The study design is described in greater detail in the parent study manuscript (Donny et al., 2015; ClinicalTrials.gov number, NCT01681875). The protocol was approved by the Institutional Review Boards of all of the participating research sites.
Measures
Withdrawal
Participant rated withdrawal symptoms using the 8-item Minnesota Nicotine Withdrawal Scale (MNWS)(Hughes & Hatsukami, 1986). Items included were angry/irritable/frustrated, anxious/nervous, depressed mood/sad, desire or craving to smoke, difficulty concentration, increased appetite/hungry/weight gain, insomnia/sleep problems/awakening at night, and restless. During the last week of the baseline period and first week of the experimental period, participants used an Interactive Voice Response (IVR) system that automatically called participants daily at a time of their choosing, instructing them to rate symptoms experienced on the prior day on 0 (“none”) to 4 (“severe”) scales. The MNWS was also completed by participants at each weekly laboratory visit and the abstinence visit.
Nicotine exposure
Nicotine exposure biomarkers were assessed at the baseline visit and at post-randomization Weeks 2 and 6 using first void urine samples or spot urine if the participant forgot the first void urine sample. Total nicotine equivalents (TNE), adjusted for creatinine, were computed as the sum of nicotine and six metabolites, which included total nicotine, total cotinine, total trans 3′-hydroxycotinine (sum of the analyte and respective glucuronide conjugate), and nicotine-N-oxide.
Nicotine dependence
Nicotine dependence was assessed at baseline as the sum of responses on the six-item Fagerström Test for Nicotine Dependence (FTND)(Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991).
Analyses
The effects of RNC cigarettes on withdrawal symptoms, assessed daily, weekly, and following overnight abstinence, were examined in a structural equation modeling (SEM) framework using Mplus 7.3 (Muthén & Muthén, 1998–2012). First, daily change during the first week of study product use for each withdrawal symptom and the total withdrawal score (i.e., average of 8 symptoms) was examined using latent growth curve models. Latent growth curve models estimated the average change over time of each withdrawal symptom starting with the first full day of product use (intercept) controlling for corresponding baseline score. Advantages of latent growth curve models versus a more simplistic approach (e.g., repeated measures ANOVA and regression) for this investigation include accounting for missing data using robust maximum likelihood estimation, separating measurement error from the true change of the symptoms over time by constructing latent variables (Curran, Obeidat, & Losardo, 2010), and ability to evaluate moderating factors of individual level withdrawal over time. Participants contributed up to 6 observations, and slope factor loadings were fixed to reflect equally-spaced assessments. Total withdrawal score was modeled as continuous and symptom levels were ordinal. Linear and quadratic functional forms for the trajectories were compared with the model having the lowest Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) being chosen. The same approach was used to examine weekly MNWS item assessments over the 6-week study; the intercept was interpreted as Week 1 symptom level, controlling for baseline.
The effect of nicotine dose on withdrawal was evaluated by regressing the slope(s) of each symptom on a dummy-coded predictor comparing the NNC control (coded as ‘0’) to each reduced nicotine condition (coded as ‘1’) separately. NNC control was also compared to usual brand (UB) control to examine effects of brand switching (i.e., symptoms due to unfamiliarity with cigarette design rather than nicotine). When nicotine dose was significantly related to the intercept or slope(s), the centering of the intercept was changed to determine at which weeks a significant nicotine effect occurred. In light of the significant reductions in CPD over time for individuals smoking 2.4 mg/g nicotine or less (Donny et al., 2015), supplemental analyses were conducted with CPD (assessed by IVR) included as a time-varying covariate. This allowed us to determine if intervention effects on withdrawal were directly attributable to nicotine (as opposed to indirect effects via reduced CPD). Lastly, using path analysis, individual withdrawal symptoms after overnight abstinence were regressed on each dummy-coded predictor, controlling for baseline level.
Moderation analyses examined whether or not the effect of nicotine reduction on withdrawal symptoms differed based on sex or baseline nicotine dependence. Product terms were included alongside the main effects as predictors. To increase power and reduce the number of comparisons, the moderation analyses were conducted comparing the combined RNC conditions (0.4 mg/g – 2.4 mg/g) to the NNC control. The 5.2 mg/g condition was excluded because of its mixed effects in the primary trial (i.e., CPD similar to control conditions but TNE was significantly reduced)(Donny, et al., 2015).
Finally, analyses were conducted to account for non-adherence (i.e., smoking non-study cigarettes) because it was prevalent (Nardone et al., 2016). Participants in the 0.4 mg/g condition who were adherent at Weeks 2 and 6 were compared with all of the individuals in the NNC control condition. Adherence status was determined using a urinary TNE cut-off (less than or equal to 6.41 nmol/ml) based on a prior study with 0.4 mg/g cigarettes (Denlinger, 2015). Age and nicotine dependence level differed between non-adherent and adherent participants (Nardone, et al., 2016), and thus were included as covariates.
We did not correct for the multiple statistical tests because we considered it more important to avoid type II error than type I error in order to identify any potential negative consequences. Thus, all tests were considered significant at α = 0.05, two-tailed.
Results
Sample Characteristics
On average, participants were 41.7 ± 13.2 years old, smoked 15.6 ± 7.6 CPD, and had a moderate severity of nicotine dependence (FTND = 5.1 ± 2.2). Furthermore, 51% of participants were white and 56.1% had attended college. Baseline sample characteristics are described in greater detail in the primary paper (Donny et al., 2015). They reported that there were no differences between the RNC and NNC conditions on any baseline variables (age, sex, race, ethnicity, college attendance, menthol cigarette use, cigarettes per day, TNE, use of other tobacco products, or FTND) except for expired CO level (Donny et al., 2015). The correlations between the individual MNWS items at baseline ranged from .20 to .60, which suggests that the items have independent explanatory value.
As previously reported in the primary paper by Donny et al. (2015), retention at Week 6 (i.e., completed Week 6 IVR call) exceeded 92%. Attrition did not differ between the study conditions. Completion rates of the six daily IVR calls in the present analyses were 99–100%. Completion rates of each in-person weekly visit after Baseline ranged from 89 – 96%. Overall, 76% of participants completed the abstinence assessment, and completion rate did not differ between study conditions.
Intervention effects
Daily withdrawal levels (first week post-randomization)
Latent growth curves of 8 symptoms assessed daily using IVR were estimated, and for each the best-fitting trajectory was linear. The best-fitting total score trajectory shape was quadratic. Individuals randomized to the 0.4 mg/g HT and usual brand cigarette conditions reported significantly increased anger/irritability/frustration relative to the NNC control on the first day of use (Table 1), which persisted through the first week for the 0.4 mg/g HT condition. No other differences were observed between RNC and NNC, or NNC and UB groups. Sex and baseline FTND score did not moderate the effects of RNC cigarette use on symptom change (ps > .05). Including daily CPD as a time-varying covariate had minimal effect on the findings (see Supplemental Table 1).
Table 1.
Effects of study cigarette conditions on withdrawal symptoms assessed over time using daily data during the first week of the experimental period
| 0.4 mg/g | 0.4 mg/g HT | 1.3 mg/g | 2.4 mg/g | 5.2 mg/g | Usual brand | |
|---|---|---|---|---|---|---|
| Group effects on the Intercept at Day 1: estimate (standard error) | ||||||
|
| ||||||
| Angry/irritable/frustrated | .04 (.35) | .75 (.34)* | .43 (.36) | .14 (.37) | .07 (.30) | .70 (.34)* |
| Anxious/nervous | .28 (.39) | .54 (.35) | .34 (.37) | .20 (.35) | .38 (.31) | .22 (.37) |
| Depressed mood/sad | .49 (.37) | .52 (.33) | .50 (.40) | .72 (.40)† | .17 (.35) | .47 (.42) |
| Desire or craving to smoke | .15 (.35) | .02 (.35) | .10 (.36) | −.002 (.37) | .32 (.33) | −.17 (.36) |
| Difficulty concentrating | .08 (.42) | .55 (.38) | .41 (.40) | .05 (.36) | .11 (.38) | .49 (.38) |
| ↑appetite/hungry/weight gain | .61 (.44) | .19 (.44) | .47 (.43) | .28 (.44) | .78 (.46) | −.38 (.40) |
| Insomnia/sleep problems/awakening at night | −.57 (.44) | .18 (.35) | −.05 (.39) | −.37 (.37) | −.12 (.38) | −.01 (.25) |
| Restless | .04 (.34) | .65 (.36)† | .06 (.35) | −.10 (.34) | .07 (.32) | −.68 (.36)† |
| Total | .05 (.46) | .94 (.53)† | .18 (.53) | −.06 (.46) | .05 (.48) | .91 (.53)† |
|
| ||||||
| Group effects on linear slope: estimate (standard error) | ||||||
|
| ||||||
| Angry/irritable/frustrated | .03 (.09) | .02 (.09) | .06 (.10) | −.01 (.09) | .02 (.07) | −.01 (.09) |
| Anxious/nervous | −.13 (.09) | .01 (.09) | −.03 (.09) | −.04 (.08) | −.04 (.07) | .16 (.09)† |
| Depressed mood/sad | .02 (.10) | .15 (.09)† | .00 (.11) | .04 (.09) | .10 (.09) | −.09 (.12) |
| Desire or craving to smoke | −.13 (.08) | −.05 (.09) | −.07 (.09) | −.03 (.08) | −.01 (.08) | .08 (.09) |
| Difficulty concentrating | .05 (.10) | −.05 (.10) | .03 (.10) | .02 (.09) | .02 (.11) | −.09 (.09) |
| ↑appetite/hungry/weight gain | −.02 (.12) | .06 (.11) | −.13 (.12) | −.07 (.12) | −.12 (.12) | .08 (.10) |
| Insomnia/sleep problems/awakening at night | .13 (.11) | .09 (.10) | .10 (.10) | .18 (.10)† | .11 (.10) | .05 (.07) |
| Restless | −.08 (.08) | −.05 (.09) | .03 (.10) | .03 (.09) | .07 (.83) | .06 (.09) |
| Total | .31 (.37) | .06 (.39) | .15 (.38) | .04 (.39) | .46 (.40) | −.25 (.41) |
|
| ||||||
| Group effects on quadratic slope: estimate (standard error) | ||||||
|
| ||||||
| Total | −.05 (.07) | .02 (.08) | −.003 (.07) | −.01 (.07) | −.08 (.08) | .07 (.08) |
Unstandardized coefficients (standard errors) are reported for the effect of each group relative to the NNC control. Significant and positive effects on the intercept can be interpreted as the group of interest having significantly higher levels of the symptom relative to the NNC control group on Day 1. Significant and positive effects on the linear slope can be interpreted as the group of interested having a more positive rate of change in the symptom from Days 1 to 6. Significant and positive effects on the quadratic slope can be similarly interpreted as the group of interest having a more positive quadratic rate of change in the symptom from Days 1 to 6.
p < .001,
p < .01,
p < .05,
p < .10; Significant coefficients are bolded using a significance level of .05.
Weekly withdrawal levels
The best-fitting functional form for most items was linear, but quadratic change was supported for desire/craving to smoke. In the 0.4 mg/g HT and 1.3 mg/g conditions, anger/irritability/frustration was higher at Week 1 relative to NNC cigarette (p = .002 and p = .03, respectively); however, a faster rate of decrease in anger/irritability/frustration scores in both groups relative to NNC control (p =.01 and p = .004, respectively; Table 2) led to no group differences in anger/irritability/frustration for Weeks 2 – 6. No other significant differences were seen for the RNC and UB conditions relative to the NNC condition. Baseline FTND score moderated effects of RNC cigarettes on Week 1 ratings of restlessness (p < .05). RNC users reported higher Week 1 restlessness levels than NNC cigarette users for those with lower baseline FTND scores as opposed to higher FTND scores; the groups did not differ from each other at Week 6. No other moderating effects were supported. The results were largely unaffected by including weekly average CPD as a time-varying covariate (see Supplemental Table 2).
Table 2.
Effects of study cigarette conditions on withdrawal symptoms assessed weekly over time during the 6-week experimental period.
| 0.4 mg/g | 0.4 mg/g HT | 1.3 mg/g | 2.4 mg/g | 5.2 mg/g | Usual brand | |
|---|---|---|---|---|---|---|
| Group effects on the Intercept at Week 1: estimate (standard error) | ||||||
|
| ||||||
| Angry/irritable/frustrated | .30 (.31) | .93 (.31)** | .77 (.35)* | .51 (.31) | .30 (.29) | −.35 (.31) |
| Anxious/nervous | −.10 (.38) | .21 (.34) | .49 (.36) | .66 (.36)† | .42 (.36) | −.01 (.36) |
| Depressed mood/sad | −.22 (.36) | .12 (.34) | .05 (.40) | .36 (.42) | −.07 (.43) | −.29 (.40) |
| Desire or craving to smoke | .12 (.37) | −.16 (.37) | .03 (.39) | −.16 (.38) | .41 (.39) | −.20 (.36) |
| Difficulty concentrating | .44 (.39) | .46 (.39) | .67 (.41) | .58 (.40) | .20 (.42) | −.35 (.38) |
| ↑appetite/hungry/weight gain | .55 (.37) | .25 (.43)† | .74 (.40) | .05 (.39) | .19 (.40) | −.30 (.41) |
| Insomnia/sleep problems/awakening at night | −.11 (.38) | .52 (.38) | −.16 (.40) | .29 (.34) | .26 (.34) | .003 (.39) |
| Restless | −.66 (.44) | .50 (.38) | −.07 (.42) | −.12 (.43) | .07 (.39) | −.27 (.37) |
|
| ||||||
| Group effects on linear slope: estimate (standard error) | ||||||
|
| ||||||
| Angry/irritable/frustrated | −.09 (.09) | −.22 (.09)* | −.28 (.10)** | −.11 (.09) | −.04 (.09) | .03 (.09) |
| Anxious/nervous | −.05 (.09) | .004 (.08) | −.15 (.09)† | −.07 (.09) | −.03 (.09) | .19 (.09)* |
| Depressed mood/sad | .08 (.11) | .04 (.09) | −.05 (.11) | −.07 (.10) | .06 (.11) | .05 (.11) |
| Desire or craving to smoke | −.34 (.24) | −.01 (.25) | −.45 (.26)† | .000 (.26) | .02 (.24) | .34 (.24) |
| Difficulty concentrating | −.10 (.10) | −.04 (.09) | −.12 (.08) | .004 (.08) | −.01 (.08) | .08 (.10) |
| ↑appetite/hungry/weight gain | −.14 (.09) | −.12 (.09) | −.17 (.09)† | −.15 (.09) | −.16 (.09)† | −.05 (.08) |
| Insomnia/sleep problems/awakening at night | .03 (.10) | −.05 (.09) | .08 (.10) | −.02 (.09) | .01 (.10) | .13 (.10) |
| Restless | .05 (.09) | −.04 (.09) | .08 (.10) | .01 (.11) | −.01 (.10) | .19 (.09)* |
|
| ||||||
| Group effects on quadratic slope: estimate (standard error) | ||||||
|
| ||||||
| Desire or craving to smoke | .04 (.04) | −.003 (.05) | .06 (.05) | −.01 (.05) | −.01 (.04) | −.01 (.04) |
Unstandardized coefficients (standard errors) are reported for the effect of each group relative to the NNC control. Significant and positive effects on the intercept can be interpreted as the group of interest having significantly higher levels of the symptom relative to the NNC control group on Week 1. Significant and positive effects on the linear slope can be interpreted as the group of interested having a more positive rate of change in the symptom from Weeks 1 to 6. Significant and positive effects on the quadratic slope can be similarly interpreted as the group of interest having a more positive quadratic rate of change in the symptom from Weeks 1 to 6.
p < .001,
p < .01,
p < .05,
p < .10; Significant coefficients are bolded using a significance level of .05.
Abstinence session withdrawal levels
When withdrawal symptoms were compared after overnight abstinence, individuals in the 0.4 mg/g (normal tar) group reported significantly less anger/irritability/frustration (p < .05) and difficulty concentrating (p < .05; Table 3) than those in the NNC condition. Those in the 1.3 mg/g group reported significantly less desire/craving to smoke than 15.8 mg/g controls (p < .05). Individuals in the UB condition reported significantly increased anxious/nervous, anger/irritability/frustration, restlessness, and desire/craving to smoke relative to NNC controls (ps < .05; Table 3). There were no other group differences. There were no significant moderators (ps > .05).
Table 3.
Effects of study cigarette conditions on withdrawal symptoms following overnight abstinence after the 6-week use period.
| 0.4 mg/g | 0.4 mg/g HT | 1.3 mg/g | 2.4 mg/g | 5.2 mg/g | Usual brand | |
|---|---|---|---|---|---|---|
| Angry/irritable/frustrated | −.64 (.29)* | .09 (.27) | −.38 (.27) | −.20 (.28) | −.35 (.27) | .67 (.27)* |
| Anxious/nervous | −.54 (.30)† | .20 (.28) | −.41 (.28) | .19 (.28) | .04 (.28) | .76 (.27)** |
| Depressed mood/sad | −.47 (.36) | .02 (.32) | −.32 (.33) | −.26 (.33) | −.18 (.33) | .04 (.32) |
| Desire or craving to smoke | −.48 (.27)† | −.08 (.27) | −.65 (.27)* | −.24 (.27) | −.32 (.27) | .58 (.28)* |
| Difficulty concentrating | −.70 (.30)* | .11 (.28) | −.38 (.28) | −.20 (.28) | −.41 (.28) | .33 (.27) |
| ↑appetite/hungry/weight gain | −.23 (.29) | .16 (.29) | .18 (.27) | −.19 (.28) | −.40 (.29) | .05 (.27) |
| Insomnia/sleep problems/awakening at night | −.09 (.31) | .16 (.30) | −.07 (.31) | .12 (.30) | −.14 (.31) | .60 (.32)† |
| Restless | −.32 (.29) | −.01 (.28) | −.24 (.28) | −.28 (.28) | −.23 (.28) | .56 (.28)* |
Unstandardized coefficients (standard errors) are reported for the effect of each group relative to the NNC control. Significant and positive effects indicate that the group of interest reported increased withdrawal relative to the NNC control during the abstinence period.
p < .001,
p < .01,
p < .05,
p < .10; Significant coefficients are bolded using a significance level of .05.
Effects of non-adherence
Daily withdrawal levels
Adjusting for age and baseline FTND, individuals who were adherent to 0.4 mg/g cigarettes based on TNE exhibited no differences in withdrawal symptoms compared to those in the NNC control condition on Day 1 (Table 4, left panel). Depressed mood/sad increased significantly in the 0.4 mg/g adherent group relative to the NNC controls over time, which resulted in significantly elevated depressed mood/sad at Days 4 (p = .04), 5 (p = .01), and 6 (p = .01; Figure 1). No other associations were detected.
Table 4.
Associations between biomarker-confirmed non-adherence with changes in individual withdrawal symptoms over time.
| Daily IVR data | Weekly data | Abstinence visit | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Intercept Day 1 | Linear slope Days 1–6 | Intercept Week 1 | Linear slope Weeks 1–6 | Quadratic Slope Weeks 1–6 | Observed level | |
| Difference between adherent in 0.4 mg/g condition and NNC controls | ||||||
| Angry/irritable/Frustrated | .02 (.48) | .17 (.12) | 1.19 (.44)** | −.06(.12) | – | −.46 (.39) |
| Anxious/nervous | −.20 (.54) | .09 (.12) | .36 (.44) | .03 (.11) | – | −.53 (.38) |
| Depressed mood/sad | .12 (.43) | .22 (.09)* | .25 (.48) | .31 (.15)* | – | .06 (.43) |
| Desire or craving to smoke | −.12 (.49) | −.21 (.12)† | −.51 (.55) | .16 (.35) | −.06 (.06) | −.41 (.34) |
| Difficulty concentrating | −.13 (.51) | .04 (.13) | .23 (.48) | .02 (.15) | – | −.48 (.39) |
| ↑appetite/hungry/weight gain | −.26 (.57) | .10 (.15) | 1.08 (.48)* | −.07 (.11) | – | .05 (.34) |
| Insomnia/sleep problems/awakening at night | −.15 (.51) | .13 (.13) | .60 (.46) | −.04 (.12) | – | −.17 (.42) |
| Restless | .22 (.47) | −.05 (.12) | .65 (.51) | −.11 (.14) | – | −.41 (.37) |
Unstandardized coefficients (standard errors) are reported, adjusting for age and baseline FTND. For the daily and weekly data, a significant, positive coefficient would be interpreted as adherent individuals in the 0.4 mg/g condition reported higher levels of the symptom than the NNC control group on Day 1 or Week 1 (significant intercept effect), respectively, or over time (linear or quadratic slope effects). For the abstinence visit, a positive coefficient would be interpreted as the adherent group reporting higher levels of the symptom than the NNC control group during abstinence.
p < .001,
p < .01,
p < .05,
p < .10; Significant coefficients are bolded using a significance level of .05.
Figure 1.
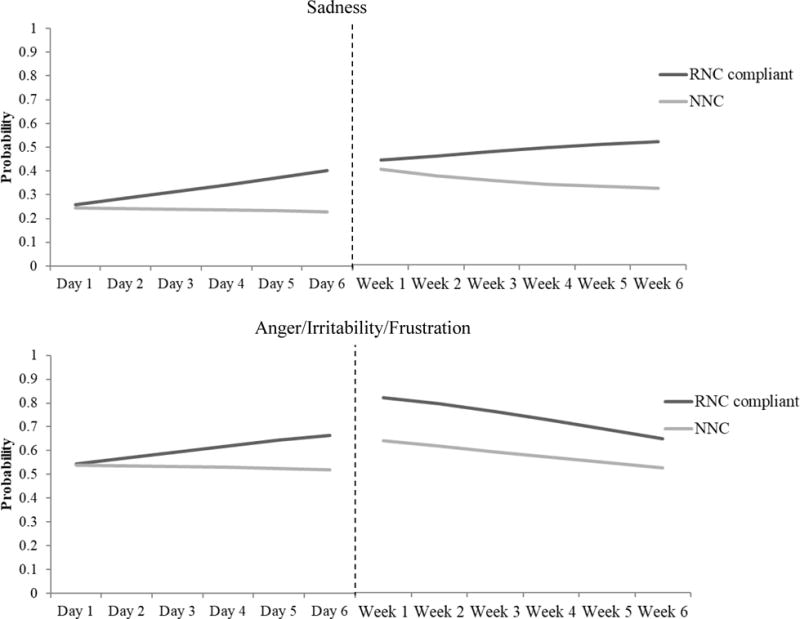
The probability of endorsing any sadness or anger/irritability/frustration overtime for individuals who smoked normal nicotine content study cigarettes versus those who were adherent when smoking RNC cigarettes
Note. The dependent variables in the analyses were scores on the withdrawal item ranging from “not at all” (0) to “severe” (4). Probabilities were estimated from latent growth curve models for the purpose of this graph. Specifically, probabilities of endorsing “slight” to “severe” were summed for each item in the RNC adherent group (.04 mg/g) versus the NNC control group during the first 6 days of the trial (using IVR daily data) and throughout the six weeks of the trial (using weekly, retrospective report data). Analyses adjusted for age and baseline FTND.
Weekly withdrawal levels
Adjusting for age and baseline FTND, relative to individuals in the NNC control condition, adherent individuals in the 0.4 mg/g conditions reported higher levels of anger/irritability/frustration (p = .01) and increased appetite/hunger/weight gain (p = .02) at Week 1, and also demonstrated an increase in depressed mood/sad over time (p = .04; Table 4). By Week 6, only ratings of depressed mood/sad were increased in the 0.4 mg/g adherent group versus NNC condition (p = .01; Figure 1).
Abstinence session withdrawal levels
Controlling for age and baseline FTND, being adherent at Weeks 2 and 6 in the 0.4 mg/g conditions based on TNE was not associated with withdrawal at the abstinence visit relative to being in the NNC condition (Table 4, right panel).
Discussion
The original analysis of this sample (Donny et al., 2015) found no effect of switching to RNC cigarettes relative to NNC control cigarettes on total withdrawal scores, including peak total withdrawal during the first week, total withdrawal at Week 6, or total withdrawal following overnight smoking abstinence. Subsequently, with this sample, smoking RNC cigarettes were shown to reduce the association between negative affect (Positive and Negative Affect Scale) and smoking over time (Robinson et al., 2017). Our present analysis extends these findings by examining dose effects of nicotine in cigarettes on individual withdrawal symptoms over 6 weeks of RNC cigarette use and during a subsequent abstinence visit and by considering moderating factors (sex, nicotine dependence, and RNC non-adherence). As described in more detail below, we found that RNC cigarettes were rarely associated with withdrawal.
As hypothesized, and consistent with prior research (Buchhalter, et al., 2005; Donny & Jones, 2009), switching from NNC to RNC cigarettes for one week increased anger/irritability/frustration, restlessness, and increased appetite/weight gain. We did not detect an increase in difficulty concentrating in the RNC condition relative to NNC condition, which is consistent with some prior research (Baldinger, et al., 1995; Donny & Jones, 2009) but not all (Buchhalter, et al., 2005). There was no apparent dose-response effect of extent of nicotine reduction on severity of withdrawal. Specifically, depending on the symptom and timing, some elevated withdrawal was identified for each RNC cigarette with 1.3 mg/g nicotine or less. This pattern of findings is consistent with prior research of withdrawal supporting a threshold for nicotine dose on withdrawal, as opposed to dose-response relationship, after overnight smoking abstinence (Faulkner, et al., 2017).
Relative to those assigned to the NNC cigarettes, withdrawal was most evident for individuals who were biochemically-verified to be adherent to smoking 0.4 mg/g cigarettes (increased anger/irritability/frustration, increased appetite/weight gain), individuals who were less nicotine dependent (increased restlessness), and those assigned to smoke the RNC cigarettes with the lowest nicotine content with high tar yield (0.4 mg/g HT; increased anger/irritability/frustration, increased appetite/weight gain). Notably, relative to the NNC condition, withdrawal did not increase among individuals assigned to the 0.4 mg/g condition with normal tar. It is possible that tar level indirectly (e.g., through compliance) or directly (e.g., via altered sensory aspects of smoking) modulated withdrawal response. Nonetheless, in all instances of withdrawal, mean severity ratings were low (i.e., mean responses corresponded with a severity rating of “slight” on the MNWS) and, as hypothesized, the symptoms returned to baseline levels within the first several weeks of RNC cigarette use. Our longer observation period that prior research allowed us to determine for the first time that symptoms largely resolved within two weeks.
Depressed mood/sadness was the sole symptom that was elevated among RNC users over time. Specifically, depressed mood/sadness in the RNC users increased in severity over time relative to NNC control group and remained elevated at Week 6. The effect size was small-to-medium (Cohen’s d = .40), and mean Week 6 ratings of depressed mood/sadness for both NNC and the adherent RNC group corresponded with a severity rating of “slight” on the MNWS (NNC control mean = .54 SD = .88; adherent group mean = .95 SD = 1.11). A prior analysis of clinical depression symptoms in this sample found no effects of adherence or nicotine reduction (Tidey et al., 2016). Thus, the increase in depressed mood/sadness may not be clinically meaningful.
Additionally, a novel contribution of this study was an examination of the importance of nicotine relative to level of cigarette smoking and brand-switching on individual withdrawal symptoms over time. Angry/irritable/frustrated, anxious/nervous, and restlessness increased in the UB condition relative to the NNC condition over time, which suggests that switching cigarette brands can impact select withdrawal symptoms. Brand-switching could produce affective/behavioral changes similar to withdrawal, for instance due to brand-specific expectancies and/or differences in the taste, feel and sensory aspects of cigarette brands. By utilizing the RNC control condition in the primary analyses, however, the aforementioned withdrawal patterns can likely be attributed to effects of nicotine reduction.
To our knowledge, this is also the first investigation of the effects of extended RNC cigarette use on individual withdrawal symptoms after at least 18-hours of smoking abstinence. Our findings supported the hypothesis that extended RNC cigarette use reduces withdrawal symptoms during smoking abstinence. Specifically, particularly in the lowest nicotine condition relative to the NNC control, there was lower anger/irritability/frustration, desire/craving to smoke, and difficulty concentrating. Reduced withdrawal in the RNC conditions relative to NNC condition would be expected for a variety of reasons, including reductions in nicotine dependence due to RNC cigarette use (Donny et al., 2015) and extinction of smoking-related stimuli after repeated exposure to cigarettes with substantially reduced nicotine. In contrast, individuals who smoked their usual brand of cigarette reported higher scores on anxious/nervous, restlessness, desire/craving to smoke, angry/irritable/frustrated relative to the NNC control. Similarly, in the primary paper for this data (Donny et al., 2015), scores for craving from the 10-item Brief Questionnaire of Smoking Urges (QSU) during abstinence were significantly reduced (ps < .001) among participants who smoked cigarettes with 2.4 mg/g nicotine or less. These results suggest that more prolonged RNC cigarette use could result in reduced withdrawal associated with smoking abstinence, which is consistent with prior findings with total withdrawal scores in treatment-seeking smokers (Hatsukami, et al., 2010).
Sex and nicotine dependence did not moderate effects of RNC cigarettes on withdrawal. In prior research, smoking NNC or RNC cigarettes suppressed withdrawal similarly for women but differently for men (Barrett, 2010; Perkins & Karelitz, 2015). These differences have been attributed to the relative importance to women of sensory aspects of smoking, such as visual or olfactory cues, in smoking response (Perkins, Fonte, & Grobe, 2000; Perkins, Jacobs, Sanders, & Caggiula, 2002). As there were no sex differences in our large sample, men and women may experience similar withdrawal suppression when RNC cigarettes are smoked over an extended period of time in real-life settings. In general, FTND also did not generally moderate the effects of RNC cigarette use relative to NNC cigarette on withdrawal. An effect was only seen for restlessness: There was a greater increase in restlessness in the RNC condition relative to the NNC condition for those with lower baseline FTND scores (as opposed to higher FTND scores). In the context of this study, the effect may be partly explained by greater adherence to RNC cigarettes among less dependent than highly dependent smokers (Nardone, et al., 2016). While sex-related and dependence-related individual differences were largely not supported, significant individual variability in the intercept and slope terms remained in most of the trajectory models even after accounting for treatment condition. This individual variability suggests that future research may benefit from considering other unexamined factors that may moderate withdrawal response to nicotine reduction.
This study had several limitations. Adherence with study cigarettes could not be guaranteed because UB cigarettes were available in day-to-day life. Adherence was actively encouraged, but widespread non-adherence (Nardone, et al., 2016) likely underestimated effects of RNC cigarettes on withdrawal. On average, TNE levels were reduced by 60-70% in the RNC users relative to the NNC users, but much greater nicotine reduction would be expected if participants fully complied. Thus, the results are encouraging, but not definitive in terms of what levels of withdrawal might be experienced by smokers if alternative nicotine sources were not used. While we attempted to address this limitation by examining withdrawal levels among those in the lowest nicotine condition who demonstrated biomarker-validated adherence assessed at Weeks 2 and 6, this approach suffers from a likely self-selection bias that could underestimate withdrawal relative to a regulatory climate where only RNC cigarettes are available.
Another limitation is that our self-report measures were subject to recall bias, which could be reduced in future research by incorporating objective withdrawal measures. For instance, we found no differences in self-reported appetite/weight gain between adherent and NNC control groups at Week 6; however, a prior analysis of this sample that focused on actual weight gain found that adherent smokers gained significantly more weight than the control group, indicating that participants may have under-reported this symptom (Rupprecht, et al., 2016). Lastly, we relied on single item measures for each symptom—an approach that has been used in prior research. While this approach supported differential effects of nicotine reduction on individual symptoms over time, this approach can reduce the ability to find significant effects if a symptom is not measured reliably. Reliance on individual items, for instance could explain why we found effects on craving at the abstinence visit in the 0.4 mg/g (marginal) and 1.3 mg/g groups only, but the primary paper that used the multi-item QSU (Donny et al., 2015) supported significant effects on craving were supported for all groups with 2.4 mg/g nicotine or less. We minimized this limitation analytically by using repeatedly-measured symptoms to construct latent variables that account for measurement error. Future psychometric research could help to determine if specific withdrawal symptoms cluster together and change similarly over time during nicotine reduction.
These limitations notwithstanding, our findings in non-treatment seeking smokers suggest that lowering the nicotine content in cigarettes to a minimally-addictive level, to reduce nicotine dependence, does not result in severe or protracted nicotine withdrawal. A reduced-nicotine standard for cigarettes would likely not lead to a complete and immediate switching to only RNC cigarettes; rather, people would likely use available NNC cigarettes that they hoarded along with RNC cigarettes, or might use other available nicotine sources (e.g., nicotine replacement therapy, e-cigarettes). Indeed, prior research suggests that withdrawal experienced with RNC cigarettes might be suppressed with other sources of nicotine, such as the nicotine patch (Donny & Jones, 2009; Hatsukami, et al., 2013b). Additional research is warranted to determine withdrawal response to RNC cigarettes when provided with alternative nicotine products.
Supplementary Material
Public Significance Statements.
We found no evidence in non-treatment seeking smokers that lowering the nicotine content in cigarettes to a less-addictive level would result in severe or protracted nicotine withdrawal.
Acknowledgments
We thank all the students, fellows, and staff members at the Center for the Evaluation of Nicotine in Cigarettes who were involved in this study and those who manufactured and characterized the investigational tobacco products used in the study.
Funding: Research reported in this publication was supported by the National Institute on Drug Abuse and FDA Center for Tobacco Products (CTP)(U54 DA031659) and a postdoctoral fellowship awarded to SSD from the Canadian Institute of Health Research (MFE-33926). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Footnotes
Contributors statement: All authors contributed in a significant way to the manuscript and have read and approved the final manuscript.
Declaration of interests: The authors have no conflicts of interest to report.
Prior dissemination of the data: Portions of the data presented in this manuscript were previously presented by SSD in a symposium at the 2017 Society for Research on Nicotine and Tobacco research meeting.
References
- Baldinger B, Hasenfratz M, Bättig K. Effects of smoking abstinence and nicotine abstinence on heart rate, activity and cigarette craving under field conditions. Human Psychopharmacology: Clinical and Experimental. 1995;10(2):127–136. doi: 10.1002/hup.470100207. [DOI] [Google Scholar]
- Barrett SP. The effects of nicotine, denicotinized tobacco, and nicotine-containing tobacco on cigarette craving, withdrawal, and self-administration in male and female smokers. Behavioural pharmacology. 2010;21(2):144–152. doi: 10.1097/FBP.0b013e328337be68. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiology Biomarkers & Prevention. 2012;21(5):761–769. doi: 10.1158/1055-9965.epi-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiology Biomarkers & Prevention. 2007;16(11):2479–2485. doi: 10.1158/1055-9965.epi-07-0393. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. The New England Journal of Medicine. 1994;331(2):123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100(4):550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Schrinel L, Eissenberg T. Withdrawal-suppressing effects of a novel smoking system: comparison with own brand, not own brand, and denicotinized cigarettes. [Article] Nicotine & Tobacco Research. 2001;3(2):111–118. doi: 10.1080/14622200110042636. [DOI] [PubMed] [Google Scholar]
- Family Smoking Prevention and Tobacco Control Act. 2009 [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine & Tobacco Research. 1999;1(1):11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Curran PJ, Obeidat K, Losardo D. Twelve frequently asked questions about growth curve modeling. Journal of Cognition and Development. 2010;11(2):121–136. doi: 10.1080/15248371003699969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger RL, Benowitz NL, Murphy SE, Hatsukami DK, Smith TT, Cwalina SN, Bennett LB, Goldstein EC, Scott C, Pacek LR, Colino CM, Donny EC. Asessing biomarkers of compliance to spectrum cigarettes using a hotel-based study protocol. Paper presented at the Society for Research on Nicotine and Tobacco; Philadelphia, PA. 2015. [Google Scholar]
- Donny EC, Denlinger RL, Tidey J, Koopmeiners JS, Benowitz NL, Vandrey RG, Hatsukami DK. Regulated reduction of nicotine in cigarettes: A randomized trial. The New England Journal of Medicine. 2015;373:1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102(2):324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug and Alcohol Dependence. 2009;104(1–2):23–33. doi: 10.1016/j.drugalcdep.2009.01.021. http://dx.doi.org/10.1016/j.drugalcdep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Walker N, Hatsukami D, Bullen C. Reducing the nicotine content of combusted tobacco products sold in New Zealand. Tobacco Control. 2016 doi: 10.1136/tobaccocontrol-2016-053186. [DOI] [PubMed] [Google Scholar]
- Faulkner P, Ghahremani DG, Tyndale RF, Cox CM, Kazanjian AS, Paterson N, Vigil C. Reduced-Nicotine Cigarettes in Young Smokers: Impact of Nicotine Metabolism on Nicotine Dose Effects. Neuropsychopharmacology. 2017;42(8):1610–1618. doi: 10.1038/npp.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. FDA announces comprehensive regulatory plan to shift trajectory of tobacco-related disease, death. 2017 Retrieved August 8, 2017. [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology. 2004;175(2):134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, Roper-Batker AN, Mackowick KM, Donny E. Dose–response effects of spectrum research cigarettes. Nicotine & Tobacco Research. 2013a;15(6):1113–1121. doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Hertsgaard LA, Vogel RI, Jensen JA, Murphy SE, Hecht SS, Allen SS. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiology Biomarkers & Prevention. 2013b;22(6):1015–1024. doi: 10.1158/1055-9965.epi-12-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Stepanov I. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. [Randomized Controlled Trial Research Support, N.I.H., Extramural] Addiction. 2010;105(2):343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus. Seventh. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Nardone N, Donny EC, Hatsukami DK, Koopmeiners JS, Murphy SE, Strasser AA, Benowitz NL. Estimations and predictors of non-compliance in switchers to reduced nicotine content cigarettes. Addiction. 2016;111(12):2208–2216. doi: 10.1111/add.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K, Fonte C, Grobe J. Sex differences in the acute effects of cigarette smoking on the reinforcing value of alcohol. [Clinical Trial Randomized Controlled Trial Research Support, U.S. Gov’t, P.H.S.] Behavioural pharmacology. 2000;11(1):63–70. doi: 10.1097/00008877-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. [Comparative Study Research Support, U.S. Gov’t, P.H.S.] Psychopharmacology. 2002;163(2):194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Sex Differences in Acute Relief of Abstinence-Induced Withdrawal and Negative Affect due to Nicotine Content in Cigarettes. Nicotine & Tobacco Research. 2015;17(4):443–448. doi: 10.1093/ntr/ntu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Nelson RA, Rohrer MS, Fant RV, Henningfield JE. Pharmacodynamic effects of new denicotinized cigarettes. Nicotine & Tobacco Research. 1999;1(4):357–364. doi: 10.1080/14622299050011491. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Kypriotakis G, Karam-Hage M, Green CE, Hatsukami DK, Cinciripini PM, Donny EC. Cigarette Nicotine Content as a Moderator of the Relationship Between Negative Affect and Smoking. Nicotine & Tobacco Research. 2017 doi: 10.1093/ntr/ntx068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacology Biochemistry and Behavior. 2000;67(1):71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Rupprecht LE, Koopmeiners JS, Dermody SS, Oliver JA, al’Absi M, Benowitz NL, Donny EC. Reducing nicotine exposure results in weight gain in smokers randomised to very low nicotine content cigarettes. Tobacco Control. 2016 doi: 10.1136/tobaccocontrol-2016-053301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Donny EC, Koopmeiners JS, Benowitz NL, Cinciripini P, Dermody SS, Drobes DJ, Luo X, McClernon FJ, Pacek LR, Vandrey R, Hatsukami DK. Baseline nicotine dependence moderates the impact of nicotine reduction on changes in nicotine dependence. Paper presented at the Society for Research on Nicotine and Tobacco Annual Meeting; Florence, Italy. 2017. Poster presentation retrieved from. [Google Scholar]
- Tidey JW, Pacek LR, Koopmeiners JS, Vandrey R, Nardone N, Drobes DJ, Denlinger RL. Effects of 6-week use of reduced-nicotine content cigarettes in smokers with and without elevated depressive symptoms. Nicotine & Tobacco Research. 2016:ntw199. doi: 10.1093/ntr/ntw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, AhnAllen CG. Separate and Combined Effects of Very Low Nicotine Cigarettes and Nicotine Replacement in Smokers with Schizophrenia and Controls. Nicotine & Tobacco Research. 2013;15(1):121–129. doi: 10.1093/ntr/nts098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services. The Health Consequences of Smoking—50Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. http://www.cdc.gov/tobacco/data_statistics/sgr/50thanniversary/index.htm (accessed June 2015) [Google Scholar]
- USDHSS. Nicotine addiction: A report of the surgeon general. Rockville Maryland: USDHSS; 1988. [Google Scholar]
- Walker N, Howe C, Bullen C, Grigg M, Glover M, McRobbie H, Whittaker R. The combined effect of very low nicotine content cigarettes, used as an adjunct to usual Quitline care (nicotine replacement therapy and behavioural support), on smoking cessation: a randomized controlled trial. Addiction. 2012;107(10):1857–1867. doi: 10.1111/j.1360-0443.2012.03906.x. [DOI] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacology, Biochemistry and Behavior. 1996;53(2):309–315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Zeller M, Hatsukami D. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the US. [Consensus Development Conference Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Tobacco Control. 2009;18(4):324–332. doi: 10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


